Abstract
Inducible regulatory T cells (iTregs, also called Tr1 cells) are generated in the periphery (circulation or tissue) of cancer patients upon the encounter of naïve CD4+ T cells with tumor-associated antigens. As p53 is often inactivated by genetic or epigenetic events during oncogenesis, p53-induced Tr1 cells might play a key role in establishing immunosuppressive networks in cancer patients. Tr1 cells were generated by co-culturing circulating CD4+CD25− T cells with autologous immature dendritic cells pulsed with a wild-type (WT) p53-derived peptide or an unrelated peptide derived from mucin 1 (MUC1). The Tr1 phenotype and the specificity for p53 of these cells were confirmed by multicolor flow cytometry. Moreover, the Tr1 cell-mediated suppression of T-cell proliferation was evaluated by CFSE-based flow cytometry, while their ability to alter the T-cell cytokine profile by ELISA and Luminex assays. The capacity of p53-induced Tr1 cells to suppress the generation and function of cytotoxic T lymphcoytes (CTLs) was assessed by flow cytometry and ELISPOT. Of note, low doses of the p53-derived peptide (p53low) induced greater numbers of Tr1 cells than the same peptide employed at high doses (p53high). Moreover, Tr1/p53low cells not secreted higher levels of interleukin-10 and transforming growth factor β1, but also mediated more robust suppressive effects on CTL proliferation than Tr1/p53high cells. Tr1/p53low cells, Tr1/p53high cells, as well as Tr1 cells generated with low doses of an unrelated MUC1-derived peptide were equally effective in suppressing the expansion and antitumor activity of p53-reactive CTLs. p53low induced the expansion of highly suppressive p53-reactive Tr1 cells. However, the capacity of these Tr1 cells to suppress the generation and function of p53-reactive CTLs was independent of their antigen-specificity.
Keywords: adaptive Treg, antigen-specific Treg, immunosuppression, p53-induced Treg, wild-type p53 peptide
Introduction
Regulatory T cells (Tregs), a small subset of CD4+ T cells, have been shown to play an essential role in maintaining immune homeostasis and tolerance.1 Studies in mice have established that the absence of Treg results in severe autoimmune reactions.2 In cancer, both the frequency and immunosuppressive activity of Tregs are increased.3-5 The accumulation of highly immunosuppressive Tregs within neoplastic lesions, in tumor-draining lymph nodes, and in the peripheral blood of cancer patients has been shown to contribute to the establishment of a local and systemic immunosuppressive environment, promoting the escape of developing neoplasms from the immune system.6 Human Tregs are a heterogenous group of cells, consisting of various subsets.7 At least 2 of these subsets have been precisely characterized: 1) thymus-derived naturally-occurring Tregs (nTregs), which mediate immunosuppressive effects mainly in a cell contact-independent manner,8,9 and 2) adaptive or inducible Tregs (iTregs), also known as Tr1 cells, which presumably originate from conventional CD4+ T (Tconv) cells in the periphery, upon interaction with cognate antigens.10,11 Recently, a more complex functional classification of circulating human Tregs has been proposed, suggesting that each subset of effector CD4+ T cells is regulated by a unique Treg counterpart.7 While this functional heterogeneity may in part explain the various immunosuppressive mechanisms attributed to Tregs,12 it may complicate the monitoring of potential changes in Treg frequency and function in pathological settings.
The existence and origin of Ag-specific Tregs have been a matter of controversy. Although antigen-specific Tregs have been reported to accumulate in the course of infectious diseases and cancer,13-17 it appears that thymus-derived nTregs can suppress all types of immune responses regardless of antigen specificity. In contrast, Tr1 cells are generally considered to be antigen-specific, mostly because their expansion strictly relies on antigenic stimulation. However, it is unclear whether this requirement also applies to the immunosuppressive functions of iTregs. Systemic therapies aimed at augmenting the activity of iTregs in vivo, given to control autoimmune disorders or transplantat rejection, result indeed in prominent off-target effects, including a generalized state of immunosuppression.18 In the course of infection and cancer, the indiscriminate depletion of Tregs might compromise the beneficial effects of therapy by promoting autoimmune reactions. Therefore, the possibility to identify and modulate the functional profile of antigen-specific Tregs would constitute a valuable tool for the development of novel and the refinement of existing immunotherepies.
In various animal models, Tregs have been shown to recognize organ-specific autoantigens.19 In addition, antigen-specific Tregs appear to be more efficient in preventing autoimmune reactions than polyclonal Tregs.20 Previous studies have demonstrated the existence of HIV-1-reactive or cytomegalovirus (CMV)-reactive Tregs in the peripheral blood of patients.21-23 Taken together, these findings provide a rationale for examining the antigen specificity of iTregs in cancer, in particular relative to the induction/expansion of iTregs as opposed to their immunosuppressive activity. There is ample evidence that tumor-associated antigens (TAAs) are often overexpressed in malignant cells and are recognized by circulating autologous T cells.24 The TP53 gene is mutated in a vast majority of cancer patients,25 resulting in the overexpression of both mutated and wild-type (WT) p53 epitopes that are recognized by T cells.26 Here, we report the phenotype and immunosuppressive functions of Tr1 cells generated in culture in response to a WT p53-derived (p53264–272) peptide. The phenotypic and functional characteristics of these cells were evaluated together with their impact on the cytotoxic activity of autologous WT p53264–272-specific cytotoxic T lymphocytes (CTLs).
Results
Phenotypic analysis of Tr1 cells generated in the presence of different amounts of a WT p53-derived peptide
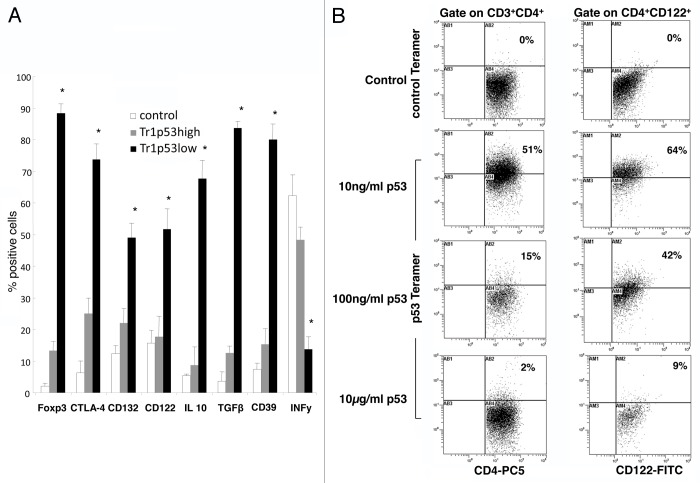
Using a modification of a previously established co-culture system, Tr1 cells were generated from CD4+CD25- T cells in the presence of immature dendritic cells (iDCs) pulsed with various concentrations of WT p53108–122. Thereafter, the phenotype of proliferating T cells was evaluated by flow cytometry. Gating on CD3+CD4+ cells, we first established the concentration of WT p53108–122 required for the optimal generation of Tr1 cells. Thus, low doses of the p53 peptide (10 ng, p53low) generated cells expressing several cell-surface and intracellular markers that are generally associated with the Tr1 phenotype, including CD39, CD132, interleukin-10 (IL-10), transforming growth factor β1 (TGFβ1), forkhead box P3 (FOXP3), and cytotoxic T-lymphocyte-associated protein 4 (CTLA4) (Fig. 1). These cells expressed low levels of interferon γ (IFNγ) (Fig. 1A and B). Of note, Tr1 cells obtained by means of iDCs pulsed with p53low (Tr1/p53low cells) expressed significantly higher levels of all Tr1 cell markers than Tr1 cells generated in the presence of high peptide concentrations (Tr1/p53high cells) (Fig. 2A). Similar results were obtained when WT p53108–122 was replaced by a mucin 1 (MUC1)-derived peptide (Fig. S1). Hereafter, Tr1/MUC1low cells are referred to as Tr1/MUC1 cells. To confirm the hypothesis that Tr1 cells generated in the presence of WT p53108–122-pulsed iDCs recognized WT p53108–122, the former were stained with a p53108–122-specific tetramer. Up to 51% of CD3+CD4+ and 64% of CD4+CD122+ Tr1/p53low cells were indeed found to be specific for WT p53108–122 (Fig. 2B). Much fewer Tr1/p53high cells were tetramer+ (P < 0.05). As expected, all Tr1 cells generated in the absence of WT p53108–122 failed to stain positively upon incubation with the p53108–122-specific tetramer, as did WT p53108–122-specific Tr1 cells stained with a control tetramer.
Figure 1. Phenotypic characteristics of p53-specific Tr1 cells generated in vitro. (A) Cytofluorometric analysis of Tr1 cells generated in the presence of autologous immature dendritic cells (iDCs) pulsed with various doses of a wild-type p53-derived (p53108–122) peptide. CD4+CD25− T cells cultured for 10 d in the presence of 150 IU/mL interleukin-2 and unpulsed iDCs served as control cells. Data are means ± SD (n = 5 experiments, performed with cells from different donors). (B) Phenotype of Tr1 cells generated in the presence of autologous iDCs pulsed with various doses of p53108–122. One representative experiment out of 5 performed with cells from different donors is shown.
Figure 2. Phenotype and antigen specificity of p53-specific Tr1 cells generated in vitro. (A) Cytofluorometric analysis of Tr1 cells generated in the presence of autologous immature dendritic cells (iDCs) pulsed with various doses of a wild-type p53-derived (p53108–122) peptide. CD4+CD25− T cells cultured for 10 d in the presence of 150 IU/mL interleukin-2 and unpulsed iDCs served as control cells. Data are means ± SD (n = 5 independent experiments); *P < 0.05. (B) Tr1 cells were stained with a control or a p53108–122-specific tetramer, followed by the quantification of tetramer+ T cells by flow cytometry. One representative experiment out of 5 performed with cells from different donors is shown.
Suppression of T-cell proliferation by p53-induced Tr1 cells
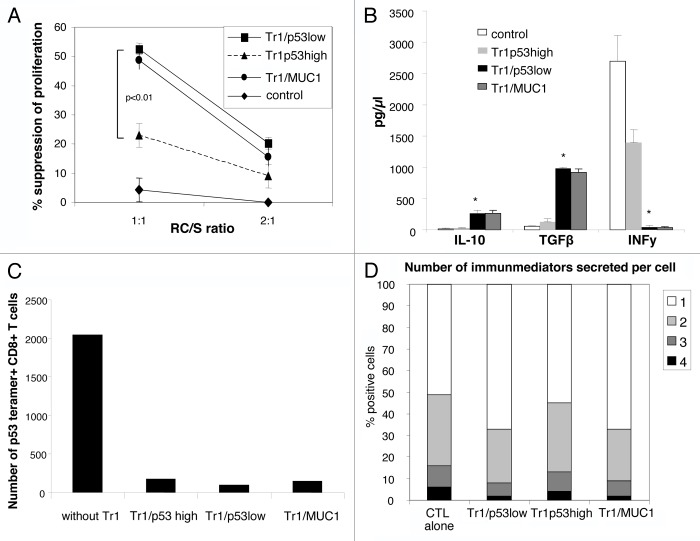
To compare the immunosuppressive activity of Tr1/p53low and Tr1/p53high cells, we incubated them with autologous CFSE-labeled CD4+CD25− responder cells (RCs) at different ratios. After 5 d of co-culture, the mean suppressor activity of Tr1/p53low cells was significantly higher than that of the Tr1/p53high counterparts (52 ± 3% vs. 20 ± 1%, P < 0.01; Fig. 3A). Tr1 cells from control cultures did not suppress the proliferarion of RCs, whereas Tr1/MUC1 cells did so nearly as well as their Tr1/p53low counterparts (Fig. 3A). These results indicate that Tr1/p53low cells, most of which are specific for WT p53108–122, mediate a higher immunosuppressive activity than Tr1/p53high cells.
Figure 3. Suppressor activity and cytokine profile of p53-specific Tr1 cells generated in vitro. (A) MACS-sorted CD4+CD25− cells were labeled with CSFE and stimulated with plate-bound OKT-3 and soluble anti-CD28 antibodies in the presence of Tr1/p53low, Tr1/p53high, Tr1/MUC1, or control cells and 150 IU/mL interleukin-2 for 5 d. Thereafter, cells were analyzed by flow cytometry upon gating on CD4+CFSE+ T cells by means of the ModFit program. The percentage of inhibition in CD4+CD25- cell proliferation as mediated by Tr1/p53low, Tr1/p53high, Tr1/MUC1, or control cells at 2 different responsder cell/suppressor (RC/S) ratios is shown. Data are means ± SD (n = 5 independent experiments). (B) Isolated Tr1/p53low, Tr1/p53high, Tr1/MUC1, or control cells were stimulated with OKT-3 and anti-CD28 antibodies and cultured in 96-well plates for 24 h. Culture supernatants were then harvested and analyzed for interleukin-10, IL-10, transforming growth factor β1 (TGFβ1), and interferon γ (IFNγ) by Luminex assays. Data are means ± SD (n = 5 independent experiments); *P < 0.05. (C) Cytotoxic T lymphocytes (CTLs) were generated in the presence of autologous dendritic cells pulsed with a wild-type p53-derived (p53108–122) peptide. Tr1 cells were added to CTL cultures on day 0. After 3 rounds of antigenic stimulation, CTLs were harvested and stained with a p53108–122-specific tetramer and analyzed by flow cytometry. One representative experiment out of 3 performed is shown. (D) Tr1 cells and CTL were co-incubated for 24 h followed by the cytofluorometric determination of cytokine profiles. Data are means ± SD (n = 5 independent experiments).
Cytokine production by p53-induced Tr1 cells
Freshly generated Tr1/p53low and Tr1/p53high cells were stimulated for 24 h with OKT-3 anti-CD28 monoclonal antibodies (mAbs), followed by the quantification of cytokine levels in colture supernatants by ELISA and Luminex assays. As expected, Tr1/p53low produced significantly higher levels of IL-10 and TGFβ1 than their Tr1/p53high counterparts. Conversely, IFNγ was secreted to much lower amounts by Tr1/p53low than by Tr1/p53high cells (Fig. 3B).
Suppression of WT p53264–272-specific CTL induction by p53-induced Tr1 cells
To test if and how p53-induced Tr1 cells would influence the induction of WT p53264–272-specific CTLs, freshly generated Tr1 cells were added to CTL cultures at the beginning of the induction period. The addition of Tr1 cells to CTL cultures (1:10 Tr1/CTL ratio) on day 0 significantly suppressed the induction of p53264–272-specific CTLs (Fig. 3C). Of note, such an immunosuppressive activity did not require the re-stimulation of Tr1 cells with peptides and was observed with Tr1/p53low, Tr1/p53high, as well as with Tr1/MUC1 cells.
Cytokine profile of CTLs suppressed p53-induced Tr1 cells
Intracellular cytokine expression was examined in CTLs that had been culture alone or together with Tr1/p53low, Tr1/p53high, or Tr1/MUC1 cells using a flow cytometry-based polyfunctional assay. In particular, we analyzed the expression of IL-2, IFNγ, tumor necrosis factor α (TNFα), and CD107a. Thus, Tr1/p53low cells suppressed polyfunctional cytokine responses (i.e., the number of cytokines expressed by a single cell) by CTLs to a significantly greater extent than their Tr1/p53high counterparts (P < 0.05). Of note, the suppressive activity of Tr1/MUC1 on cytokine expression was similar to that of Tr1/p53low cells (Fig. 3D).
p53-induced Tr1 cells suppress p53264–727-specific CTL functions
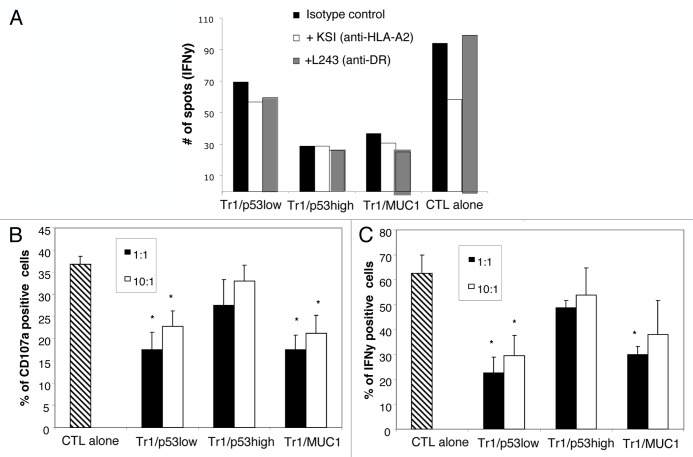
Next, we studied the influence of p53-induced Tr1 cells on the ability of p53264–272-specific CTLs to recognize malignant cells by means of IFNγ-specific ELISPOT assays. To this aim, we incubated p53264–272-specific CTLs and IFNγ-pretreated cancer cells in the presence or absence of Tr1 cells (1:10 Tr1/CTL ratio), either in direct contact or by means of transwells (in selected assays). The anticancer effector functions of p53264–272-specific CTLs were signficiantly suppressed by admixed Tr1 cells (Fig. 4A). Similar results were obtained when transwells were employed (data not shown), suggesting that the immunosuppressive activitiy of Tr1 cells in this context was cell-contact independent. When p53264–272-specific CTLs were incubated with IFNγ-pretreated cancer cells in the absence of Tr1 cells, tumor cells were recognized, leading to the secretion of IFNγ. Such a functional response was blocked by a HLA-A2-specific mAb (KS1), confirmed that the recognition of tumor cells by CTLs was HLA-A2-restricted. Conversely, no alterations in the CTL response were recorded upon the administration of isotype-matched control or anti-HLA Class II (L243) mAbs (Fig. 4A). The antitumor activity of p53264–272 peptide-specific CTLs was inhibited almost equally by Tr1/p53low, Tr1/p53high, and Tr1/MUC1 cells. The immunosuppressive activity of all these Tr1 cells could not be influenced by the KS1 antibody (Fig. 4A).
Figure 4. Suppression of CTL functions by p53-specific Tr1 cells generated in vitro. (A) Cytotoxic T lymphocytes (CTLs) were incubated with Tr1/p53low, Tr1/p53high, or Tr1/MUC1 cells, followed by the quantification of interferon γ (IFNγ) secretion by ELISPOT assays. Data are from one out of 3 independent experiments performed. (B and C) CTLs were cultured in the presence or in absence of p53-specific Tr1 cells generated in vitro. CTL degranulation (CD107a exposure) and intracellular IFNγ expression levels were then analyzed by flow cytometry. Data are means ± SD (n = 5 independent experiments); *P < 0.05.
p53-induced Tr1 cells suppress p53264–272-specific CTL degranulation and IFNγ expression
To further confirm our results, we quantified the degranulation of CTLs and their intracellular IFNγ expression levels upon co-incubation with p53-induced Tr1 cells. Thus, both CTL degranulation and intracellular IFNγ expression levels were more robustly reduced by Tr1/p53low cells than by their their Tr1/p53high counterparts (P < 0.05). Tr1/MUC-1 cells suppressed CTL degranulation and IFNγ expression similarly to Tr1/p53low cells (Fig. 4B and C). Of note, the addition of KSI or L243 mAbs did not alter the ability of p53-induced Tr1 cells to suppress CTL degranulation and reduce IFNγ expression levels (data not shown).
Discussion
In this study, aimed at determining the HLA-DR4-restricted immunosuppressive reactivity of human iTregs generated in vitro, we characterized the phenotype and functional profile of Tr1 cells arising from circulating CD4+CD25- T cells exposed to iDCs pulsed with a WT p53-derived peptide. CD4+CD25− T cells were isolated from the PBMCs of healthy individuals and cultured under conditions that had previously been optimized for Tr1 cell expansion.27 In particular, this study was undertaken to gain insights into the antigen specificity of the immunosuppressive effects mediate by Tr1 cells that—at the time of induction and expansion—were recognize a self-antigen. Using a well-defined system for the generation of Tr1 cells in vitro,28 we compared the effects mediated by WT p53108–122 peptide-specific Tr1 cells vs. those mediated by Tr1 cells specific for an unrelated, MUC1-derived peptide. Interestingly, while the induction of Tr1 cells was found to be peptide-specific, the immunosuppression mediated by expanded Tr1 cells was not.
Our study also shows that p53-specific Tr1 cells generated in the presence of iDCs pulsed with a low peptide dose strongly suppress the generation of CTLs and their immunological activity. Indeed, the expression levels of several established Tr1 cell markers were higher in p53-specific cells generated with low (as opposed to high) peptide doses. These data strongly suggest that the antigen dose influences the frequency and functions of Tr1 cells expanding in vitro. Other groups have observed an inverse correlation between the antigen dose and the generation of Tr1 cells in mice28 and humans.29
Once the antigen-specific Tr1 cells are generated, they are expected to suppress the functions of effector T cells in an antigen-restricted manner. Interestingly, we observed that MUC1-induced Tr1 cells were as effective as their p53-induced counterparts in suppressing both the accumulation and antitumor activitiy of p53-specific CTLs. Further, neither HLA-A2-specific nor HLA DR-specific antibodies blocked the immunosuppressive actovity of Tr1 cells, suggesting that these effects are neither HLA-restricted nor (presumably) antigen-specific.
While our in vitro model of Tr1 cell induction and function have limitations, especially when compared with in vivo system, it nevertheless allows for the analysis of the antigen specificity of Tr1 cells generated in a microenvironment that approximates realistic conditions. The presence of TAAs recognized by T cells in cancer patients is established, and TAA-specific CTLs, including p53-specific CTLs, are often detectable within neoplastic lesions as well as in the peripheral blood of cancer patients.30 It has been suggested that iTregs play a key role in suppressing anticancer functions of tumor-reactive CTLs.31 However, it remains unclear whether this clinically relevant acvitity is mediated by all iTregs or a TAA-specific subset of iTregs. Our study provides convincing evidence in support of a TAA-driven generation and expansion of iTregs. However, once generated, TAA-specific iTregs fall under the microenvironmental control and may rapidly be converted into “universal” suppressor cells. In this context, iTregs lose their HLA restriction and T-cell receptor (TCR)-determined specificity to acquire the capability to suppress a wide range (if not all) of adaptive immune responses. Neoplastic lesions attempting to limit antitumor immunity presumably play a critical role in this context.
Thus, the nature of the local inflammatory microenvironment appears to significantly affect (if not entirely control) the activity of tumor-infiltrating suppressor cells. The remarkable plasticity of these cells guarantees that not just a single TAA-specific immune response, but the responses to several TAAs, are effectively suppressed, providing the tumor with a means to escape immunosurveillance. While speculative, the scenario of a tumor-mediated conversion of initially TAA-specific iTregs into broadly immunosuppressive, HLA-unrestricted suppressors is an excellent example of how human neoplasms may subvert adaptive immune responses to their own benefit.
Materials and Methods
Healthy volunteers
Peripheral venous blood samples were obtained from HLA-DR4+HLA2+ healthy donors. Prior to phlebotomy, all subjects signed an informed consent approved by the Institutional Review Board of the University of Pittsburgh.
PBMC collection
Blood samples (20–30 mL) were drawn into heparinized tubes and centrifuged on Ficoll-Hypaque gradients (GE Healthcare Bioscience). PBMCs were recovered, washed in AIM-V medium (Invitrogen), quantified by upon staining with trypan blue (to obtain information on viability), and stored frozen until used for experiments.
HLA typing
PBMCs were phenotyped for the expression of HLA-DR4 and HLA-A2 by flow cytometry using anti-HLA-DR4 and anti-HLA-A2 mAbs (Beckman Coulter) as well as isotype-matched (IgG) control antibodies.
Antibodies
The following fluorochrome-conjugated anti-human mAbs were used in flow cytometry assessments: anti-CD3-ECD, anti-CD4-PC5, anti-CD25-FITC, anti-CD25-PE, anti-IFNα-PE (all from Beckman Coulter), anti-CD39-PE, anti-FOXP3-FITC (both from eBioscience), and anti-CD122-FITC, anti-CD132-FITC, anti-CTLA4-PE, anti-GITR-FITC, anti-IL-10-PE, anti-TGFβ1-PE (all from R&D Systems). Isotype-matched antibodies, which served as negative controls for surface as well as intracellular stainings, were purchased from Beckman Coulter. Before use, all mAbs were titrated to determine the optimal staining dilutions using activated as well as non-activated PBMCs. The p53108–122-specific tetramer and the scrambled control tetramer were manufactured and labeled with phycoerythrin (PE) by the NIH.
Surface and intracellular staining
Cells were stained for cytofluorometric assessments as previously described.5 Briefly, cells were incubated with mAbs specific for surface markers for 30 min at 4 °C (in the dark) and then fixed with 2% (w/v) paraformaldehyde (PFA) in PBS for 15 min. Thereafter, cells were permeabilized with 0.1% (w/v) saponin in PBS for 30 min and stained with mAbs specific for intracellular markers for 30 min at 4 °C (in the dark). Cells were washed twice with 0.1% saponin in PBS, resuspended in flow buffer (1× PBS, 2% fetal bovine serum, 0.2% azide) and immediately analyzed by flow cytometry. Appropriate isotype-matched control mAbs were included for each sample.
Flow cytometry
Flow cytometry was performed on a EPICS® XL-MCL flow cytometer (Beckman Coulter). Acquisition and analysis were restricted to the lymphocyte gate, based on characteristic forward scatter (FSC) and side scatter (SSC) properties. FSC and SSC were set in a linear scale, and at least 105 cells were acquired for analysis, which was performed using the Coulter Expo 32 v l.2 software. Additional analyses were performed upon gating on the CD4+ or CD8+ T-cell subset.
Tr1 in vitro system
PBMCs from HLA-A2+/DR4+ healthy subjects were processed for the generation of Tr1 cells using an in vitro co-culture system previously established in our laboratory.27 Briefly, upon the isolation of PBMCs, monocytes were separated from the lymphocyte fraction based on their capacity to adhere to plastic. Monocytes were differentiated into iDCs by culture in AIM-V medium supplemented with 1000 IU/mL granulocyte macrophage colony-stimulating factor (GM-CSF) and 4 ng/mL IL-4 for 7 d. CD4+CD25− cells were isolated from the lymphocyte fraction using the Regulatory T-cell Isolation Kit (Miltenyi Biotech), and AutoMACS. iDCs were pulsed with various doses of the WT p53108–122 peptide or MUC1-derived peptide for 4 h at 4 °C and kept on ice for 1 h before harvesting. CD4+CD25− cells (1 × 106) were then co-incubated in flat-bottom 24-well plates with autologous pulsed iDCs (1 × 105) using complete AIM-V medium supplemented with 10 IU/mL IL-2, 20 IU/mL IL-10, and 20 IU/mL IL-15. Medium was changed on days 3 and 6. On day 9, the culture medium was replaced with fresh medium supplemented with 1 μg/mL OKT-3 and 1 μg/mL Brefeldin A. On day 10, lymphocytes and cell supernatants were separately harvested. In parallel, control CD4+CD25− cells were cultured for 10 d in complete medium supplemented with 150 IU/mL IL-2 (in 24-well plates), under standard conditions (37 °C, 5% CO2 in air).
In vitro generation of peptide-specific CD8+ T cells using peptide-pulsed autologous iDCs
Autologous CD8+ T cells were negatively selected from nonadherent PBMCs using the CD8+ T-cell Isolation Kit (Miltenyi Biotech) and co-cultured with peptide-loaded iDCs in 96-well plates for 6 d, as previously described.32,33 To generate the HLA-A2.1-restricted peptide-specific CTLs, iDCs were exposed to either WT p53264–272 (LLGRNSFEV) or an unrelated MUC1-derived peptide. On day 7, RCs were re-stimulated with peptide-pulsed iDCs in AIM-V medium supplemented with 20 IU/mL IL-2, 10 ng/mL IL-7, and 10 ng/mL IL-21. RCs were then stimulated with irradiated, peptide-pulsed autologous PBMCs. After 2 or more rounds of stimulation, responsive effector cells were tested. To determine the immunosuppressive effects of Tr1 cells on CTL generation, Tr1 cells generated as previously described were added to the co-cultures at different Tr1/CTL ratios (1:1, 1:2, and 1:10) on day 0.
Tetramer staining and cytofluorometric analysis of in vitro generated CTLs
CD8+ cells stimulated with HLA-A2-restricted WT p53264–272 were incubated with the corresponding HLA-A2-restricted p53-specific tetramer (1:100 dilution) for 45 min at room temperature and in the dark. CTLs were then stained with anti-CD8-FITC, anti-CD5-APC, anti-CCR7-PC7, and anti-CD45RA-ECD mAbs for 30 min at 4 °C, washed twice with flow buffer, fixed with 2% (w/v) PFA in PBS, and analyzed by flow cytometry as previously described.28,29
ELISPOT assay
CTLs recognizing HLA-A2.1-restricted WT p53264–272 or the MUC1-derived peptide (control conditions) as well as Tr1/CTL co-cultures were maintained for 24 h prior to being processed for ELISPOT assays. Different Tr1/CTL ratios were used (2:1, 1:1, or 0:1). ELISPOT assays were performed using HLA-A2+ head and neck squamous cell carcinoma PCI-13 cells as stimulators, upon pre-treatment with 1000 U IFNγ (to upregulate HLA-A2 expression).34
Suppression assays
Tr1 cells obtained from the in vitro system were tested for their capacity to suppress the proliferation of CD4+CD25− RCs, as previously described.13 Briefly, 1 × 105 CFSE-labeled autologous CD4+CD25− cells were co-incubated in flat-bottom 96-well plates with Tr1 suppressor (S) cells at a 1:1 or 2:1 ratio. To induce proliferation, RCs were stimulated with 2 μg/mL plate-bound OKT-3 and 2 μg/mL soluble anti-CD28 mAbs (Miltenyi), in the presence of 150 IU/mL IL-2 for 5 d. Thereafter, cells were harvested and analyzed by flow cytometry. CFSE data were analyzed using the ModFit software (Vertity Software House; Topsham) as previously described.4
CTL cytokine profile
Tr1 cells obtained from co-cultures were evaluated for the ability to suppress the expression of cytokines by CTLs by using a previously established cytofluorometric assay.35 To this aim, CTLs were incubated with freshly generated Tr1 cells, and the following molecules were studied using multicolor flow cytometry: IFNγ, IL-2, TNFα, and +CD107a. Cytokine profiles were analyzed using the SPICE software v 4.3 (Bioinformatics and Computational Biosciences Branch, NIAID).
ELISA and Luminex
Freshly generated Tr1 cells were placed in fresh medium supplemented with 1 μg/mL OKT-3 and 1 μg/mL anti-CD28 mAbs. Culture supernatants were collected after 24 h and stored frozen until analysis. TGFβ levels in acidified supernatants were analyzed by ELISA (R&D Systems), while the concentration of IFNγ and IL-10 was evaluated by the Luminex technology, using a human cytokine 10-plex mAb bead kit (Biosource/Invitrogen). ELISA and Luminex assays were performed according to the manufacturers’ instructions.
CTL degranulation assays
Ten thousand freshly generated CTLs were incubated with peptide-pulsed iDCs in the presence of a CD107a-specific antibody (Beckman Coulter) and Tr1/p53low, Tr1/p53high, or Tr1/MUC1 cells for 4 h. When appropriate, anti-HLA-A2 (KS1) or anti-HLA-DR (L243) mAbs were added to the cultures. At the end of the incubation period, cells were harvested and processed for the quantification of CD8+IFNγ+ and CD8+CD107a+ T cells by flow cytometry.
Statistical analyses
Data are presented as means ± SD (n = at least 3 experiments). Student’s t-tests with the threshold value set to P < 0.05 were used to determine statistical significance.
Supplementary Material
Glossary
Abbreviations:
- Ag
antigen
- FSC
forward scatter
- iDC
immature dendritic cell
- iTreg
inducible regulatory T cell
- mAb
monoclonal antibody
- nTreg
naturally-occuring Treg
- PBMC
peripheral blood mononuclear cell
- RC
responder cell
- SSC
side scatter
- TAA
tumor-associated antigen
- Tconv
conventional CD4+ T cells
- Treg
regulatory T cell
- WT
wild-type
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
Supplementary materials may be found here:
http://www.landesbioscience.com/journals/oncoimmunology/article/25514
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25514
References
- 1.Whiteside TL, Schuler P, Schilling B. Induced and natural regulatory T cells in human cancer. Expert Opin Biol Ther. 2012;12:1383–97. doi: 10.1517/14712598.2012.707184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol. 2011;187:2061–6. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–12. [PubMed] [Google Scholar]
- 4.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Lenzner DE, Jackson EK, et al. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin Cancer Res. 2009;15:6348–57. doi: 10.1158/1078-0432.CCR-09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6301–11. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- 6.Whiteside TL, Mandapathil M, Szczepanski M, Szajnik M. Mechanisms of tumor escape from the immune system: adenosine-producing Treg, exosomes and tumor-associated TLRs. Bull Cancer. 2011;98:E25–31. doi: 10.1684/bdc.2010.1294. [DOI] [PubMed] [Google Scholar]
- 7.Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. 2012;119:4430–40. doi: 10.1182/blood-2011-11-392324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 11.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065X.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 12.Whisler RL, Stobo JD. Heterogeneity of murine regulatory T cells. I. Subpopulations of amplifier and suppressor T cells. J Exp Med. 1976;144:398–413. doi: 10.1084/jem.144.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betts G, Jones E, Junaid S, El-Shanawany T, Scurr M, Mizen P, et al. Suppression of tumour-specific CD4⁺ T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut. 2012;61:1163–71. doi: 10.1136/gutjnl-2011-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addey C, White M, Dou L, Coe D, Dyson J, Chai JG. Functional plasticity of antigen-specific regulatory T cells in context of tumor. J Immunol. 2011;186:4557–64. doi: 10.4049/jimmunol.1003797. [DOI] [PubMed] [Google Scholar]
- 15.Hindley JP, Ferreira C, Jones E, Lauder SN, Ladell K, Wynn KK, et al. Analysis of the T-cell receptor repertoires of tumor-infiltrating conventional and regulatory T cells reveals no evidence for conversion in carcinogen-induced tumors. Cancer Res. 2011;71:736–46. doi: 10.1158/0008-5472.CAN-10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–18. doi: 10.1016/S1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 17.Brusko TM, Koya RC, Zhu S, Lee MR, Putnam AL, McClymont SA, et al. Human antigen-specific regulatory T cells generated by T cell receptor gene transfer. PLoS One. 2010;5:e11726. doi: 10.1371/journal.pone.0011726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fourcade J, Sun Z, Kudela P, Janjic B, Kirkwood JM, El-Hafnawy T, et al. Human tumor antigen-specific helper and regulatory T cells share common epitope specificity but exhibit distinct T cell repertoire. J Immunol. 2010;184:6709–18. doi: 10.4049/jimmunol.0903612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seddon B, Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J Exp Med. 1999;189:877–82. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol. 2011;186:6329–37. doi: 10.4049/jimmunol.1100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angin M, King M, Altfeld M, Walker BD, Wucherpfennig KW, Addo MM. Identification of HIV-1-specific regulatory T-cells using HLA class II tetramers. AIDS. 2012;26:2112–5. doi: 10.1097/QAD.0b013e328358cc75. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kared H, Lelièvre JD, Donkova-Petrini V, Aouba A, Melica G, Balbo M, et al. HIV-specific regulatory T cells are associated with higher CD4 cell counts in primary infection. AIDS. 2008;22:2451–60. doi: 10.1097/QAD.0b013e328319edc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwele S, Fischer AM, Brestrich G, Wlodarski MW, Wagner L, Schmueck M, et al. Cytomegalovirus-specific regulatory and effector T cells share TCR clonality--possible relation to repetitive CMV infections. Am J Transplant. 2012;12:669–81. doi: 10.1111/j.1600-6143.2011.03842.x. [DOI] [PubMed] [Google Scholar]
- 24.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–65. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 25.Royds JA, Iacopetta B. p53 and disease: when the guardian angel fails. Cell Death Differ. 2006;13:1017–26. doi: 10.1038/sj.cdd.4401913. [Review] [DOI] [PubMed] [Google Scholar]
- 26.Ito D, Albers A, Zhao YX, Visus C, Appella E, Whiteside TL, et al. The wild-type sequence (wt) p53(25-35) peptide induces HLA-DR7 and HLA-DR11-restricted CD4+ Th cells capable of enhancing the ex vivo expansion and function of anti-wt p53(264-272) peptide CD8+ T cells. J Immunol. 2006;177:6795–803. doi: 10.4049/jimmunol.177.10.6795. [DOI] [PubMed] [Google Scholar]
- 27.Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion of human T regulatory type 1 cells in the microenvironment of cyclooxygenase 2 overexpressing head and neck squamous cell carcinoma. Cancer Res. 2007;67:8865–73. doi: 10.1158/0008-5472.CAN-07-0767. [DOI] [PubMed] [Google Scholar]
- 28.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–11. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long SA, Rieck M, Tatum M, Bollyky PL, Wu RP, Muller I, et al. Low-dose antigen promotes induction of FOXP3 in human CD4+ T cells. J Immunol. 2011;187:3511–20. doi: 10.4049/jimmunol.1003880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandruzzato S, Rossi E, Bernardi F, Tosello V, Macino B, Basso G, et al. Large and dissimilar repertoire of Melan-A/MART-1-specific CTL in metastatic lesions and blood of a melanoma patient. J Immunol. 2002;169:4017–24. doi: 10.4049/jimmunol.169.7.4017. [DOI] [PubMed] [Google Scholar]
- 31.Yong X, Xiao YF, Luo G, He B, Lü MH, Hu CJ, et al. Strategies for enhancing vaccine-induced CTL antitumor immune responses. J Biomed Biotechnol. 2012;2012:605045. doi: 10.1155/2012/605045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann TK, Loftus DJ, Nakano K, Maeurer MJ, Chikamatsu K, Appella E, et al. The ability of variant peptides to reverse the nonresponsiveness of T lymphocytes to the wild-type sequence p53(264-272) epitope. J Immunol. 2002;168:1338–47. doi: 10.4049/jimmunol.168.3.1338. [DOI] [PubMed] [Google Scholar]
- 33.Ito D, Visus C, Hoffmann TK, Balz V, Bier H, Appella E, et al. Immunological characterization of missense mutations occurring within cytotoxic T cell-defined p53 epitopes in HLA-A*0201+ squamous cell carcinomas of the head and neck. Int J Cancer. 2007;120:2618–24. doi: 10.1002/ijc.22584. [DOI] [PubMed] [Google Scholar]
- 34.Connolly N, Riddler S, Stanson J, Gooding W, Rinaldo CR, Ferrone S, et al. Levels of antigen processing machinery components in dendritic cells generated for vaccination of HIV-1+ subjects. AIDS. 2007;21:1683–92. doi: 10.1097/QAD.0b013e32825eabbc. [DOI] [PubMed] [Google Scholar]
- 35.Macatangay BJ, Szajnik ME, Whiteside TL, Riddler SA, Rinaldo CR. Regulatory T cell suppression of Gag-specific CD8 T cell polyfunctional response after therapeutic vaccination of HIV-1-infected patients on ART. PLoS One. 2010;5:e9852. doi: 10.1371/journal.pone.0009852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.