Abstract
Giardia duodenalis is the most prevalent intestinal protozoan infection especially in children. In Portugal scarce data are available relative to this infection in preschoolers. The present study was conducted from April to July 2009 in public preschools in Lisbon enrolling 316 children. Stool examination was performed through microscopy. Molecular analysis was conducted in all positive samples for G. duodenalis in order to determine the assemblage and subassemblage of this parasite. Eight of the preschoolers studied children (2.5%, 8/316) were infected with G. duodenalis. Additionally the brother of one of the infected children was also infected. Genotyping analysis targeting ssu-rRNA and β-giardin loci revealed six infections with assemblage A and 3 with assemblage B. Sub-assemblage determination was possible in four of the samples, with three A2 and one A3. The limited number of cases precluded an association of a determined symptom with an assemblage. The data presented here show the relevance of considering G. duodenalis analysis in children with intestinal complaints even in developed countries.
1. Introduction
Giardiasis is a widespread intestinal disease caused by Giardia duodenalis. This protozoan parasite has a global distribution, infecting humans and a wide range of mammalian hosts [1]. The prevalence of giardiasis in humans in developed countries is 2–7% [2], while it may vary between 20 and 30% in developing countries [3].
The spectrum of clinical manifestations in human giardiasis is relatively variable, ranging from the absence of symptoms to acute or chronic diarrhea, dehydration, abdominal pain, nausea, vomiting, and weight loss [4]. Children are especially affected, with more severe consequences than adults. However, the impact of Giardia infection in children development is not clear. Some studies showed detrimental effects on nutritional status and poorer cognitive function on children with giardiasis [5–8], while others showed that giardiasis did not affect childhood growth [9]. Host factors, such as immune status, nutritional status, and age, are recognized as important determinants for the severity of infection [10]. However, studies on the possible association between G. duodenalis assemblages and the severity of the disease have proved thus far to be inconsistent [11].
Molecular tools have demonstrated that G. duodenalis is a species complex comprising at least eight assemblages (A–H), among which only A and B were found infecting humans [12].
Previous studies focused on the prevalence of giardiasis in preschool children (3-4%) [13, 14], on G. duodenalis assemblages determination in humans [13, 15], animals, and water [15, 16], and, more recently, on prevalence and risk factors for G. duodenalis infection among children [17].
The aim of this study was to determine whether there was any G. duodenalis in preschool children enrolled, the assemblages and their relation to clinical data from the city of Lisbon.
2. Material and Methods
2.1. Study Design, Population, and Sample
A cross-sectional study was conducted from April to July 2009. The population in study were all preschoolers (3306 children) attending that year the public preschools under the supervision of the Lisbon City Hall. The selection of the kindergartens where the study was conducted was decided by the Lisbon City aiming at reflecting a wider socioeconomic of the families and geographic dispersion of the children. The number of children involved was of 685.
A total of 316 (46.1%, 316/685) preschool children, aged 3–6, attending the selected kindergartens of the network of public schools, were enrolled in this study. All children in the schools were invited to participate. The enrolled children were those whose parents collected the stool samples and signed the informed consent.
2.2. Sample Collection
A meeting was held with the director of each school as well as with the parents to explain the study objectives, prior to sample collection.
The stool containers were delivered to the parents or guardians on a Friday. The parents/guardians were told to collect three stool samples in consecutive days without any pharmacologic induction and stored at 4°C till Monday morning, when the team went to the schools to collect all the samples.
2.3. Microscopy
The fresh stool samples were screened for G. duodenalis and other intestinal parasites through microscopic analysis in saline and also in iodine. Furthermore, the formolether concentration method was also performed to increase the sensitivity of the detection. All samples were screened by three different microscopists, for cross-check results. Positive samples for G. duodenalis were kept in filter paper (Generation Card Kit, Qiagen) and preserved at −20°C for further analysis.
2.4. DNA Extraction and PCR Amplification
DNA was extracted from samples preserved in filter paper. A DNA extraction protocol for dried blood spots (Generation Capture Card Kit, Qiagen) was adapted for stool samples. Changes to the original protocol included tripling the solution volumes used, except for the final elution step (100 μL) [18]. For some samples, DNA was reextracted using elution volumes of 25 μL. All samples were amplified using primers targeting the small subunit ribosomal RNA (ssu-rRNA) [19, 20] and β-giardin (bg) loci [21, 22].
Amplification reactions were performed using 2 μL of DNA template in a final volume of 25 μL, using illustra PuReTaq Ready-To-Go PCR beads (GE Healthcare, UK). Both positive (DNA isolated from the Portland-1 strain (ATCC 30888DLGC Promochem) and negative controls (no template added) were included in each series of PCR reactions. PCR products were visualized on 2% agarose gel stained with ethidium bromide.
2.5. Sequence Analysis
For sequence analysis, PCR products were purified using JETQUICK Gel Extraction Spin Kit/50 (Genomed, Germany) according to the manufacturer's instructions. DNA sequencing reactions were carried out in both directions using primers GiarF/GiarR for ssu-rRNA gene fragment (175 bp) [20] and those described previously [22] for β-giardin gene fragment (511 bp). Sequences obtained in this study were aligned with previously published sequences of G. duodenalis isolates available in the GenBank database, using ClustalW.
2.6. Clinical Data and Treatment
A questionnaire for clinical data relative to the children was filled by each participant's parent or guardian.
All children infected with G. duodenalis were assisted by a paediatric doctor from the Tropical Diseases Clinic of the Institute of Hygiene and Tropical Medicine and treated for the infection with 15 mg/kg/day of metronidazole divided in three daily doses, for seven days. Three weeks after the treatment new stool samples were obtained in order to confirm the treatment efficacy. If a child was infected with any intestinal parasite, the remaining members of the household were invited to collect their stools that were also screened for intestinal parasites.
2.7. Ethical Considerations
The present study was submitted and approved by the Ethical Committee of the Institute of Hygiene and Tropical Medicine, Lisbon. Written informed consent was obtained from all parents or the legal guardians of the children participating in the study.
3. Results
3.1. Parasitological Results
From the 316 children participating in this study, 166 (52.5%) were males and 150 (47.5%) were females. The mean age was 5.03, ranging between three and six years old. G. duodenalis was the only pathogenic parasite found in the faeces. G. duodenalis cysts were found in 8 out of the 316 samples examined by microscopy (2.5%), corresponding to four of the schools included in this work. Furthermore one family member (brother), from one of the infected children, was also infected with G. duodenalis.
3.2. Genotyping Characterization
The nine positive samples for G. duodenalis were successfully amplified for ssu-rRNA fragment gene, and for the bg gene only seven samples were amplified (77.8%, 7/9) (Figures 1 and 2).
Figure 1.
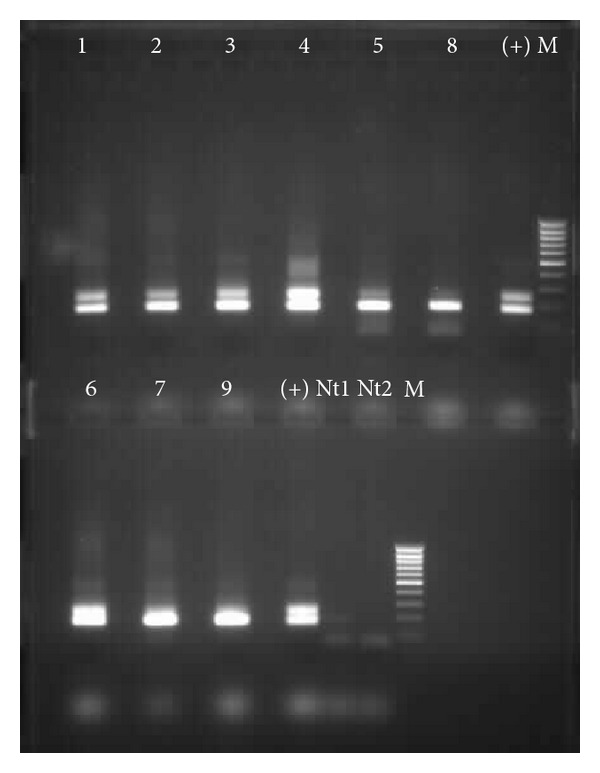
Electrophoretic separation of ssu-rRNA PCR products (175 bp). Lanes 1–9, G. duodenalis positive samples through microscopy; lanes (+), positive control (G. duodenalis DNA, strain Portland-1, ATCC 30888DTM LGC Promochem); lanes M, 100 bp ladder; lanes Nt1 and Nt2, negatives controls (no DNA) from the first and the second PCR reaction, respectively.
Figure 2.

Electrophoretic separation of β-giardin PCR products (511 bp). Lanes 1–9, G. duodenalis positive samples through microscopy; lanes (+), positive control (G. duodenalis DNA, strain Portland-1, ATCC 30888DTM LGC Promochem); lanes M, 100 bp ladder; lanes Nt1 and Nt2, negatives controls (no DNA) from the first and the second PCR reaction, respectively.
Ssu-rRNA sequences obtained in this study were compared with homologous sequences found in GenBank using BLAST. Six samples belong to assemblage A and three to B (Table 1). Sequences obtained for bg gene were also compared with public sequences from GenBank using BLAST. Additionally, bg sequences were analysed for subassemblage discrimination according to the genetic polymorphisms described elsewhere [23]. Isolates 3, 4 and 5 belong to subassemblage A2, while the other isolate (6) belongs to subassemblage A3 (Table 1).
Table 1.
Clinical data and G. duodenalis assemblages of the infected children.
| Case | Age (years) | School | Present symptoms | Medical examination | Assemblages | |
|---|---|---|---|---|---|---|
| ssu | bg | |||||
| 1 | 5.8 | Musgueira (JI77) | Lack of appetite | Abdominal distension | B | NA |
| 2 | 4.8 | Horta Nova | No symptoms observed | Abdominal distension | A | NA |
| 3 | 6.0 | Horta Nova | Flatulence | Normal | A | A2 |
| 4* | 9.5 | Horta Nova | Abdominal pain, lack of appetite, and flatulence | Pain on deep palpation and abdominal distention | A | A2 |
| 5 | 6.3 | Alto da Faia | Abdominal pain, lack of appetite | Abdominal distension | A | A2 |
| 6 | 6.3 | Alto da Faia | No symptoms observed | Normal | A | A3 |
| 7 | 3.9 | Ameixoeira | No symptoms observed | Normal | B | B** |
| 8 | 5.8 | Ameixoeira | No symptoms observed | Normal | B | B** |
| 9 | 4.3 | Ameixoeira | No symptoms observed | Abdominal distension | A | NI |
*This children was a family member (brother) of participant 3.
**It was not possible to subtype assemblage B due to high level of polymorphism observed.
NA: not amplified.
NI: not identified. Although successfully amplified for bg gene, this sample did not present enough quality for sequentiation.
For the remaining two isolates, 7 and 8, belonging to assemblage B, it was not possible to determine the respective subassemblage due to the high nucleotide variability observed in the chromatogram.
New stool samples were collected from all infected children after the complete treatment and analyzed. No parasite was detected by microscopy.
3.3. Clinical Data
At the time of the stool sample collection only four children reported symptoms (1, 3, 4, 5), including lack of appetite, abdominal pain, and flatulence (Table 1).
Case number 9, the one with intermittent diarrhea, was the only case of moderate malnutrition (BMI 12; −2 < z score < −3).
The medical examination was normal in four children (3, 6, 7, and 8). Abdominal distension and pain on deep palpation were observed in the remaining five (Table 1).
3.4. Genetic Assemblage and Clinical Presentation
From the three children infected with G. duodenalis assemblage B, two presented a normal medical examination with no symptoms (7 and 8), while the other one (1) presented abdominal distension and referred lack of appetite as a symptom. For the six children infected with assemblage A, two had a normal medical examination (3 and 6) and three referred no symptoms (2, 6, and 9). All these results are described in Table 1.
4. Discussion
The number of children found infected with G. duodenalis in this study (8/316; 2.5%) was similar to other studies conducted in Northern and Centre of Portugal where 3%, 3.7%, and 1.9%, respectively [13, 14, 17] were infected. These results are in agreement with the reported prevalence for this parasite in developed countries [12].
The use of microscopy as the only diagnostic procedure for detecting G. duodenalis may be considered as a limitation of this study. However, the use of three stool samples allow the detection of over 90% of infection [24], which is very similar to the sensitivities recently reported for rapid diagnostic tests [25], while one stool sample will allow the detection of 60 to 80%, and the analysis of two stool samples will allow the detection of 80 to 90% [24]. In this study at least two stool samples were obtained from all the children and three samples for more than 80% of the enrolled children. Furthermore microscopy has the additional advantage of allowing the detection of other intestinal parasites [26].
In our study assemblage A was more frequent, with six isolates, while assemblage B was detected in three children, which is the opposite pattern reported worldwide with assemblage B appearing more common [11, 12]. Subassemblage determination was only possible for assemblage A positive samples, as B samples were impossible to subtype due to the presence of double peaks at specific position in the chromatogram. The difficulty of subtyping assemblage B has been reported [18, 27]. The importance of being able to subtype is especially relevant when a source of infection must be traced or when a distinction between reinfection/new infection is mandatory for therapeutic control. For instance, in this study, two brothers were infected with the same subassemblage (A2) of G. duodenalis, suggesting a common source of infection.
Another interesting data obtained from this work was the finding of a child (case 9) with malnutrition (moderate), which is a common situation in developing countries [6]. This child also corresponds to the only one complaining of intermittent diarrhea. In this case chronic infection could have contributed to the child nutritional status.
While there has been a growing interest in the molecular characterization of G. duodenalis, there is still a lack of clear association between the assemblage and the clinical outcome, with contradictory results. A study conducted in the Netherlands found a strong correlation between assemblage A and mild, intermittent diarrhea and assemblage B with severe and persistent diarrhea [10]. Other studies conducted in Ethiopia and Saudi Arabia also suggest a correlation between the presence of symptoms and infection with the assemblage [28, 29]. On the other hand, a study performed in Australia revealed that children infected with G. duodenalis isolates from assemblage A were 26 times more likely to have diarrhea than children with assemblage B [20]. Supporting these results other works in Bangladesh and Spain also showed a statistical association between assemblage A and symptomatic infections and between assemblage B and asymptomatic infections [30, 31]. In what concerns to Portugal, the data obtained by Sousa et al. [15] supports that G. duodenalis belonging to assemblage A is strongly associated with symptomatic cases. In agreement with this, other work revealed a higher prevalence of assemblage B in asymptomatic children [13]. None of the children studied presented diarrhea at the moment of the stool sample collection. However, of the four symptomatic children at enrolment, three were infected with A2 subassemblage with more evident symptoms when compared with the other symptomatic children carrying B assemblage.
5. Conclusions
This study contributed with new and relevant data for the epidemiology of giardiasis in Portugal and challenged the pattern of assemblage B being more frequent than assemblage A, eventually suggesting that a different epidemiological profile is detected in developed countries. The fact that a brother from an infected child was also infected reinforced the importance of when testing a child or a family member; if positive, then the entire household should be screened. The study did not show an association between the clinical pattern observed and the assemblages. The low number of children infected with G. duodenalis does not justify the need for consistent request of stool parasitological analysis. However, special attention should be given to children reporting abdominal complains, considering that 4/9 of the infected children were symptomatic.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgments
The authors thank Rosalia Vargas, deputy mayor for education of the Lisbon City Hall, for allowing and supporting this work; all head directors of the participating kindergartens for their support; and Laura Cravo for technical assistance.
References
- 1.Thompson RCA, Monis PT. Variation in Giardia: implications for taxonomy and epidemiology. Advances in Parasitology. 2004;58:69–137. doi: 10.1016/S0065-308X(04)58002-8. [DOI] [PubMed] [Google Scholar]
- 2.Furness BW, Beach MJ, Roberts JM. Giardiasis surveillance—United States, 1992–1997. Morbidity and Mortality Weekly Report. 2000;49(7):1–13. [PubMed] [Google Scholar]
- 3.Ortega YR, Adam RD. Giardia: overview and update. Clinical Infectious Diseases. 1997;25(3):545–550. doi: 10.1086/513745. [DOI] [PubMed] [Google Scholar]
- 4.Eckmann L. Mucosal defences against Giardia . Parasite Immunology. 2003;25(5):259–270. doi: 10.1046/j.1365-3024.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 5.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359(9306):564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 6.Botero-Garcés JH, García-Montoya GM, Grisales-Patiño D, Aguirre-Acevedo DC, Álvarez-Uribe MC. Giardia intestinalis and nutritional status in children participating in the complementary nutrition program, Antioquia, Colombia, May to October 2006. Revista do Instituto de Medicina Tropical de Sao Paulo. 2009;51(3):155–162. doi: 10.1590/s0036-46652009000300006. [DOI] [PubMed] [Google Scholar]
- 7.Newman RD, Moore SR, Lima AAM, Nataro JP, Guerrant RL, Sears CL. A longitudinal study of Giardia lamblia infection in north-east Brazilian children. Tropical Medicine and International Health. 2001;6(8):624–634. doi: 10.1046/j.1365-3156.2001.00757.x. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Intestinal Parasites Control: Burden and Trends. Geneva, Switzerland: WHO Division of Control of Tropical Diseases, World Health Organization; 1998. [Google Scholar]
- 9.Hollm-Delgado MG, Gilman RH, Bern C, et al. Lack of an adverse effect of Giardia intestinalis infection on the health of Peruvian children. American Journal of Epidemiology. 2008;168(6):647–655. doi: 10.1093/aje/kwn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Homan WL, Mank TG. Human giardiasis: genotype linked differences in clinical symptomatology. International Journal for Parasitology. 2001;31(8):822–826. doi: 10.1016/s0020-7519(01)00183-7. [DOI] [PubMed] [Google Scholar]
- 11.Cacciò SM, Ryan U. Molecular epidemiology of giardiasis. Molecular and Biochemical Parasitology. 2008;160(2):75–80. doi: 10.1016/j.molbiopara.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Yaoyu F, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clinical Microbiology Reviews. 2011;24(1):110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almeida AA, Delgado ML, Soares SC, et al. Genotype analysis of Giardia isolated from asymptomatic children in northern Portugal. Journal of Eukaryotic Microbiology. 2006;53(1):S177–S178. doi: 10.1111/j.1550-7408.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 14.Sarmento A, Costa JM, Valente CAP, Teixeira ME. Infecção por parasitas intestinais na população pediátrica. Acta Pediátrica Portuguesa. 2004;35:307–311. [Google Scholar]
- 15.Sousa MC, Morais JB, Machado JE, Poiares-Da-Silva J. Genotyping of Giardia lamblia human isolates from Portugal by PCR-RFLP and sequencing. Journal of Eukaryotic Microbiology. 2006;53(1):S174–S176. doi: 10.1111/j.1550-7408.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira FS, Pereira-Baltasar P, Parreira R, et al. Intestinal parasites in dogs and cats from the district of Évora, Portugal. Veterinary Parasitology. 2011;179(1–3):242–245. doi: 10.1016/j.vetpar.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Júlio C, Vilares A, Oleastro M, et al. Prevalence and risk factors for Giardia duodenalis infection among children: acase study in Portugal. Parasites and Vectors. 2012;5(1, article 22) doi: 10.1186/1756-3305-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira FS, Centeno-Lima S, Gomes J, et al. Molecular characterization of Giardia duodenalis in children from the Cufada Lagoon Natural Park, Guinea-Bissau. Parasitology Research. 2012;111(5):2173–2177. doi: 10.1007/s00436-012-3068-6. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins RM, Meloni BP, Groth DM, Wetherall JD, Reynoldson JA, Thompson RCA. Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. Journal of Parasitology. 1997;83(1):44–51. [PubMed] [Google Scholar]
- 20.Read C, Walters J, Robertson ID, Thompson RCA. Correlation between genotype of Giardia duodenalis and diarrhoea. International Journal for Parasitology. 2002;32(2):229–231. doi: 10.1016/s0020-7519(01)00340-x. [DOI] [PubMed] [Google Scholar]
- 21.Cacciò SM, De Giacomo M, Pozio E. Sequence analysis of the β-giardin gene and development of a polymerase chain reaction-restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. International Journal for Parasitology. 2002;32(8):1023–1030. doi: 10.1016/s0020-7519(02)00068-1. [DOI] [PubMed] [Google Scholar]
- 22.Lalle M, Jimenez-Cardosa E, Cacciò SM, Pozio E. Genotyping of Giardia duodenaiis from humans and dogs from Mexico using a β-giardin nested polymerase chain reaction assay. Journal of Parasitology. 2005;91(1):203–205. doi: 10.1645/GE-293R. [DOI] [PubMed] [Google Scholar]
- 23.Cacciò SM, Beck R, Lalle M, Marinculic A, Pozio E. Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. International Journal for Parasitology. 2008;38(13):1523–1531. doi: 10.1016/j.ijpara.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Gardner TB, Hill DR. Treatment of giardiasis. Clinical Microbiology Reviews. 2001;14(1):114–128. doi: 10.1128/CMR.14.1.114-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swierczewski B, Odundo E, Ndonye J, Kirera R, Odhiambo C, Oaks E. Comparison of the triage micro parasite panel and microscopy for the detection of Entamoeba histolytica/Entamoeba dispar, Giardia lamblia, and Cryptosporidium parvum in stool samples collected in Kenya. Journal of Tropical Medicine. 2012;2012:5 pages. doi: 10.1155/2012/564721.564721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuurman T, Lankamp P, van Belkum A, Kooistra-Smid M, van Zwet A. Comparison of microscopy, real-time PCR and a rapid immunoassay for the detection of Giardia lamblia in human stool specimens. Clinical Microbiology and Infection. 2007;13(12):1187–1191. doi: 10.1111/j.1469-0691.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 27.Bonhomme J, Le Goff L, Lemée V, Gargala G, Ballet JJ, Favennec L. Limitations of tpi and bg genes sub-genotyping for characterization of human Giardia duodenalis isolates. Parasitology International. 2011;60(3):327–330. doi: 10.1016/j.parint.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Gelanew T, Lalle M, Hailu A, Pozio E, Cacciò SM. Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Tropica. 2007;102(2):92–99. doi: 10.1016/j.actatropica.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Al-Mohammed HI. Genotypes of Giardia intestinalis clinical isolates of gastrointestinal symptomatic and asymptomatic Saudi children. Parasitology Research. 2011;108(6):1375–1381. doi: 10.1007/s00436-010-2033-5. [DOI] [PubMed] [Google Scholar]
- 30.Haque R, Roy S, Kabir M, Stroup SE, Mondal D, Houpt ER. Giardia assemblage A infection and diarrhea in Bangladesh. Journal of Infectious Diseases. 2005;192(12):2171–2173. doi: 10.1086/498169. [DOI] [PubMed] [Google Scholar]
- 31.Sahagún J, Clavel A, Goñi P, et al. Correlation between the presence of symptoms and the Giardia duodenalis genotype. European Journal of Clinical Microbiology and Infectious Diseases. 2008;27(1):81–83. doi: 10.1007/s10096-007-0404-3. [DOI] [PubMed] [Google Scholar]


