T cells from RA patients are hypoglycolytic due to insufficient induction of the glycolytic activator PFKFB3, resulting in impaired autophagy and reduced ROS production.
Abstract
In the HLA class II–associated autoimmune syndrome rheumatoid arthritis (RA), CD4 T cells are critical drivers of pathogenic immunity. We have explored the metabolic activity of RA T cells and its impact on cellular function and fate. Naive CD4 T cells from RA patients failed to metabolize equal amounts of glucose as age-matched control cells, generated less intracellular ATP, and were apoptosis-susceptible. The defect was attributed to insufficient induction of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3), a regulatory and rate-limiting glycolytic enzyme known to cause the Warburg effect. Forced overexpression of PFKFB3 in RA T cells restored glycolytic flux and protected cells from excessive apoptosis. Hypoglycolytic RA T cells diverted glucose toward the pentose phosphate pathway, generated more NADPH, and consumed intracellular reactive oxygen species (ROS). PFKFB3 deficiency also constrained the ability of RA T cells to resort to autophagy as an alternative means to provide energy and biosynthetic precursor molecules. PFKFB3 silencing and overexpression identified a novel extraglycolytic role of the enzyme in autophagy regulation. In essence, T cells in RA patients, even those in a naive state, are metabolically reprogrammed with insufficient up-regulation of the glycolytic activator PFKFB3, rendering them energy-deprived, ROS- and autophagy-deficient, apoptosis-sensitive, and prone to undergo senescence.
T lymphocytes are key drivers of the chronic inflammatory process that leads to rheumatoid arthritis (RA), a prototypic autoimmune syndrome manifesting with destruction of synovial joints, accelerated cardiovascular disease, and shortened life expectancy (Weyand and Goronzy, 2006; Naz and Symmons, 2007; Goronzy and Weyand, 2009). CD4 T cells are the major cellular component in synovitis, where they form complex tertiary lymphoid architectures and provide help for the production of signifying autoantibodies (Takemura et al., 2001; Goronzy and Weyand, 2005; Seyler et al., 2005). RA occurs in genetically predisposed hosts. The strongest inherited risk derives from genes in the MHC class II region, intimately connected to the antigen recognition process of CD4 T cells (Kochi et al., 2010). Patients with RA have a phenotype of premature immune aging, exemplified in the accumulation of CD4+CD28− T cells, contraction of T cell diversity, and shortening of T cell telomeres (Schmidt et al., 1996; Koetz et al., 2000; Weyand et al., 2009). The responsiveness of CD4 T cells to activating signals is altered in RA patients, with some tolerance defects originating in membrane-proximal signaling events (Singh et al., 2012). RA T cells express low levels of ataxia telangiectasia mutated, a protein kinase involved in sensing DNA double-strand breaks, orchestrating cell cycle checkpoints and facilitating DNA damage repair (Shao et al., 2009). In response to unattended DNA lesions and genomic stress, RA T cells chronically activate the JNK–stress kinase pathway (Shao et al., 2010).
Chronic T cell activation in RA imposes cellular energy demands that deviate from conditions where most T cells are in a resting state. Exposure to antigen elicits rapid and extensive clonal expansion, and T cells respond to their fairly unique energy needs by greatly enhancing metabolic activities and up-regulating aerobic glycolysis (Heikamp and Powell, 2012; MacIver et al., 2013), as well as autophagy (Fox et al., 2005; Walsh and Bell, 2010). This shift from a primarily respiratory energetic pathway to a less conservative but more strident glycolytic metabolism with lactate production (known as the Warburg effect), coupled with increased glucose uptake, is used by proliferating cells to promote the efficient conversion of glucose into the macromolecules needed to construct new cells (Pearce, 2010; Wang et al., 2011). Triggering of the T cell antigen receptor not only leads to rapid cell replication and clonal expansion, it also induces the T cell differentiation program (Wang and Green, 2012), including the synthesis of large amounts of effector cytokines and a shift in T cell trafficking patterns. Notably, functionally distinct T cell subsets are characterized by distinct metabolic programs (Finlay and Cantrell, 2011; Michalek et al., 2011).
The metabolic fate of glucose and the pathways to which it is committed is tightly regulated by a cascade of enzymes and metabolites (Mor et al., 2011). Cells catabolize glucose through glycolysis; some tissues use it to build glycogen. Under conditions of high glucose flux, cells can divert glucose to the pentose phosphate pathway (PPP). A key event in the glycolytic breakdown of glucose is the phosphorylation of fructose 6-phosphate to fructose 1,6 bisphosphate through 6-phosphofructo-1-kinase (PFK1), an irreversible reaction which commits glucose to glycolysis. As a gatekeeper in the metabolic degradation of glucose, PFK1 is controlled by downstream metabolites, most importantly by its allosteric activator fructose 2,6-bisphosphate (F2,6BP; Van Schaftingen et al., 1980). F2,6BP can enhance glycolysis even in the presence of glucose and can overcome the inhibitory effects of ATP, effectively uncoupling the glycolytic flux from cellular bioenergetics (Okar et al., 2001). Cellular levels of F2,6BP are essentially set by the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase (PFKFB), which catalyzes both the production and degradation of F2,6BP through its kinase and phosphatase functions (Okar et al., 2001; Rider et al., 2004). The family of PFKFBs includes four isoenzymes, PFKFB1–4, which are regulated through diverse mechanisms, including tissue-specific expression, alternative splicing, alternative promoter usage, and enzymatic regulation through covalent and allosteric interactions. Rapidly proliferating cells, including tumors, have the inducible isoform of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3), which allows them to promptly attend to heightened energy demands (Chesney et al., 1999). Numerous human malignancies have high expression of PFKFB3 (Atsumi et al., 2002; Bando et al., 2005; Kessler et al., 2008). PFKFB3 generates F2,6BP and, hence, critically regulates the glycolytic rate under normal and pathophysiological conditions (Okar and Lange, 1999; Seo et al., 2011; Telang et al., 2012). PFKFB3 is distinguished from the other isoenzymes by its high kinase to bisphosphatase ratio, emphasizing its critical role in giving energy-stressed cells access to the essential biofactor F2,6BP (Yalcin et al., 2009b; Colombo et al., 2010).
Given the energetic and biosynthetic demands of T cells undergoing activation, they also resort to autophagy, a tightly regulated catabolic mechanism maintaining homeostasis through recycling building blocks for basal macromolecular synthesis and eliminating damaged proteins or organelles (Pua et al., 2009; Jia et al., 2011; Kovacs et al., 2012). During autophagy, portions of the cytoplasm are sequestered in double-membrane vesicles, the autophagosomes, and then degraded after fusing with lysosomes, committing macromolecules to the recycling process. A screen of yeast mutants incapable of surviving nitrogen starvation has identified a network of autophagy-related (Atg) genes all involved in the formation of autophagosomes (Tsukada and Ohsumi, 1993). A mammalian homolog of yeast Atg8 is the microtubule-associated protein 1 light chain 3 (LC3). LC3 undergoes posttranscriptional modification by a ubiquitination-like process to transfer into a soluble form, LC3-I. LC3-I is then lapidated with phosphatidylethanolamine into LC3-II, which associates with the outer and inner autophagosome membranes (Kabeya et al., 2000; Tanida et al., 2004). Accordingly, LC3 levels provide a good quantification of autophagic activity (Kabeya et al., 2000).
In this study, we have investigated how the metabolic competence of T lymphocytes is reprogrammed in a disease setting that exposes these long-lived cells to a chronic inflammatory milieu and how metabolic reprogramming affects T cell survival. We found that naive CD4 T cells from RA patients are in a state of energy deprivation, mostly because they are unable to fully mobilize aerobic glycolysis. They divert glucose to the PPP, as indicated by the excess production of NADPH, resulting in the decline of cellular reactive oxygen species (ROS) levels. They also fail to tap into autophagy to access internal biosynthetic precursors. The two defects synergize in maintaining the energy deprivation of the T cells and are linked to the same molecular underpinning: the inability to up-regulate the glycolytic rate-limiting enzyme PFKFB3. This enzyme, mechanistically linked to the Warburg effect in cancer cells, is robustly induced by TCR activation, in anticipation of the enormous energy demands that result from clonal expansion and effector molecule synthesis. PFKFB3 silencing in healthy CD4 T cells mimics the conditions encountered in RA and forced overexpression of PFKFB3 in RA T cells repairs the glycolytic insufficiency and the autophagic activity. As a functional consequence of impaired glucose metabolism, reduced autophagy, and reduced ROS levels, RA T cells become much more sensitive to limitations in glucose utilization and are prone to undergo apoptosis. The metabolic reprogramming in naive RA T cells exposes the patients to chronic T cell loss, exhaustion of their T cell reserve, and the development of lymphopenia, now recognized as a risk factor for autoimmunity.
RESULTS
Reduced glucose consumption in RA CD4 T cells
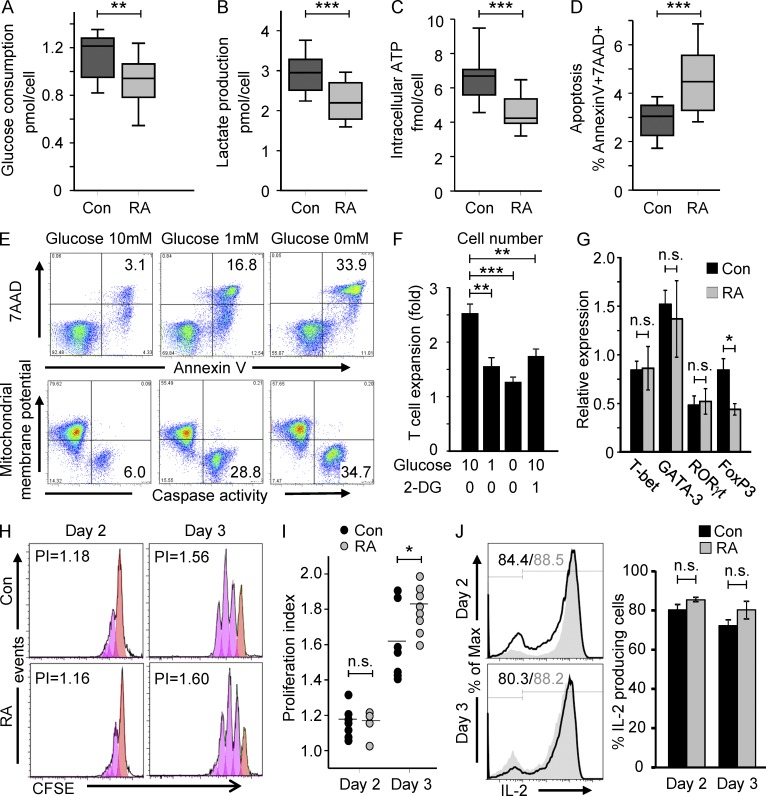
TCR triggering initiates an activation cascade that leads to robust T cell proliferation, clonal expansion, rapid mobility, and effector molecule production, all associated with a sudden increase in metabolic demand. To meet the biosynthetic needs, T cells undergoing activation switch their glucose metabolism from oxidative phosphorylation to aerobic glycolysis (Finlay, 2012). To compare glycolytic flux in healthy CD4 T cells and in autoimmune-prone T cells from RA patients, we quantified glucose utilization (Fig. 1 A), lactate production (Fig. 1 B), and generation of intracellular ATP levels (Fig. 1 C) in naive CD4 T cells 72 h after stimulation. All three parameters were reduced in RA T cells by 20–30%. The lowered lactate and ATP production in RA T cells was maintained over a wide range of TCR signal strengths which was modified by titrating the ratio of anti-CD3–coated beads per T cell from 0.25 to 2. The failure of RA T cells to consume glucose at a level of normal T cells was associated with a significant increase in apoptosis (Fig. 1 D). The proportion of 7AAD+ and Annexin V+ cells 3 d after TCR cross-linking was 50% higher in RA T cells. As in previous studies (Fujii et al., 2009; Singh et al., 2009), the frequencies of T cells expressing activation markers was even higher in RA T cells than in control T cells, excluding insufficient stimulation of the patient-derived cells as a cause of reduced glucose consumption.
Figure 1.
Glucose hypometabolism in RA T cells. Naive CD4 (CD4+CD45RO−) T cells were isolated from RA patients (RA) and age-matched controls (Con) and stimulated with anti-CD3/CD28 microbeads. On day 3, cells were harvested, washed, and recultured for a period of 4 h. Culture medium was collected at 0 and 4 h to measure glucose (A, 14 RA patients and 11 healthy controls) and lactate concentration (B, 21 RA patients and 18 healthy controls). Intracellular ATP was determined in cell pellets (C, 17 RA patients and 18 healthy controls). Frequencies of apoptotic (7AAD+ and Annexin V+) T cells were assessed by flow cytometry (D). Results are shown as box plots. Median, 25th, and 75th percentiles (box), and 10th and 90th percentiles (whiskers) are displayed. T cell apoptosis and caspase activity (E) in relation to glucose availability were evaluated by stimulating T cells for 2 d, washing them and reculturing them in the absence or presence of glucose (10 or 1 mM), n = 3. To block glycolytic activity, 2-deoxy-d-glucose (2-DG) was added to T cell cultures on day 0. T cell expansion (F) was measured after 72 h, n = 4. T cell responsiveness was compared in RA and control CD4+CD45RO− T cells 48 and 72 h after stimulation. Expression of the lineage commitment transcription factors T-bet, Gata-3, FoxP3, and RORγt was measured by RT-PCR after 48 h (G, 10 RA patients and 11 healthy controls). T cell proliferation was quantified by CFSE dilution and representative histograms are shown (H). Proliferation indices from four to eight RA patients and seven age-matched controls are presented (I). Frequencies of IL-2–producing T cells were quantified by cytometric analysis of intracellular staining (J). Results from 4 RA patients and 4 age-matched controls are shown as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., non-significant.
Withdrawal of proliferating CD4 T cells from glucose as an energy source revealed several phenotypes that all confirmed the dependence of clonally expanding T cells on the fermentation of glucose. Glucose deprivation of activated CD4 T cells induced massive T cell apoptosis and caspase activity (Fig. 1 E). The frequencies of 7AAD+ and Annexin V+ cells and caspase-active cells increased fivefold when cells were cultured in low-glucose media and further increased under glucose-free conditions. Cell expansion was abrogated under glucose deprivation and attenuated by 2-deoxy-d-glucose, a competitive inhibitor of hexokinase which catabolically blocks glycolysis (Fig. 1 F).
Dependent on their differentiation state, T cells differ in their aerobic glycolysis. Glucose uptake and glycolysis are increased in Th1, Th2, and Th17 cells, whereas regulatory T cells exhibit oxidative phosphorylation with more limited glycolytic rates (O’Neill and Hardie, 2013). To rule out that the naive CD4 T cells isolated from RA patients had already entered a T cell differentiation program, the T cell lineage-specific transcription factors T-bet, GATA-3, RORγt, and FoxP3 were measured 48 h after T cell stimulation (Fig. 1 G). T-bet, GATA-3, and RORγt expression was indistinguishable in control and patient-derived T cells. In RA T cells, FoxP3 was less abundantly expressed, suggesting that the patients’ T cells are less likely to differentiate into regulatory T cells. A reduction in FoxP3+ T reg cells should have led to overall increased glycolysis and could not explain the hypoglycolytic state of naive CD4 T cells in the patients.
To examine whether the patient-derived T cells were anergic, thus consuming less glucose, several parameters of T cell responsiveness were monitored 48–72 h after TCR stimulation. RA cells responded as vigorously as control T cells and proliferated even faster (Fig. 1, H and I). Frequencies of IL-2–producing T cells in RA and control samples were similar (Fig. 1 J), confirming that the patients’ T cells entered the activation cascade and up-regulated the TCR-induced gene programs. In essence, naive RA T cells were uncommitted to a T cell lineage and were highly responsive to TCR-mediated signaling but displayed abnormalities in glucose utilization, ATP production, and apoptotic susceptibility.
Induction of PFKFB3 is impaired in RA T cells
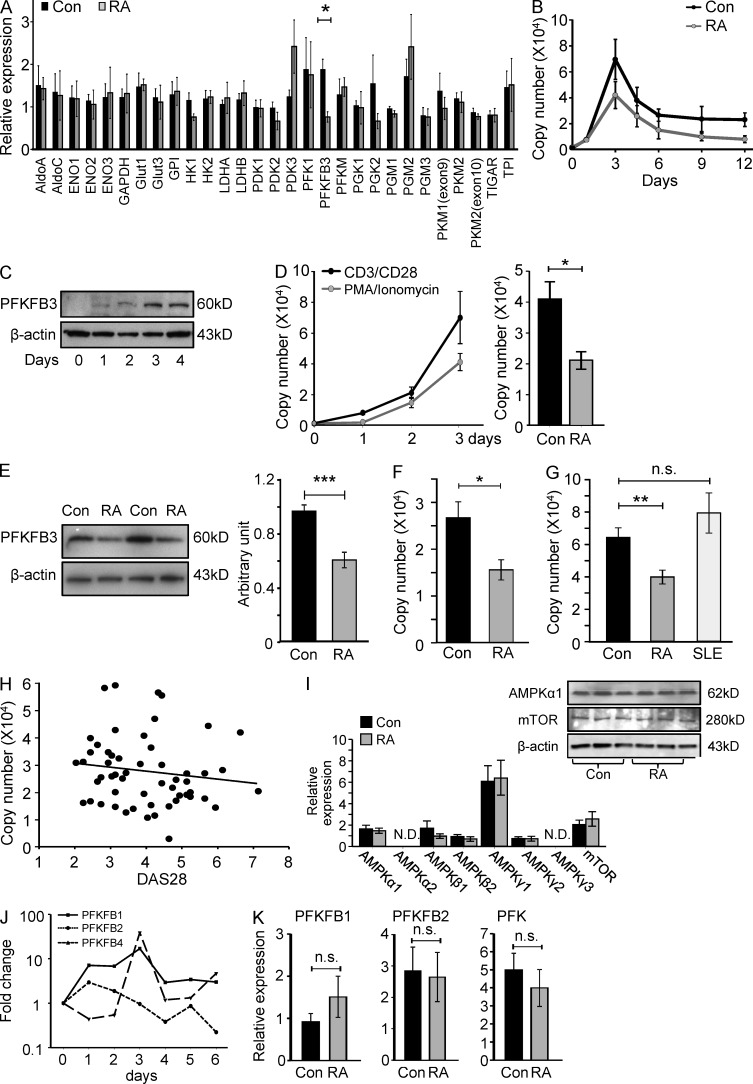
The ability of cells to utilize glucose as an energy source is tightly regulated through key glucose transporters and a series of enzymes which determine the metabolic fate of intracellular glucose. Critical adjustments in glycolytic flux are set by F2,6BP, which activates PFK1 and is by itself synthesized and degraded by a family of bifunctional enzymes, the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases (PFKFB; Moncada et al., 2012). To explore whether RA T cells have an intrinsic defect in glycolysis, we measured the expression of 29 glycolysis-related genes in activated CD4 T cells from RA patients and matched controls (Fig. 2 A). Expression levels were quantified 72 h after T cell activation. Most of the markers critically involved in glucose metabolism were indistinguishable between RA and control T cells, including the glucose transporters. However, RA T cells had a defect in up-regulating PFKFB3, a rate-limiting enzyme in glycolysis. Transcripts for PFKFB3 were 50% lower in T cells from patients compared to controls. To assess the impact of TCR-mediated signaling on PFKFB3 expression, we monitored mRNA levels over a period of 12 d after TCR cross-linking (Fig. 2 B) in control and patient-derived T cells. Resting naive CD4 T cells had low levels of PFKFB3-specific transcripts and no detectable protein (Fig. 2, B and C). T cell stimulation induced massive up-regulation of PFKFB3 transcription up to 30-fold compared with prestimulation levels. Both transcript and protein levels were maximal 72 h after T cell triggering (Fig. 2, B and C). PFKFB3 transcript levels subsequently declined but remained above steady-state for >2 wk (Fig. 2 B). In RA T cells, poststimulation PFKFB3 peaked after 72 h but remained below levels in control T cells over the entire activation cycle. Bypassing the TCR and stimulating T cells with 20 ng/ml PMA and 200 µg/ml ionomycin provided a potent PFKFB3-inducing signal, albeit not quite as efficient as TCR ligation (Fig. 2 D). Reduced PFKFB3 induction in RA T cells was maintained after TCR-independent activation.
Figure 2.
PFKFB3 induction is suppressed in RA T cells. Naive CD4 (CD4+CD45RO−) T cells were isolated from RA patients and age-matched controls and stimulated with anti-CD3/CD28 microbeads for 3 d. Glycolysis-related gene transcripts were quantified by qPCR (A, n = 3). Kinetics of the expression of PFKFB3 in T cells following TCR ligation were monitored by RT-PCR over 12 d (B, 6 RA patients and 6 healthy controls) or by Western blotting over 4 d (C). Naive CD4 T cells were stimulated with either anti-CD3/CD28 microbeads or 20 ng/ml PMA and 200 µg/ml ionomycin and PFKFB3 transcript levels were monitored over 72 h in six samples. PMA/ionomycin-induced PFKFB3 transcripts from 5 RA patients and 6 controls are shown (D). Protein levels of PFKFB3 were quantified by Western blotting. Representative data for 2 patients and 2 controls are shown. Quantification of band densities from five independent experiments in 10 patients and 10 control samples are given as mean ± SEM (E). Expression of PFKFB3 in CD4+CD45RO+ memory T cells was quantified in 10 controls and 5 RA patients by RT-PCR (F). Activation-induced up-regulation of PFKFB3 was compared in control T cells, RA T cells, and SLE T cells on day 3 after TCR ligation. PFKFB3 mRNA levels were determined by RT-PCR in n = 16 RA patients, n = 33 controls, and n = 11 SLE patients. Results are given as mean ± SEM (G). PFKFB3 transcripts quantified by qPCR on day 3 after T cell stimulation were correlated with RA disease activity (DAS28; r2 = 0.020, P = 0.306; H). Expression of AMPK family members and mTOR was determined by qPCR and Western blotting, respectively. Data from 6 RA patients and 6 age-matched controls are presented as mean ± SEM and representative immunoblots are shown (I). Transcripts of PFKFB1, 2, and 4 were quantified by qPCR over 6 d (J). Activation-induced up-regulation of PFKFB1, 2, and 4 were compared in control and RA T cells (n = 8 each) on day 3 after TCR ligation. Results are given as mean ± SEM (K). *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., non-significant.
In a cohort of RA patients, TCR-mediated PFKFB3 up-regulation was significantly diminished (Fig. 2 E). RA T cells reached only 60–65% of the protein concentration in control T cells. Reduced induction of PFKFB3 was not limited to the naive subset but also involved memory CD4+CD45RO+ T cells (Fig. 2 F). CD4+CD45RO+ memory T cells expressed overall lower levels of PFKFB3 transcripts with patient-derived memory CD4 T cells generating ∼50% of the transcripts found in controls.
To rule out that the suppression of PFKFB3 is a direct consequence of the inflammatory activity in RA, we compared transcript levels in three cohorts: patients with RA, patients with systemic lupus erythematosus (SLE), and age-matched healthy individuals. Patients with the autoimmune syndrome SLE were clearly distinct from RA patients, as their CD4 T cells expressed even higher levels of PFKFB3 transcripts than the controls (Fig. 2 G). To assess the impact of RA activity on PFKFB3 induction in individual RA patients, TCR-induced PFKFB3 transcript levels in CD4+CD45RA+ T cells were correlated with a disease activity score (Fig. 2 H). PFKFB3 mRNA levels were essentially independent from the intensity of the joint inflammation. Patients with low disease activity had no advantage over patients with many inflamed joints. PFKFB3 copy numbers <40,000 occurred frequently even among patients with DAS28 scores <3, indicating that PFKFB3 deficiency in this cohort of 55 cases correlated with the diagnosis of RA but not with the inflammatory milieu.
Collectively, RA CD4 T cells have low efficiency in adapting to the metabolic demands of TCR stimulation and insufficiently induce the glycolytic enzyme PFKFB3. The defect does not occur in another autoimmune disease, SLE, and appears to be independent from the inflammatory milieu.
Insufficient induction of PFKFB3 could possibly be a consequence of abnormal energy sensing in the RA T cells. The adenosine monophosphate-activated protein kinase (AMPK) complex and mammalian target of rapamycin (mTOR) are master regulators of cellular energy homeostasis. AMPK functions as part of an evolutionarily conserved energy-sensing pathway that couples cellular bioenergetics to metabolic control and cell growth (Blagih et al., 2012). Low cellular energy stores activate AMPK via phosphorylation to promote ATP-producing catabolic pathways and limit ATP-consuming processes. mTOR, a downstream target of AMPK, acts as an intracellular nutrient sensor and ultimately controls protein synthesis and cell growth (Chi, 2012). To assess the intactness of the energy-sensing apparatus, expression of the different components of the AMPK complex, as well as protein levels of AMPKα1 and mTOR, were determined (Fig. 2 I). Transcriptome analysis for AMPKα1, α2, β1, β2, γ1, γ2, γ3, and mTOR revealed no differences between patient-derived and control T cells. AMPKγ1 transcripts were abundant, and AMPKα2- and γ3-specific sequences were not detected. Immunoblotting for the major AMPK component, AMPKα1, and mTOR protein demonstrated similar expression levels in naive CD4 T cells from RA patients and healthy controls, suggesting that nutrient-sensing mechanisms were intact.
The defect in producing glycolytic enzyme was selective for the PFKFB3 isoform. Overall, PFKFB1 and PFKFB4 responded to T cell stimulation, and PFKFB2 transcripts were unaffected (Fig. 2 J). Kinetics of the three isoforms were indistinguishable between RA and control T cells (Fig. 2 K). Thus, human T cells regulate the energetic demands associated with antigen recognition and T cell differentiation by intensifying aerobic glycolysis and boost glucose metabolism through the induction of PFKFB regulatory enzymes. The metabolic switch and adaptation to biosynthetic needs involving PFKFB3 is defective in the autoimmune syndrome RA but well maintained in patients with active SLE.
PFKFB3 deficiency renders CD4 T cells apoptosis susceptible
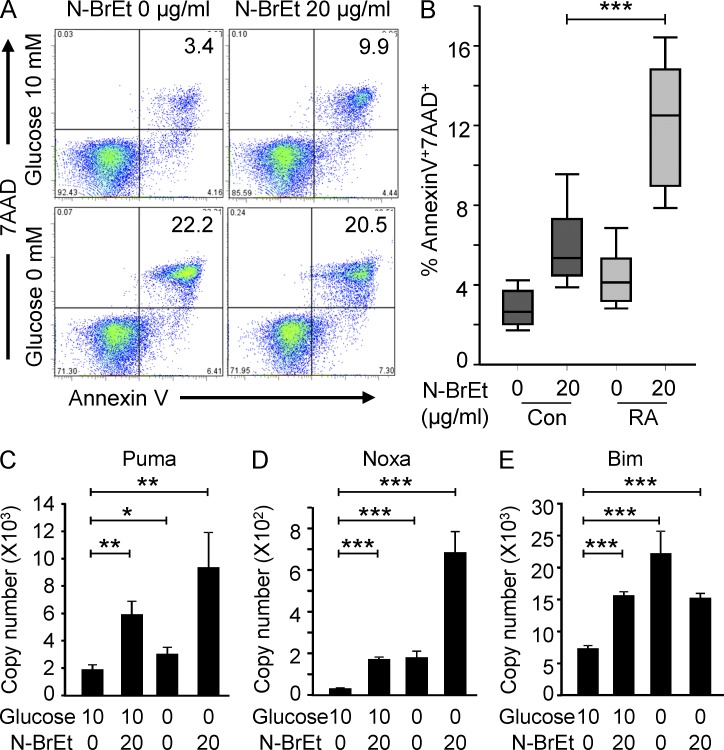
To assess the functional impact of diminished glycolytic flux caused by insufficient up-regulation of PFKFB3, we exploited pharmacologic and genetic inhibition of the enzyme through the PFKFB inhibitor N-bromoacetylethanolamine phosphate (N-BrEt; Harada et al., 1997; Hirata et al., 2000) or PFKFB3 shRNA. Healthy naive CD4 T cells were activated by TCR ligation, and 48 h later, PFKFB enzyme activity was inhibited by treating with 20 µg/ml N-BrEt. The frequency of Annexin V+ and 7AAD+ cells more than doubled to 10% of the T cell population (Fig. 3 A). In glucose-free medium, 20% of the T cells underwent apoptosis and addition of the PFKFB3 inhibitor no longer had an effect on T cell survival (Fig. 3 A). Notably, naive CD4 T cells from RA patients were more sensitive to PFKFB inhibition. Treatment with the inhibitor increased the frequencies of apoptotic T cells to 15% as compared with 5% in controls (Fig. 3 B). Lack of glucose rapidly induced up-regulation of the apoptotic genes Puma (Fig. 3 C), Noxa (Fig. 3 D), and Bim (Fig. 3 E), connecting the apoptotic susceptibility of RA T cells to their inability to metabolize glucose with the same efficiency as healthy T cells (Fig. 1 D).
Figure 3.
PFKFB3 deficiency renders CD4 T cells apoptosis susceptible. Naive CD4 T cells were purified and activated as in Fig. 1. On day 2, T cells were washed and treated with 20 µg/ml of the PFK-2 inhibitor N-BrEt for an additional 48 h in the absence or presence of 10 nM glucose. Apoptotic cells were detected by flow cytometry; representative data are shown (A). Inhibitor-induced increases in death rates for control and RA T cells are presented for 18 patients and 15 controls (B). Data are shown as box plots. Median, 25th, and 75th percentiles (box), and 10th and 90th percentiles (whiskers) are displayed. Transcripts of Puma (C), Noxa (D), and Bim (E) were quantified by qPCR and are shown as mean ± SEM from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
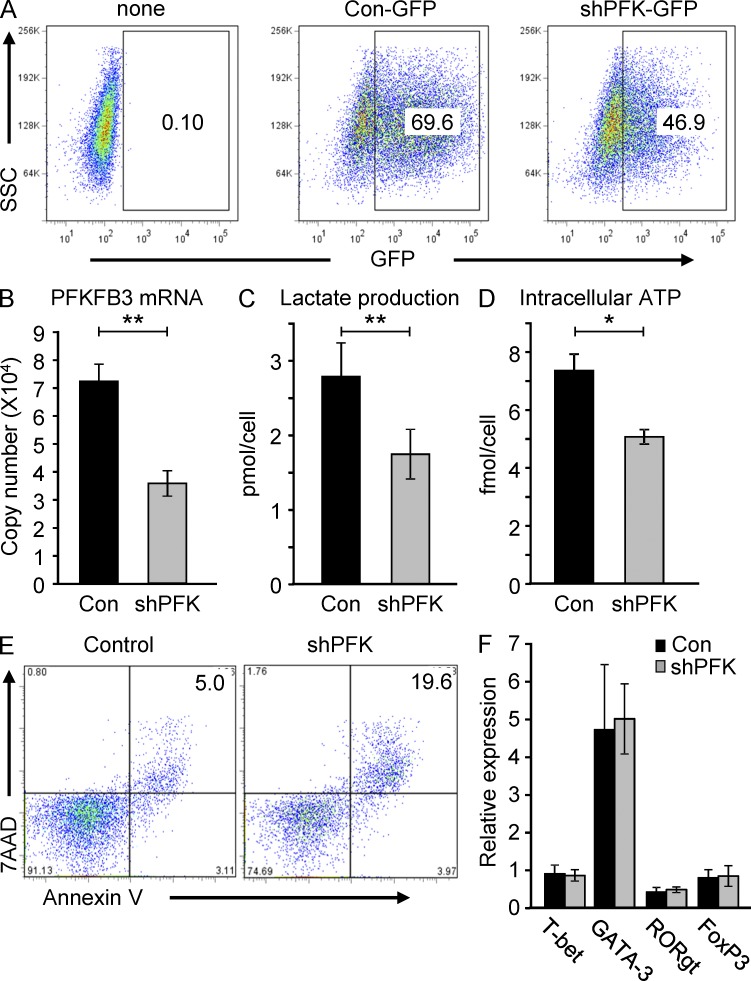
The critical role of PFKFB3 in regulating the response pattern of antigen-activated T cells was confirmed by gene-specific RNA interference. Healthy CD4 T cells were transfected with pSuper-shPFKFB3-GFP or control plasmids. Intracellular GFP expression traced the vectors in about 45–70% of cells (Fig. 4 A). shRNA interference reduced PFKFB3 transcripts to 50% (Fig. 4 B), mimicking conditions in RA T cells. Three outcome parameters were evaluated after PFKFB3 knockdown: lactate production (Fig. 4 C), intracellular ATP levels (Fig. 4 D), and apoptotic susceptibility (Fig. 4 E). Generation of intracellular ATP and output of lactate were reduced by 25–35%, again resembling the spontaneous PFKFB3-deficient RA T cells (Fig. 1). Frequency of Annexin V+ and 7AAD+ cells markedly increased from 5 to 20% when PFKFB3 was knocked down (Fig. 4 E). To explore whether PFKFB3 knockdown is able to redirect the T cell differentiation program toward the selection of low glycolytic lineages, the lineage-specific transcription factors T-bet, GATA-3, RORγt, and FoxP3 were quantified in T cells transfected with pSuper-shPFKFB3-GFP or control plasmids (Fig. 4 F). Interfering with glycolytic activity early in the T cell activation program did not affect T cell lineage assignment.
Figure 4.
Silencing PFKFB3 constrains glucose metabolism in T cells. T cells were transfected with PFKFB3 shRNA plasmids (shPFKFB3-GFP) or control plasmids on day 2 after stimulation. Transfection efficiency was monitored by flow cytometry for GFP-expressing cells (A), and PFKFB3 transcripts were quantified 24 h later in control and shPFK-transfected cells by RT-PCR (B). Lactate production (C) and concentrations of intracellular ATP (D) were compared in five independent experiments, and results are given as mean ± SEM. Apoptotic cells were detected by flow cytometry; representative dot blots are shown (E). Expression of the lineage commitment transcription factors T-bet, Gata-3, FoxP3, and RORγt was measured in T cells 24 h after transfection (F), n = 8. *, P < 0.05; **, P < 0.01.
Ectopically expressed PFKB3 restores apoptotic resistance in RA T cells
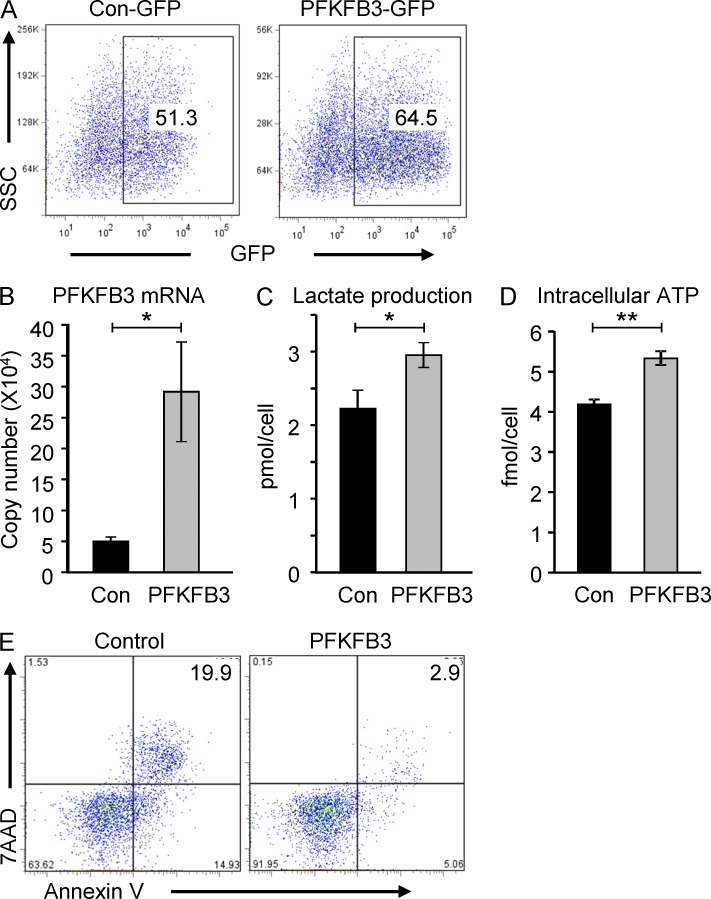
To support the hypothesis that the metabolic and functional abnormalities of RA T cells were causally related to PFKFB3 deficiency, we overexpressed PFKFB3 in RA T cells. FACS detected intracellular GFP in 50–65% of the T cells transfected with either pIRES-PFKFB3-GFP or control GFP constructs (Fig. 5 A). Aberrant expression was confirmed by RT-PCR of PFKFB3 transcripts (Fig. 5 B). Forced overexpression of the enzyme reconstituted glycolytic flux in RA T cells and improved both lactate production (Fig. 5 C) and intracellular ATP generation (Fig. 5 D). Repairing PFKFB3 expression had a direct effect on cell survival (Fig. 5 E) and protected cells from apoptotic death. Thus, defects in T cell longevity encountered in autoimmune T cells can be directly attributed to the metabolic failure caused by PFKFB3 insufficiency.
Figure 5.
Overexpression of PFKFB3 in RA T cells restores glucose metabolism and protects from apoptosis. CD4+CD45RA− T cells were isolated from RA patients, stimulated with anti-CD3/CD28 beads, and 48 h later transfected with PFKFB3 overexpression plasmids (pIRES-PFKFB3-GPF) or control plasmids. Cells were kept in culture for an additional 48 h. Flow cytometric analysis of a representative experiment analyzing GFP expression is shown (A). The following parameters were determined in transfected T cells: (B) PFKFB3 mRNA level by qPCR; (C) lactate production; and (D) intracellular ATP concentration quantified as in Fig. 1. Data from five independent experiments are presented as mean ± SEM. Apoptotic susceptibility was tested in PFKFB3 and control transfected T cells by measuring 7AAD+ and Annexin V+ cells (E). *, P < 0.05; **, P < 0.01.
The loss of PFKFB3 in CD4 T cells enhances the PPP and imposes reductive stress
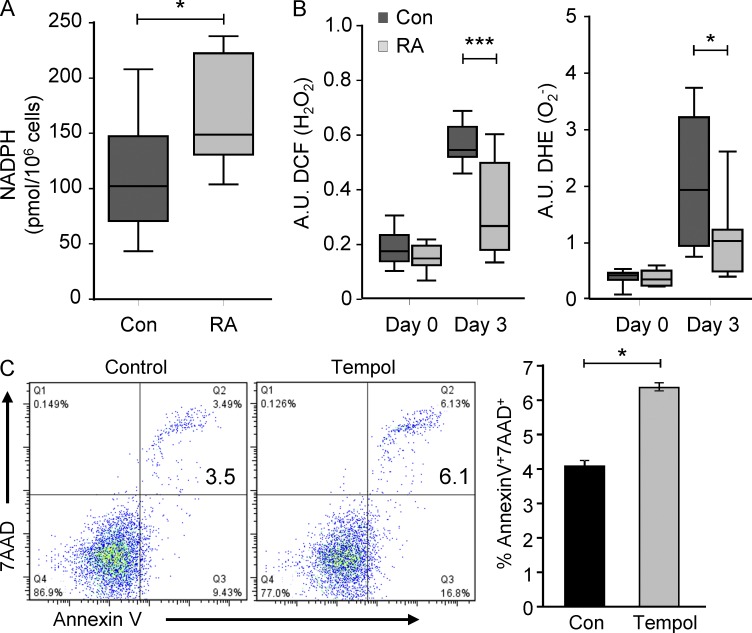
Intracellular glucose is phosphorylated to glucose-6-phosphate (G6P) to enter glycolysis and be metabolized into ATP, the coenzyme NADH, and pyruvate. Alternatively, G6P can proceed through the PPP, resulting in the production of ribose-5-phosphate and NADPH. The PPP serves as one of the main antioxidant cellular defense systems and provides ribose phosphate to the cell (Stanton, 2012). To explore whether the hypoglycolytic activity of RA T cells had any impact on the utilization of the PPP and the cellular redox state, NADPH and ROS levels were compared in patient and control T cells stimulated for 72 h. NADPH levels were 50% higher in the RA-derived cells (Fig. 6 A), indicating that G6P was shunted toward the PPP. A major function of the PPP is the regeneration of reduced glutathione at the expense of NADPH, which scavenges ROS and protects the cytoplasmic compartment from oxidative stress. To examine whether ROS levels were altered in hypoglycolytic, PPP-utilizing RA T cells, cellular H2O2 and superoxide levels were assessed by cytometric analysis of profluorescent redox-sensitive indicators (Fig. 6 B). After activation, H2O2 and superoxide levels increased robustly about three- to fivefold in healthy T cells. In RA T cells, the TCR-dependent ROS release was dampened, increasing only about twofold (Fig. 6 B). In essence, PFKFB3-deficient RA T cells overutilize the PPP, ultimately depleting the cytoplasm of ROS and imposing reductive stress.
Figure 6.
Naive CD4 T cells were purified and activated as in Fig. 1. NADPH levels were measured in T cell extracts 72 h after activation. Data from 8 RA patients and 10 age-matched controls are presented as box plots. Median, 25th, and 75th percentiles (box), and 10th and 90th percentiles (whiskers) are displayed (A). T cells were loaded with fluorogenic dyes (DCF and DHE) and H2O2 and O2− production was quantified by flow cytometry on days 0 and 3 after stimulation. Data from 11–18 RA patients and 15–22 healthy controls are presented as box plots. Median, 25th, and 75th percentiles (box), and 10th and 90th percentiles (whiskers) are displayed (B). CD4+CD45RO− T cells were stimulated and cultured in the absence or presence of the ROS scavenger Tempol (50 µM) for 72 h. Frequencies of apoptotic (7AAD+ and Annexin V+) T cells were assessed by flow cytometry. Representative dot blots are shown and data from three independent experiments are presented as mean ± SEM (C). *, P < 0.05; ***, P < 0.001.
ROS are critically involved in signaling events that support T cell expansion and survival (Sena et al., 2013). Initial experiments exploring the metabolic intactness of RA T cells had already demonstrated higher apoptotic susceptibility (Fig. 1 D). To connect the scavenging of cytoplasmic ROS with apoptotic susceptibility, T cells were stimulated with anti-CD3/CD28 beads in the absence and presence of 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (Tempol), a cell-permeable and stable free radical nitroxide with strong antioxidant properties. Tempol treatment rendered T cells apoptosis-prone (Fig. 6 C), assigning prosurvival functions to optimally controlled ROS concentrations and implicating PPP hyperactivity in shortening the survival of RA T cells.
RA T cells fail to use autophagy to tap into cell-internal energy sources
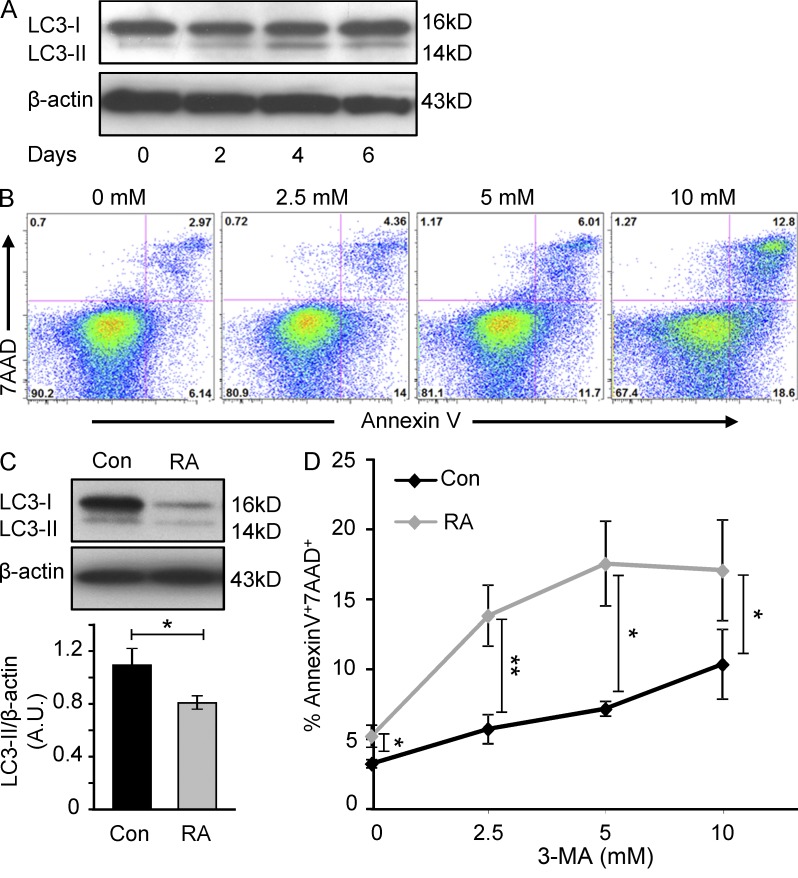
After antigen encounter, naive CD4 T cells initiate massive clonal expansion, launch a cellular differentiation program, synthesize large amounts of effector molecules, and adapt their cellular mobility apparatus. To accommodate the dual demand for energy as well as biosynthetic precursor molecules, naive T cells shift from oxidative phosphorylation to aerobic glycolysis to drive ATP production and provide pyruvate. In parallel, naive T cells tap into cell-internal storage, induce autophagy, and break down their own components and reuse them. To understand to what degree naive CD4 T cells rely on autophagy induction, we monitored the autophagy marker LC3-II over the 1st wk after stimulation (Fig. 7 A). Resting naive CD4 T cells have very low levels of LC3-II. Following TCR cross-linking, they activate autophagy which peaks at day 4. As the activation cycle declines on day 6, LC3-II protein levels remain above background, indicating persistent, low-level autophagy. Evidence for a critical contribution of autophagy to T cell survival came from experiments applying the autophagy inhibitor 3-methyladenine (3-MA; Bell et al., 2008). In the presence of 3-MA, frequencies of dying T cells were up to fourfold higher (Fig. 7 B), confirming a critical contribution of autophagy in securing T cell survival. Comparison of LC3-II protein levels in stimulated RA and control T cells revealed a 30–40% reduction in the patient-derived cells (Fig. 7 C), indicating lower autophagic activity despite the repressed glycolytic flux. To test the dependence of RA and control T cells on autophagy as an energy source, we cultured activated T cells in increasing concentrations of the autophagy inhibitor 3-MA (Fig. 7 D). Apoptosis rates were higher in resting RA T cells, as previously seen. 3-MA increased the proportion of apoptotic cells markedly; RA T cells were much more prone to die than control cultures. These data support the notion that RA T cells are less able to rely on cell-internal energy sources and that they fail to compensate for their energy-deficient state by tapping into internal stores.
Figure 7.
Impaired induction of autophagy in RA T cell. Freshly isolated naive CD4 (CD4+CD45RO−) T cells were prepared from healthy individuals and stimulated with anti-CD3/CD28–coated beads. Cells were harvested on days 0, 2, 4, and 6, and cell extracts were analyzed for the autophagy marker LC3-II by Western blot (A). On day 2 after stimulation, cells were treated with the autophagy inhibitor 3-MA for 24 h. Frequencies of apoptotic cells were determined by flow cytometry as 7AAD and Annexin V double-positive cells. Representative data from five independent experiments are shown (B). Expression of LC3-II protein in RA and control T cells was compared by Western blot analysis on day 4 after TCR stimulation. Protein levels were quantified by densitometry. Western blot results from a representative experiment are shown and results from six different control-patient pairs are presented as mean ± SEM (C). Apoptotic sensitivity was tested in control and RA T cells by treating with increasing doses of 3-MA. Frequencies of 7AAD+/Annexin V+ cells from 6 patients and 5 controls are given as mean ± SEM (D). *, P < 0.05; **, P < 0.01.
PFKFB3 regulates not only glycolysis, but also autophagy
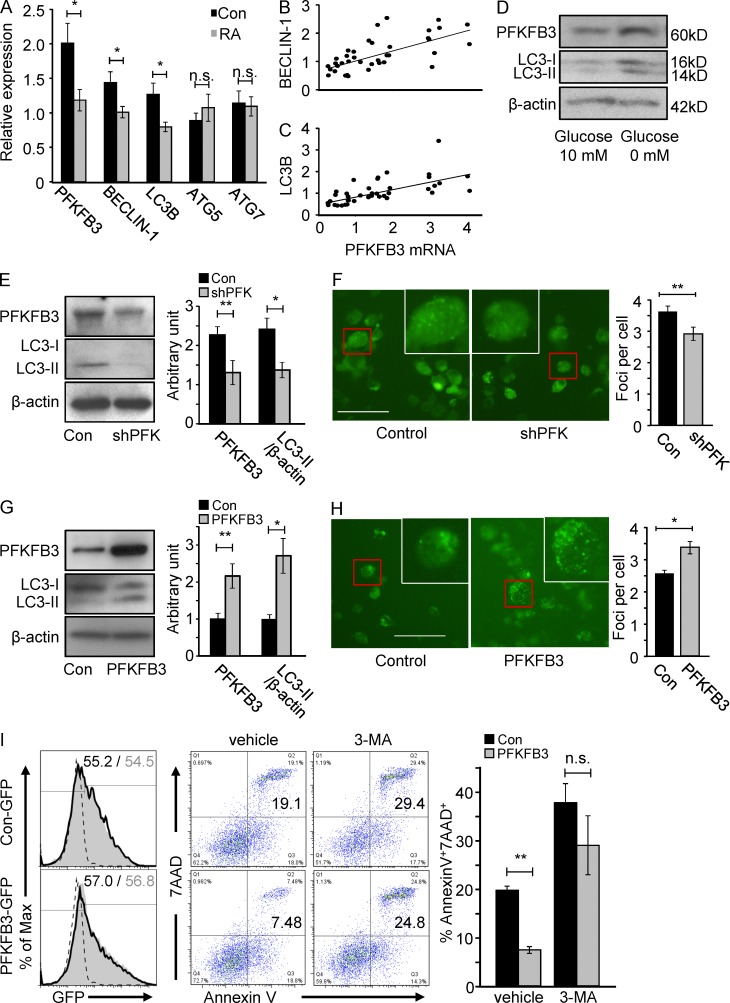
The combination of repressed glycolytic flux and reduced autophagic activity in autoimmune T cells raised the question whether the two effects were mechanistically linked. Comparative profiling of RA and control T cells 3 d after TCR cross-linking demonstrated not only reduced PFKFB3 transcript levels but also a significant reduction of the autophagy gene beclin-1 and LC3B (Fig. 8 A). mRNA concentrations for ATG5 and ATG7 did not distinguish between RA and normal T cells (Fig. 8 A). PFKFB3 and beclin-1 or LC3B transcripts were closely correlated in individual samples (R2 = 0.445, P < 0.001 and R2 = 0.405, P < 0.001, respectively; Fig. 8, B and C), suggesting interdependence of glycolytic and autophagy control in human T cells. Under conditions of glucose deprivation, kinetics of PFKFB3 and LC3-II induction were linked (Fig. 8 D).
Figure 8.
PFKFB3 is a regulator of autophagy in human T cells. Naive CD4 (CD4+CD45RO−) T cells were isolated from RA patients and age-matched controls and stimulated with anti-CD3/CD28 microbeads. Autophagy-related gene transcripts (Beclin-1, LC3B, Atg5, and Atg7) were quantified by qPCR on day 3 (A, 20 RA patients and 19 healthy controls), and correlated with the induction of PFKFB3 (B and C). Freshly isolated naive CD4 T cells were stimulated with CD3/CD28 beads. On day 2, cells were washed and then cultured in the absence and presence of 10 mM glucose for an additional 48 h. Concentrations of PFKFB3 and LC3-II were measured by Western blotting. Representative data from one of three independent experiments are shown (D). T cells were transfected with pSuper-PFKFB3-GFP (shPFK) plasmids (E) or pIRES-PFKFB3-GFP (PFKFB3) plasmids (G) 48 h after stimulation and incubated for an additional 48 h. PFKFB3 and LC3-II protein levels were detected by Western blotting. Representative blots are shown and data from five independent experiments are presented as mean ± SEM. For microscopic analysis of LC3 foci, T cells were transfected with pSuper-PFKFB3-H2Kk (shPFK) plasmids (F) or pIRES-PFKFB3-H2Kk (PFKFB3) plasmids (H) 48 h after stimulation and incubated for an additional 48 h. In each experiment, LC3-GFP foci were determined in >50 cells. Representative pictures are shown and data from three to four independent experiments are presented as mean ± SEM. Bars, 50 µM. CD4+CD45RO− T cells from RA patients were transfected with PFKFB3 or control plasmids and tested for apoptotic susceptibility by culturing them in the absence and presence of 2.5 µM 3-MA. Frequencies of 7AAD+ and Annexin V+ cells were determined cytometrically (I). Representative dot plots are presented. Frequencies of apoptotic cells from three independent experiments are given as mean ± SEM. *, P < 0.05; **, P < 0.01; n.s., non-significant.
To provide unequivocal evidence for a regulatory role of PFKFB3 in setting the threshold for autophagy induction, we silenced and overexpressed PFKFB3 in TCR-stimulated T cell blasts. Western blot quantification and microscopic analysis of LC3-II illustrated that PFKFB3-specific RNA interference suppressed autophagy (Fig. 8, E and F). Conversely, forced overexpression of PFKFB3 promptly accelerated autophagic activity as indicated by LC3-II doubling (Fig. 8, G and H). To test whether PFKFB3 overexpression could protect RA T cells from autophagy-related apoptosis, reconstitution with exogenous PFKFB3 and treatment with the autophagy inhibitor 3-MA were combined. PFKFB3 overexpression partially rescued RA T cells from 3-MA–induced apoptosis (Fig. 8I), confirming the upstream placement of PFKFB3 in autophagy regulation.
DISCUSSION
T cells almost exclusively rely on glycolysis to support their unusual life style, such as long periods of quiescence alternating with sudden demand for explosive growth and synthetic hyperactivity (MacIver et al., 2013). Upon triggering of their antigen receptor, T cells switch their cellular ATP production from oxidative phosphorylation to high-throughput aerobic glycolysis despite access to adequate oxygen to support complete oxidation of glucose, often termed the Warburg effect (Warburg, 1956; Wang et al., 1976; Greiner et al., 1994). Just as tumor cells, T cells use this mechanism to secure their survival and, in parallel, have access to an essential carbon source for the synthesis of macromolecules mediating their effector functions. At a fundamental level, it is glucose metabolism that regulates T cell function and differentiation and therefore influences the final outcome of adaptive immune responses. Here, we find that T cells from patients with the autoimmune syndrome RA have lost the ability to make efficient use of the Warburg effect, putting them under considerable energetic stress and shortening T cell longevity. This metabolic reprogramming is associated with at least three major consequences: increased shunting of glucose into the PPP, a shift in the redox balance imposing reductive stress, and limited utilization of autophagy. The metabolic abnormalities affected naive CD4 T cells, which are antigen-nonexposed and appear independent from the inflammatory milieu, suggesting a fundamental defect in T cell metabolism in this autoimmune disease.
Given their dependence on glucose, T cells exploit several mechanisms to optimize utilization of this energy source. Sustainable high levels of glucose uptake can be achieved by increasing the expression of the primary glucose transporter Glut1 (Frauwirth et al., 2002; Vander Heiden et al., 2009). Expression of T cell glucose transporters remains intact in RA T cells. T cell activation also triggers the rapid transcription of genes encoding glycolytic enzymes, including glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, aldolase, and enolase, as well as PFKFB (Okar et al., 2001). The four members of the PFKFB family, PFKFB1–4, appear to respond differently to TCR stimulation. mRNA levels for PFKFB1 and 4 increased upon stimulation, whereas PFKFB2 was unchanged (Fig. 2 I). In contrast, PFKFB3 was robustly up-regulated, reaching peak values after 3 d and staying at a 10-fold higher level for almost 2 wk, compatible with a tight link between T cell cycle progression and metabolic adaptation. Such a link is supported by recent studies that have implicated c-myc and HIF-1a in metabolic control of T cell fate (Nakamura et al., 2005; Wang et al., 2011). However, this link seems to be no longer maintained in RA T cells, which fail to up-regulate PFKFB3 to the same extent as healthy T cells yet undergo full activation and progress through the cell cycle even faster than control T cells (Fig. 1). T cell metabolism is closely connected to their functional commitment (O’Neill and Hardie, 2013). To avoid indirect effects attributable to altered T cell lineage assignment in RA, experiments in this study were conducted in naive CD4 T cells and lineage commitment was monitored by the induction of appropriate transcription factors (Fig. 1G).
PFKFB3 is distinguished from the other isoenzymes by its high kinase to bisphosphatase ratio; overexpression in transformed cells has identified it as a powerful glycolytic activator (Yalcin et al., 2009b; Colombo et al., 2010). Our data suggest that transcriptional control of PFKFB3 is critical in T cell homeostasis as the reduction of PFKFB3 transcript levels by 30–40% in RA T cells was sufficient to noticeably reduce lactate and ATP production. Resting naive CD4 T cells had low levels of PFKFB3 protein and were already more susceptible to spontaneous apoptosis. However, apoptotic death rates were particularly elevated in RA T cells on day 3 after stimulation, when metabolic demands peaked and PFKFB3 levels were significantly lower than in healthy T cells. Pharmacologic and genetic inhibition of PFKFB3 in healthy T cells was sufficient to accelerate apoptotic T cell loss (Fig. 3 A and Fig. 4 E) but never reached the excessive T cell death as in glucose-free medium, compatible with PFKFB3 fine-tuning glucose utilization. Partial PFKFB3 silencing intended to recapitulate the partial reduction of the enzyme in RA T cells reproduced the metabolic consequences and the impact on T cell survival in normal T cells.
The impaired PFKFB3 induction in RA T cells had major consequences for metabolic regulation as well as long-term survival. The shunting of glucose to the PPP is likely an adaptive mechanism dealing with insufficient progression of glycolysis. As a consequence, RA T cells produced higher levels of NADPH and consumed cytoplasmic ROS, as evidenced in the reduced rise of activation-induced H2O2 and superoxide (Fig. 6, A and B). ROS have an integral part in the early and delayed activation cascade triggered by TCR ligation and thus ultimately regulate T cell differentiation and protective and pathogenic immunity. Appropriate studies need to address the long-term outcome of reductive stress in naive CD4 T cells.
The current study did not fully delineate the molecular pathways that connect glycolytic deficiency and apoptosis induction. Expectedly, energy deprivation rendered T cells apoptosis-prone, and we demonstrated marked up-regulation of three proapoptotic Bcl-2 family proteins, PUMA, NOXA, and BIM. NOXA has been implicated as the main mediator of apoptosis in energy-starved cells (Alves et al., 2006). However, two more pathways are critically involved in determining T cell expansion and longevity, and both are regulated by PFKFB3. Inhibition of autophagy resulted in prompt enhancement of cell death, with increased susceptibility of RA T cells at least partially reversible by restoration of PFKFB3 expression. Importantly, ROS availability also affected the fate decisions in poststimulation T cells (Fig. 6 C).Thus, several mechanisms bias RA T cells toward shortened expansion and survival.
Knockin and knockdown experiments confirmed that PFKFB3 had functions that extended beyond its immediate impact as a glycolysis activator. A role for PFKFB3 in regulating the process of autophagy was unexpected and initially appeared counterintuitive. Restraining energy resources by repressing PFKFB3 induction should diminish the ATP pool and drive the cell toward utilization of alternative energy resources. Autophagy was initially identified as a lysosomal-mediated degradative mechanism activated mainly by starvation. However, recent discussions have emphasized that autophagy serves to secure biosynthetic precursors needed for the production of T cell cytokines and to prepare the T cell for the trafficking requirements associated with differentiation of naive into memory and effector T cells. TCR ligation in naive CD4 T cells serves as an inducer of autophagic activity (Nakamura et al., 2005), confirming that autophagy is an integral part of the T cell activation program. The knockout of different autophagy genes has demonstrated the critical role of autophagy proteins in the maintenance of normal numbers of B cells, CD4+ T cells, CD8+ T cells, and fetal hematopoietic stem cells (Virgin and Levine, 2009; Liu et al., 2010; Mizushima and Levine, 2010). However, induction of PFKFB3 preceded the up-regulation of LC3-II in CD4 T cells positioning the enzyme as a potential regulator of the autophagic process. LC3-II concentrations were significantly lower in RA T cells, reiterating the parallel course of PFKFB3 and autophagy. RA T cells died more rapidly than healthy T cells when autophagic signaling pathways were inhibited via 3-MA, emphasizing that the patient-derived cells had a lower ATP reserve and less resistance to energy deprivation. The hypersensitivity to the autophagy inhibitor could be partially reverted by forced overexpression of PFKFB3 in RA T cells (Fig. 8 I), again placing the glycolytic enzyme upstream of autophagy regulation.
Gene expression profiling supported the notion that RA T cells fail to commit to autophagy as efficiently as control T cells. TCR triggering reliably induced expression of genes specific for proteins involved in the assembly of the autophagosome, such as beclin-1 and LC3B. Evidence for a role of PFKFB3 in regulating expression of autophagic genes derived from silencing as well as overexpression studies. These studies did not define whether the glycolytic enzyme directly or indirectly, via a downstream metabolite, influences transcription of several autophagy-associated genes. PFKFB3 has been reported to accumulate both in the cytoplasm as well as the nucleus of proliferating cells with the nuclear fraction interacting with the E3 ubiquitin ligase APC/C-Cdh1 (Almeida et al., 2010). Ectopic expression of PFKFB3 increases the expression of several key cell cycle proteins and decreases the expression of the cell cycle inhibitor p27 independent from its role in enhancing glycolytic flux (Yalcin et al., 2009a), all suggestive of an extra-glycolytic role of the enzyme. More recent studies suggest that the synchronization between increased glycolysis and cell cycle control reflects the action of the E3 ubiquitin ligase APC/C-Cdh1 which actively degrades PFKFB3 in the nucleus (Almeida et al., 2009). Under conditions of inhibition of this degradation step, PFKFB3 overflows into the cytosol where it activates glycolysis. Defining the kinase also as a regulator of autophagy would support the concept that PFKFB3 is not only responsible for heating up glucose metabolism but also for enhancing formation of a new biomass (Vander Heiden et al., 2009).
Whereas the Warburg effect is essential in serving the proliferative needs of neoplastic and non-neoplastic cells, the reversed Warburg effect in RA T cells shortens T cell longevity by lowering the apoptosis threshold. Apoptotic death of proliferating T cells is considered a critical determinant of peripheral T cell homeostasis. Apoptosis of T cells is a mechanism through which clonal expansion is counterbalanced. In contrast, lymphopenia has been implicated in triggering autoimmunity, possibly through the selection of autoreactive T cell clones in a host that favors autoproliferation to fill an empty space (King et al., 2004; Goronzy and Weyand, 2012, 2013). The signals inducing T cell death in RA patients are multifold, including insufficiency of telomerase (Fujii et al., 2009), lack of DNA damage repair (Shao et al., 2009), and activation of JNK stress kinases (Shao et al., 2010). The current study adds energy deprivation as well as dysbalanced ROS consumption to the signals that deplete T cells in RA. Excessive attrition of naive T cells will eventually force the system to replace naive cells with hyperproliferated, end-differentiated, and apoptosis-resistant T cells typically found in RA patients (Schmidt et al., 1996; Weyand et al., 2009) and provides an explanation for the age-inappropriate immunosenescence phenotype of patients affected with this disease (Goronzy et al., 2010; Hohensinner et al., 2011; Cavanagh et al., 2012; Magnoni et al., 2012). Recent work connecting cellular senescence to the senescence-associated secretory phenotype (SASP) is emphasizing the potential role that senescence plays in inflammatory tissue damage (Campisi, 2013; Tchkonia et al., 2013).
In RA, autoimmunity precedes clinical symptoms by a decade and persists unabated even when downstream inflammation is well controlled, exposing lymphocytes to chronic stimulation and enforcing adaptations to persistent stress. Therefore, it is possible that the deficiency of PFKFB3 results from continuous cytokine and antigen signals. Several findings in the current study question that PFKFB3 deficiency is a consequence of the inflammatory milieu. Patients with aggressive and systemic inflammation in the setting of SLE, a clinically distinct type of autoimmune disease, are similar to age-matched controls in PFKFB3 transcription (Fig. 2 D). Equally important, we could not demonstrate a relationship between the disease activity score and the levels of TCR activation–induced PFKFB3 in a cohort of RA patients (Fig. 2 G). Alternatively, the metabolic defect in T cells precedes the manifestations of RA and participates in the disease process. Systematic examination of metabolic capacity of T cells from patients with preclinical disease and comparative analysis of T cell metabolism in patients with different types of autoimmunity will be informative. Independent from the signals that lead to altered expression and function of PFKFB3, our data have implications for the therapeutic management of RA as targeting of the insufficiency in energy and biomass production, and the dysregulation of the cytoplasmic redox environment may be beneficial in restoring T cell homeostasis in RA.
MATERIALS AND METHODS
Patients and controls.
Demographic characteristics of 128 patients and 103 healthy control subjects are given in Tables 1 and 2. All patients fulfilled the diagnostic criteria for RA (American College of Rheumatology 1987 revised criteria) and were positive for rheumatoid factor and anti-CCP antibodies. Demographically matched healthy individuals served as controls and were enrolled if they had no personal or family history of autoimmune disease, cancer, chronic viral infection, or any other inflammatory syndrome. Disease activity scores (DAS 28) were determined as previously described (Prevoo et al., 1995; van Gestel et al., 1998). The study was approved by the Institutional Review Board and written informed consent was obtained from all participants.
Table 1.
Demographic characteristics of study populations
| Characteristics | control | RA | P-value |
| Number of subjects | 103 | 128 | |
| Sex, female/male | 63/37% | 73/27% | 0.57 |
| Age, mean ± SD | 50.2 ± 12 yr | 52.8 ± 11 yr | 0.18 |
| Ethnicity | 0.62 | ||
| African American | 28.2% | 37.5% | |
| White | 42.7% | 39.8% | |
| Hispanic | 22.3% | 18.0% | |
| Asian | 6.8% | 4.7% |
Table 2.
Clinical characteristics of study populations
| Characteristics | Values |
| Disease duration, mean ± SD | 8.05 ± 7.11 yr |
| Active diseasea | 83.6% |
| Extra-articular manifestations | 25.8% |
| Nodules | 24.2% |
| Sicca | 6.3% |
| Pulmonary fibrosis | 4.7% |
| Joint replacements | 21.1% |
| Tobacco yes/no | 23/77% |
| DMARDs naïve | 18.0% |
| Medications | |
| Corticosteroids | 51.6% |
| Methotrexate | 62.5% |
| Hydroxychloroquine | 38.3% |
| Sulfasalazine | 8.6% |
| TNF inhibitors | 21.1% |
Active disease defined by FDA criteria (presence of three or more of the following: morning stiffness (>45 min), swollen joints (>3), tender joints (>6), and sed rate (>28 mm).
Cells and culture.
CD4 naive T cell subsets were purified from PBMC using CD45RO and CD4 microbeads (Miltenyi Biotec). Subset purity was monitored by flow cytometry and reached >95%. Naive CD4 (CD4+CD45RO−) T cells (1.0 × 105/well) were stimulated with CD3/CD28-coated beads (Life Technologies) at a ratio of 1:1. Cells were enumerated by flow cytometry or trypan blue exclusion.
For cell proliferation assays, naive CD4 T cells were labeled with CFSE and stimulated with anti-CD3/CD28 beads (1:1 ratio). T cell proliferation was determined by flow cytometry after 48 and 72 h. To detect intracellular IL-2, cells were harvested on day 3 after TCR ligation, and incubated with PMA and ionomycin in the presence of brefeldin A for 5 h before intracellular staining with anti–IL-2 antibodies as previously described (Li et al., 2012).
For experiments examining the impact of PFKFB3 on T cell growth and survival, cells were harvested 48 h after stimulation and treated with N-BrEt (provided by J.D. Foster, University of North Dakota, Grand Forks, ND) in the absence/presence of 10 mM glucose for an additional 48 h. Frequencies of apoptotic T cells were measured by cytometric analysis of 7AAD and PE-Annexin V (BD). Apoptotic cells with high caspase activity were detected with MitoCasp kits (Cell Technology, Inc.). Increased cell death rates were calculated as follows: delta percentage = % dead cell at 20 µg/ml N-BrEt − % dead cell at 0 µg/ml N-BrEt.
In the autophagy inhibition experiments, CD4 T cells were activated for 48 h and then treated with indicated doses of the autophagy inhibitor 3-MA (Sigma-Aldrich) for an additional 48 h. In the PFKFB3 rescue experiments, 3-MA was added to the transfected cells after 24 h and cells were cultured for 1 d more. ROS dependence of apoptosis induction was tested by treating CD4 T cells with the ROS scavenger Tempol (Sigma-Aldrich) for 24 h.
Plasmids.
pIRES-PFKFB3-GFP (PFKFB3) plasmids containing the complete PFKFB3 coding sequence (GenBank accession no. NM_004566) were generated by PCR using CD4 T cells cDNA for template amplification. The following primers were used: 5′-CGAAGATGCCGTTGGAACTGA-3′ (forward) and 5′-TGGAATGGAACCGACACGTCT-3′ (reverse), with EcoRI and SalI restriction sites at the NH2 and COOH terminals, respectively. An EcoRI–SalI PFKFB3 expression cassette was subcloned into pIRES-GFP plasmid (Takara Bio Inc.). The pIRES-H2Kk plasmid (courtesy of J. Tahvanainen, Turku University, Turku, Finland) has been previously described (Tahvanainen et al., 2006). For silencing of the PFKFB3 gene, pSUPER-shPFKFB3-GFP (shPFK) plasmids or pSuper-shPFKFB3-H2Kk plasmids were constructed that contained the 19-nt sequence CTGAAACTGACGCCTGTCG, derived from the mRNA transcript of PFKFB3.
Plasmid transfections.
Naive CD4 T cells were stimulated with anti-CD3/CD28 beads for 48 h and then transfected with empty-pIRES-GFP plasmids or pIRES-PFKFB3-GFP (PFKFB3) plasmids. Transfection efficiencies were monitored by measuring the frequency of GFP-positive cells using flow cytometer.
To knock down PFKFB3 expression, 3 µg pSuper-PFKFB3-GFP/neo plasmids (shPFK) or control vector were transfected into activated CD4 T cells using the Amaxa Nucleofector system and the Human T cell Nucleofector kit (Lonza). Cells were cultured for 24 h before further experiments.
To assess autophagic activity, cells were cotransfected with GFP-LC3 plasmids and pIRES-PFKFB3-H2Kk or pSuper-shPFKFB3-H2Kk plasmids on day 2 after TCR ligation. At day 4, H2Kk-positive cells were purified with MACSelect Kk MicroBeads (Miltenyi Biotec) and analyzed by fluorescent microscopy (BX41TF; Olympus).
Glucose consumption, lactate production, and intracellular ATP concentration.
Purified CD4+CD45RO− T cells (2.5 × 105/well) were stimulated for 48 h, washed twice, and then cultured with fresh complete medium containing glucose (1,000 mg/liter). After 4 h of incubation, T cells were counted and intracellular ATP concentrations were measured using ATP assay kits (Abcam). To quantify lactate concentrations, cell-free supernatants were analyzed in triplicate using a lactate assay kit (Abcam). In brief, 20 µl of sample or standard were incubated with 50 µl of lactate reagent solution for 30 min at 37°C, and then the reaction was terminated by adding 50 µl 0.5 M acetic acid. Absorbance was measured at 490 nm.
For determination of glucose concentrations, cell-free supernatants were analyzed using a glucose assay kit (Sigma-Aldrich) according to the manufacturer’s instructions. In brief, samples were prepared at a total volume of 50 µl/well with glucose assay buffer and mixed with 50 µl reaction mixture containing 46 µl glucose assay buffer, 2 µl glucose probe, and 2 µl glucose enzyme mix. After 30 min of incubation, absorbance was measured at 570 nm. Glucose consumption was expressed as the decrease in glucose concentration in culture medium over a 4-h culture period.
NADPH level and ROS production assay.
CD4+CD45RO− T cells from both RA patients and age-matched controls were stimulated for 72 h and then washed with cold PBS. NADPH levels were assayed by using NADPH assay kits (Abnova) according to the manufacturer’s instruction. The assay system is based on a glucose dehydrogenase cycling reaction in which the formed NADPH reduces a formazan reagent, the optical density of which is read at 570 nm.
For ROS production assays, freshly isolated CD4+CD45RO− T cells or T cells stimulated with anti-CD3/CD28 microbeads for 72 h were incubated with 10 µM of DHE or DCF (Molecular Probes) for 30 min at 37°C. Cells were washed and analyzed by flow cytometry. In each experiment, calibration beads (Molecular Probes) were included to standardize the fluorescence intensity readings.
RT-PCR.
Total RNA was extracted with RNeasy kits (QIAGEN) and cDNA was synthesized with AMV-reverse transcriptase (Roche) and random hexamers (Roche). Reaction solutions for the qRT-PCR consisted of 2.0 µl of diluted RT-PCR product, 0.2 µM of each primer, and SYBR green PCR master mix (Thermo Fisher Scientific). The primers used were as follows: PFKFB3, 5′-CTCGCATCAACAGCTTTGAGG-3′ (forward) and 5′-TCAGTGTTTCCTGGAGGAGTC-3′ (reverse); and 18S ribosomal RNA, 5′-GCCTCACTAAACCATCCAA-3′ (forward) and 5′-TCAGTGTTTCCTGGAGGAGTC-3′ (reverse). Copy numbers were calculated by comparing each sample with a standard curve generated by amplifying serially diluted plasmids containing relevant sequences. cDNA copies were adjusted for 1 × 108 ribosomal RNA copies. Other primers used for glycolysis-related genes, AMPKs, mTOR, and autophagic genes are listed in Table 3. All PCR results were normalized to 18S rRNA.
Table 3.
List of primers
| Gene name | GenBank | Forward primer (5′-3′) | Reverse primer (5′-3′) |
| AldoA | NM_000034 | AGATGAGTCCACTGGGAGCAT | CACGCCCTTGTCTACCTTGAT |
| AldoC | NM_005165 | GGATGAGTCTGTAGGCAGCAT | GAGTGGTGGTTTCTCCATCAG |
| AMPKα1 | NM_006251 | TGAAAATGTCCTGCTTGATG | TACTTCTGGTGCAGCATAGT |
| AMPKα2 | NM_006252 | GTTGCGGATCTCCAAATTAT | GACGTTAGCATCATAGGAAG |
| AMPKβ1 | NM_006253 | GCTTGGCACAGTTAACAACATC | TTCGGGTTTGCAGACGTAG |
| AMPKβ2 | NM_005399 | TACCAGTCAGCTTGGCACA | CCTCAGATCGAAACGCAT |
| AMPKγ1 | NM_002733 | GGACTCCTTTAAACCGCTTG | CTTGGACATGAACTCTGGCT |
| AMPKγ2 | NM_024429 | ATCCAGACACTCCCATCATC | GCACTTCACAACACCTTCAA |
| AMPKγ3 | NM_017431 | CCACATCCTCACACACAAAC | GAATCACATCAAAGCGGG |
| ATG5 | NM_004849 | GACAAAGATGTGCTTCGAGATGTG | GTAGCTCAGATGTTCACTCAG |
| ATG7 | NM_006395 | ACATCATTGCAGAAGTAGCAGCCA | ATGCCTGGGCATCCAGTGAACTTC |
| Beclin-1 | NM_003766 | CAGACAGATGTGGATCACCC | ATCAGCCTCTCCTCCTCTAGTG |
| Bim | NM_138621 | ACGCTTACTATGCAAGGAGGG | GGTCTTCGGCTGCTTGGTAAT |
| ENO1 | NM_001428 | GCCCTGGTTAGCAAGAAACTG | TTCTCAACGGCACCAGCTTTG |
| ENO2 | NM_001975 | AACAGTGAAGCCTTGGAGCTG | TCCTCAATGGAGACCACAGGA |
| ENO3 | NM_053013 | CTATCCTGTGGTCTCCATCGA | CTCCTCGATCCTCATGAGTTG |
| GAPDH | NM_002046 | GGAGTCAACGGATTTGGTCGT | TCTCGCTCCTGGAAGATGGT |
| Glut1 | NM_006516 | GCAGTTTGGCTACAACACTGG | TTTCGAGAAGCCCATGAGCAC |
| Glut3 | NM_006931 | TGGCTACAACACTGGGGTCAT | TTGAATTGCGCCTGCCAAAGC |
| GPI | NM_000175 | ATCAACTACACCGAGGGTCGA | CCAATGTTGATGACGTCCGTG |
| HK1 | NM_000188 | CGAGAGTGACCGATTAGCACT | AGACAGGAGGAAGGACACGTT |
| HK2 | NM_000189 | ACGGAGCTCAACCATGACCAA | AAGATCCAGAGCCAGGAACTC |
| LC3B | NM_022818 | GTCCGACTTATTCGAGAGCAG | CTGAGATTGGTGTGGAGACG |
| LDHA | NM_005566 | GATTCCAGTGTGCCTGTATGG | CTACAGAGAGTCCAATAGCCC |
| LDHB | NM_002300 | GCGTGTGCTATCAGCATTCTG | TTCTCTGCACCAGATTGAGCC |
| MTOR | NM_004958 | AGCCGGTGTTGCAGAGACTT | TCAGACCTCACAGCCACAGAA |
| NOXA | NM_021127 | CCTGGGAAGAAGGCGCG | TCAGGTTCCTGTGCAGAAG |
| PDK1 | NM_002610 | GCTAGGCGTCTGTGTGATTTG | AACACCTCTGTTGGCATGGTG |
| PDK2 | NM_002611 | CAACCAGCACACCCTCATCTT | GCCCTCATGGCATTCTTGAAG |
| PDK3 | NM_005391 | TCGCCGCTCTCC ATCAAACAA | CTGAACCAATCCCACTGAAGG |
| PFK1 | NM_002626 | CTGTACTCATCAGAGGGCAAG | TGCCAGCATCTTCAGCATGAG |
| PFKFB1 | NM_002625 | CTCCATCTACCT TTGCCGACA | GCCCTGGGACTGAATGAAGTT |
| PFKFB2 | NM_006212 | CACCAATACAACCCGGGAGA | GCAGCAATGACATCAGGATCA |
| PFKFB4 | NM_004567 | CCAACTGCCCAACTCTCATTG | GCGATACTGGCCAACATTGAA |
| PFKM | NM_000289 | GAAGAGCACCATGCAGCCAAA | TCGTTCCCGAAAGTCCTTGCA |
| PGK1 | NM_000291 | GGGTCGTTATGAGAGTCGACT | AGGTGGCTCATAAGGACTACC |
| PGK2 | NM_138733 | AAGTCAGCCATGTCAGCACTG | GCCTGCTGCTTGTCCATTACA |
| PGM1 | NM_002633 | TCCAGAGTATCATCTCCACCG | ATGATGCAGGATACAGCAGGG |
| PGM2 | NM_018290 | AGCAGAAGGTTTGCCCGACTT | TTCACGCTCCTGTGGAAACAG |
| PGM3 | NM_015599 | CTCCTGGTGGAGATTGGAGAA | CTAAACAGTGCAGTGCCATGC |
| PKM1(exon9) | NM_182470 | GGGGTTCGGAGGTTTGATGAA | AGGTCTGTGGAGTGACTTGAG |
| PKM2 | NM_182471 | TCTGTACCATTGGCCCAGCTT | TGGCTGTGCGCACATTCTTGA |
| PKM2(exon10) | NM_002654 | GGGGTTCGGAGGTTTGATGAA | TTGCAAGTGGTAGATGGCAGC |
| PUMA | NM_014417 | GGACGACCTCAACGCACAGTA | GGCAGGAGTCCCATGATGAGA |
| TIGAR | NM_020375 | GGACAAAGCAGACCATGCATG | ACCCCGTATTTCCTTTCCCGA |
| TPI | NM_000365 | GTCAGATGAGCTGATTGGGCA | GGTGTTGCAGTCTTGCCAGTA |
Western blotting.
Cellular proteins were extracted using an extraction kit from Active Motif, and expression levels were examined following a standard Western blotting protocol. In brief, 50 µg protein of each sample was electrophoresed in 10% or 4–15% Tris-Glycine gel (Bio-Rad Laboratories) and transferred to PVDF blot membrane. The membrane was blocked with 5% nonfat milk (Bio-Rad Laboratories) solution and incubated with primary antibody at 1:1,000 (anti-PFKFB3, Abcam; anti-LC3B, anti-AMPK, and anti-mTOR, Cell Signaling Technology) overnight in the cold room followed by secondary horseradish peroxidase–conjugated anti-Ig antibody (Santa Cruz Biotechnology, Inc.) for 2 h. The enhanced chemiluminescence detection system (GE Healthcare) was used to detect bands with peroxidase activity. β-Actin (1:5,000; Santa Cruz Biotechnology, Inc.) served as internal control.
Statistical analysis.
All data are presented as mean ± SEM. Data were analyzed using SPSS 10.0 software. Statistical significance was assessed by ANOVA and unpaired Student’s t test as appropriate. A p-value of <0.05 was considered significant.
Acknowledgments
This work was supported by National Institutes of Health grants AR042547, EY011916, AI044142, and HL058000 (C.M. Weyand), and AI090019 and AI057266 (J.J. Goronzy). Fellowship support for Z. Yang came from the Govenar Discovery Fund.
The authors declare no conflict of interest.
Footnotes
Abbreviations used:
- 3-MA
- 3-methyladenine
- AMPK
- adenosine monophosphate-activated protein kinase
- F2
- 6BP, fructose 2,6-bisphosphate
- G6P
- glucose-6-phosphate
- LC3
- microtubule-associated protein 1 light chain 3
- mTOR
- mammalian target of rapamycin
- N-BrEt
- N-bromoacetylethanolamine phosphate
- PFK1
- 6-phosphofructo-1-kinase
- PFKFB3
- 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
- PPP
- pentose phosphate pathway
- RA
- rheumatoid arthritis
- ROS
- reactive oxygen species
- SLE
- systemic lupus erythematosus
- Tempol
- 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl
References
- Almeida A., Bolaños J.P., Moncada S. 2010. E3 ubiquitin ligase APC/C-Cdh1 accounts for the Warburg effect by linking glycolysis to cell proliferation. Proc. Natl. Acad. Sci. USA. 107:738–741 10.1073/pnas.0913668107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves N.L., Derks I.A., Berk E., Spijker R., van Lier R.A., Eldering E. 2006. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity. 24:703–716 10.1016/j.immuni.2006.03.018 [DOI] [PubMed] [Google Scholar]
- Atsumi T., Chesney J., Metz C., Leng L., Donnelly S., Makita Z., Mitchell R., Bucala R. 2002. High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res. 62:5881–5887 [PubMed] [Google Scholar]
- Bando H., Atsumi T., Nishio T., Niwa H., Mishima S., Shimizu C., Yoshioka N., Bucala R., Koike T. 2005. Phosphorylation of the 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase/PFKFB3 family of glycolytic regulators in human cancer. Clin. Cancer Res. 11:5784–5792 10.1158/1078-0432.CCR-05-0149 [DOI] [PubMed] [Google Scholar]
- Bell B.D., Leverrier S., Weist B.M., Newton R.H., Arechiga A.F., Luhrs K.A., Morrissette N.S., Walsh C.M. 2008. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc. Natl. Acad. Sci. USA. 105:16677–16682 10.1073/pnas.0808597105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagih J., Krawczyk C.M., Jones R.G. 2012. LKB1 and AMPK: central regulators of lymphocyte metabolism and function. Immunol. Rev. 249:59–71 10.1111/j.1600-065X.2012.01157.x [DOI] [PubMed] [Google Scholar]
- Campisi J. 2013. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75:685–705 10.1146/annurev-physiol-030212-183653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh M.M., Weyand C.M., Goronzy J.J. 2012. Chronic inflammation and aging: DNA damage tips the balance. Curr. Opin. Immunol. 24:488–493 10.1016/j.coi.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney J., Mitchell R., Benigni F., Bacher M., Spiegel L., Al-Abed Y., Han J.H., Metz C., Bucala R. 1999. An inducible gene product for 6-phosphofructo-2-kinase with an AU-rich instability element: role in tumor cell glycolysis and the Warburg effect. Proc. Natl. Acad. Sci. USA. 96:3047–3052 10.1073/pnas.96.6.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H. 2012. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 12:325–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo S.L., Palacios-Callender M., Frakich N., De Leon J., Schmitt C.A., Boorn L., Davis N., Moncada S. 2010. Anaphase-promoting complex/cyclosome-Cdh1 coordinates glycolysis and glutaminolysis with transition to S phase in human T lymphocytes. Proc. Natl. Acad. Sci. USA. 107:18868–18873 10.1073/pnas.1012362107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D.K. 2012. Regulation of glucose metabolism in T cells: new insight into the role of Phosphoinositide 3-kinases. Front Immunol. 3:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D., Cantrell D.A. 2011. Metabolism, migration and memory in cytotoxic T cells. Nat. Rev. Immunol. 11:109–117 10.1038/nri2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C.J., Hammerman P.S., Thompson C.B. 2005. Fuel feeds function: energy metabolism and the T-cell response. Nat. Rev. Immunol. 5:844–852 10.1038/nri1710 [DOI] [PubMed] [Google Scholar]
- Frauwirth K.A., Riley J.L., Harris M.H., Parry R.V., Rathmell J.C., Plas D.R., Elstrom R.L., June C.H., Thompson C.B. 2002. The CD28 signaling pathway regulates glucose metabolism. Immunity. 16:769–777 10.1016/S1074-7613(02)00323-0 [DOI] [PubMed] [Google Scholar]
- Fujii H., Shao L., Colmegna I., Goronzy J.J., Weyand C.M. 2009. Telomerase insufficiency in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 106:4360–4365 10.1073/pnas.0811332106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J.J., Weyand C.M. 2005. Rheumatoid arthritis. Immunol. Rev. 204:55–73 10.1111/j.0105-2896.2005.00245.x [DOI] [PubMed] [Google Scholar]
- Goronzy J.J., Weyand C.M. 2009. Developments in the scientific understanding of rheumatoid arthritis. Arthritis Res. Ther. 11:249 10.1186/ar2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J.J., Weyand C.M. 2012. Immune aging and autoimmunity. Cell. Mol. Life Sci. 69:1615–1623 10.1007/s00018-012-0970-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J.J., Weyand C.M. 2013. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 14:428–436 10.1038/ni.2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J.J., Shao L., Weyand C.M. 2010. Immune aging and rheumatoid arthritis. Rheum. Dis. Clin. North Am. 36:297–310 10.1016/j.rdc.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner E.F., Guppy M., Brand K. 1994. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J. Biol. Chem. 269:31484–31490 [PubMed] [Google Scholar]
- Harada Y., Tominaga N., Watanabe M., Shimokawa R., Ishiguro M., Sakakibara R. 1997. Inhibition of fructose-6-phosphate,2-kinase by N-bromoacetylethanolamine phosphate in vitro and in vivo. J. Biochem. 121:724–730 10.1093/oxfordjournals.jbchem.a021646 [DOI] [PubMed] [Google Scholar]
- Heikamp E.B., Powell J.D. 2012. Sensing the immune microenvironment to coordinate T cell metabolism, differentiation & function. Semin. Immunol. 24:414–420 10.1016/j.smim.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T., Watanabe M., Miura S., Ijichi K., Fukasawa M., Sakakibara R. 2000. Inhibition of tumor cell growth by a specific 6-phosphofructo-2-kinase inhibitor, N-bromoacetylethanolamine phosphate, and its analogues. Biosci. Biotechnol. Biochem. 64:2047–2052 10.1271/bbb.64.2047 [DOI] [PubMed] [Google Scholar]
- Hohensinner P.J., Goronzy J.J., Weyand C.M. 2011. Telomere dysfunction, autoimmunity and aging. Aging Dis. 2:524–537 [PMC free article] [PubMed] [Google Scholar]
- Jia W., Pua H.H., Li Q.J., He Y.W. 2011. Autophagy regulates endoplasmic reticulum homeostasis and calcium mobilization in T lymphocytes. J. Immunol. 186:1564–1574 10.4049/jimmunol.1001822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. 2000. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19:5720–5728 10.1093/emboj/19.21.5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R., Bleichert F., Warnke J.P., Eschrich K. 2008. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB3) is up-regulated in high-grade astrocytomas. J. Neurooncol. 86:257–264 10.1007/s11060-007-9471-7 [DOI] [PubMed] [Google Scholar]
- King C., Ilic A., Koelsch K., Sarvetnick N. 2004. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 117:265–277 10.1016/S0092-8674(04)00335-6 [DOI] [PubMed] [Google Scholar]
- Kochi Y., Suzuki A., Yamada R., Yamamoto K. 2010. Ethnogenetic heterogeneity of rheumatoid arthritis-implications for pathogenesis. Nat Rev Rheumatol. 6:290–295 10.1038/nrrheum.2010.23 [DOI] [PubMed] [Google Scholar]
- Koetz K., Bryl E., Spickschen K., O’Fallon W.M., Goronzy J.J., Weyand C.M. 2000. T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 97:9203–9208 10.1073/pnas.97.16.9203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J.R., Li C., Yang Q., Li G., Garcia I.G., Ju S., Roodman D.G., Windle J.J., Zhang X., Lu B. 2012. Autophagy promotes T-cell survival through degradation of proteins of the cell death machinery. Cell Death Differ. 19:144–152 10.1038/cdd.2011.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Yu M., Lee W.W., Tsang M., Krishnan E., Weyand C.M., Goronzy J.J. 2012. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat. Med. 18:1518–1524 10.1038/nm.2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Lee J.Y., Wei H., Tanabe O., Engel J.D., Morrison S.J., Guan J.L. 2010. FIP200 is required for the cell-autonomous maintenance of fetal hematopoietic stem cells. Blood. 116:4806–4814 10.1182/blood-2010-06-288589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver N.J., Michalek R.D., Rathmell J.C. 2013. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 31:259–283 10.1146/annurev-immunol-032712-095956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnoni L.J., Vraskou Y., Palstra A.P., Planas J.V. 2012. AMP-activated protein kinase plays an important evolutionary conserved role in the regulation of glucose metabolism in fish skeletal muscle cells. PLoS ONE. 7:e31219 10.1371/journal.pone.0031219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek R.D., Gerriets V.A., Jacobs S.R., Macintyre A.N., MacIver N.J., Mason E.F., Sullivan S.A., Nichols A.G., Rathmell J.C. 2011. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186:3299–3303 10.4049/jimmunol.1003613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N., Levine B. 2010. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 12:823–830 10.1038/ncb0910-823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs E.A., Colombo S.L. 2012. Fulfilling the metabolic requirements for cell proliferation. Biochem. J. 446:1–7 10.1042/BJ20120427 [DOI] [PubMed] [Google Scholar]
- Mor I., Cheung E.C., Vousden K.H. 2011. Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb. Symp. Quant. Biol. 76:211–216 10.1101/sqb.2011.76.010868 [DOI] [PubMed] [Google Scholar]
- Nakamura H., Makino Y., Okamoto K., Poellinger L., Ohnuma K., Morimoto C., Tanaka H. 2005. TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J. Immunol. 174:7592–7599 [DOI] [PubMed] [Google Scholar]
- Naz S.M., Symmons D.P. 2007. Mortality in established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 21:871–883 10.1016/j.berh.2007.05.003 [DOI] [PubMed] [Google Scholar]
- O’Neill L.A., Hardie D.G. 2013. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 493:346–355 10.1038/nature11862 [DOI] [PubMed] [Google Scholar]
- Okar D.A., Lange A.J. 1999. Fructose-2,6-bisphosphate and control of carbohydrate metabolism in eukaryotes. Biofactors. 10:1–14 10.1002/biof.5520100101 [DOI] [PubMed] [Google Scholar]
- Okar D.A., Manzano A., Navarro-Sabatè A., Riera L., Bartrons R., Lange A.J. 2001. PFK-2/FBPase-2: maker and breaker of the essential biofactor fructose-2,6-bisphosphate. Trends Biochem. Sci. 26:30–35 10.1016/S0968-0004(00)01699-6 [DOI] [PubMed] [Google Scholar]
- Pearce E.L. 2010. Metabolism in T cell activation and differentiation. Curr. Opin. Immunol. 22:314–320 10.1016/j.coi.2010.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevoo M.L., van’T Hof M.A., Kuper H.H., van Leeuwen M.A., van de Putte L.B., van Riel P.L. 1995. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 38:44–48 10.1002/art.1780380107 [DOI] [PubMed] [Google Scholar]
- Pua H.H., Guo J., Komatsu M., He Y.W. 2009. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J. Immunol. 182:4046–4055 10.4049/jimmunol.0801143 [DOI] [PubMed] [Google Scholar]
- Rider M.H., Bertrand L., Vertommen D., Michels P.A., Rousseau G.G., Hue L. 2004. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem. J. 381:561–579 10.1042/BJ20040752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D., Goronzy J.J., Weyand C.M. 1996. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J. Clin. Invest. 97:2027–2037 10.1172/JCI118638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena L.A., Li S., Jairaman A., Prakriya M., Ezponda T., Hildeman D.A., Wang C.R., Schumacker P.T., Licht J.D., Perlman H., et al. 2013. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 38:225–236 10.1016/j.immuni.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M., Kim J.D., Neau D., Sehgal I., Lee Y.H. 2011. Structure-based development of small molecule PFKFB3 inhibitors: a framework for potential cancer therapeutic agents targeting the Warburg effect. PLoS ONE. 6:e24179 10.1371/journal.pone.0024179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyler T.M., Park Y.W., Takemura S., Bram R.J., Kurtin P.J., Goronzy J.J., Weyand C.M. 2005. BLyS and APRIL in rheumatoid arthritis. J. Clin. Invest. 115:3083–3092 10.1172/JCI25265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L., Fujii H., Colmegna I., Oishi H., Goronzy J.J., Weyand C.M. 2009. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J. Exp. Med. 206:1435–1449 10.1084/jem.20082251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L., Goronzy J.J., Weyand C.M. 2010. DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol Med. 2:415–427 10.1002/emmm.201000096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Deshpande P., Pryshchep S., Colmegna I., Liarski V., Weyand C.M., Goronzy J.J. 2009. ERK-dependent T cell receptor threshold calibration in rheumatoid arthritis. J. Immunol. 183:8258–8267 10.4049/jimmunol.0901784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Deshpande P., Li G., Yu M., Pryshchep S., Cavanagh M., Weyand C.M., Goronzy J.J. 2012. K-RAS GTPase- and B-RAF kinase-mediated T-cell tolerance defects in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 109:E1629–E1637 10.1073/pnas.1117640109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton R.C. 2012. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 64:362–369 10.1002/iub.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahvanainen J., Pykäläinen M., Kallonen T., Lähteenmäki H., Rasool O., Lahesmaa R. 2006. Enrichment of nucleofected primary human CD4+ T cells: a novel and efficient method for studying gene function and role in human primary T helper cell differentiation. J. Immunol. Methods. 310:30–39 10.1016/j.jim.2005.11.024 [DOI] [PubMed] [Google Scholar]
- Takemura S., Klimiuk P.A., Braun A., Goronzy J.J., Weyand C.M. 2001. T cell activation in rheumatoid synovium is B cell dependent. J. Immunol. 167:4710–4718 [DOI] [PubMed] [Google Scholar]
- Tanida I., Ueno T., Kominami E. 2004. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 36:2503–2518 10.1016/j.biocel.2004.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T., Zhu Y., van Deursen J., Campisi J., Kirkland J.L. 2013. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 123:966–972 10.1172/JCI64098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telang S., Clem B.F., Klarer A.C., Clem A.L., Trent J.O., Bucala R., Chesney J. 2012. Small molecule inhibition of 6-phosphofructo-2-kinase suppresses t cell activation. J. Transl. Med. 10:95 10.1186/1479-5876-10-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada M., Ohsumi Y. 1993. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 333:169–174 10.1016/0014-5793(93)80398-E [DOI] [PubMed] [Google Scholar]
- van Gestel A.M., Haagsma C.J., van Riel P.L. 1998. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 41:1845–1850 [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H.G. 1980. Fructose 2,6-bisphosphate, the probably structure of the glucose- and glucagon-sensitive stimulator of phosphofructokinase. Biochem. J. 192:897–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M.G., Cantley L.C., Thompson C.B. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 324:1029–1033 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H.W., Levine B. 2009. Autophagy genes in immunity. Nat. Immunol. 10:461–470 10.1038/ni.1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.M., Bell B.D. 2010. T cell intrinsic roles of autophagy in promoting adaptive immunity. Curr. Opin. Immunol. 22:321–325 10.1016/j.coi.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Green D.R. 2012. Metabolic checkpoints in activated T cells. Nat. Immunol. 13:907–915 10.1038/ni.2386 [DOI] [PubMed] [Google Scholar]
- Wang R., Dillon C.P., Shi L.Z., Milasta S., Carter R., Finkelstein D., McCormick L.L., Fitzgerald P., Chi H., Munger J., Green D.R. 2011. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 35:871–882 10.1016/j.immuni.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Marquardt C., Foker J. 1976. Aerobic glycolysis during lymphocyte proliferation. Nature. 261:702–705 10.1038/261702a0 [DOI] [PubMed] [Google Scholar]
- Warburg O. 1956. On the origin of cancer cells. Science. 123:309–314 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- Weyand C.M., Goronzy J.J. 2006. T-cell-targeted therapies in rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 2:201–210 10.1038/ncprheum0142 [DOI] [PubMed] [Google Scholar]
- Weyand C.M., Fujii H., Shao L., Goronzy J.J. 2009. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 5:583–588 10.1038/nrrheum.2009.180 [DOI] [PubMed] [Google Scholar]
- Yalcin A., Clem B.F., Simmons A., Lane A., Nelson K., Clem A.L., Brock E., Siow D., Wattenberg B., Telang S., Chesney J. 2009a. Nuclear targeting of 6-phosphofructo-2-kinase (PFKFB3) increases proliferation via cyclin-dependent kinases. J. Biol. Chem. 284:24223–24232 10.1074/jbc.M109.016816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin A., Telang S., Clem B., Chesney J. 2009b. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp. Mol. Pathol. 86:174–179 10.1016/j.yexmp.2009.01.003 [DOI] [PubMed] [Google Scholar]