Abstract
By analyzing over 2000 samples from a randomized clinical trial, we have recently associated high levels of tumor-infiltrating lymphocytes with an excellent prognosis among triple negative breast cancer patients as well with improved clinical responses to immunogenic chemotherapy among patients bearing HER2 over-expression. These findings suggest that immunomodulation could represent a new approach to treat these aggressive breast cancer subtypes.
Keywords: breast cancer, immunotherapies, biomarkers, tumor infiltrating lymphocytes, prognosis, trastuzumab efficacy
Breast cancer (BC) has not been traditionally considered as an “immunogenic” malignancy. The incidence of BC is not increased among transplanted (i.e., immunosuppressed) vs. non-transplated patients, yet disease outcome has been reported to be worse among the former. Nevertheless, tumor-infiltrating lymphocytes (TILs) have been consistently documented in BC lesions and have been associated with prognosis. In a seminal paper published in 1992; Aaltoma and colleagues reported that lymphocytic infiltration was associated with a good prognosis, but only among rapidly proliferating tumors (i.e., those that manifested a high number of mitoses).1
We have recently evaluated the prognostic and predictive value of TILs in a large cohort of newly-diagnosed, primary lymph node-positive BC patients that—in the context of a prospective clinical trial—were randomized to receive either high-dose anthracycline-based chemotherapy or a combinatorial regimen involving anthracyclines and docetaxel.2 The use of tumor specimens archived during clinical trials has significant advantages in that (1) the quality of the clinical data are exceptional, (2) patients are treated homogeneously (exception made for the randomization, obviously) and (3) pathological features are assessed in a centralized fashion. Hence, the results of such biomarker studies can be considered more reliable than those of ad hoc or retrospective studies.3 Furthermore, the randomization allowed us to determine if the levels of TILs were associated with improved therapeutic responses to "immunogenic" chemotherapy. We hypothesized that high-dose anthracyclines would be more immunogenic than the combinatorial regimen involving anthracyclines (given at a comparatively lower dose) and docetaxel, as they would directly exert immunostimulatory functions and/or docetaxel (which is usually given together with steroids) would inhibit antitumor immune responses. TILs were evaluated in samples of the primary tumor that was removed at surgery, before chemotherapy.4
High levels of TILs were strongly associated with a good prognosis among patients affected by triple negative (estrogen receptor-, progesterone receptor- and HER2-negative) BC. In contrast, TILs had no prognostic value among patients bearing HER2-overexpressing (HER2+) lesions (irrespective of where overexpression was due to gene amplification or to transcriptional/post-transcriptional mechanisms). Rather, in patient with HER2+ disease, increasing amounts of TILs were associated with improved therapeutic responses to high-dose anthracyclines.2
These data raise interesting considerations. Both triple negative and HER2+ BC are associated with high rates of cancer cell proliferation and genomic instability. TIL levels were significantly higher in these BC subtypes than in luminal (ER+/HER2−) BC lesions. This supports the hypothesis that genomic instability can promote antitumor immune responses owing to the availability of a large number of tumor-associated antigens. Yet, genomic instability might also facilitate tumor progression as instable malignant cells can easily adapt and escape immunosurveillance. Genomic instability is indeed generally associated with high levels of clonal heterogeneity, resistance to therapy and poor prognosis. However, it seems that if an immune response against triple negative BC had developed, which can be determined by the presence of TILs at diagnosis, disease outcome is improved independent of chemotherapeutic regimen. This suggests that non-chemotherapy specific mechanisms combined with natural immunity can restore antitumor responses that perhaps operate synergistically in the presence of robust antigenic loads.5 The key to treat triple negative BC may therefore consist of finding how to promote immune recognition in patients who do not manifest high TIL levels at diagnosis. As this disease has limited therapeutic options upon recurrence and is associated with a dismal prognosis, immunomodulatory regimens for the treatment of triple negative BC undoubtedly warrant further investigation.
In contrast, the type of treatment seems to significantly influence clinical outcomes among BC patients bearing HER2+ lesions and high levels of TILs at diagnosis. Oncogene-addicted tumors have been shown to upregulate a number of immunosuppressive signaling pathways6 and this may sustain tumor-meditated immunosuppression even in micrometastatic disease. As we quantified the amount of TILs in primary lesions before chemotherapy, our data suggest that high-dose anthracyclines may somehow relieve or modulate immunosuppression in HER2+ tumors.
Given that trastuzumab, a monoclonal antibody targeting HER2, not only is the standard of care for BC patients with HER2+ lesions but also enhances immune responses,7,8 we evaluated whether TIL levels were associated with differences in therapeutic responses to trastuzumab. Increasing evidence suggests that an intact immune system is required for tumor regression upon oncogene inhibition, forming the rationale for combinatorial regimens involving targeted anticancer agents and immunotherapy.6 By analyzing tumor samples collected in the context of the FinHER trial,9 we found high levels of TILs to be associated with improved therapeutic responses to trastuzumab-based chemotherapy.10 This data suggest that trastuzumab is most efficacious in the presence of TILs. The HER2-inhibitory effect of trastuzumab probably plays a role, though the ERBB1/2 tyrosine kinase inhibitor lapatinib has been shown to be inferior to trastuzumab in this respect. Determining how HER2 promotes immunosuppression and the mechanisms whereby trastuzumab can relieve it is currently under investigation. As the TILs accumulating in BC lesions predominantly reflect a TH1 immune response, investigating the therapeutic potential of agents that inhibit T-cell-targeting immunosuppressive mechanisms stands out as a promising therapeutic approach.4 Preclinical data obtained in murine models of BC support this notion.8
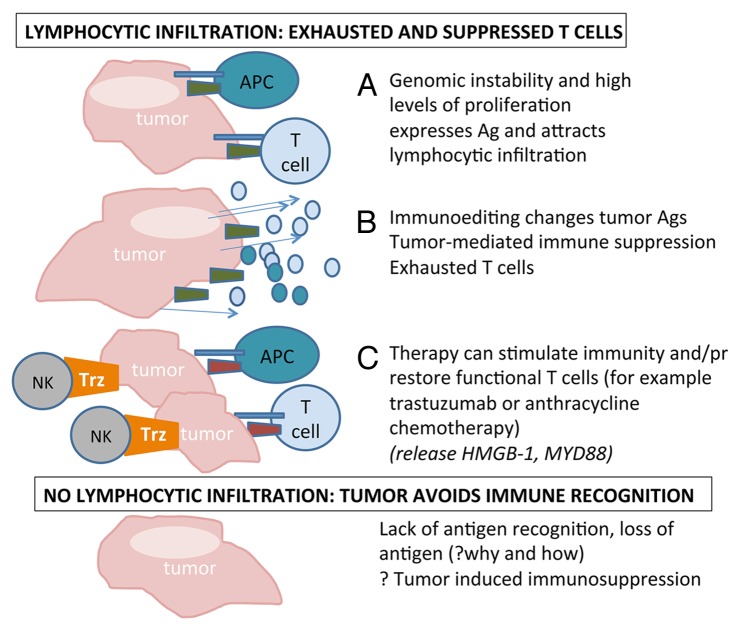
However, in view of our data, one could conclude that HER2+ and triple negative BCs should perhaps be subdivided into tumors that contain high levels of TILs at diagnosis and tumors that do not, and treatment be determined accordingly (Fig. 1). The use of immunomodulatory strategies to treat these two BC subtypes warrants further investigation, as—should clinical findings support this approach—immunotherapy may open a new era for the clinical management of BC.
Figure 1. Possible links between the presence of lymphocytic infiltration at diagnosis and the clinical outcome of breast cancer patients. (A) Tumors that are genetically unstable and/or exhibit high rates of proliferation are associated with high levels of lymphocytic infiltration at diagnosis. (B) Genomic instability facilitates tumor progression as it can help malignant cells to avoid immune recognition by altering their antigenic properties; neoplastic cells establish immunosuppressive mechanisms; T cells are subject to regulation or become exhausted and gradually lose their functions. (C) Chemotherapy can restore functional T cells through high mobility group box 1 (HMGB1)- or myeloid differentiation 88 (MYD88)-mediated signaling cascades; trastuzumab (Trz) can induce functional immune effectors by interacting with Fc receptor-expressing cells such as natural killer cells. Elucidating how tumors that do not present with lymphocytic infiltration at diagnosis avoid immune recognition is critical for the development of efficient therapeutic regimens.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest have been disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24720
References
- 1.Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28A:859–64. doi: 10.1016/0959-8049(92)90134-N. [DOI] [PubMed] [Google Scholar]
- 2.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 3.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–52. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 5.Kang TH, Mao CP, Lee SY, Chen A, Lee JH, Kim TW, et al. Chemotherapy acts as an adjuvant to convert the tumor microenvironment into a highly permissive state for vaccination-induced antitumor immunity. Cancer Res. 2013;73:2493–504. doi: 10.1158/0008-5472.CAN-12-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 2010;18:485–98. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 8.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108:7142–7. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joensuu H, Bono P, Kataja V, Alanko T, Kokko R, Asola R, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27:5685–92. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 10.Loi S, Michiels S, Lambrechts D, et al. Tumor PIK3CA mutations, lymphocyte infiltration, and recurrence-free survival (RFS) in early breast cancer (BC): Results from the FinHER trial. Journal of Clinical Oncology 2012; 30:no. 15_suppl 507 [Google Scholar]