Abstract
Poorly biodegradable, incomplete Freund’s adjuvant (IFA)-based anticancer vaccines primed CD8+ T cells that did not localize to the tumor site but to the persisting, antigen-rich vaccination site, which became a T-cell graveyard. Short-lived, water-based formulations and the provision of immunostimulatory molecules overcame this issue, resulting in tumor suppression. Here, we discuss the implications of these findings for the development of therapeutic anticancer vaccines.
Keywords: sequestration, T-cell dysfunction, T-cell deletion, vaccine depot, T-cell trafficking, immunotherapy, antigen persistence
Peptide antigens emulsified in incomplete Freund’s adjuvant (IFA) are widely used to vaccinate cancer patients. While several clinical trials testing peptide/IFA-based vaccines documented an increase in circulating antigen-specific T cells,1 objective therapeutic benefits have been rare. Why do vaccination-induced T cells often fail to mediate robust anti-tumor effects? While part of the answer lies with tumor-induced immunoregulatory cells and immunosuppressive factors,2 we tested the hypothesis that the efficacy of IFA-based vaccines is intrinsically limited.3
IFA-based vaccines are water-in-paraffin oil emulsions that cause local inflammation and form poorly biodegradable depots, de facto protecting antigens from degradation as they are slowly released.4,5 While this is beneficial for the induction of B-cell responses, we found it to be profoundly detrimental when used to induce CD8+ T-cell responses. In short, the administration of a gp100-derived peptide in IFA resulted in the robust priming of gp100-specific CD8+ T cells that were detectable in the circulation but not within tumor lesions. Instead, gp100-specific T cells accumulated at the vaccination site, which eventually became a T-cell graveyard. In this setting, T cells responded to the chronic release of gp100-derived peptides by producing cytokines including interferon γ (IFNγ), which upregulated host FASL and hence caused the apoptotic demise of FAS+ T cells. Spared T cells became exhausted, memory responses were limited and—most importantly—the therapeutic impact of the vaccine was minimal. A few gp100-specific T cells did reach the tumor, perhaps explaining the occasional activity of peptide/IFA vaccines in patients. However, most vaccination-elicited T cells never did so and hence their anti-tumor potential remained unrealized.3
Replacing IFA with saline reduced vaccine persistence and abolished T-cell priming, perhaps due to the lack of dendritic cell activation. We therefore decided to use an immunostimulatory cocktail called covax, consisting of a Toll-like receptor 7 (TLR7) agonist (imiquimod cream), an agonistic anti-CD40 antibody, and interleukin-2 (IL-2). The gp100-derived peptide emulsified in IFA and combined with covax induced strong T-cell priming, but again this was followed by T-cell sequestration at the vaccination site and minimal antitumor activity. In contrast, the gp100-derived peptide suspended in saline and combined with covax induced T cells that did not accumulate at the vaccination site but reached the tumor instead, efficiently suppressing tumor growth. T-cell sequestration correlated with the local presentation of the gp100-derived peptide, which lasted less than 2 d when saline was employed vs. up to 3 mo when IFA was used.
Our findings highlight several issues that are relevant for anticancer immunotherapy:
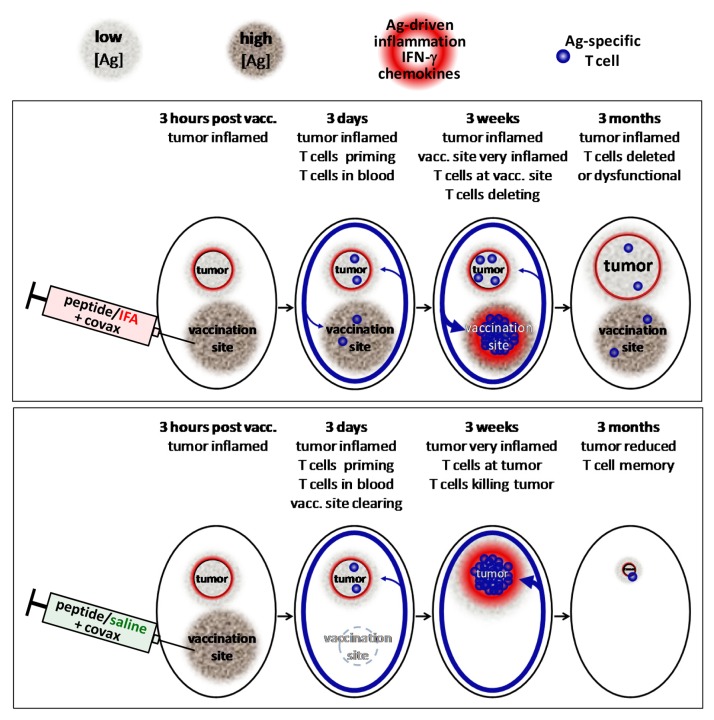
(1) Location, location, location. We never questioned whether vaccination-induced T cells in the circulation would behave as we wished, i.e., traffic to the tumor. In retrospect, it seems obvious that effector T cells would recognize cells presenting vaccine-derived antigens as targets and release cytokines that induce local inflammation and chemokine production, thereby causing the recruitment of additional effectors (Fig. 1). Thus, cells at the vaccination site present the peptides chronically released from the IFA depot and compete with the tumor for “attention,” and—owing to the large amount of injected antigen—may well “win.”
Figure 1. Proposed model of immune response after vaccination with peptide/IFA + covax vs. peptide/saline + covax. Vaccination with peptide in IFA + covax (top) results in a persistent, antigen-rich vaccine depot that primes T cells to become effector cells that enter the circulation. Effector T cells reaching vaccination sites encounter high densities of peptide antigen (high [Ag]), prompting high IFN-γ release, inflammation and chemokine production and stronger T-cell accumulation than comparatively low [Ag] tumors. Eventually, most T cells at vaccination sites are deleted while remaining T cells are dysfunctional and poorly control tumor growth. Vaccination with peptide in saline + covax (bottom) also primes T cells, but vaccine antigen is cleared rapidly, resulting in T-cell accumulation at the most antigen-dense remaining site (tumor). Remaining memory cells are functional to control recurrence and respond to booster vaccination.
(2) Measuring T cells in the circulation provides limited, and sometimes deceiving, information. Our study provides one more possible reason why monitoring immune responses in the circulation, while convenient, often fails to predict clinical responses.6 The time has therefore come to actually implement routine tumor sampling in the context of immunotherapy-based clinical trials. Some accessible tumors, such as cutaneous melanoma, allow for biopsies. For less accessible tumors, sampling can be achieved with fine-needle aspirates or by means of specific study designs. For instance, in neoadjuvant settings, immunotherapy is administered prior to surgical tumor resection, representing an abundant source of material.7 Identifying intratumoral immune responses that correlate with clinical outcome will inform the design of next-generation immunotherapies.
(3) Adding immunomodulators to IFA-based vaccines may increase T-cell responses in the blood without promoting tumor regression. The addition of covax to our IFA-based vaccine increased the abundance and survival of antigen-specific T cells but did not overcome the graveyard effect. In this context, we observed an increased reactogenicity at the vaccination site, while tumor growth was not inhibited. Conversely, adding covax to our water-based vaccine strongly boosted the priming of T cells that trafficked to the tumor and suppressed its growth, yet were not associated with increased reactogenicity at the vaccination site.
(4) A vaccine is not a vaccine is not a vaccine. Anticancer vaccines are often discussed as a single entity: as in “cancer vaccines (don’t) work.” However, not all vaccines are equal. Simply switching from IFA to water abolished the graveyard effect associated with our vaccine, enhanced the trafficking of T cells to tumors, and increased the therapeutic impact. Besides promoting the recruitment of antigen-specific T cells at the vaccination site, IFA induced the IFNγ-driven accumulation of FASL+PDL1+ immunosuppressive myeloid cells.3 Thus, lessons learnt from one particular formulation may not necessarily apply to “cancer vaccines” in general.
(5) Optimal antigen persistence may be a critical feature for the therapeutic effects of anticancer vaccines. Presumably, an optimal time of antigen presentation exists that is neither too short (causing weak T-cell priming) nor too long (inducing a graveyard). Our immune system has evolved to eliminate most acute infections rapidly, often within a week, and perhaps such a time frame of antigen presentation elicits optimal immune responses.8,9 The duration of antigen presentation and the local microenvironment upon vaccination can be manipulated in several ways, offering a promising avenue for the optimization of anticancer vaccines.
(6) The therapeutic efficacy of anticancer vaccines requires a broad range of immunostimulatory agents. A synergistic combination of immunostimulatory agents that per se are rather weak (a TLR7 agonist, a CD40 agonist and IL-2) was absolutely required for the elicitation of effective antitumor immune responses by our vaccine. However, this combination has never tested in cancer patients, partly due to their unavailability on the market.10 Forward-thinking owners of immunomodulators recognize that single-agent vaccine adjuvants rarely induce sufficiently potent immune responses; we hope to see more of them working together to develop curative cancer vaccines that can save the lives of patients with cancer.
Acknowledgments
The work was supported by the National Institute of Health (NIH) grants RO1 1CA143077 (W.W.O) and PO1 CA128913 (W.W.O.) and a Melanoma Research Alliance Established Investigator Award (W.W.O.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24743
References
- 1.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–76. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 3.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–72. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonhoure F, Gaucheron J. Montanide ISA 51 VG as adjuvant for human vaccines. J Immunother. 2009;29:647–8. [Google Scholar]
- 5.Reinhardt RL, Bullard DC, Weaver CT, Jenkins MK. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J Exp Med. 2003;197:751–62. doi: 10.1084/jem.20021690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16:2861–71. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zehn D, King C, Bevan MJ, Palmer E. TCR signaling requirements for activating T cells and for generating memory. Cell Mol Life Sci. 2012;69:1565–75. doi: 10.1007/s00018-012-0965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blair DA, Turner DL, Bose TO, Pham QM, Bouchard KR, Williams KJ, et al. Duration of antigen availability influences the expansion and memory differentiation of T cells. J Immunol. 2011;187:2310–21. doi: 10.4049/jimmunol.1100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]