Abstract
Background
Nosocomial infections (NI) increase morbidity and mortality. Studies of their prevalence in single institutions can reveal trends over time and help to identify risk factors.
Methods
In March and April 2010, data were prospectively recorded from all inpatients at the Hannover Medical School (Germany) except those treated in the pediatric, psychosomatic, and psychiatric services. The data were acquired systematically by chart review and by interviews with the medical staff. Infections were classified according to the definitions of the Centers for Disease Control and Prevention (CDC). Information was obtained on underlying diseases, invasive procedures, the use of antibiotics, devices (the application of specific medical techniques such as drainage, vascular catheters, etc.), and detected pathogens.
Results
Of the 1047 patients studied, 117 (11.2%) had a total of 124 nosocomial infections, while 112 (10.7%) had 122 community-acquired infections. The most common NI were surgical site infections (29%), infections of the gastrointestinal tract (26%) and respiratory tract (19%), urinary tract infections (16%), and primary sepsis (4%). The most common pathogens were Escherichia coli, coagulase-negative staphylococci, Candida spp., Enterococcus spp., and Pseudomonas aeruginosa. Multivariable regression analysis revealed the following independent risk factors for NI: antibiotic treatment in the last 6 months (odds ratio [OR] = 2.9), underlying gastrointestinal diseases (OR = 2.3), surgery in the last 12 months (OR = 1.8), and more than two underlying diseases (OR = 1.7). Each additional device that was used gave rise to an OR of 1.4. Further risk factors included age, length of current or previous hospital stay, trauma, stay on an intensive care unit, and artificial ventilation.
Conclusion
In this prevalence study, NI were a common complication. Surgical site infections were the single most common type of NI because of the large number of patients that underwent surgical procedures in our institution. More investigation will be needed to assess the benefit of prevalence studies for optimizing appropriate, effective preventive measures.
Data from Germany’s Hospital Infection Surveillance System (Krankenhaus-Infektions-Surveillance-System, KISS) (www.nrz-hygiene.de) and the national prevalence study NIDEP-1 conducted in 1994 (1) show that 400 000 to 600 000 nosocomial infections (NI) occur annually in Germany, with 10 000 to 15 000 deaths (mortality = 2.6%; up to 10% in intensive care units) (2). The length of stay in an intensive care unit is prolonged by an average 5.3 (± 1.6) days if the patient acquires an NI (3). Apart from the high morbidity and mortality, NI is associated with higher costs: Graf et al. calculated additional expenditure of € 22 905 for surgical site infection following sternotomy (4). An investigation of the costs incurred by nosocomial pneumonia from Staphylococcus aureus revealed that additional charges of € 17 281 per patient could be attributed to methicillin resistance in S. aureus pneumonia (5).
Prevention of NI is therefore crucial, and adequate preventive measures have to be established. Particularly important in this regard is knowledge of the distribution of NI, the risk areas, and the patient-related risk factors. These efforts are supported by the 2011 amendment of the German Protection against Infection Act (Infektionsschutzgesetz, IfSG) and the related establishment and alignment of the hygiene regulations in the German federal states. These regulations created the conditions necessary for improvement of hygiene and medical quality in patient care.
Prevalence studies can reveal weaknesses which allow needed measures such as quality and process parameters to be established. This in turn allows good standards of hygiene to be secured, for example via the implementation of guidelines.
Studies of NI prevalence in various European countries show rates between 3.5% and 11.6% (1, 6– 10). Urinary tract infections (UTI) are the most frequent NI, followed by pneumonia, surgical site infection, and primary sepsis. These prevalence studies are multicenter investigations and seldom reflect the individual distribution of the different NI or the respective risk factors in medical facilities with particularly high rates of NI.
In the knowledge of the impending amendment of the IfSG and the lack of data on NI at high-level university hospitals with a focus on surgery, we decided to conduct a prospective study of the prevalence of NI. Our aims were to detect all infections (nosocomial and community-acquired), identify the risk factors for NI, and accordingly modify the practices of infection control in our own institution, introducing new prevention measures if necessary.
Methods
All inpatients treated at a university hospital with 1411 beds (Hannover Medical School) between 1 March 2010 and 30 April 2010, with the exception of those in the departments of pediatrics and psychiatry and psychosomatic diseases, were included in the prevalence study.
Infections were classified according to the definitions of the Centers for Disease Control and Prevention (CDC) (11). An infection was defined as nosocomial if the first signs of infection occurred more than 48 h after admission.
On every weekday members of the infection control team recorded, for each patient, all investigations, results, and notes made by nurses and physicians. The nursing and medical staff were interviewed on the day of the patient’s inclusion in the survey. Furthermore, patient-specific parameters such as demographic data, underlying diseases, invasive interventions, recent hospital admissions, and antibiotic treatment were recorded.
Descriptive statistical evaluation was accompanied by univariate and multivariate risk factor analysis, with the aim of identifying independent risk factors for the presence of at least one NI compared with patients without NI.
The methods are described in more detail in the eBox.
eBox. Methods.
Study design and definitions
A prospective prevalence study was carried out to determine the frequency of infections at the 1411-bed University Hospital of Hannover Medical School, where the focus is on surgery. In the year 2010 Hannover Medical School had 612 beds for surgical specialties (including gynecology, otorhinolaryngology, ophthalmology, and urology), 387 beds for internal medicine, and 150 intensive care beds. The study included all inpatients treated between 1 March 2010 and 30 April 2010. Patients admitted to the departments of psychiatry and psychosomatic diseases (136 beds) and pediatrics (181 beds) were excluded from the study.
Infections were classified according to the definitions of the centers for Disease Control and Prevention (CDC) (11). An infection was defined as nosocomial if the first signs of infection occurred more than 48 h after admission to the hospital. The primary endpoint was defined as occurrence of a community-acquired infection (CAI) or nosocomial infection (NI). Secondary endpoints were length of hospital stay and death for patients with NI. The risk factor analysis focused on establishment of the factors associated with the presence of NI. To this end, patients with NI were compared to patients without NI (including patients with CAI).
Data acquisition
All investigations and examinations, results, and notes written by nurses and physicians were recorded prospectively for every individual patient. The nursing and medical staff were interviewed on the day of the patient’s inclusion in the survey in order to ensure full data were obtained on the health and infection status of each patient.
Recording of the following data for each patient was laid down in advance in the study protocol: demographic data, reason for admission, underlying diseases, use of certain medical devices (e.g., urinary catheters, gastric and other tubes, vascular catheters, and drains), operations, recent hospital admissions, and antibiotic treatment. Trained members of the infection control team recorded data for the patients of three wards on each weekday. All infections were then classified exclusively by two trained members of the infection control team.
Statistical analysis
In the descriptive analysis, absolute numbers and percentages were calculated for categorical variables, median and interquartile range for continuous variables. The aim of further analysis was to identify independent risk factors for patients with one or more NI compared with all patients without NI. To this end, the univariate analysis compared these two groups and tested the differences. Fisher’s exact test or the chi-square test was used for categorical variables, the Wilcoxon rank-sum test for continuous variables.
In the multivariate analysis a logistic regression model was calculated with stepwise forward selection of variables. The p value for inclusion of a parameter in the model was set at 0.05, the limit for retention of variables in the model at 0.06. The multivariate analysis took account of all parameters/all patient data from the protocol/the univariate analysis with the exception of the parameter “current antibiotic treatment”. The resulting model was then adjusted according to the parameters age and sex.
The level of significance was set at 0.05. All analyses were performed using SPSS software (IBM SPSS statistics version 19, Somer, NY, USA).
Results
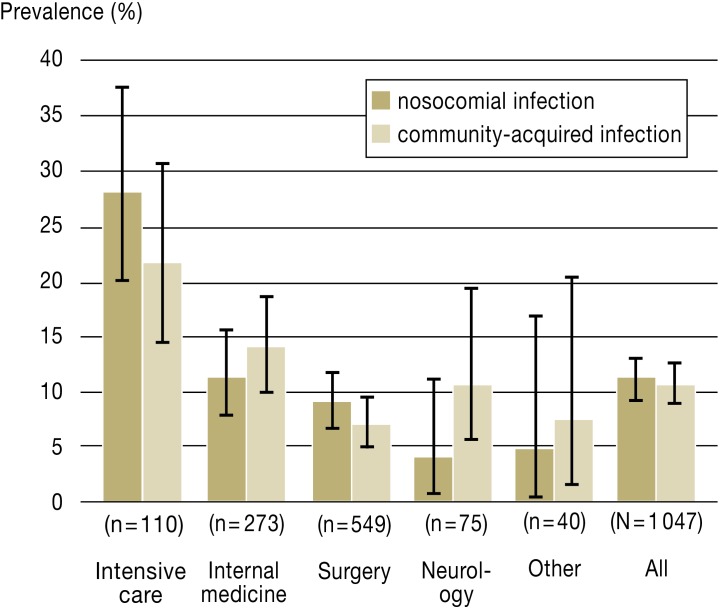
At the time of the prevalence study there was 96% occupancy of the 1094 beds included, meaning that 1047 patients could be evaluated. A total of 247 infections were found in 226 patients (overall prevalence 22%, 95% confidence interval [95% CI] 19.2 to 24.2). This included 112 patients (10.7%, 95% CI 8.9 to 12.7) with 122 community-acquired infections (CAI). There were 117 patients (11.2%, 95% CI 9.3 to 13.2) with 124 NI. The highest prevalence was found in intensive care units (NI 28.2%, 95% CI 20.2 to 37.7; CAI 21.8%, 95% CI 14.5 to 30.7), followed by internal medicine wards (NI 11.4%, 95% CI 7.8 to 15.7; CAI 13.9%, 95% CI 10 to 18.6) and surgery (NI 9.1%, 95% CI 6.8 to 11.8; CAI 7.1%, 95% CI 5.1 to 9.6). Only in intensive care units and on surgical wards was the prevalence of NI higher than that of CAI (Figure).
Figure.
Prevalence of infections among inpatients
The distribution of the most frequent kinds of infections is shown in Table 1. The predominant CAI were pneumonia (n = 28) and infections of the gastrointestinal tract (n = 25).
Table 1. Distribution of the types of infection.
| Type of infection | Number (%) (n=246) |
|---|---|
| Nosocomial infections | 124 (50) |
|
36 (29) |
|
32 (26) |
|
24 (19) |
|
20 (16) |
|
5 (4) |
|
7 (6) |
| Community-acquired infections | 122 (50) |
|
28 (23) |
|
25 (21) |
|
22 (18) |
|
11 (9) |
|
7 (6) |
|
29 (24) |
The most frequently encountered type of NI was surgical site infections (n = 36), followed by gastrointestinal infections (n = 32) and pneumonia (n = 24). The surgical site infections were predominantly deep incisional surgical site infections (n = 17) or organ infections (n = 14). Most of the gastrointestinal infections were accounted for by infectious gastroenteritis (n = 23), caused in the majority of cases by noroviruses or Clostridium difficile. Nosocomial pneumonia was observed principally in intensive care units (Table 2).
Table 2. Distribution of the prevalence and type of nosocomial infections in specific areas.
| Area | Number of patients | NI prevalence % (number of NI patients) | Number of postoperative SSI | Number of GI | Number of cases of pneumonia | Number of UTI | Number of cases of primary sepsis |
|---|---|---|---|---|---|---|---|
| Intensive care units | 110 | 28.2 (31) | 8 | 4 | 15 | 9 | 0 |
| Internal medicine*1 | 273 | 11.4 (31) | 1 | 17 | 4 | 5 | 3 |
| Surgery | 549 | 9.3 (51) | 26 | 10 | 3 | 6 | 1 |
|
320 | 13.4 (43) | 24 | 10 | 3 | 4 | 0 |
|
229 | 3.5 (8) | 2 | 0 | 0 | 2 | 1 |
| Neurology | 75 | 4.0 (3) | 0 | 1 | 1 | 0 | 1 |
| Others*4 | 40 | 5.0 (2) | 1 | 0 | 1 | 0 | 0 |
*1Hematooncology/radiotherapy, pneumology, gastroenterology, cardiology, nephrology and infectiology;
*2cardiothoracic, transplantation and vascular surgery, trauma surgery, visceral surgery, plastic, hand and restorative surgery and neurosurgery;
*3otorhinolaryngology, gynecology, ophthalmology, and urology;
*4sleep laboratory, dentistry, maxillofacial surgery; GI, gastrointestinal infections; UTI, urinary tract infections; NI, nosocomial infections; SSI, surgical site infections
Among the 124 NI the most frequent pathogens were Escherichia coli, coagulase-negative staphylococci and Candida spp. (Table 3). Eighteen (15%) of the bacterial pathogens showed conspicuous (multi)resistance. This group included:
Table 3. Distribution of the 162 nosocomial infections in 124 patients by pathogen.
| Pathogen | Number (%)*1 |
|---|---|
| Escherichia coli | 23 (19) |
| Coagulase-negative staphylococci | 22 (18) |
| Candida spp. | 19 (15) |
| Enterococcus faecium | 16 (13) |
| Pseudomonas aeruginosa | 12 (10) |
| Enterococcus faecalis | 10 (8) |
| Norovirus | 5 (4) |
| Clostridium difficile | 5 (4) |
| Streptococcus spp. | 5 (4) |
| Klebsiella pneumoniae | 4 (3) |
| Staphylococcus aureus | 3 (2) |
| Other pathogens | 25 (20) |
| Unidentified | 22 (18) |
| Polymicrobial infection*2 | 37 (30) |
*1Multiple entries possible; *2Infection with >1 pathogen
Ten multiresistant gram-negative bacteria (MRGN)
Three members of the family Enterobacteriaceae with an exclusively extended spectrum of beta-lactamases (ESBL)
Three vancomycin-resistant enterococci (VRE)
Two methicillin-resistant strains of Staphylococcus aureus (MRSA)
The sex distribution of the patients studied was 54% male, 46% female (p = 0.061). Their mean age was 57 ± 18 years. The median age of the patients with NI was similar to that of patients without NI (59 years [49 to 70] versus 59 years [44 to 71]). Patients with NI stayed in hospital longer than those without NI (median 37 days [19 to 58] versus 10 days [5 to 21]). We did not investigate NI as a cause of death, but the death rate was considerably higher in patients with NI than without NI (11% versus 2%; p < 0.001). Demographic data and other patient characteristics are shown in Table 4.
Tabel 4. Patient characteristics of the 1047 inpatients included in the prevalence survey.
| Parameter | All patients (N=1047) | Patients without NI (n=930) | Patients with NI (n=117) | p value |
|---|---|---|---|---|
| Age >59 years (median) | 518 (50%) | 461 (50%) | 57 (49%) | 0.922 |
| Men | 564 (54%) | 491 (53%) | 73 (62%) | 0.061 |
| Type of underlying disease | ||||
| Cardiological | 420 (40%) | 363 (39%) | 57 (49%) | 0.046 |
| Endocrinological | 225 (22%) | 194 (21%) | 31 (27%) | 0.188 |
| Malignant neoplasm | 213 (20%) | 187 (20%) | 26 (22%) | 0.626 |
| Gastrointestinal | 200 (19%) | 159 (17%) | 41 (35%) | <0.001 |
| Nephrological | 176 (17%) | 154 (17%) | 22 (19%) | 0.514 |
| Neurological | 140 (13%) | 126 (14%) | 14 (12%) | 0.773 |
| Trauma surgery | 136 (13%) | 117 (13%) | 19 (16%) | 0.306 |
| Pulmonological | 132 (13%) | 107 (12%) | 25 (21%) | 0.005 |
| Metabolic | 103 (10%) | 86 (9%) | 17 (15%) | 0.097 |
| Angiological | 100 (10%) | 86 (9%) | 14 (12%) | 0.32 |
| Hematological | 55 (5%) | 45 (5%) | 10 (9%) | 0.119 |
| Rheumatological | 42 (4%) | 40 (4%) | 2 (2%) | 0.218 |
| >2diseases | 359 (34%) | 301 (32%) | 58 (50%) | <0.001 |
| Other patient characteristics | ||||
| Devices | 709 (68%) | 605 (65%) | 104 (89%) | <0.001 |
|
211 (20%) | 159 (17%) | 52 (44%) | <0.001 |
|
207 (20%) | 160 (17%) | 47 (40%) | <0.001 |
|
154 (15%) | 114 (12%) | 40 (34%) | <0.001 |
|
78 (7%) | 51 (6%) | 27 (23%) | <0.001 |
|
42 (4%) | 23 (3%) | 19 (16%) | <0.001 |
|
553 (53%) | 478 (51%) | 75 (64%) | 0.01 |
|
162 (16%) | 115 (12%) | 47 (40%) | <0.001 |
| Antibiotic treatment in previous 6 months*1 | 672 (64%) | 567 (61%) | 105 (90%) | <0.001 |
| Current antibiotic treatment | 532 (51%) | 440 (47%) | 92 (79%) | <0.001 |
| Surgery in previous 6 months | 593 (57%) | 502 (54%) | 91 (78%) | <0.001 |
| Hospital admission in previous 12 months | 563 (54%) | 481 (52%) | 82 (70%) | <0.001 |
| Presence of wounds | 251 (24%) | 204 (22%) | 47 (40%) | <0.001 |
| Dialysis | 57 (5%) | 49 (5%) | 8 (7%) | 0.514 |
*1Not including antibiotics for treatment of NI; p value, level of significance; NI, nosocomial infection
Logistic regression identified the following independent risk factors for the development of NI (Table 5):
Table 5. Multivariable risk factor analysis for the occurrence of nosocomial infections.
| Parameter | OR | 95% CI | p value |
|---|---|---|---|
| Antibiotic treatment in previous 6 months | 2.9 | 1.5–5.7 | 0.001 |
| Underlying gastrointestinal disease | 2.3 | 1.4–3.6 | <0.001 |
| Surgery in previous 12 months | 1.8 | 1.1–3.0 | 0.023 |
| More than 2 underlying diseases | 1.7 | 1.1–2.6 | 0.016 |
| Per device | 1.4 | 1.2–1.5 | <0.001 |
Area under receiver operating characteristic (ROC) curve = 0.78 OR, odds ratio; 95% CI, 95% confidence interval; p value, level of significance
Antibiotic treatment in the previous 6 months (OR = 2.9, 95% CI 1.5 to 5.7)
Gastrointestinal diseases (OR = 2.3, 95% CI 1.4 to 3.6)
Surgery in the previous 12 months (OR = 1.8, 95% CI 1.1 to 3.0)
Presence of > 2 underlying diseases (OR = 1.8, 95% CI 1.1 to 2.6)
Per device used (OR = 1.4, 95% CI 1.2 to 1.5)
Discussion
One in every five patients (22%) in this prevalence study was found to have an infection. Nosocomial infections and community-acquired infections (CAI) were found in similar frequency (11.2% versus 10.7%). Recent investigations of the prevalence of infections in hospital have focused exclusively on NI, so that older studies have to be consulted for data on the overall prevalence of infections in hospital inpatients. The multicenter study conducted by Emmerson et al. in 1994 described a similar overall prevalence (23.7%), but there were notably more CAI than NI (14.7% versus 9%) (12). Also in 1994, Rüden et al. found an overall rate of infections of 13.5% in Germany (1); again, the CAI predominated (10% versus 3.5%). The 11.2% prevalence of NI observed at the University Hospital of Hannover Medical School is at the high end of the range reported from hospitals with more than 600 beds or university hospitals in various European countries (4.4 to 13.5%) (Table 6). However, the 28.2% prevalence of NI in intensive care units in our study was comparable with that in other investigations (25 to 48%) (Table 6).
Table 6. Results of studies of the prevalence of nosocomial infections in European hospitals.
| Authors | Study year | Country | Number of hospitals | Number of patients | NI prevalence | (Range) | NI prevalence in intensive care units | NI prevalence in university hospitals and hospitals with >600 beds |
|---|---|---|---|---|---|---|---|---|
| Emmerson et al. (12) | 1993 | UK | 57 | 37111 | 9.0% | (8.4–11.2) | 34.2% | 10.7% |
| Rüden et al. (1) | 1994 | DE | 72 | 14966 | 3.5% | (0.0–8.9) | 15.3% | 4.4% |
| Pittet et al. (8) | 1996 | CH | 4 | 1349 | 11.6% | (9.8–13.5) | 25% | 13.5% |
| Gikas et al. (16) | 1999 | GR | 14 | 3925 | 9.3% | (5.0–13.4) | 48.4% | 13.4% |
| Klavs et al. (6) | 2001 | SI | 19 | 6695 | 4.6% | (3.1–5.4) | 26.9% | 5.4% |
| Eriksen et al. (15) | 2002 | NO | 65 | 12314 | 5.3% | (5.1–5.4) | 25.4% | 5.4% |
| Lanini et al. (38) | 2002 | IT | 51 | 9609 | 6.1% | (5.6–6.7)*2 | 34.3% | – |
| Pellizzer et al. (10) | 2003 | IT | 21 | 6352 | 7.6% | (2.6–17.6) | 25.8% | – |
| Reilly et al. (9) | 2005 | SCO | 45 | 11608 | 9.5% | (8.8–10.2)*2 | – | – |
| Fitzpatrick et al. (21) | 2006 | IE | 59 | 11185 | 5.2% | – | – | – |
| Valinteliene et al. (32) | 2007 | LT | 30 | 6288 | 3.4% | (0.0–12.3) | 10% | – |
| Ilic et al. (20) | 2009*1 | RS | 1 | 764 | 6.2% | (5.6–6.8)*2 | – | – |
| Zarb et al. (19) | 2010 | EU | 66 | 19888 | 7.1% | (5.8–7.8) | 28.1% | 7.4% |
| Present study | 2010 | DE | 1 | 1047 | 11.2% | (9.3–13.2)*2 | 28.2% | 11.2% |
*1Publication year; *295% confidence interval;
NI, nosocomial infections
UK: United Kingdom; DE: Germany; CH: Switzerland; GR: Greece; SI: Slovenia; NO: Norway; IT: Italy; SCO: Scotland; IE: Ireland; LT: Lithuania; RS: Serbia; EU: European Union
Caution is necessary when comparing the findings of such prevalence studies because of the potential differences in the way they were conducted. The factors with the greatest influence on the results are:
The number and types of hospitals
The patient collective
The definition of the infections
The number and types of infections
The nature and experience of the study personnel
In the present study we excluded patients from the departments of psychiatry and psychosomatic diseases and pediatrics. The results of prevalence studies in pediatrics (3.4 to 7.5%) (13– 17) and psychiatry (0 to 3.5%) (9, 12, 17) suggest that inclusion of these specialties would have lowered the prevalence of NI at the University Hospital of Hannover Medical School.
Surgical site infections were the type of NI most frequently detected in our study. Multicenter studies, among them the recent point prevalence survey of the European Centre for Disease Prevention and Control (ECDC), classify surgical site infections as the second to fourth most common kind of NI (11 to 28%) (1, 9, 10, 12, 15, 16, 18, 19). In the German NIDEP-I study of 1994, surgical site infections were second to urinary tract infections (UTI) even in hospitals with a focus on surgery (1). More recent prevalence studies by Ilic et al. and Fitzpatrick et al. found that surgical site infections were the most frequent NI (20, 21). In the multicenter survey of NI prevalence by Magill et al., surgical site infections were again the most frequent and UTI only the third-ranked NI in hospitals with a focus on surgery (22). The situation is similar in our study: UTI, at 16%, were only the fourth most frequent NI. Decreasing prevalence of UTI over the years has already been noted by other authors (12, 15). The reason may be improved preventive measures, e.g., appropriate use of urinary catheters. The relatively young study group (mean age 57 years) may also play a part. Studies with a high prevalence of UTI often include a high proportion of patients well over 60 (1, 9, 10, 18).
The second-ranked group of NI in our survey were gastrointestinal infections, predominantly infectious gastroenteritis. Emmerson et al. found an increase in the prevalence of such infections from 0.13% in 1980 to 0.51% in 1993 (12). Gastrointestinal infections were the third most frequent group in a study of the prevalence of NI conducted in Scotland in 2005 and 2006 (9). The principal reasons were observed to be an increase in C. difficile-related infections (CDI) (23), which can also be seen in Germany (24), and infections with noroviruses (25). The high prevalence of infectious gastroenteritis is associated among other things with the season of investigation, because norovirus infections (26) and CDI (27) occur particularly in cold weather. Without norovirus enteritis (n = 15) the overall prevalence of NI in our survey would have been 9.7%; gastrointestinal infections, at 17%, would still have been in third place.
The distribution of pathogens corresponds to the pattern in previous studies, with E. coli, Pseudomonas aeruginosa, enterococci, and coagulase-negative staphylococci as the most frequent causes of NI (6, 8, 10, 16). In contrast to those earlier surveys, however, we found only a small number of NI due to S. aureus. This may be explained, particularly with regard to surgical site infections, by the preventive measures routinely taken at the Cardiothoracic, Transplantation, and Vascular Surgery Department of the University Hospital of Hannover Medical School, where all patients are washed with antiseptic lotion and have mupirocin ointment applied to the nares immediately before surgery (28). Moreover, a higher proportion of NI were caused by multiresistant gram-negative bacteria (MRGN) (8%) than by methicillin-resistant S. aureus (MRSA) (2%) and vancomycin-resistant enterococci (VRE) (2%). The trend towards an increase in MRGN is already apparent in German intensive care units (29, 30). The observation of a low number of infections involving MRSA is not in agreement with previous publications (31).
Alongside the noncontrollable patient-related factors—such as diseases of the gastrointestinal tract, presence of more than two underlying diseases, and operations in the previous 12 months—controllable exogenous factors were identified: the risk of an NI increased by a factor of 1.4 with every device used.
Other studies identified isolated urinary catheters, vascular catheters, and invasive ventilation as independent risk factors (17, 18, 21, 32). When one also considers that UTI, pneumonia, surgical site infections, and primary sepsis account on average for 80% of the NI in the publications listed (1, 22, 32), it seems plain that prevention of device-associated infections is highly important. Unexpectedly, antibiotic treatment in the previous 6 months that was not given for management of the current NI had the strongest association with presence of an NI (OR = 2.9). Fitzpatrick et al. and Ilic et al. found inter alia that systemic administration of antibiotics was associated with NI (20, 21). On one hand this could indicate a patient’s high morbidity; on the other, it may point to an immunomodulatory effect of antibiotics. This aspect requires clarification in more detailed studies. The prevalence of systemic antibiotic administration (n = 473, 45%) in our survey was relatively high compared with other European studies (range 19 to 59%) (19, 33– 35).
Limitations
Our prevalence survey was a single-center study at a top-level university hospital with a focus on surgery, so its findings are not transferable in toto to hospitals with other foci or to the situation in Germany in general.
The relatively large collective of 1047 patients means the results are likely to be robust, however, and the survey is comparable in size with the single-center study of 1501 patients by Ciofa degli Atti et al. (13) and the multicenter study by Magill et al. with 851 patients (22). Under-reporting of NI seems unlikely in our study, because all kinds of NI were recorded. The results can deviate by up to 34% when focusing exclusively on device-related NI (1, 22, 32), and other important and/or frequent types of infection, e.g., gastrointestinal infections, can be overlooked. The time of year chosen for the survey may have affected the distribution of pathogens and also the prevalence of NI, particularly gastroenteritis caused by noroviruses and C. difficile, which are found more frequently in the cooler months (26, 27). Prevalence of NI can also be considerably affected if there happens to be an outbreak of infection at the time of the survey, and this has to be taken into account when interpreting the data. Rates of NI may also be overestimated; such overestimations are ascertained much more often in prevalence studies than in longitudinal incidence studies.
However, incidence studies involve high costs in terms of time and personnel; prevalence studies therefore represent a valuable, economical alternative means of delineating trends.
Conclusion
According to § 23 of the German Protection against Infection Act, hospitals in Germany are obliged to record and evaluate nosocomial infections. For reasons of economy, many hospitals often analyze the data for only one type of infection. Especially for large hospitals or for university hospitals with a particular focus, single-center prevalence surveys can be an important tool in the improvement of surveillance and other preventive measures. They highlight specific trends in the frequency of and risk factors for nosocomial infections relatively quickly and with low personnel costs. Our prevalence study showed the predominance of surgical site infections and gastrointestinal infections among the NI at a top-level university hospital with a focus on surgery.
Moreover, it emerged that antibiotic treatment and device use were strongly associated with NI. Patient safety can therefore be considerably improved by prevention of:
Surgical site infections
Device-related infections, especially in intensive care units
Nosocomial gastroenteritis from C. difficile.
Based on the results of our survey, surveillance of surgical site infections and obligatory training for all members of staff involved in surgery at our institution commenced in 2011. Furthermore, an antibiotic stewardship program was set up to counter, among other things, C. difficile-related infections and the development of multiresistant pathogens.
Key Messages.
A prevalence survey showed that 11.2% of 1047 inpatients had a nosocomial infection and 10.7% a previously acquired infection.
The most common types of infection were surgical site infections (29%), gastrointestinal tract infections (26%), and lower respiratory tract infections (19%).
The most important independent risk factors for a nosocomial infection were antibiotic treatment in the previous 6 months (odds ratio [OR] = 2.9) and underlying gastrointestinal disease (OR = 2.3).
Multiresistant gram-negative bacteria accounted for a higher number of nosocomial infections than resistant gram-positive bacteria (methicillin-resistant S. aureus and vancomycin-resistant Enterococcus species).
Acknowledgments
Translated from the original German by David Roseveare.
The authors are grateful to Angela Legarth (data management), Britt-Christin Hilbig, Jonathan Joshi, Simone Valentin (data collection) and Dr. Cornelia Henke-Gendo (data interpretation) for their valuable assistance.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Rüden H, Gastmeier P, Daschner FD, Schumacher M. Nosocomial and community-acquired infections in Germany. Summary of the results of the First National Prevalence Study (NIDEP). Infection. 1997;25:199–202. doi: 10.1007/BF01713142. [DOI] [PubMed] [Google Scholar]
- 2.Gastmeier P, Geffers C. Nosokomiale Infektionen in Deutschland: Wie viele gibt es wirklich? Dtsch Med Wochenschr. 2008;133:1111–1115. doi: 10.1055/s-2008-1077224. [DOI] [PubMed] [Google Scholar]
- 3.Beyersmann J, Gastmeier P, Grundmann H, et al. Use of multistate models to assess prolongation of intensive care unit stay due to nosocomial infection. Infect Control Hosp Epidemiol. 2006;27:493–499. doi: 10.1086/503375. [DOI] [PubMed] [Google Scholar]
- 4.Graf K, Ott E, Vonberg RP, Kuehn C, Haverich A, Chaberny IF. Economic aspects of deep sternal wound infections. Eur J Cardiothorac Surg. 2010;37:893–896. doi: 10.1016/j.ejcts.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Ott E, Bange FC, Reichardt C, et al. Costs of nosocomial pneumonia caused by meticillin-resistant Staphylococcus aureus. J Hosp Infect. 2010;76:300–303. doi: 10.1016/j.jhin.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Klavs I, Bufon Luznik T, Skerl M, et al. Prevalance of and risk factors for hospital-acquired infections in Slovenia-results of the first national survey, 2001. J Hosp Infect. 2003;54:149–157. doi: 10.1016/s0195-6701(03)00112-9. [DOI] [PubMed] [Google Scholar]
- 7.Di Pietrantonj C, Ferrara L, Lomolino G. Multicenter study of the prevalence of nosocomial infections in Italian hospitals. Infect Control Hosp Epidemiol. 2004;25:85–87. doi: 10.1086/502299. [DOI] [PubMed] [Google Scholar]
- 8.Pittet D, Harbarth S, Ruef C, et al. Prevalence and risk factors for nosocomial infections in four university hospitals in Switzerland. Infect Control Hosp Epidemiol. 1999;20:37–42. doi: 10.1086/501554. [DOI] [PubMed] [Google Scholar]
- 9.Reilly J, Stewart S, Allardice GA, et al. Results from the Scottish National HAI Prevalence Survey. J Hosp Infect. 2008;69:62–68. doi: 10.1016/j.jhin.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Pellizzer G, Mantoan P, Timillero L, et al. Prevalence and risk factors for nosocomial infections in hospitals of the Veneto region, north-eastern Italy. Infection. 2008;36:112–119. doi: 10.1007/s15010-007-7092-x. [DOI] [PubMed] [Google Scholar]
- 11.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Emmerson AM, Enstone JE, Griffin M, Kelsey MC, Smyth ET. The Second National Prevalence Survey of infection in hospitals-overview of the results. J Hosp Infect. 1996;32:175–190. doi: 10.1016/s0195-6701(96)90144-9. [DOI] [PubMed] [Google Scholar]
- 13.Ciofi degli Atti ML, Cuttini M, Rava L, et al. Trend of healthcare-associated infections in children: annual prevalence surveys in a research hospital in Italy, 2007-2010. J Hosp Infect. 2012;80:6–12. doi: 10.1016/j.jhin.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Muhlemann K, Franzini C, Aebi C, et al. Prevalence of nosocomial infections in Swiss children’s hospitals. Infect Control Hosp Epidemiol. 2004;25:765–771. doi: 10.1086/502474. [DOI] [PubMed] [Google Scholar]
- 15.Eriksen HM, Iversen BG, Aavitsland P. Prevalence of nosocomial infections in hospitals in Norway, 2002 and 2003. J Hosp Infect. 2005;60:40–45. doi: 10.1016/j.jhin.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Gikas A, Pediaditis J, Papadakis JA, et al. Prevalence study of hospital-acquired infections in 14 Greek hospitals: planning from the local to the national surveillance level. J Hosp Infect. 2002;50:269–275. doi: 10.1053/jhin.2002.1181. [DOI] [PubMed] [Google Scholar]
- 17.Askarian M, Yadollahi M, Assadian O. Point prevalence and risk factors of hospital acquired infections in a cluster of university-affiliated hospitals in Shiraz, Iran. J Infect Public Health. 2012;5:169–176. doi: 10.1016/j.jiph.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Gravel D, Taylor G, Ofner M, et al. Point prevalence survey for healthcare-associated infections within Canadian adult acute-care hospitals. J Hosp Infect. 2007;66:243–248. doi: 10.1016/j.jhin.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Zarb P, Coignard B, Griskeviciene J, et al. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill. 2012;17 doi: 10.2807/ese.17.46.20316-en. [DOI] [PubMed] [Google Scholar]
- 20.Ilic M, Markovic-Denic L. Nosocomial infections prevalence study in a Serbian university hospital. Vojnosanit Pregl. 2009;66:868–875. doi: 10.2298/vsp0911868i. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick F, McIlvenny G, Oza A, et al. Hospital infection society prevalence survey of Healthcare Associated Infection 2006: comparison of results between Northern Ireland and the Republic of Ireland. J Hosp Infect. 2008;69:265–273. doi: 10.1016/j.jhin.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Magill SS, Hellinger W, Cohen J, et al. Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida. Infect Control Hosp Epidemiol. 2012;33:283–291. doi: 10.1086/664048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubberke ER, Butler AM, Yokoe DS, et al. Multicenter study of Clostridium difficile infection rates from 2000 to 2006. Infect Control Hosp Epidemiol. 2010;31:1030–1037. doi: 10.1086/656245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gastmeier P, Weitzel-Kage D, Behnke M, Eckmanns T. Surveillance of Clostridium difficile-associated diarrhoea with the German nosocomial infection surveillance system KISS (CDAD-KISS) Int J Antimicrob Agents. 2009;33:19–23. doi: 10.1016/S0924-8579(09)70011-1. [DOI] [PubMed] [Google Scholar]
- 25.Atmar RL, Estes MK. The epidemiologic and clinical importance of norovirus infection. Gastroenterol Clin North Am. 2006;35:275. doi: 10.1016/j.gtc.2006.03.001. 90, viii. [DOI] [PubMed] [Google Scholar]
- 26.Matthews JE, Dickey BW, Miller RD, et al. The epidemiology of published norovirus outbreaks: a review of risk factors associated with attack rate and genogroup. Epidemiol Infect. 2012;140:1161–1172. doi: 10.1017/S0950268812000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kern WV, Dettenkofer M. Nosokomiale Infektionen. Internist (Berl) 2009;50:691–703. doi: 10.1007/s00108-009-2389-8. [DOI] [PubMed] [Google Scholar]
- 28.Graf K, Sohr D, Haverich A, Kuhn C, Gastmeier P, Chaberny IF. Decrease of deep sternal surgical site infection rates after cardiac surgery by a comprehensive infection control program. Interact Cardio vasc Thorac Surg. 2009;9:282–286. doi: 10.1510/icvts.2009.205286. [DOI] [PubMed] [Google Scholar]
- 29.Mattner F, Bange FC, Meyer E, Seifert H, Wichelhaus TA, Chaberny IF. Preventing the spread of multidrug-resistant gram-negative pathogens: recommendations of an expert panel of the German Society For Hygiene and Microbiology. Dtsch Arztebl Int. 2012;109(3):39–45. doi: 10.3238/arztebl.2012.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geffers C, Gastmeier P. Nosocomial infections and multidrug-resistant organisms in Germany: epidemiological data from KISS (the Hospital Infection Surveillance System) Dtsch Arztebl Int. 2011;108(6):87–93. doi: 10.3238/arztebl.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kock R, Mellmann A, Schaumburg F, Friedrich AW, Kipp F, Becker K. The epidemiology of methicillin resistant Staphylococcus aureus (MRSA) in Germany. Dtsch Arztebl Int. 2011;108(45):761–767. doi: 10.3238/arztebl.2011.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valinteliene R, Gailiene G, Berzanskyte A. Prevalence of healthcare-associated infections in Lithuania. J Hosp Infect. 2012;80:25–30. doi: 10.1016/j.jhin.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Ansari F, Erntell M, Goossens H, Davey P. The European surveillance of antimicrobial consumption (ESAC) point-prevalence survey of antibacterial use in 20 European hospitals in 2006. Clin Infect Dis. 2009;49:1496–1504. doi: 10.1086/644617. [DOI] [PubMed] [Google Scholar]
- 34.de With K, Bestehorn H, Steib-Bauert M, Kern WV. Comparison of defined versus recommended versus prescribed daily doses for measuring hospital antibiotic consumption. Infection. 2009;37:349–352. doi: 10.1007/s15010-008-8138-4. [DOI] [PubMed] [Google Scholar]
- 35.Willemsen I, van der Kooij T, van Benthem B, Wille J, Kluytmans J. Appropriateness of antimicrobial therapy: a multicentre prevalence survey in the Netherlands, 2008-2009. Euro Surveill. 2010;15 doi: 10.2807/ese.15.46.19715-en. [DOI] [PubMed] [Google Scholar]
- 36.Gastmeier P, Brauer H, Sohr D, et al. Converting incidence and prevalence data of nosocomial infections: results from eight hospitals. Infect Control Hosp Epidemiol. 2001;22:31–34. doi: 10.1086/501821. [DOI] [PubMed] [Google Scholar]
- 37.Ustun C, Hosoglu S, Geyik MF, Parlak Z, Ayaz C. The accuracy and validity of a weekly point prevalence survey for evaluating the trend of hospital-acquired infections in a university hospital in Turkey. Int J Infect Dis. 2011;15:e684–e677. doi: 10.1016/j.ijid.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Lanini S, Jarvis WR, Nicastri E, et al. Healthcare-associated infection in Italy: annual point-prevalence surveys, 2002-2004. Infect Control Hosp Epidemiol. 2009;30:659–665. doi: 10.1086/597596. [DOI] [PubMed] [Google Scholar]