Abstract
Several recent reports have identified TET1 as the main enzyme modulating DNA methylation and gene transcription via hydroxylation of 5-methylcytosine. However, little is known about the protein network that controls TET1 activity. By using a new proximity ligation in situ assay, we identified MeCP2, HDAC1/6/7, EZH2, mSin3A, PCNA, and LSD1 as TET1-interacting proteins. We also discerned that TET1/PCNA acts as a demethylator of the cyclical methylation/demethylation process, the perturbation of which promotes the aberrant methylation hallmarks frequently observed in cancer cells.
Keywords: DNA methylation, demethylation, TET1, glioma
Introduction
Methylcytosine dioxygenase TET1 is an enzyme participating in DNA demethylation by catalyzing the conversion of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC).1,2 Like many proteins involved in chromatin and DNA modification, TET1 contains a CXXC zinc finger domain. However, the CXXC domain of TET1 has no DNA binding activity and is dispensable for its catalytic activity in vivo.3 This implies that other proteins are involved in the DNA binding of TET1, a necessary step to promote the conversion of methylcytosine to 5-hmC. To identify these proteins, we used a recently described assay based on a proximity ligation in situ assay (P-LISA), which has allowed us to visualize and quantify the in vitro Dnmt1/PCNA interaction.4-6 Then, we observed that the couple TET1/partner has a nonredundant role in the epigenetic regulation of genes. We also noted that TET1/PCNA acts as a demethylator of the cyclical methylation/demethylation process.
Results/Discussion
Because TET1 is instrumental in the conversion of 5mC to 5hmC, we postulated that it was preferentially associated to genome regions enriched in 5mC and thus could interact with proteins that bind methylated DNA.7,8 To address this thesis, we have taken a candidate approach and investigated whether TET1 could form complexes with proteins, binding fully methylated DNA such as MBD1, MeCP2, or MBD4 or hemimethylated DNA such as UHRF1. More generally, we also examined whether TET1 could form complexes with protein-binding chromatin such as the EZH2, Sin3A, HDAC1, HDAC2, HDAC6, and LSD1 proteins.
For this purpose, we performed 3 sets of experiments: 1) P-LISA to analyze stable and transient interactions (very close proximity <40 nm) at endogenous protein levels directly in situ, 2) co-immunoprecipitation (Co-IP) to analyze the co-presence of 2 proteins in the same protein complex, and 3) pull-down to analyze the direct interaction of 2 proteins in an acellular assay.
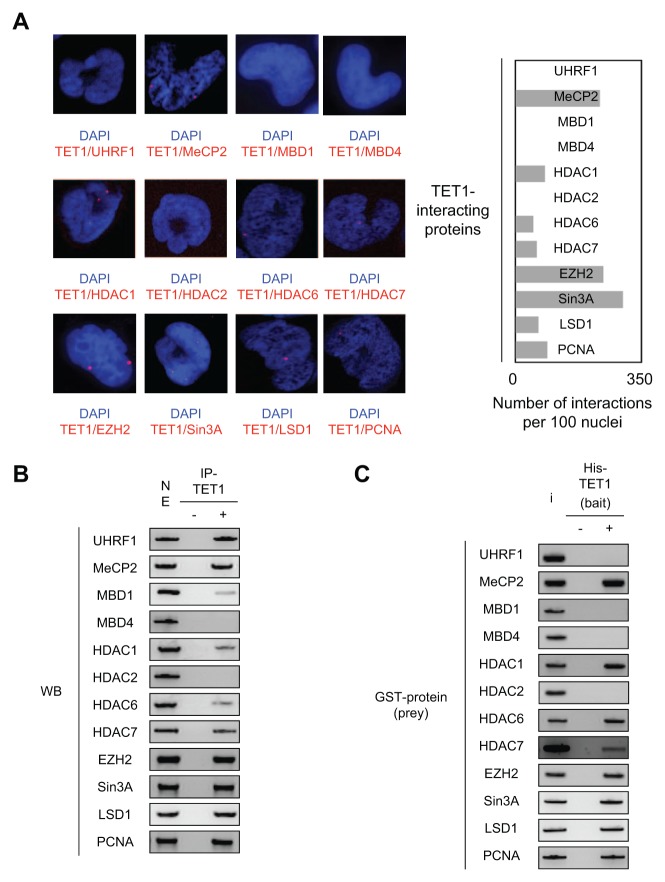
After validation of the TET1 antibody in P-LISA (Suppl. Fig. S1), we observed that TET1 interacts or is in close proximity with HDAC1, HDAC6, HDAC7, LSD1, EZH2, MeCP2, PCNA, and Sin3A but not with UHRF1, MBD1, MBD4, and HDAC2 (Fig. 1A). Quantification of these interactions suggests that TET1/mSin3A, TET1/MeCP2, and TET1/EZH2 are the most frequent and important observed interactions (Fig. 1A).
Figure 1.
Description of TET1-interacting proteins. (A) Monitoring of TET1-interacting proteins in U251 cells by using P-LISA. The nucleus appears in blue after DAPI coloration, and each red dot represents one interaction between TET1 and the considered protein. For each interaction, we quantified the number of interactions by using the freeware BlobFinder. Each bar on the graph represents the mean ± SD of considered interactions quantified from 100 nuclei in 3 independent experiments. (B) Monitoring of TET1-interacting proteins in U251 cells by using the immunoprecipitation (IP) method. “NE” represents gel loading with 50% of the nuclear extract used to perform the IP of TET1. “–” indicates the IP performed in the absence of the TET1 antibody, and “+” indicates the IP performed in the presence of the TET1 antibody. Gel pictures are representative of 3 independent experiments. (C) Monitoring of TET1-interacting proteins by using the pull-down method. The His-tag TET1 recombinant protein was used as bait, while indicated glutathione S-transferase proteins were used as prey in the pull-down assay. “I” (input) represents the deposit of 30% of the total amount of protein used in the considered assay. “–” indicates the pull-down performed in the absence of the His-tag TET1 recombinant protein, and “+” indicates the pull-down performed in the presence of the His-tag TET1 recombinant protein. Gel pictures are representative of 3 independent experiments.
Co-IP indicated that TET1 was co-immunoprecipitated with HDAC1, HDAC6, HDAC7, LSD1, EZH2, PCNA, Sin3A, UHRF1, MeCP2, and MBD1 but not with MBD4 and HDAC2 (Fig. 1B). Pull-down indicated that TET1 directly interacted with HDAC1, HDAC6, HDAC7, LSD1, EZH2, MeCP2, PCNA, and Sin3A but not with UHRF1, MBD1, MBD4, and HDAC2 (Fig. 1C).
Thus, by using these 3 methods, our data support the idea that the MeCP2, HDAC1, HDAC6, HDAC7, EZH2, and Sin3A proteins directly interact with TET1 to form multiprotein complexes (since we observed positive results in P-LISA, Co-IP, and pull-down experiments); that UHRF1 and MBD1 proteins do not directly interact with TET1 but form multiprotein complexes with TET1 (since we noted negative results in pull-down and P-LISA but positive results in Co-IP); and that the HDAC2 and MBD4 proteins do not directly interact with TET1 and do not form protein complexes with TET1 (since we noted negative results in P-LISA, Co-IP, and pull-down experiments).
By reporting that TET1 can form multiprotein complexes with a large panel of chromatin-binding proteins, our data introduce the idea that each TET1-including complex could be implicated in the epigenetic regulation of a specific group of genes. To investigate this point, we focused our study on TET1/EZH2 and TET1/Sin3A to determine whether these TET1-including complexes are redundant for the epigenetic regulation of 2 TET1-targeted genes: NES1 and HOXD12.9,10
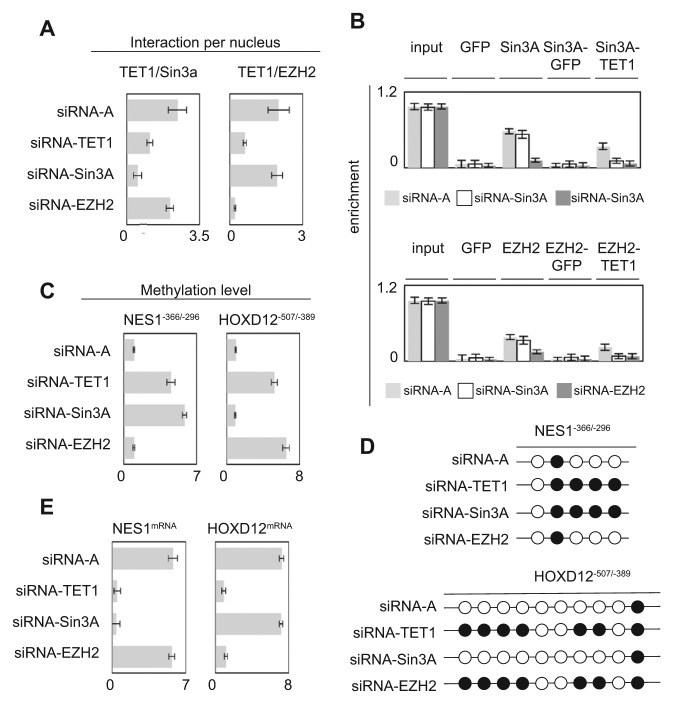
To examine this issue, we used U251 cells presenting a low level of Sin3A or EZH2 induced by the down-regulation of Sin3A or EZH2 via an siRNA approach (Suppl. Fig. S2). First, we noted that Sin3A down-regulation affected the TET1/Sin3A interactions but not the TET1/EZH2 interactions and that EZH2 down-regulation affected the TET1/EZH2 interactions but not the TET1/Sin3A interactions (Fig. 2A).
Figure 2.
Nonredundant functionality of TET1/EZH2- and TET1/Sin3A-including complexes to the NES1 and HOXD12 genes. (A) Impact of TET1, EZH2, or Sin3A down-regulation on the formation of TET1/EZH2 and TET1/Sin3A interactions. P-LISA experiments were performed 48 hours after siRNA treatment. Graphs represent the mean ± SD of considered interactions quantified by using the freeware BlobFinder from 100 nuclei in 3 independent experiments. (B) Impact of TET1, EZH2, or Sin3A down-regulation on TET1/EZH2 and TET1/Sin3A recruitment on the NES1−366/−296 and HOXD12−507/−389 genes by ChIP and reChIP experiments. Graphs represent the mean ± SD of 3 independent experiments. (C) Impact of TET1, EZH2, or Sin3A down-regulation on methylation of the NES1−366/−296 and HOXD12−507/−389 genes by using the MeDIP method. Graphs represent the mean ± SD of 3 independent experiments. (D) Impact of TET1, EZH2, or Sin3A down-regulation on methylation of the NES1−366/−296 and HOXD12∓507/∓389 genes by using the bisulfite sequencing method. A black circle represents a CpG having a methylation percentage >50%, and an open circle represents a CpG having a methylation percentage <50% (according to data illustrated by Suppl. Fig. S3). (E) Impact of TET1, EZH2, or Sin3A down-regulation on methylation of the NES1−366/−296 and HOXD12−507/−389 genes by using the qPCR method. Graphs represent the mean ± SD of 3 independent experiments.
Chromatin immunoprecipitation (ChIP) and sequential chromatin immunoprecipitation (reChIP) indicated that the down-expression of Sin3A and EZH2 decreased TET1 recruitment on the NES1 and HOXD12 genes, respectively (Fig. 2B). These data suggest that Sin3A and EZH2 play a role as anchors for TET1 recruitment on the NES1 and HOXD12 genes.
Methylated DNA immunoprecipitation (MeDIP) and bisulfite sequencing experiments showed that the decrease of TET1/Sin3A recruitment on the NES1 gene in cells treated with siRNA-Sin3A induced an increase in the methylation of this gene, without promoting a change to methylation of the HOX12D gene (Fig. 2C and 2D and Suppl. Fig. S3). Conversely, we noted that the decrease of TET1/EZH2 recruitment on the HOXD12 gene in cells treated with siRNA-EZH2 produced an increase in methylation of the HOX12D gene, without promoting a change to methylation of the NES1 gene (Fig. 2C and 2D and Suppl. Fig. S3).
Finally, qPCR analysis revealed that the variation of methylation of the NES1 and HOXD12 genes influenced the expression level of these genes (Fig. 2E). Based on this example, we conclude that the TET1 interaction partners can confer a distinct and specific influence on the epigenetic regulation of certain TET1-targeted genes.
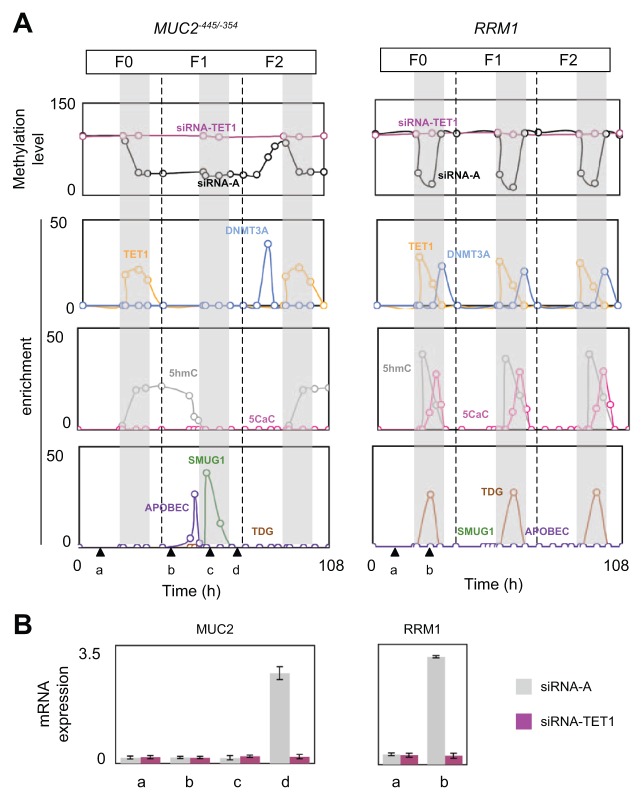
Next, we analyzed the putative participation of TET1 in a dynamic process of methylation/demethylation. For this purpose, we analyzed the kinetics of methylation/demethylation of the MUC2 and RRM1 genes (i.e., 2 TET1-targeted genes).10 U251 cells were arrested in G0/G1 by serum deprivation and thymidine incorporation, and the cell number was quantified for the next 108 hours. As shown in Supplementary Figure S4, the doubling time of U251 cells was 36 hours. Thus, during a 108-hour period, we were able to study 3 generations or 3 cell cycles (referred to as F0, F1, and F2). For each generation, the S phase was identified by thymidine incorporation (Suppl. Fig. S4).
MeDIP experiments were performed to establish the kinetics of methylation/demethylation of the MUC2 and RRM1 genes. Thus, we noted that RRM1 was demethylated and remethylated during each S phase of each cell generation, while MUC2−445/−354 was demethylated in the S phase of F0 (S/G0) and then methylated in G1/F2 and demethylated in S/F2 (Fig. 3A). Bisulfite sequencing experiments also indicated that MUC2 is methylated in G1/F0 and unmethylated in G1/F2 and that RMM1 is methylated in G1/F0 and unmethylated in S/G0 (Suppl. Fig. S5).
Figure 3.
Cyclical epigenetic regulation of the MUC2 and RRM1 genes. (A) The kinetics of MeDIP, hMeDIP, and ChIP experiments were analyzed at the indicated time. The numbers of immunoprecipitated genes or methylated genes were quantified by qPCR and normalized to inputs according to the previous description. The number of generations (F0, F1, and F2) was determined by counting the cells after an initial synchronization in the G0/G1 phase (serum deprivation, 72 hours) (Suppl. Fig. S3). S phases (gray areas) were determined by measuring the 3H-thymidine incorporation in the DNA of U251 cells (Suppl. Fig. S3). (B) qPCR monitoring of expression levels of the MUC2 and RRM1 genes at certain times (as explained in A).
Similar experiments performed with U251/siRNA-TET1 cells indicated that TET1 down-expression abolished the demethylation affecting the MUC2 and RRM1 genes (Fig. 3A). These findings support the idea that methylation/demethylation processes cyclically regulated the MUC2 and RRM1 genes.
The role of TET1 in the cyclical methylation/demethylation processes of the MUC2 and RRM1 genes was also demonstrated by the fact that the demethylation periods coincide with the period of TET1 recruitment on the MUC2 and RRM1 genes. In addition, ChIP experiments identified DNMT3A as the DNA methyltransferase implicated in the cyclical methylation/demethylation processes of the MUC2 and RRM1 genes (Fig. 3A).
Because TET1-mediated demethylation promotes 5mC/5hmC/5-carboxylcytosine (5caC) conversion,7 we next analyzed the kinetics of the appearance of 5hmC and 5caC on the MUC2 and RRM1 genes. Hydroxymethylated DNA immunoprecipitation (hMeDIP) experiments revealed that 5hmC appears on MUC2 in S/F0 and then continues in G2/F0 and at the beginning of G1/F1 before disappearing at the end of G1/F1 (Fig. 3A). Next, 5hmC reappears in S/F2. 5caC was not detected on MUC2. On the other hand, on RRM1, hMeDIP and IP of 5caC indicated that 5hmC and 5caC appear in each S phase of the F0, F1, and F2 generations (Fig. 3A).
Because TDG, APOBEC3, and SMUG1 are enzymes putatively implicated in the 5hmC/5caC-mediated DNA demethylation process, we next analyzed the recruitment of these enzymes on the MUC2 and RRM1 genes.11 The kinetics of ChIP experiments indicated that APOBEC3 was recruited on MUC2 at the end of G1/F1, that is, when 5hmC disappears from MUC2. SMUG1 was recruited on MUC2 in S/F1 (Fig. 3A). These data make sense in that SMUG1 is a single-stranded selective monofunctional uracil–DNA glycosylase implicated in 5-hydroxymethyluracil/5hmC conversion.12 Contrary to MUC2, only TDG was recruited on RRM1 (Fig. 3A). Thus, we conclude that TDG recruitment on RRM1 is cyclical since it occurs in each S phase of the F0, F1, and F2 generations.
Next, we studied the impact of the presence of 5mC, 5hmC, and 5caC on MUC2 and RRM1 gene expression. For this purpose, qPCR experiments were performed when MUC2 was positive for 5mC and 5hmC and negative for 5mC and 5hmC. Figure 3B indicated that MUC2 was expressed in G2/F1, that is, when MUC2 was negative for 5mC and 5hmC. Similar experiments indicated that RRM1 was expressed in S/F0, that is, when RRM1 was negative for 5mC, 5hmC, and 5caC.
To summarize, by examining the modification of cytosines in the MUC2 and RRM1 genes and the recruitment of certain epigenetic enzymes, our data clearly indicate that the MUC2 and RRM1 genes are cyclically and epigenetically regulated by TET1/PCNA-including complexes since reChIP experiments demonstrated that TET1 and PCNA were co-recruited on the MUC2 and RRM1 genes (Suppl. Fig. S6). In addition, the cyclic and epigenetic regulation of RRM1 appears at each cell cycle and can be qualified as “classic,” while the regulation of MUC2 appears more “surprising or nonclassic,” with an inheritance of DNA methylation/demethylation processes extended on 3 cellular generations.
Finally, we hypothesized that the inheritance of DNA methylation/demethylation processes could be a cause of aberrant DNA hypermethylation or hypomethylation in cancer cells. Indeed, the deficiency of a demethylation-involved enzyme (such as TET1/PCNA) could promote the hypermethylation of certain genes, while the deficiency of a DNMT of interest could promote the hypomethylation of certain genes.
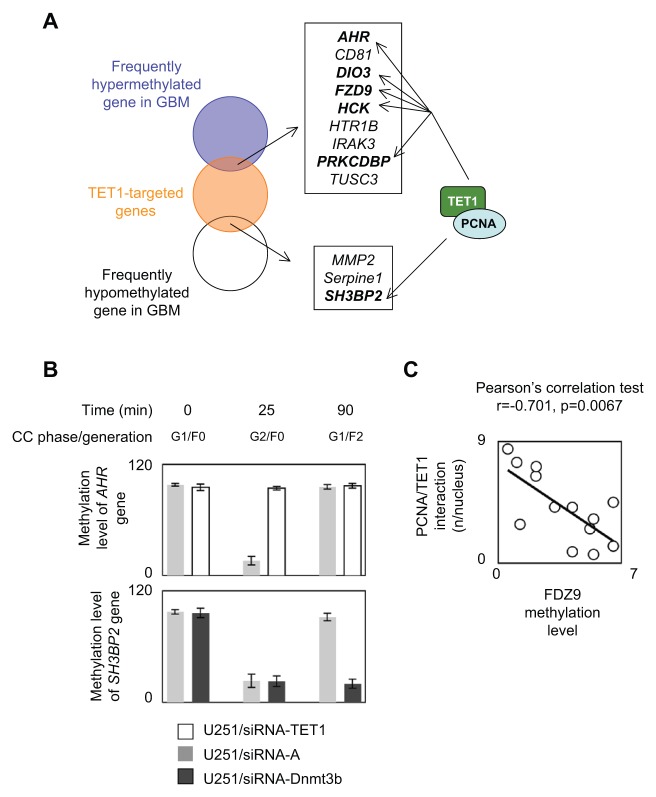
To verify this idea, we initially analyzed whether TET1/PCNA was recruited on genes characterized as being TET1-targeted genes and as being frequently hypomethylated or hypermethylated in glioblastoma multiforme (GBM).10,13,14 According to this hypothesis, we focused our analysis on 3 genes frequently hypomethylated in GBM (i.e., the MMP2, SH3BP2, and Serpine1 genes) and on 9 genes frequently hypermethylated in GBM (i.e., the AHR, CD81, DIO3, FZD9, HTR1B, HCK, IRAK3, PRKCDBP, and TUSC3 genes) (Fig. 4A). reChIP experiments of PCNA/TET1 revealed that the PCNA and TET1 proteins were co-recruited on the AHR, DIO3, FZD9, HCK, PRKCDBP, and SH3BP2 genes (Fig. 4A and Suppl. Fig. S7). In other words, our data indicate that TET1/PCNA is implicated in the aberrant DNA methylation status of 6 of 12 genes that are observed as being aberrantly methylated (hypomethylated or hypermethylated) in GBM.
Figure 4.
Implication of cyclical methylation/demethylation on the methylation hallmarks of glioma. (A) Identification of genes aberrantly methylated in glioma and a target of TET1/PCNA. (B) Impact on the methylation status of the AHR and SH3BP2 genes of DNMT3B or TET1 down-regulation. CC = cell cycle. (C) Correlation between the number of PCNA/TET1 interactions (according to P-LISA experiments) and the methylation level of FZD9. Each open circle represents 1 cell line or 1 PCTC.
To validate the idea that AHR could be hypermethylated in GBM cells presenting a deficiency of PCNA/TET1-including complexes, we analyzed the impact on the methylation level of AHR of the siRNA-induced down-regulation of TET1 in U251 cells. As illustrated by Figure 4B, we noted that the methylation level of AHR decreased in G2/F0 of U251/siRNA-A cells but not in U251/siRNA-TET1 cells. This finding confirms the idea that the deficiency of TET1 is the cause of the hypermethylation of genes that are described as being frequently hypomethylated in GBM.
To determine whether the loss of the methylation actor of PCNA/TET1-targeted genes could be a source for the frequently hypermethylated genes in GBM, we evaluated, by the MeDIP method, the methylation level of SH3BP2 in U251 cells harboring or not harboring DNMT3B down-regulation since this DNMT is identified as being recruited on SH3BP2 (Suppl. Fig. S8). As expected, we noted that the siRNA-induced down-expression of DNMT3B decreased the recruitment of this enzyme on SH3BP2 and vice versa promoted the hypomethylation of SH3BP2 (Fig. 4B and Suppl. Fig. S8). Through the example of the SH3BP2 and AHR genes, our data support the idea that the presence of PCNA/TET1 as a demethylator constitutes a cause for the presence of certain aberrant DNA methylation processes frequently observed in GBM.
By extrapolating these results, we finally supposed that the number of PCNA/TET1 interactions could be inversely correlated with the degree of methylation of a PCNA/TET1-targeted gene. To investigate this point, we analyzed in parallel the number of PCNA/TET1 interactions by P-LISA and the methylation level of FZD9 in a panel of 14 cell lines or primary cultured tumor cells (PCTCs): Du145, MCF7, MDA-MD231, H1975, H358, Meso96, Meso56, U251, PCTC-GBM#1, PCTC-GBM#2, PCTC-GBM#3, PCTC-GBM#4, PCTC-GBM#5, and PCTC-GBM#6. The graph in Figure 4C and the Pearson test indicated the presence of inverse correlations between the number of PCNA/TET1 interactions by P-LISA and the methylation of FZD9. Thus, these data confirm that the presence of PCNA/TET1 in cells is associated with the level of methylation of its targeted genes.
To summarize, our data described new interaction partners of TET1. More importantly, our results also identified TET1/PCNA as a demethylator of the methylation/demethylation process of the inheritance of DNA methylation extended on 3 cellular generations, of which the perturbation explains the presence of aberrantly methylated genes in cancer cells.
Materials and Methods
Methods are available in the supplementary material.
P-LISA
Cells were fixed with 4% paraformaldehyde in PBS (pH 7.4) for 15 minutes at room temperature. Permeabilization was performed with PBS containing 0.5% Triton X-100 for 20 minutes at room temperature, and staining was performed according to the manufacturer’s instructions (Olink Bioscience, Uppsala, Sweden). Fluorescence was visualized with ApoTome (Zeiss, Marly-le-Roi, France). A 3-dimensional view was obtained by using the Amira 4.1.1 program (Visualization Sciences Group, Bordeaux, France). The number of interactions was determined by using the freeware BlobFinder (available for download from www.cb.uu.se/~amin/BlobFinder), as previously described.5
Investigation of cell cycle progression
U251 cells were initially blockaded in G0/G1 by serum deprivation (72 hours). Next, the number of cells and 3H-thymidine incorporation were measured at indicated times to assess cell cycle progression. Indeed, the measure of the number of cells permits us to evaluate the doubling time of cells, and the measure of 3H-thymidine incorporation permits us to determine the S phase since 3H-thymidine is incorporated into DNA during the DNA replication process occurring during the S phase. Briefly, 5 µCi/mL of 3H-thymidine (GE Healthcare, Little Chalfont, UK) was incubated with cells. Cells were then washed 3 times with cold side scatter (SSC) (0.15 M sodium chloride, 0.015 M sodium citrate) and trypsinized. One half of the sample from each time was removed, and a carrier DNA was added. Cells were then disrupted sonically and precipitated with 15 N of HCL containing 6% pyrophosphate. The precipitate was filtered through Whatman GF/C filters (Sigma, Saint-Quentin-Fallavier, France), which were washed with 4% perchloric acid, 70% ethanol, 95% ethanol, and 100% ethanol and dried, and 3H radioactivity was counted by liquid scintillation spectrometry (Beckman Coulter, Brea, CA). The other half of the sample from each time point was diluted in SSC, and the cell number was determined in a cell counter (Life Technologies, Carlsbad, CA).
Supplementary Material
Acknowledgments
The authors thank Philippe Hulin for his technical assistance (MicroPICell, Plateau technique d’imagerie cellulaire, IFR26, Centre de Recherche en Cancérologie Nantes-Angers, INSERM U892). They also thank Dr Philippe Juin for the MCF7 and MDA-MD231 cells and Dr Marc Grégoire and Christophe Blanquart for the Meso96 and Meso56 cells.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplementary material for this article is available on the Genes & Cancer website at http://ganc.sagepub.com/supplemental.
References
- 1. Guo J, Su Y, Zhong C, Ming G, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cells. 2011;145:423-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang H, Zhang X, Clark E, Mulcahey M, Huang S, Shi Y. TET1 is a DNA-binding protein that modulates DNA methylation and gene transcription via hydroxylation of 5-methylcytosine. Cell Res. 2010;20:1390-3 [DOI] [PubMed] [Google Scholar]
- 3. Frauer C, Rottach A, Meilinger D, et al. Different binding properties and function of CXXC zinc finger domains in Dnmt1 and Tet1. PLoS One. 2011;6:e16627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hervouet E, Debien E, Cheray M, et al. Disruption of Dnmt1/PCNA/UHRF1 interactions promotes tumorigenesis by inducing genome and gene-specific hypomethylations and chromosomal instability. PLoS One. 2010;5:e11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hervouet E, Hulin P, Vallette FM, Cartron PF. Proximity ligation in situ assay for monitoring the global DNA methylation in cells. BMC Biotechnol. 2011;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Söderberg O, Gullberg M, Jarvius M, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995-1000 [DOI] [PubMed] [Google Scholar]
- 7. Kinney S, Pradhan S. Ten eleven translocation enzymes and 5-hydroxymethylation in mammalian development and cancer. Adv Exp Med Biol. 2013;754:57-79 [DOI] [PubMed] [Google Scholar]
- 8. Wu H, Zhang Y. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev. 2011;25:2436-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Margueron R, Li G, Sarma K, et al. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell. 2008;32:503-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williams K, Christensen J, Pedersen M, et al. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473:343-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Branco M, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet. 2011;13:7-13 [DOI] [PubMed] [Google Scholar]
- 12. Boorstein R, Cummings AJ, Marenstein D, et al. Definitive identification of mammalian 5-hydroxymethyluracil DNA N-glycosylase activity as SMUG1. J Biol Chem. 2001;276:41991-7 [DOI] [PubMed] [Google Scholar]
- 13. Laffaire J, Everhard S, Idbaih A, et al. Methylation profiling identifies 2 groups of gliomas according to their tumorigenesis. Neuro-Oncology. 2010;164:141-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez R, Martin-Subero J, Rohde V, et al. A microarray-based DNA methylation study of glioblastoma multiforme. Epigenetics. 2009;4:255-64 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.