Abstract
Background
During the H1N1 pandemic of 2009 and 2010, the large number of patients with severe respiratory failure due to H1N1 infection strained the capacities of treatment facilities for extracorporeal membrane oxygenation (ECMO) around the world. No data on this topic have yet been published for Germany.
Methods
During the pandemic, the German ARDS Network (a task force of the DIVI’s respiratory failure section) kept track of the availability of ECMO treatment facilities with a day-to-day, Internet-based capacity assessment. In cooperation with the Robert Koch Institute, epidemiological and clinical data were obtained on all patients treated for influenza in intensive care units.
Results
116 patients were identified who had H1N1 disease and were treated in the intensive care units of 9 university hospitals and 3 other maximum medical care hospitals. 61 of them received ECMO. The overall mortality was 38% (44 of 116 patients); among patients receiving ECMO, the mortality was 54% (33 of 61 patients). The mortality was higher among patients who had an accompanying malignancy or immune deficiency (72.2%).
Conclusion
Even persons without any other accompanying disease developed life-threatening respiratory failure as a result of H1N1 infection, and many of these patients needed ECMO. This study reveals for the first time that the mortality of H1N1 infection in Germany is comparable to that in other countries. H1N1 patients with acute respiratory failure had a worse outcome if they also had serious accompanying diseases.
Compared with the historically significant 1918 flu pandemic and common seasonal influenza, the seasonal 2009/2010 H1N1 influenza pandemics did not hit Germany as hard as many had feared. With 2.9 million estimated doctor visits and 5000 hospital admissions (1), the 2009/2010 numbers were in line with those of previous (seasonal) influenza waves. The Robert Koch Institute (RKI) was notified of altogether 258 confirmed H1N1 influenza deaths. Strikingly, 85% of the H1N1 pandemic patients who died in 2009 (H1N1pdm09 = 2009 pandemic influenza A virus subtype H1N1) were younger than age 65 (U. Buchholz, Robert Koch Institute, personal communication) and the average age was only 44 years. In contrast, during the 1976–1999 seasonal influenza waves in the United States only 10% of the influenza deaths were younger than age 65 (2).
Similarly, during the 2009/2010 H1N1 pandemic, cases of H1N1 pneumonia were reported worldwide where young, previously completely healthy adults had to be admitted to an intensive care unit (ICU) for treatment because of the severity of their conditions. According to a US study, 25% of the hospital-admitted patients diagnosed with H1N1pdm09 required intensive care treatment; of these, 36% developed severe acute respiratory distress syndrome (ARDS) (3).
Southern hemisphere countries, where the pandemic started six months earlier, reported that 34% of these patients required, due to the severity of their acute respiratory failure, the use of an extracorporeal lung support system for extracorporeal membrane oxygenation and removal of CO2 (ECMO) (4). Until recently, extracorporeal lung support systems were only cautiously used for the treatment of acute life-threatening hypoxemia in ARDS patients. The reason is that earlier prospective, randomized, controlled clinical trials (RCTs) (5, 6) reported complications and no evidence of survival advantages in specialized treatment centers (7). The idea, proposed as early as 1980 especially by Gattinoni et al. (8), to use complementary extracorporeal CO2 removal to be able to apply lung-protective mechanical ventilation with limited positive airway in ARDS patients, experienced a renaissance (9– 11). Ventilation with lower tidal volumes (VT 6 mL/kg predicted body weight [PBW]) increases the probability of survival among ARDS patients (12). To address the issue of at times insufficient CO2 removal and to learn from the experience that low tidal volumes not necessarily protect individual lung areas from overinflation (13), concepts of ultra-protective ventilation with VT < 6mL/kg PBW involving extracorporeal lung support systems have increasingly been put forward (14, 15). Apart from the fact that extracorporeal lung support is indicated in cases of life-threatening hypoxemia, this background helps to understand why ECMO was used worldwide for the treatment of large numbers of ARDS patients during the H1N1 influenza pandemic. This approach is supported by recent data from an RCT (16) and national observations (17), showing comparatively low mortality rates among ECMO-treated patients, and have contributed to a new view of the role of extracorporeal lung support in the context of ARDS therapy.
The number of patients who developed ARDS along with the H1N1 influenza pandemic has led to high levels of utilization of available extracorporeal lung support beds in many countries of the world (18, 19). Studies documenting the severity and course of patients with H1N1 pneumonia provoked ARDS in Germany that would allow for international comparison are not available as yet. When the 2009/2010 pandemic started, no system was in place to provide information about the level of utilization of intensive care resources and systematic data on H1N1 influenza patients with acute respiratory failure requiring intensive care treatment.
To collect these data, the German ARDS network was founded in 2008 at the initiative of the German Society of Anesthesiology and Intensive Care Medicine (DGAI, Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin) and then developed into an interdisciplinary network supported by various scientific societies within the “respiratory failure“ section of the German Interdisciplinary Association of Intensive Care and Emergency Medicine (DIVI, Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin).
Of particular interest were the following questions:
Characterization of (ICU-treated) ventilated H1N1 patients regarding age, sex distribution, concomitant conditions, intensive care scores). Were there any differences between cases in Germany and cases reported in other countries?
How frequently was extracorporeal lung support required in these patients?
Were there any independent risk factors with an impact on the mortality rate among these patients?
Some of these data were presented in an oral presentation at the annual meeting of the DGAI (HAI) in autumn 2010.
Methods
First, a web-based registry to record ECMO therapy beds and the level of their utilization was established within this network during the German 2009/2010 H1N1 influenza pandemics. Using an individual login, participating hospitals were able to report their free capacities on the ARDS Network’s website (www.ardsnetwork.de) on a daily basis.
Via the German ARDS Network’s website, referring hospitals had open access to the traffic light style status indicator:
green = capacity for transfer patients
yellow = limited capacity for transfer patients
red = no capacity for transfer patients.
The scope of this web-based service was limited to posting the capacity status; patients were not actively distributed or assigned to centers.
In collaboration with the Robert Koch Institute (RKI, Department of Infectious Disease Epidemiology, Unit for Respiratory Infections), the web-based registration of new patients with influenza and respiratory failure with and without extracorporeal lung support was started within the network. The web-based questionnaire comprised approximately 120 variables in total, which were collected on admission to and discharge from intensive care units. The primary objective was to collect data on the severity of respiratory failure from patients with ARDS as a consequence of H1N1 pneumonia by recording the items of intensive care scores on disease severity (simplified acute physiology score II [SAPS-II]; acute physiology and chronic health evaluation [APACHE-II]) and organ dysfunction (sepsis-related organ failure assessment [SOFA]), as well as ventilation parameters and oxygenation index (paO2/FiO2) on admission to the intensive care unit. Furthermore, the question whether the use of ECMO was required to treat hypoxemic respiratory failure was asked to assess the severity of the gas exchange disruption. Data on comorbidities potentially associated with the severity of the H1N1 provoked respiratory failure were also collected. With regard to the vital and ventilation parameters on admission day, the worst readings within 24 hours after admission to the respective center were selected and recorded. Data analysis was performed in an anonymized fashion.
Statistics
All analyses were conducted using SPSS Statistics 20.0 software. Results are represented as absolute values or mean values ± standard deviation (SD) or 95% confidence intervals (CI); for comparisons, the Mann-Whitney U test (independent variables) and the chi-square test (frequencies) were used. The influence of various variables (body-mass index, age, ECMO therapy performed, SOFA score, and paO2/FiO2 ratio on ICU admission, as well as presence of concomitant malignant or immunological diseases) on mortality was investigated using logistic regression analysis.
Results
During the time of the 2009/2010 H1N1 influenza pandemic, a high number of patients with H1N1 provoked acute respiratory failure required intensive care in Germany, resulting in an increased utilization of extracorporeal lung support systems (ECMO) in German hospitals.
Capacity data collection
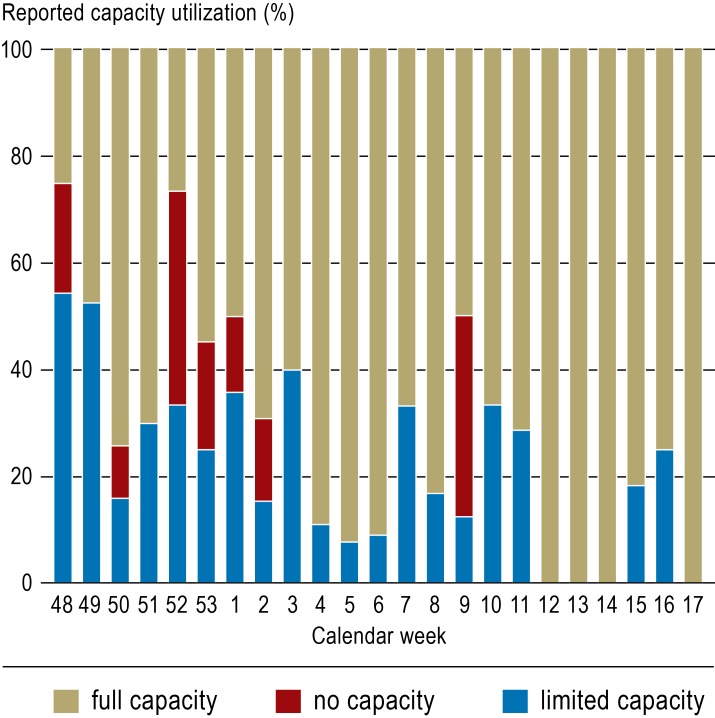
During the H1N1 influenza pandemic, German centers of excellence provided extracorporeal lung support therapy for up to eight patients at the same time. Figure 1 provides an overview of the reported capacities for transferred patients by calendar week during the observation period. At times, the utilization of the centers’ resources reached levels where it was not possible to accept ARDS patients referred for ECMO therapy (red traffic light). The web-based capacity recording system, which received data from a total of 40 contributing hospitals, helped not only participating centers, but also referring hospitals to find out where at a specific point in time treatment for a patient could be provided.
Figure 1.
Capacity data collection for extracorporeal lung support systems
(“traffic light“) within the “Acute Respiratory Distress Syndrome“ (ARDS) network for the period from 2009 (calendar week 48) to 2010 (calendar week 14). Blue represents limited admission capacity and red no admission capacity. The heights of the bars represent the percentage of the respective reported data (blue or red) of the reported data total (yellow, red or green) within the respective calendar week. Altogether 40 hospitals contributed to the capacity data collection
The average length of stay for these patients was 36 days (95% CI: 31–41), with a resulting additional ICU burden of altogether 3135 intensive care days.
Patient-related data collection
Based on information reported by 9 university hospitals and 3 maximum medical care hospitals with 450 ventilator beds in total, data from 116 patients with H1N1 provoked ARDS were analyzed. These patients were admitted to the ICUs between July 2009 and April 2010.
Patient characteristics
The time period between onset of disease and initiation of mechanical ventilation was typically short (mean: 5.7 days, 95% CI: 4.8–6.7). Table 1 shows the characteristics and pre-existing illnesses of these patients. Remarkably, the majority of these patients (62 of 116; 53.4%) had no pre-existing conditions, in contrast to public announcements made in the press and on radio and TV stations. In 84% of cases, the patients were transferred from other hospitals to the respective centers.
Table 1. Characteristics of and common concomitant diseases among H1N1 pneumonia patients with ARDS in the German ARDS Network’s cohort*.
| Total n = 116 |
no ECMO n = 55 |
ECMO n = 61 |
p value | |
|---|---|---|---|---|
| Female | 50 (43%) | 23 | 27 | 0.503 |
| Mean age (min/max) | 43 (40–45) [5/66] |
43 (39–47) [5/65] |
42 (39–45) [13/66] |
0.191 |
| Mean BMI (kg/m2) (min/max) | 32 (31–34) [18–68] |
31 (29–34) [18/63] |
32 (28–35) [18/68] |
0.508 |
| Health profession | 2 (1.7%) outpatient nurse; nurse |
1 | 1 | 0.096 |
| H1N1-vacinated | 2 (1.7%) | 1 | 1 | 0.890 |
| Pregnancy | 8 (7%) | 4 | 4 | 0.982 |
| Asthma | 4 (3.4%) | 0 | 4 | 0.148 |
| COPD | 11 (9.5%) | 6 | 5 | 0.278 |
| Other chronic lung diseases | 3 (2.6%) | 2 | 1 | 0.492 |
| Heart disease | 8 (6.9%) | 5 | 3 | 0.328 |
| Chronic kidney disease | 8 (6.9%) | 5 | 3 | 0.369 |
| Chronic liver disease | 1 (0.9%) | 0 | 1 | 0.592 |
| Hemato-oncological disease | 13 (11.2%) | 7 | 6 | 0.734 |
| Diabetes mellitus | 14 (12.1%) | 6 | 8 | 0.190 |
| HIV infection | 2 (1.7%) | 1 | 1 | 0.879 |
| Immunological disease | 6 (5.2%) | 1 | 5 | 0.218 |
| Malignancy | 3 (2.6%) | 0 | 3 | 0.128 |
*Values are reported as mean values (95% confidence interval) and minimum/maximum, absolute numbers or % of total. ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation (extracorporeal lung support therapy)
Lung findings on admission
Almost all patients (105 of 116, 91%) tested positive for H1N1, using a polymerase chain reaction (PCR) assay; in the remainder of the patients, the diagnosis had already been established in the referring hospital; in the ECMO centers, no repeat PCR testing was undertaken to confirm the diagnosis. The majority of samples were throat swaps (62%) and respiratory secretions (44%) obtained by bronchoalveolar lavage. In 11% of patients, direct microbiological testing revealed an additional bacterial infection.
Chest CT scans showed a typical combination of areas of consolidation and ground glass–type increases in density. These changes regularly had a bilateral and diffuse distribution pattern (Figure 2).
Figure 2.
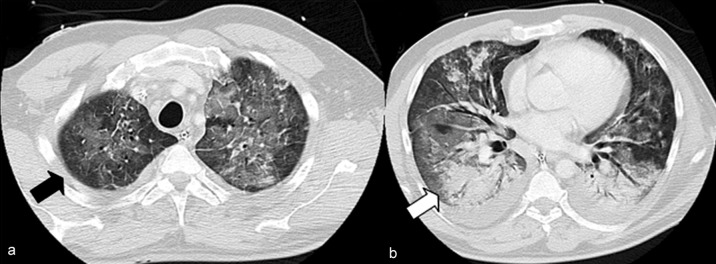
IV contrast-enhanced chest CT scan (lung window) in a 47-year-old male patient with influenza provoked acute respiratory distress syndrome (ARDS). Ground glass–type increases in density (a, black arrow) and areas of consolidation (b, white arrow) are recognizable. Reprinted from: Grieser C, Goldmann A, Steffen I et al.: Computed tomography findings from patients with ARDS due to Influenza A (H1N1) virus-associated pneumonia. Eur J Radiol 2012; 81: 389–94. Used by permission of the publisher (Elsevier)
At the time of admission to the center of excellence, 103 patients (89%) were on invasive ventilation. The mean duration of ventilation prior to admission to the ARDS center was 2.6 days (95% CI: 1.1–1.4). This comparatively early patient transfer to an ARDS center of excellence certainly reflects the severity of the condition, and, above all, its rapid progression.
Severity of the condition, mechanical ventilation, and pulmonary gas exchange
On ICU admission, patients showed, in addition to acute respiratory failure, significant other organ system dysfunctions (Table 2). Despite full deployment of lung-protective ventilation strategies, all patients were on admission in a state of severe to most severe hypoxemia which is reflected in the Horovitz index (paO2/FiO2 = arterial oxygen partial pressure/inspiratory oxygen fraction; normal range >350 mm Hg), among others.
Table 2. Intensive care scores and variables of mechanical ventilation on ICU admission (total study population and stratified for extracorporeal membrane oxygenation [ECMO] therapy)*.
| Total n = 116 |
no ECMO n = 55 |
ECMO n = 61 |
p value | |
|---|---|---|---|---|
| APACHE-II | 21.5 (20.1–23.1) | 19.5 (17.4–21.8) | 23.5 (21.7–25.4) | 0.006 |
| SAPS-II | 41.9 (38.6–45.2) | 44.0 (38.6–49.5) | 40.0 (36.0–44.0) | 0.301 |
| Predictive mortality (%) (SAPS-II) | 33.7 (28.6–39.0) | 37.8 (29.3–46.2) | 30.1 (23.7–36.6) | 0.301 |
| SOFA | 11.2 (10.5–11.9) | 9.9 (8.7–11.1) | 12.4 (11.5–13.2) | 0.001 |
| paO2/FiO2 (mm Hg) | 112 (99–126) | 141 (118–163) | 87 (74–101) | <0.001 |
| PEEP (mbar) | 18 (17–19) | 16 (15–18) | 20 (18–21) | 0.001 |
| Plateau pressure (mbar) | 32 (31–34) | 31 (29–33) | 34 (32–36) | 0.048 |
| Tidal volume/ ideal body weight (mL/kg) | 5.7 (5.1–6.2) | 5.9 (4.9–6.9) | 5.6 (4.9–6.2) | 0.624 |
| Prone position | 55 (47%) | 25 (46%) | 29 (48%) | 0.921 |
| Inhalative nitric oxide (NO) | 32 (28%) | 10 (18%) | 22 (37%) | 0.086 |
| Inhalative iloprost | 13 (11%) | 5 (9%) | 8 (13%) | 0.389 |
*Values are reported as mean values (95% confidence interval) or as absolute values with percentage. APACHE, Acute Physiology And Chronic Health Evaluation; PEEP, Positive End Expiratory Pressure; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment
As part of a multimodality treatment algorithm, 47% of patients (55 of 116) were stabilized in prone position after ICU admission to recruit non-ventilated lung areas. In addition, 28% of patients received inhalative nitric oxide (NO) and 11% an inhalative prostacyclin analogue (iloprost) to improve the ventilation/perfusion ratio by inducing selective pulmonary vasodilation and to treat the severe pulmonary hypertension with associated right heart burden (20) frequently seen in ARDS patients.
Despite this regimen, 61 patients (53%) showed a degree of oxygenation impairment, requiring, under consideration of a lung-protective ventilation strategy, extracorporeal lung support to ensure adequate and secure oxygen supply.
The mortality rate among the total study population was 38% (44 of 116 patients) and 30.6% among patients without any identified pre-existing condition (n = 62) (Table 3). Of the ECMO patients without pre-existing conditions in their medical histories, 41.9% died compared with 19.4% of patients without ECMO and without pre-existing conditions (p = 0.049). These differences are explained by the severity of the condition, especially with respect to ARDS. In contrast, the mortality among patients with concomitant malignant or immunological diseases was 72.2%. All of the ECMO patients with concomitant malignant diseases (n = 10) died.
Table 3. Treatment outcomes in patients without and with ECMO therapy*.
| no ECMO (n = 55) |
ECMO (n = 61) |
p value | |
|---|---|---|---|
| Length of ICU stay (d) | 27 (20–34) | 33 (27–39) | 0.094 |
| Length of hospital stay (d) | 32 (26–37) | 39 (31–47) | 0.582 |
| Duration of mechanical ventilation (d) | |||
| Duration of invasive ventilation (n = 46) (d) | 23 (16–30) | 32 (26–38) | 0.008 |
| Duration of non-invasive ventilation (n = 21) (d) | 4 (1–7) | 7 (2–12) | 0.189 |
| Mortality | 11 (20%) | 33 (54%) | <0.001 |
*Values are reported as mean values (95% confidence interval) or as absolute values with percentage. ECMO, extracorporeal membrane oxygenation (extracorporeal lung support therapy)
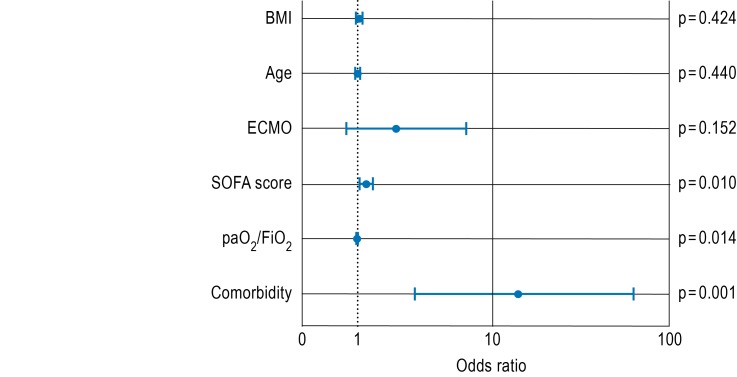
Multivariate analysis (Figure 3) revealed a strong association between a concomitant malignant or immunological condition and patient death during the course of the disease (odds ratio [OR]: 13.9; 95% CI: 3.1–62.9). Based on our clinical and scientific experiences, it seems reasonable to assume that this represents a critical causal influence. Apart from this, the severity of organ dysfunction (SOFA score) on admission (OR 1.2; 95% CI: 1.1–1.4) and, to a lesser extent, the paO2/FiO2 ratio (OR 0.99; 95% CI: 0.98–1.0) had an impact on lethality, too.
Figure 3.
Odds ratios for the outcome variable ’mortality’, dependent on body mass index, age, performance of an ECMO therapy, SOFA score or PaO2/FIO2 ratio on ICU admission, as well as presence of concomitant malignant or immunological diseases (“comorbidities”). Logarithmic scale, logistic regression, level of significance: p<0.05. ECMO, extracorporeal membrane oxygenation (extracorporeal lung support therapy); SOFA, Sequential Organ Failure Assessment
Discussion
The ARDS Network’s registry data represent the first data that have ever become available on treated patients with H1N1 provoked severe respiratory failure in Germany; a significant percentage of these patients were treated with extracorporeal lung support systems. Recording of free treatment capacities for therapies requiring highly sophisticated specialized equipment and expertise, has so far been introduced for e.g. burn patients. With the recording of the capacities of ECMO centers, information about available therapy spaces for patients with severe ARDS is now available.
International comparison of the ARDS Network’s data
Similar to the approach taken in Germany, worldwide severely ill patients with H1N1 pneumonia were transferred to centers of excellence with ECMO therapy facilities (3, 4, 17, 21). Published study data reveal comparatively high survival rates in the total population of H1N1 influenza patients with acute respiratory failure; especially the improved survival rates related to the use of extracorporeal support were confirmed. Mortality, in particular among ECMO-treated patients, was lower compared with the German patient population. However, the severity of acute disease was also significantly lower in some of these cohorts (17). Furthermore, the definition of the exclusion criteria set for the use of ECMO therapy may represent another conceivable reason for a lower mortality. For example, the Australian/New Zealand cohort had only few patients with severe comorbidities, such as AIDS, hemato-oncological and metastatic diseases, acute or chronic liver diseases or immunosuppression (4). In Germany, the number of ECMO patients with severe pre-existing diseases (n = 10 of 61, 16%) was significantly higher. None of these patients survived. Multivariate analysis (Figure 3) clearly shows that the mortality rate in the ECMO group does not appear to be causally related to the method per se, but rather to a large extent to the presence of concomitant malignant or immunological disease. The mortality among all patients without documented concomitant diseases was by contrast with 30.6% similar to that in current international studies (4, 17). This once again raises the question whether extracorporeal lung support therapy in patients with such severe concomitant diseases is justified. However, because of the small number of cases in our cohort, final conclusions cannot be drawn (22).
Capacity data collection and H1N1-related capacity utilization
The newly established traffic light–type, web-based capacity data collection system showed at times maximum use of capacities in German ARDS centers for ECMO resources. The high utilization of intensive care resources is demonstrated by the 116 documented H1N1 influenza patients, generating a total of 3135 intensive care therapy days with an average length of stay of 36 days.
We assume that the provision of web-based information about free capacities available for requesting centers has facilitated the referral of patients to centers of excellence. However, with a lack of surveys investigating this effect, no direct evidence of it is available.
Since time is a critical factor influencing the indication to use extracorporeal lung support systems, the ARDS Network’s national capacity data collection initiative will in future provide the opportunity to support the fast allocation of critically ill patients.
Limitations
A limitation of this study is that neither all hospitals providing care for ventilated patients with respiratory failure participated in the ARDS Network’s capacity data collection initiative, nor were data of all patients with H1N1 provoked acute respiratory failure captured. Therefore, it cannot be excluded that the clinical data of the total patient population treated in Germany are biased by this. Regretfully, no generally valid incidence or prevalence data can be derived from the available data. A further limitation is the restrictedness of the collected data set. As a result of the voluntary nature of participation in the German ARDS Network’s registry and the lack of time resources in the centers, the questionnaire was limited to a few variables. Therefore, it is not possible to make statements about the special H1N1 therapy, any complications during hospital stay, or the cause of death.
Outlook
Healthy patients, too, may develop a life-threatening H1N1 provoked respiratory failure which in many cases has required the use of ECMO. The mortality among patients with severe pre-existing diseases was high. Future studies and guidelines should be designed to develop inclusion and exclusion criteria for the use of ECMO to ensure that this sophisticated technology continues to be deployed in patients who benefit from it. The posting of the daily updated free capacities on the ARDS Network’s website (www.ardsnetwork.de) may facilitate early patient transfer. In principal, the organized allocation of patients is conceivable to prevent excessive burden on individual centers.
Key Messages.
Patients with H1N1 pneumonia typically were started on mechanical ventilation not long after the onset of the disease (approximately after 6 days).
Young, healthy individuals may develop life-threatening H1N1 provoked respiratory failure too.
Patients with H1N1 pneumonia frequently required extracorporeal lung support.
Mortality among H1N1 patients with severe concomitant diseases was higher compared with previously healthy individuals.
The survival rates in Germany are in line with those internationally published.
eBox. ARDS Network collaborators*.
Prof. Dr. med. Michael Adamzik, Klinik für Anästhesiologie und Intensivmedizin, Universitätsklinikum Essen, Hufelandstraße 55, 45147 Essen
Prof. Dr. med. Thomas Bein, Klinik für Anästhesiologie und Operative Intensivmedizin, Universitätsklinikum Regensburg, Franz-Josef-Strauss-Allee 11, 93053 Regensburg
PD Dr. med. Henning Ebelt, Klinik für Innere Medizin III, Universitätsklinikum Halle (Saale), Ernst-Grube-Straße 40, 06097 Halle
Dr. med. Ingolf Eichler, Klinik für Herz- und Gefäßchirurgie, Klinikum Dortmund, Beurhausstraße 40, 44137 Dortmund
Prof. Dr. med. Götz Geldner, Klinik für Anästhesiologie, Intensivmedizin und Schmerztherapie, Klinikum Ludwigsburg-Bietigheim gGmbH, Posilipostraße 4, 71640 Ludwigsburg
Dr. med. Christian Lojewski, Klinik für Anästhesiologie m. S. operative Intensivmedizin, Charité – Universitätsmedizin Berlin, Campus Virchow Klinikum & Campus Mitte, Augustenburger Platz 1, 13353 Berlin
PD Dr. med. Ralf Michael Muellenbach, Klinik und Poliklinik für Anästhesiologie, Universitätsklinikum Würzburg, Zentrum Operative Medizin, Oberdürrbacher Straße 6, 97080 Würzburg
Prof. Dr. med. Rolf Rossaint, Klinik für Anästhesiologie und Klinik für Operative Intensivmedizin Erwachsene, Universitätsklinikum Aachen, Pauwelsstr. 30, 52074 Aachen
Prof. Dr. med. Claudia D. Spies, Klinik für Anästhesiologie mit Schwerpunkt operative Intensivmedizin, Charité – Universitätsmedizin Berlin, Campus Virchow Klinikum & Campus Mitte, Augustenburger Platz 1, 13353 Berlin
Dr. med. Claus Steuernagel, Klinik für Anästhesiologie, Intensivmedizin und Schmerztherapie, Elisabeth-Krankenhaus Essen, Klara-Kopp-Weg 1, 45138 Essen
*The German ARDS Network is an interdisciplinary working group within the “respiratory failure“ section of the German Interdisciplinary Association of Intensive Care and Emergency Medicine (DIVI)
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
We are grateful for the kind support and cooperation of the Robert Koch Institute, and would like to thank especially Dr. Udo Buchholz, Robert Koch Institute, DGZ-Ring 1, 13086 Berlin.
Footnotes
Conflict of interest statement
PD Dr. Weber-Carstens has received funding for a research project he initiated from Novalung.
Prof. Quintel has received fees for consultancy from Maquet, Novalung, and hemodec. For the preparation of continuing medical education events, he has received fees from Novalung. University Medicine Göttingen, the employer of Mr. Quintel, is an ECMO center.
PD Dr. Kalenka has received fees for oral presentations from Maquet and Novalung.
PD Dr. Kluge is a member of the Advisory Board of Novalung and has received material support for a research project initiated by him from Novalung.
PD Dr. Müller has received reimbursement for travel and accommodation expenses from Maquet.
Dr. Rosseau has received fees for the preparation of continuing medical education events from Novalung.
PD Dr. Moerer is a member of the ECMO team in Göttingen.
Prof. Peters, Prof. Putensen, Prof. Zwißler, and Dr. Goldmann declare that no conflict of interest exists.
References
- 1.RKI, Arbeitsgemeinschaft Influenza. Bericht zur Epidemiologie der Influenza in Deutschland, Saison 2009/10. Accessible at: www.influenza.rki.de. [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Jain S, Benoit SR, Skarbinski J, et al. Influenza-Associated Pneumonia Among Hospitalized Patients With 2009 Pandemic Influenza A (H1N1) Virus—United States, 2009. Clin Infect Dis. 2012;54:1221–1229. doi: 10.1093/cid/cis197. [DOI] [PubMed] [Google Scholar]
- 4.The Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 5.Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–2196. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 6.Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295–305. doi: 10.1164/ajrccm.149.2.8306022. [DOI] [PubMed] [Google Scholar]
- 7.Ma DS, Kim JB, Jung SH, et al. Outcomes of venovenous extracorporeal membrane oxygenation support for acute respiratory distress syndrome in adults. Korean J Thorac Cardiovasc Surg. 2012;45:91–94. doi: 10.5090/kjtcs.2012.45.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gattinoni L, Agostoni A, Pesenti A, et al. Treatment of acute respiratory failure with low-frequency positive-pressure ventilation and extracorporeal removal of CO2. Lancet. 1980;2:292–294. doi: 10.1016/s0140-6736(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 9.Kaushik M, Wojewodzka-Zelezniakowicz M, Cruz DN, et al. Extracorporeal carbon dioxide removal: the future of lung support lies in the history. Blood Purif. 2012;34:94–106. doi: 10.1159/000341904. [DOI] [PubMed] [Google Scholar]
- 10.Lindskov C, Jensen RH, Sprogoe P, et al. Extracorporeal membrane oxygenation in adult patients with severe acute respiratory failure. Acta Anaesthesiol Scand. 2013;57:303–311. doi: 10.1111/aas.12050. [DOI] [PubMed] [Google Scholar]
- 11.Terragni P, Maiolo G, Ranieri VM. Role and potentials of low-flow CO(2) removal system in mechanical ventilation. Curr Opin Crit Care. 2012;18:93–98. doi: 10.1097/MCC.0b013e32834f17ef. [DOI] [PubMed] [Google Scholar]
- 12.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 13.Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175:160–166. doi: 10.1164/rccm.200607-915OC. [DOI] [PubMed] [Google Scholar]
- 14.Bein T, Weber-Carstens S, Goldmann A, et al. Lower tidal volume strategy (≈3 mL/kg) combined with extracorporeal CO(2) removal versus ’conventional’ protective ventilation (6 ml/kg) in severe ARDS : The prospective randomized Xtravent-study. Intensive Care Med. 2013;39:847–856. doi: 10.1007/s00134-012-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moerer O, Quintel M. Protective and ultra-protective ventilation: using pumpless interventional lung assist (iLA) Minerva Anestesiol. 2011;77:537–544. [PubMed] [Google Scholar]
- 16.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 17.Noah MA, Peek GJ, Finney SJ, et al. Referral to an Extracorporeal Membrane Oxygenation Center and Mortality Among Patients With Severe 2009 Influenza A(H1N1) JAMA. 2011;306:1659–1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 18.Kaisers U, Rossaint R. New pandemic A (H1N1) influenza: Are our intensive care resources sufficient? Anaesthesist. 2010;59:9–10. doi: 10.1007/s00101-009-1668-z. [DOI] [PubMed] [Google Scholar]
- 19.Bürkle MA, Frey L, Zwissler B. Pandemic influenza A/H1N1 2009: Challenge for intensive care medicine. Anaesthesist. 2010;59:11–22. doi: 10.1007/s00101-009-1667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beiderlinden M, Kuehl H, Boes T, Peters J. Prevalence of pulmonary hypertension associated with severe acute respiratory distress syndrome: predictive value of computed tomography. Intensive Care Med. 2006;32:852–857. doi: 10.1007/s00134-006-0122-9. [DOI] [PubMed] [Google Scholar]
- 21.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 22.Combes A, Bacchetta M, Brodie D, et al. Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care. 2012;18:99–104. doi: 10.1097/MCC.0b013e32834ef412. [DOI] [PubMed] [Google Scholar]