Abstract
The daily intake of low-dose aspirin lowers the risk of several cancers among the adults. The continuous administration of low-dose aspirin to TH-MYCN mice (a model of pediatric neuroblastoma) delays tumor outgrowth and decreases tumor-promoting inflammation by inhibiting regulatory cells of the innate immune system as well as immunosuppressive mediators such as transforming growth factor β (TGFβ) and thromboxane A2. These findings pave novel avenues for the clinical management of neuroblastoma.
Keywords: MYCN, aspirin, cyclooxygenase 1, dendritic cell, myeloid-derived suppressor cell, neuroblastoma, prostaglandin D2, thromboxane A2, tumor-associated macrophage
Developing neoplasms elicit inflammatory responses that can become chronic and be harnessed by malignant cells, eventually translating into cancer-related inflammation.1 Inflammation contributes to tumor progression by disabling immunosurveillance, by stimulating angiogenesis and by providing neoplastic cells with growth factors. As such, cancer-related inflammation constitutes a potential therapeutic target, a concept that has already been intensively investigates. Recent studies have revealed that the daily intake of low doses of aspirin reduce the incidence of several cancers of the adult, including breast, prostate and colon carcinoma.2,3 Aspirin irreversibly inactivates cyclooxygenase 1 (COX1) (and to a lower extent COX2), in turn affecting the production of arachidonic acid-derived inflammatory mediators such as the prostaglandins (PGs) and thromboxanes (TXs) (Fig. 1). The anticancer effects of aspirin have been suggested to depend on the inhibition of platelet activation, directly stemming from a reduction in TXA2 levels. This would be paralleled by a reduction in the metastatic potential of cancer cells and in the levels of COX2, by angiogenesis inhibition as well as by the attenuation of pro-inflammatory pathways involving, among several mediators, PGE2.2 Previous studies have not investigated the precise composition of the tumor microenvironment in response to low-dose aspirin.
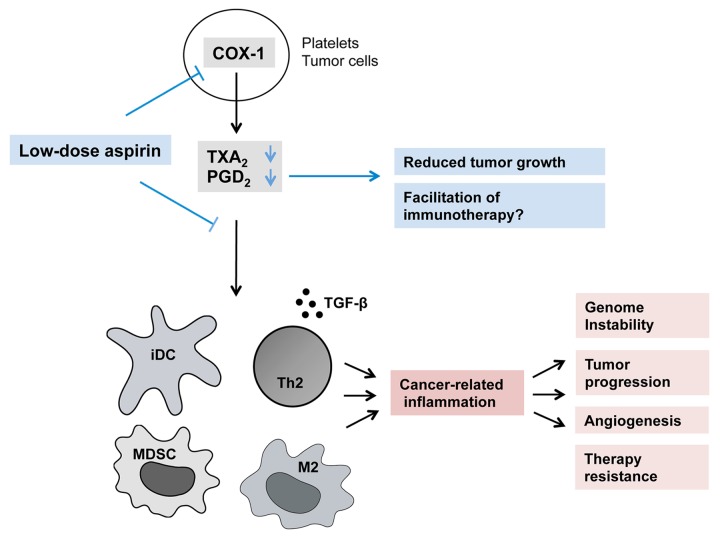
Figure 1. Immunological mechanisms underlying the antineoplastic effects of low-dose aspirin. By inactivating cyclooxygenase 1 (COX1) in platelets and/or tumor cells, the conversion of arachidonic acid into thromboxane A2 (TXA2) and prostaglandin D2 (PGD2) is reduced, leading to a decrease in the number of immature immune cells—including immature dendritic cells (iDCs), myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs)—that infiltrate neoplastic lesions, as well as in the expression of immunosuppressive mediators, such as transforming growth factor β (TGFβ). Ultimately, such immunomodulatory effects limit tumor growth, suggesting that low-dose aspirin might be successfully employed in combinatorial regimens to improve the therapeutic efficacy of immunotherapy.
In comparison to adult cancers, little is known about pediatric neoplasms in terms of cancer-related inflammation. Neuroblastoma, a malignant embryonic tumor of the sympathetic nervous system, affects 10.5 out of 106 children below 15 y of age, and is the most common tumor among infants, exhibiting a large spectrum of clinical behaviors. Improving the long-term survival or patients affected by high-risk neuroblastoma remains a challenge, even though an immunotherapeutic protocol based on GD2-specific monoclonal antibodies has successfully been introduced in the clinical practice a few years ago.4 The tumor microenvironment has been shown to significantly influence the clinical response to immunotherapy of several distinct cancer. In particular, cancer-related inflammation is expected to hamper the clinical efficacy of immunotherapy.
COX2, which is involved in the production of pro-inflammatory prostaglandins, has previously been shown to be expressed by neuroblastoma cells and to constitute a therapeutic target in this setting.5 We have recently characterized in qualitative and quantitative terms the inflammatory response that accompany the development of aggressive neuroblastomas in transgenic TH-MYCN mice, addressing the question whether oncogenesis and tumor progression would be stimulated by cancer-related inflammation also in a pediatric setting.6 In this model, which is appreciated for its similarities with human neuroblastoma, all mice homozygous for the MYCN transgene develop palpable aggressive intraabdominal tumors at a median age of 5.4 weeks.7
We documented a striking evolution in the composition of hematopoietic cells infiltrating TH-MYCN-driven neuroblastomas. Indeed, while in early lesions effectors of the adaptive immune system such as CD8+ T lymphocytes prevailed, during tumor progression these cells were progressively outnumbered by cells of the innate immune system such as myeloid-derived suppressor cells (MDSCs), dendritic cells (DCs) and tumor-associated macrophages (TAMs). Along with tumor progression, DCs and TAMs acquired a phenotype resembling that of immunosuppressive (and hence tumor-promoting) immature DCs and M2 TAMs, exhibiting a reduced expression of MHC Class II molecules (DCs, TAMs) and an elevated expression of the mannose receptor CD206 (TAMs).
Driven by the observation that an inflammatory response accompanies tumor progression in this model, we investigated the cyclooxygenase/prostaglandin axis, finding that COX1 is expressed by lesions of all stage. Consequently, we administered low doses of aspirin (10 mg/kg, corresponding to 60 mg for an adult human, when translated using body surface area) to homozygous mice once daily for ten days, starting at the age of 4.5 weeks. Aspirin-treated mice presented with a significantly reduced tumor burden at sacrifice, and the intratumoral hematopoietic compartment of these animals was significantly deprived of innate immune cells. Along similar lines, low-dose aspirin reduced the expression of immunosuppressive mediators such as transforming growth factor β (TGFβ). When assessing COX metabolites, we found that low-dose aspirin significantly decreased the intratumoral levels of both TXA2 and PGD2 (Fig. 1).
Our data provide novel insights into cancer-related inflammation in pediatric tumors, as exemplified by high-risk neuroblastoma. Our study highlights the presence of cancer-related inflammation in these tumors and its gradual deterioration toward a progressively more immunosuppressive state, an unfavorable setting for (immuno)therapy. In addition, our findings elucidate the molecular and cellular mechanisms whereby low-dose aspirin exerts anticancer effects, as analyzed ex vivo (Fig. 1). To our knowledge, ours represents the first study investigating the responses of the tumor microenvironment to low-dose aspirin.
In addition, we have recently shown that active T-cell responses prevail in human neuroblastoma samples,8 suggesting that a least some degree of immunosurveillance persist in this setting. Other studies have demonstrated the clinical benefits of chimeric antigen receptor (CAR)-expressing cells for neuroblastoma patients9 and, as mentioned above, an immunotherapeutic protocol based on anti-GD2 antibodies has recently been incorporated into the clinical routine.4 Our findings suggest that low-dose aspirin might be used as a standalone therapeutic intervention in high-risk neuroblastoma patients.6 Moreover, mechanistic data plead in favor of low-dose aspirin as a measure to enhance the antineoplastic effects of immunotherapy, suggesting that a combinatorial approach might also be beneficial. The addition of low-dose aspirin to the current therapeutic regimen based on anti-GD2 antibodies, granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin-2 (IL-2) could generate a favorable tumor microenvironment for these immunomodulatory agents, and hence enhance response rates. The long-term usage of low-dose aspirin as a single maintenance therapy for children with potential minimal residual disease and at risk of relapse is another option. In this context, it should be kept in mind that the contribution of aspirin to Reye’s syndrome in children with viral infections has been suggested and feared, but also questioned.10
In conclusion, further studies are warranted on the possibility of using low-dose aspirin in individuals affected by high-risk neuroblastoma as well as on the hypothesis that cancer-related inflammation would represent a suitable therapeutic target for pediatric cancer patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest have been disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24658
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9:259–67. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 4.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Children’s Oncology Group Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnsen JI, Lindskog M, Ponthan F, Pettersen I, Elfman L, Orrego A, et al. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Res. 2004;64:7210–5. doi: 10.1158/0008-5472.CAN-04-1795. [DOI] [PubMed] [Google Scholar]
- 6.Carlson LM, Rasmuson A, Idborg H, Segerström L, Jakobsson PJ, Sveinbjörnsson B, et al. Low-dose aspirin delays an inflammatory tumor progression in vivo in a transgenic mouse model of neuroblastoma. Carcinogenesis. 2013;34:1081–8. doi: 10.1093/carcin/bgt009. [DOI] [PubMed] [Google Scholar]
- 7.Rasmuson A, Segerström L, Nethander M, Finnman J, Elfman LH, Javanmardi N, et al. Tumor development, growth characteristics and spectrum of genetic aberrations in the TH-MYCN mouse model of neuroblastoma. PLoS One. 2012;7:e51297. doi: 10.1371/journal.pone.0051297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson LM, De Geer A, Sveinbjornsson B, Orrego A, Martinsson T, Kogner P, Levitskaya J. The microenvironment of human neuroblastoma supports the activation of tumor-associated T lymphocytes. OncoImmunology. 2013;2:3, e23618. doi: 10.4161/onci.23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casteels-Van Daele M, Van Geet C, Wouters C, Eggermont E. Reye syndrome revisited: a descriptive term covering a group of heterogeneous disorders. Eur J Pediatr. 2000;159:641–8. doi: 10.1007/PL00008399. [DOI] [PubMed] [Google Scholar]