Abstract
Homeostatic TLR4 signaling protects the intestinal epithelium in health. Evidence suggests that perturbed TLR4 signaling is linked to carcinogenesis. We have recently demonstrated that the common human TLR4 variant D299G exerts pro-inflammatory effects and drives malignant tumor progression in human colon cancer.
Keywords: Toll-like receptors, cancer-associated inflammation, innate immunity, colon cancer, pattern-recognition receptors, tumor microenvironment, lipopolysaccharide, intestinal epithelial cells, metastasis
Colon cancer is the second leading cause of cancer death in the Western world. Innate immunity influences tumor cell behavior, representing an important component of tumor development and progression in the intestine. Genetic, commensal and environmental factors may interact to modulate innate immune reactions, resulting in variable tumor phenotypes.
Toll-like receptors (TLR) play a key role in innate immunity of the intestinal mucosa, crucially involved in maintaining mucosal as well as commensal homeostasis through control of milieu influences. TLR4 is the major receptor for LPS activation which requires the presence of accessory molecules, CD14, LBP, and MD-2. Downstream, TLR4 signals via MyD88-dependent and MyD88-independent pathways. Basal TLR4 signaling exerts host-protective responses in the intestinal mucosa.1 TLR4 expression is low in healthy intestinal epithelium, but significantly upregulated in inflamed mucosa (e.g., in inflammatory bowel diseases (IBD)2).
Evidence suggests that aberrant TLR4 signaling is linked to colonic carcinogenesis. TLR4 activation differentially modulates proliferation of the intestinal epithelium and its crypt progenitors.3 A recent study demonstrated in a chemically-induced colitis-associated tumorigenesis-model (AOM/DSS) that complete loss-of-function of the murine TLR4 gene protects against the development of colonic tumors.4 The TLR4 gene is significantly mutated in some human gastrointestinal cancers, including esophageal adenocarcinoma5 and noncardia gastric carcinoma.6
The 2 most common missense polymorphisms, D299G and T399I, which occur in exon 4 of the human TLR4 gene (A896G and C1196T, respectively), are located within the extracellular domain of the receptor.7 The co-segregated TLR4-D299G/T399I haplotype is predominantly found in populations of European descent (at frequencies up to 10–18%). Both mutations result in conformational changes, which may impair ligand binding8 and render carriers hypo-responsive to LPS challenge.7 So far, the functional role of these two TLR4 mutations in human colon cancer has not been addressed. In a recent study, we investigated the effects of TLR4-D299G and TLR4-T399I on intestinal epithelial cell (IEC) biology and molecular function. Our results, which have been published in reference9 and are briefly summarized here, imply that TLR4-D299G drives inflammation-associated malignant progression in human colon cancer. Our findings9 were based upon 2 experimental models:
First, we generated IEC lines (Caco-2) that stably overexpress wild-type (WT) TLR4, or mutant TLR4-D299G or TLR4-T399I. Caco-2 cells (non-invasive enterocyte/adenoma cells) represent an accepted in vitro human IEC model to study differentiation, barrier integrity and tumorigenesis. Stable expression of TLR4-D299G in Caco-2 cells caused aberrant actin cytoskeletal disorganisation with nuclear atypia (multipolar spindles and misaligned chromosomes), suggestive of malignant transformation. These alterations were confirmed in several individual TLR4–299G IEC clones, but were not present in IEC controls (TLR4-WT, TLR4-T399I or mock). We found that TLR4-D299G, but not TLR4-WT, activated the Wnt/β-catenin pathway, leading to IEC de-differentiation and a mesenchymal phenotype. TLR4-D299G IEC showed highly invasive behavior, whereas control clones did not. Mechanistically, TLR4-D299G induced IEC invasion via Wnt-dependent STAT3 activation. The major TLR4-D299G targets were dominated by pro-inflammatory and pro-tumorigenic genes. In addition, TLR4-D299G constitutively secreted large protein amounts of pro-inflammatory mediators (involved in acute phase, coagulation and complement responses). Furthermore, xenografts in mice demonstrated TLR4-D299G-induced acceleration of intestinal tumor growth, which was blocked by STAT3 inhibition. By contrast, TLR4-WT, TLR4-T399I, or mock xenografts failed to grow.
Second, to assess the role of TLR4-D299G in the pathophysiology of human primary colon cancer, we examined human colonic specimens (214 cases) in a proof-of-concept approach.9 Primary human colon cancers endogenously carrying the TLR4-D299G mutation showed enhanced malignant progression, when compared with TLR4-WT colon cancer at the time of diagnosis. Advanced disease (UICC ≥ III with invasion and distant organ metastasis at initial diagnosis) was more frequent in TLR4-D299G colon cancer patients than TLR4-WT. This observation correlated with increased expression levels of STAT3 mRNA in TLR4-D299G colon cancers compared with TLR4-WT. However, TLR4 mRNA was significantly induced in most sporadic human colon cancers, regardless of genotype.
In conclusion, our results9 suggest that TLR4-D299G in established tumorigenic cells of the colon may accelerate the transition to invasion and metastasis (Fig. 1). Thus, we have identified a previously unknown role for the TLR4-D299G polymorphism as a gain-of-function mutation with enhanced oncogenic potential in the intestinal epithelium. TLR4-D299G promotes a pro-inflammatory microenvironment, which may be responsible for triggering cancer progression. Our data imply a novel mechanistic link between aberrant innate immune signaling and malignant progression via STAT3 in colon cancer.
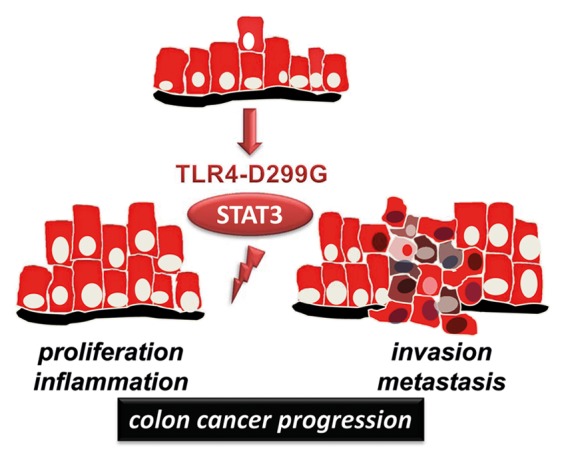
Figure 1. Model of TLR4-D299G-mediated cancer progression in tumorigenic IEC.
Future studies will need to examine larger patient cohorts from different geographical origin and ethnicity. While TLR4-D299G may modify the intestinal tumor cell phenotype, it remains unclear whether carriers may actually have a significantly increased overall risk of developing colon cancer, - or other types of cancer. Notably, patients with IBD are at greater risk of colon cancer than the general population. TLR4-D299G has been associated with IBD susceptibility.10 Whether IBD patients carrying the TLR4-D299G variant are at increased risk of developing a more aggressive phenotype of colitis-associated neoplasia remains to be determined.
In our in vitro cell culture model, cancer progression was due to mutation-driven and cell-autonomous, but TLR4 ligand-independent, activation. It will be essential to investigate in vivo whether environmental factors (e.g., commensal bacteria) may influence the IEC phenotype in TLR4-D299G mutation carriers. In addition, it must be examined whether abnormal signaling via TLR4-D299G in vivo skews the commensal composition, leading to changes in mucosal immune tolerance that may contribute to tumorigenesis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24890
References
- 1.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7. doi: 10.1128/IAI.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neal MD, Sodhi CP, Jia H, Dyer M, Egan CE, Yazji I, et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J Biol Chem. 2012;287:37296–308. doi: 10.1074/jbc.M112.375881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–81. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulak AM, Stojanov P, Peng S, Lawrence MS, Fox C, Stewart C, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet. 2013;45:478–86. doi: 10.1038/ng.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hold GL, Rabkin CS, Chow WH, Smith MG, Gammon MD, Risch HA, et al. A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology. 2007;132:905–12. doi: 10.1053/j.gastro.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–91. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 8.Ohto U, Yamakawa N, Akashi-Takamura S, Miyake K, Shimizu T. Structural analyses of human Toll-like receptor 4 polymorphisms D299G and T399I. J Biol Chem. 2012;287:40611–7. doi: 10.1074/jbc.M112.404608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyking A, Ey B, Rünzi M, Roig AI, Reis H, Schmid KW, et al. Toll-like receptor 4 variant D299G induces features of neoplastic progression in Caco-2 intestinal cells and is associated with advanced human colon cancer. Gastroenterology. 2011;141:2154–65. doi: 10.1053/j.gastro.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Jager PL, Franchimont D, Waliszewska A, Bitton A, Cohen A, Langelier D, et al. Quebec IBD Genetics Consortium. NIDDK IBD Genetics Consortium The role of the Toll receptor pathway in susceptibility to inflammatory bowel diseases. Genes Immun. 2007;8:387–97. doi: 10.1038/sj.gene.6364398. [DOI] [PubMed] [Google Scholar]


