Epidermal IL-15Rα, shed by keratinocytes upon stimulation with inflammatory cytokines, counteracts IL-15–induced proliferation of IL-17–producing T cells to dampen psoriatic skin disease.
Abstract
Stromal cells at epithelial surfaces contribute to innate immunity by sensing environmental danger signals and producing proinflammatory cytokines. However, the role of stromal cells in controlling local inflammation is unknown. We show that endogenous soluble IL-15 receptor α (IL-15Rα) derived from epidermal stroma, notably keratinocytes, protects against dendritic cell/IL-15-mediated, T cell-driven skin inflammation in vivo, and is relevant to human psoriasis. Selective lack of IL-15Rα on stromal epidermal cells exacerbated psoriasiform inflammation in animals. Epidermal IL-15Rα was shed by keratinocytes via proteolytic cleavage by matrix metalloproteinases upon stimulation with proinflammatory cytokines to counteract IL-15–induced proliferation of IL-17+ αβ and γδ T cells and production of TNF, IL-23, IL-17, and IL-22 during skin inflammation. Notably, administration of soluble IL-15Rα was able to repress secretion of IL-1β, IL-6, and TNF by keratinocytes, dampen expansion of IL-17+ αβ and γδ T cells in vivo, and prevent psoriasis in two mouse models, including human xenograft AGR mice. Serum levels of soluble IL-15Rα negatively correlated with disease severity, and levels rose upon successful treatment of psoriasis in patients. Thus, stressed epidermal stromal cells use soluble IL-15Rα to dampen chronic inflammatory skin disease.
Psoriasis is a chronic, relapsing-remitting inflammatory disorder mediated by effector T cells, including CD4+ and CD8+ αβ and γδ T cells (Lowes et al., 2007; Nestle et al., 2009). These T cells secrete an array of proinflammatory cytokines, including tumor necrosis factor-α (TNF), interferon-γ (IFN-γ), IL-17, and IL-22, to stimulate proliferation of keratinocytes and recruit inflammatory cells (Di Meglio et al., 2011; Lowes et al., 2007). Notably, αβ T cells appear to be crucial in the chronic-relapsing phase of the disease, where these cells become resident in the skin, presumably as memory T cells dependent on IL-15 and other homeostatic cytokines (Boyman et al., 2007). Upon induction of a psoriatic lesion, CD8+ T cells, notably α1β1 integrin+ cells, home to the epidermis and correlate with epidermal thickening (acanthosis) and elongation of dermal papillae (papillomatosis), leading to enhanced interdigitation of epidermis and dermis (Boyman et al., 2004; Conrad et al., 2007). As for γδ T cells, the involvement of these cells has recently been implicated in the pathogenesis of psoriasis (Laggner et al., 2011), especially during the early stages of psoriasiform skin inflammation in mouse models (Cai et al., 2011; Pantelyushin et al., 2012).
Furthermore, DCs appear to be involved very early in the pathogenesis of psoriasis by producing type I IFNs (Nestle et al., 2005). Interestingly, treatment with imiquimod (IMQ), an agonist of Toll-like receptors (TLR) 7 and 8, can stimulate DCs to produce type I IFNs, and thus exacerbate psoriasis in patients (Gilliet et al., 2004). Moreover, treatment of normal mice with IMQ results in psoriasiform skin inflammation, which is characterized by epidermal thickening and interdigitation of epidermis and dermis, thus resembling papillomatosis (van der Fits et al., 2009). Cutaneous inflammation in the IMQ model appears to share many pathophysiologic pathways with (early) psoriasis plaque formation (Nestle et al., 2009). Hence, the immune cascade involved in this IMQ-induced psoriasis model hinges on the stimulation of DCs via TLR7 and 8, leading to activation of the IL-23−IL-17 axis with stimulation of T cells capable of IL-17 and IL-22 production (van der Fits et al., 2009; Cai et al., 2011; Pantelyushin et al., 2012; Tortola et al., 2012). The importance of IL-17 in psoriasis is further highlighted by the recent success of biologics targeting IL-17 in patients (Krueger, 2012; Leonardi et al., 2012; Papp et al., 2012).
In addition to the immune system, keratinocytes have long been known to produce proinflammatory cytokines, including TNF and IL-1β, thus contributing to the inflammatory milieu in psoriatic skin plaques (Barker et al., 1991). Moreover, a publication examining the expression of IL-15 and IL-IL-15Rα on keratinocytes in vitro, as well as IL-15 and IL-15–binding sites in skin biopsies from psoriasis patients, suggested that keratinocytes might stimulate neighboring keratinocytes and immune cells by presenting IL-15 via membrane-bound IL-15Rα to these cells (Rückert et al., 2000). Recently, production of the proinflammatory IL-1 family member IL-36 by keratinocytes has been shown to play a role in inducing psoriasiform inflammation in the IMQ mouse model (Tortola et al., 2012). Furthermore, keratinocytes are known to secrete different antimicrobial peptides, including β-defensins, LL-37 cathelicidin, and S100 proteins. Some of these antimicrobial peptides are able to form complexes with self-DNA molecules, thereby leading to the activation of DCs, as has been shown for LL-37–DNA complexes stimulating plasmacytoid DCs via TLR9 engagement to produce IFN-α (Lande et al., 2007). Moreover, antimicrobial peptides exert chemotactic activities toward innate and adaptive immune cells. Thus, keratinocytes, through the production of antimicrobial peptides and proinflammatory cytokines, are contributing to cutaneous inflammation in immune defense, as well as in psoriasis (Nestle et al., 2009). However, whether keratinocytes or other stromal cells are involved in modulating or dampening inflammation in the skin is unknown.
Here, we show that stromal cells, most notably keratinocytes in the skin, express high levels of IL-15Rα and release soluble IL-15Rα (sIL-15Rα) via proteolytic cleavage to suppress the inflammatory response in murine and human psoriasis. These data reveal a crucial role for stroma-derived sIL-15Rα in counter-regulating inflammation in this common chronic inflammatory disorder.
RESULTS
Opposing effects of IL-15 and IL-15Rα in psoriasiform skin inflammation
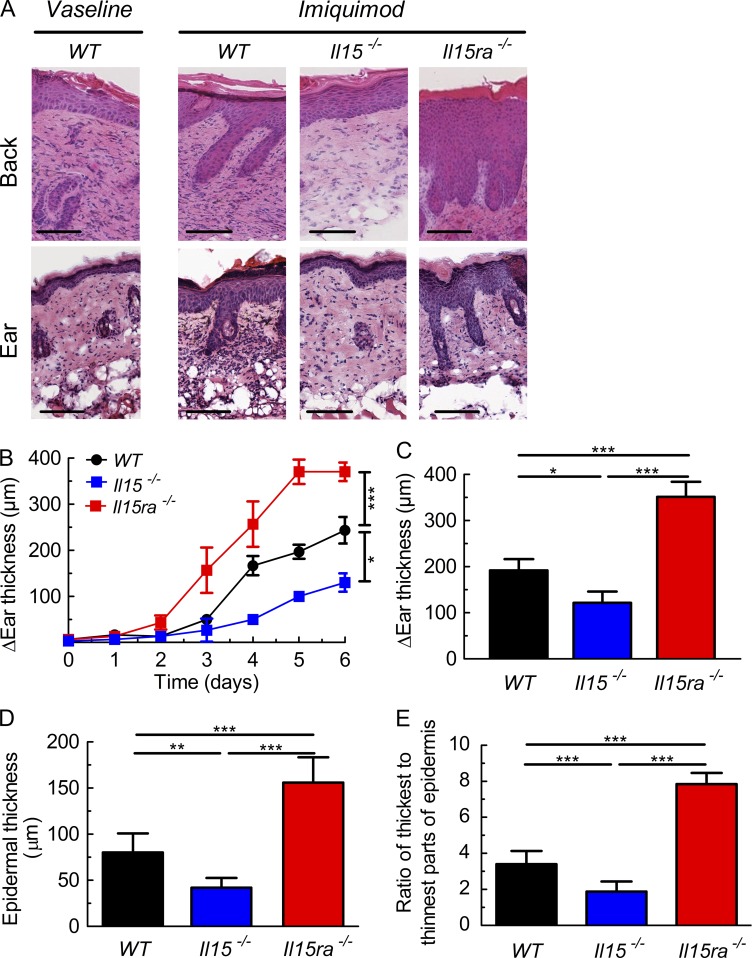
IL-15 can induce the production of proinflammatory cytokines involved in the pathogenesis of psoriasis (McInnes and Liew, 1998). Hence, it was not surprising that administration of IMQ to Il15−/− mice failed to induce a psoriasiform skin pathology, in contrast to WT mice, which showed marked thickening of epidermis and interdigitation of epidermis and dermis of the treated back or ear skin compared with control WT mice treated with Vaseline (Fig. 1 A). Il15−/− mice showed consistently less thickening of the IMQ-treated ears compared with WT, which was already evident on day 4 of IMQ application (Fig. 1 B). On day 6, ear thickness, epidermal thickness, and interdigitation of epidermis and dermis of Il15−/− mice was 35–50% lower than in WT animals (Fig. 1, C–E).
Figure 1.
Opposing effects of IL-15 and IL-15Rα in psoriasiform skin inflammation. WT, Il15−/−, and Il15ra−/− mice were treated for six consecutive days with IMQ cream on their shaved back skin or right ear, whereas WT animals receiving Vaseline served as controls. (A) Representative hematoxylin-eosin staining is presented of the back skin (top) and ear (bottom) of indicated mice on day 6. Bars, 100 µm. (B and C) The difference in ear thickness in micrometers between IMQ-treated and control (Vaseline)-treated animals, as determined daily (B) or on day 6 (C). Symbols represent mean difference in ear thickness ± SD for three to six mice per group. (D and E) Shown are (interfollicular) epidermal thickness (D) and ratio of maximal/minimal epidermal thickness (E). Data are representative of four independent experiments. P-values were determined using one-way ANOVA; *, P < 0.01; **, P < 0.001; ***, P < 0.0001.
These results were in line with a previous study reporting the use of a mAb interfering with the binding of IL-15 to IL-15Rα, thus inhibiting IL-15R signaling and the maintenance of psoriasiform inflammation in a xenograft mouse model (Villadsen et al., 2003). Thus, we expected to see a similar phenotype as in Il15−/− mice when treating Il15ra−/− mice with IMQ. Very surprisingly, however, Il15ra−/− animals were not only able to develop a psoriasiform disease upon IMQ application, but the inflammatory skin reaction was significantly more pronounced than in WT mice (Fig. 1 A). Ear thickness of IMQ-treated Il15ra−/− animals was increased as early as on day 3 (Fig. 1 B), and, on day 6 of IMQ treatment, ear thickness, epidermal thickness and interdigitation of epidermis and dermis was 75–130% higher in Il15ra−/− compared with WT mice (Fig. 1, C–E).
Psoriasiform skin disease depends on IL-15 production by DCs
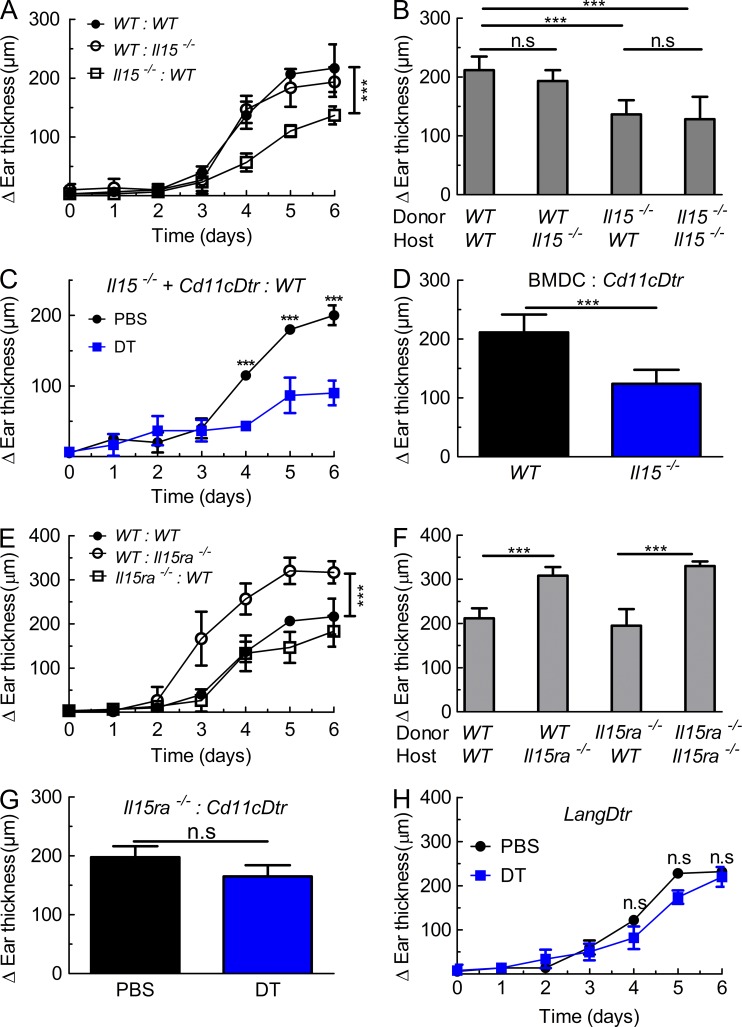
Immunohistochemical analysis of skin sections from WT and Il15ra−/− animals for IL-15 revealed the presence of IL-15+ cells in the dermis (unpublished data). To gain insight into which cells produced IL-15 upon cutaneous IMQ application, we generated BM chimeras using WT or Il15−/− BM cells adoptively transferred into lethally irradiated WT or Il15−/− hosts. Upon de novo reconstitution of the immune compartment and IMQ treatment, these BM chimeras showed that absence of IL-15 on radio-resistant host stromal cells was dispensable for skin inflammation (Fig. 2, A and B). Conversely, selective deficiency of IL-15 on hematopoietic cells as found in Il15−/−–WT chimeras was sufficient to replicate the phenotype of Il15−/− mice (Fig. 2, A and B).
Figure 2.
IL-15Rα expression by stromal cells dampens DC-IL-15-mediated psoriasiform skin inflammation. (A and B) BM chimeras were generated using WT donor BM adoptively transferred to lethally irradiated WT (WT:WT) or Il15−/− (WT:Il15−/−) hosts, or by transferring Il15−/− BM to lethally irradiated WT (Il15−/−:WT) or Il15−/− (Il15−/−:Il15−/−) mice, followed by treatment for 6 consecutive days with IMQ cream on their right ear. Shown is the difference in ear thickness as in Fig. 1 B, either determined daily (A) or on day 6 (B). (C) Chimeras were generated using a 1:1 mixture of Il15−/− and Cd11cDtr BM adoptively transferred to lethally irradiated WT hosts. After reconstitution, mice received either PBS (black line) or DT (blue line), followed by treatment and assessment as in A. (D) Cd11cDtr animals, injected with DT, received 1 d before IMQ treatment intradermal injection of 106 BMDC from either WT or Il15−/− mice, followed by treatment and assessment as in B. (E and F) Chimeras were generated using WT donor BM adoptively transferred to lethally irradiated WT (WT:WT) or Il15ra−/− (WT:Il15ra−/−) hosts, or by transferring Il15ra−/− BM to lethally irradiated WT (Il15ra−/−:WT) or Il15ra−/− (Il15ra−/−:Il15ra−/−) mice, followed by treatment and evaluation as in A and B. (G) BM chimeras, generated using Il15ra−/− BM adoptively transferred to lethally irradiated Cd11cDtr mice, received either PBS or DT, followed by treatment and evaluation as in B. (H) LangDtr mice received either PBS or DT, followed by treatment and assessment as in A. Symbols represent mean difference in ear thickness ± SD for two to four mice per group. Data are representative of two to three independent experiments. P-values were determined using one-way ANOVA; ns, not significant; ***, P < 0.0001.
As IMQ is a TLR7 and 8 agonist, and these TLRs are abundant in DCs, we reasoned that IL-15 production by these cells might be crucial for IMQ-mediated psoriasiform pathology. Thus, selective depletion of DCs in Cd11cDtr mice receiving diphtheria toxin (DT) significantly reduced skin inflammation upon IMQ application (unpublished data), in line with a recent publication (Tortola et al., 2012). To exclude the possibility that CD11c+ DCs produced another intermediary cytokine acting on another cell subset to induce IL-15 production by the latter, we generated chimeras carrying a 1:1 mixture of Il15−/− and Cd11cDtr BM adoptively transferred to lethally irradiated WT hosts. In these mice, Il15−/− DCs are resistant to DT treatment but unable to produce IL-15, whereas Cd11cDtr are the only DCs able to secrete IL-15. Upon IMQ treatment, mixed chimeras receiving DT showed a similar deficiency to develop psoriasiform inflammation (Fig. 2 C), as did Il15−/− mice and DT-treated Cd11cDtr animals. Conversely, mixed Il15−/−–Cd11cDtr chimeras receiving PBS developed robust ear thickening (Fig. 2 C), which was indistinguishable from WT–WT chimeras (Fig. 2, A and B), suggesting that IL-15 production by roughly half of CD11c+ DCs was sufficient to induce inflammatory skin disease. Further evidence for a crucial role of DCs producing IL-15 in this model came from DT-treated Cd11cDtr mice receiving intradermal injection of BM-derived DCs (BMDCs) from either WT or Il15−/− mice. Upon IMQ treatment, only WT and not Il15−/− BMDCs were able to induce a level of psoriasiform pathology (Fig. 2 D), which was comparable to IMQ-treated WT mice.
IL-15Rα expression by stromal cells dampens psoriasiform inflammation
Similar to the aforementioned experiments, we generated BM chimeras to investigate the cellular source of IL-15Rα. Interestingly, selective absence of IL-15Rα on radio-resistant cells in WT-Il15ra−/− chimeras nicely replicated the exaggerated inflammatory skin response found in Il15ra−/− mice (Fig. 2, E and F). This was not caused by the persistence of radio-resistant skin DCs expressing lower CD11c levels, including Langerhans cells, the latter of which have been shown to resist high-dose irradiation (Merad et al., 2002). Thus, upon IMQ treatment, Il15ra−/−–Cd11cDtr chimeras receiving DT showed an inflammatory skin response similar to that seen in control chimeras receiving PBS (Fig. 2 G). Moreover, Langerin-Dtr (LangDtr) animals treated with DT were indistinguishable from PBS-treated LangDtr (Fig. 2 H), suggesting that Langerhans cells were not implicated in IMQ-mediated psoriasiform skin pathology, neither in the IL-15–induced inflammation nor in the IL-15Rα-mediated dampening of disease.
Keratinocytes express high surface IL-15Rα and release IL-15Rα upon stimulation
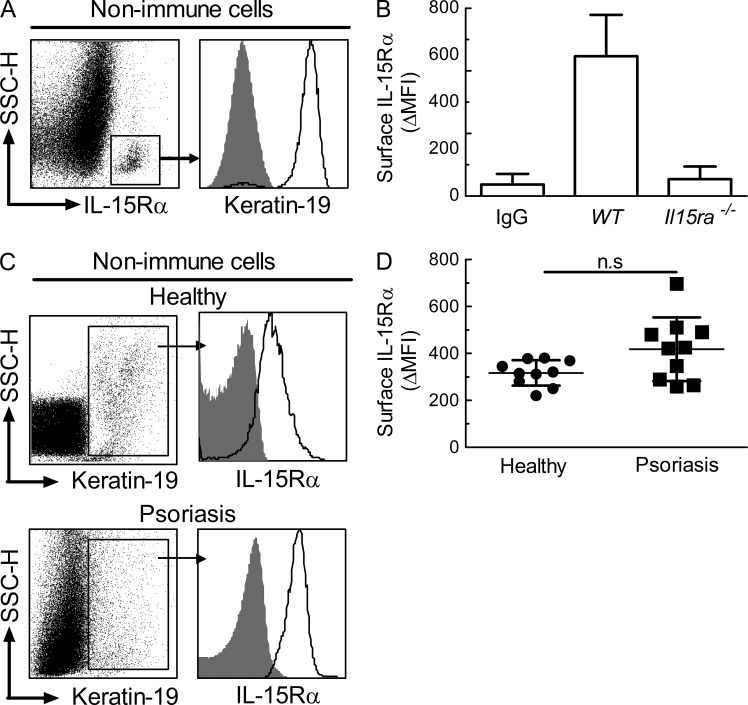
The aforementioned results suggested that stromal skin cells expressed significant levels of IL-15Rα to dampen IL-15–induced cutaneous inflammation. We assessed IL-15Rα protein levels in single-cell suspensions of murine total skin using flow cytometry. Upon gating on immune lineage-negative (nonimmune) cells, there was a clear population of cells expressing high levels of IL-15Rα, which was present in the epidermal, but not dermal, fraction (Fig. 3 A, left; and not depicted). Strikingly, this IL-15Rαhigh stromal cell subset was >95% positive for the keratinocyte-specific marker (cyto-) keratin-19 (Fig. 3 A, right). Isotype-matched control staining of WT murine keratinocytes gave only background signals, comparable to IL-15Rα staining of Il15ra−/− murine keratinocytes (Fig. 3 B).
Figure 3.
Primary human and mouse keratinocytes express high levels of surface IL-15Rα. (A) Primary epidermal skin cells from WT mice were analyzed by flow cytometry. Shown are dot plot profiles of side scatter (SSC-H) versus IL-15Rα expression on CD45− CD3− CD11b− CD11c− CD207− MHC class II− nonimmune cells, as well as histograms of isotype-matched control staining (gray shaded area) versus (cyto-) keratin 19 expression (solid line) on gated IL-15Rαhigh nonimmune epidermal cells. (B) Mean fluorescence intensity (MFI) of surface IL-15Rα on keratin-19+ epidermal cells was determined by flow cytometry using skin from WT and Il15ra−/− mice; IgG designates isotype-matched control staining. (C and D) Surface IL-15Rα expression on keratin-19+ human epidermal cells was determined by flow cytometry using skin from healthy subjects (n = 10) versus psoriasis patients (n = 10). Shown are histograms of surface IL-15Rα expression (solid line) versus isotype-matched control staining (gray shaded area) in healthy (C, top) and psoriasis (C, bottom), as well as MFI values, including mean ± SD, of these groups (D). Data are representative of three independent experiments. P-values were determined using one-way ANOVA; ns, not significant.
Similar to murine cells, keratin-19+ epidermal cells obtained from healthy human individuals also expressed significant IL-15Rα levels (Fig. 3 C, top), as was the case for keratin-19+ cells from skin plaques of psoriasis patients (Fig. 3 C, bottom). Although keratinocytes from skin plaques of psoriasis patients showed a tendency toward higher expression levels of cell surface IL-15Rα compared with keratinocytes from healthy individuals, upon subtraction of background staining, IL-15Rα levels were not significantly different between these groups (Fig. 3 D).
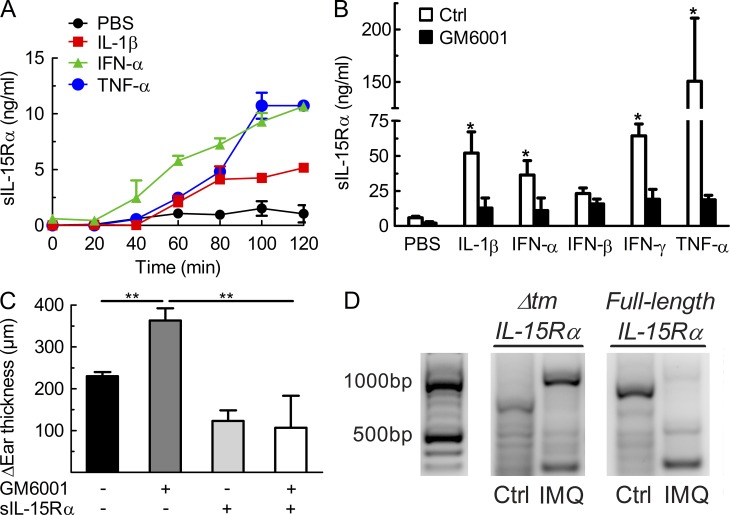
In primary normal human keratinocyte cultures, stimulation with proinflammatory cytokines, including IL-1β, IFN-α, IFN-γ, and TNF, induced the release of soluble IL-15Rα (sIL-15Rα) into the supernatant (Fig. 4, A and B). Similar results were obtained using primary murine keratinocytes and human keratinocyte cell lines (unpublished data). Interestingly, the secretion of sIL-15Rα by human keratinocytes upon stimulation with the aforementioned cytokines was almost absent in the presence of the matrix metalloproteinase (MMP) inhibitor GM6001 (Fig. 4 B). In line with these in vitro data, administration of GM6001 to WT mice receiving topical IMQ exacerbated cutaneous inflammation, in comparison to IMQ-treated WT mice receiving PBS, whereas co-administration of recombinant sIL-15Rα was able to correct the GM6001-mediated exacerbation (Fig. 4 C). Moreover, there was no evidence of increased alternative splicing and generation of IL-15Rα lacking the transmembrane part (Δtm) encoded by exon 6 (Dubois et al., 1999), as shown by reverse transcription polymerase chain reaction using specific primers for full-length and Δtm IL-15Rα (Fig. 4 D).
Figure 4.
Release of soluble IL-15Rα depends on proteolytic cleavage by MMPs, rather than alternative splicing of IL-15Rα. (A) Primary keratinocytes from healthy subjects were stimulated using PBS, IL-1β, IFN-α, and TNF, and supernatant was collected at different time points for measuring levels of soluble IL-15Rα (sIL-15Rα). (B) Primary human keratinocytes were treated as in A using PBS, IL-1β, IFN-α, IFN-β, IFN-γ, and TNF, without or with the MMP inhibitor GM6001, followed by determining levels of sIL-15Rα in the supernatant 2 h later. (C) WT mice received IMQ cream on their right ear for 6 consecutive days, along with intraperitoneal injections of either PBS, GM6001, sIL-15Rα, or GM6001 plus sIL-15Rα. Shown is the difference in ear thickness as in Fig. 1 C determined on day 6. (D) WT mice were treated using Vaseline (Ctrl) or IMQ on their right ear for 6 consecutive days, followed by sacrificing mice, harvesting the right ears for mRNA extraction, and performing RT-PCR for full-length IL-15Rα (around 800 bp) versus IL-15Rα lacking the transmembrane domain (Δtm) due to alternative splicing (around 500 bp). Data are representative of two to three independent experiments. P-values were determined using one-way ANOVA; *, P < 0.01; **, P < 0.001.
Collectively, these data show that murine and human keratinocytes express significant levels of IL-15Rα, both during homeostasis and inflammation and that upon stimulation with proinflammatory cytokines, keratinocytes are able to release sIL-15Rα via proteolytic cleavage by MMPs.
IL-15 drives T cell expansion and production of proinflammatory cytokines
As previously mentioned, IL-15 is able to induce the production of different proinflammatory cytokines implicated in the pathogenesis of psoriasis (McInnes and Liew, 1998). Moreover, IL-15 is a common γ chain (γc) cytokine and, similar to other γc cytokine members, IL-15 is involved in the survival and maintenance of T cells, especially memory CD8+ and, to a lesser extent, memory CD4+ T cells (Boyman et al., 2012).
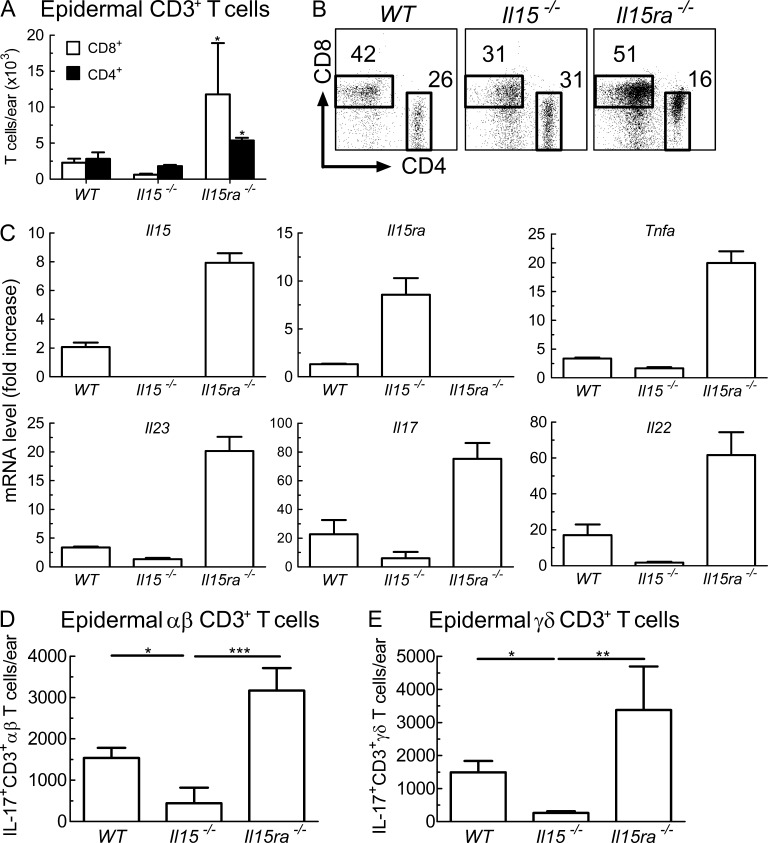
Paralleling the degree of psoriasiform inflammation, Il15ra−/− animals showed the strongest expansion of CD3+ T cells upon IMQ treatment, followed by WT and Il15−/− mice (Fig. 5, A and B). The increase in cell counts and percentages was most notable for CD8+ T cells, which were over 5-fold more prominent in Il15ra−/− animals compared with WT and over 20-fold in comparison to Il15−/− mice. CD4+ T cells were also increased in IMQ-treated Il15ra−/− animals, being twice as abundant as in WT and three times more than in Il15−/− mice.
Figure 5.
IL-15 drives T cell expansion and production of proinflammatory cytokines. (A and B) WT, Il15−/−, and Il15ra−/− mice were treated for 6 consecutive days with IMQ cream on their right ear. Epidermal skin cells were isolated and analyzed by flow cytometry for T cell subsets. Shown are absolute numbers (A) and dot plots (B) of CD8+ (open bars) and CD4+ (filled bars) CD3+ T cells in indicated animals. (C) mRNA levels of IL-15, IL-15Rα, TNF, IL-23p19, IL-17, and IL-22 were measured by quantitative RT-PCR in ears of WT, Il15−/−, and Il15ra−/− mice treated for 3 consecutive days with IMQ. Shown is fold increase in mRNA normalized to ribosomal protein L27 versus control skin of WT mice. Data are displayed as mean ± SD. (D and E) WT, Il15−/−, and Il15ra−/− mice received IMQ for 3 d, followed by direct ex vivo analysis of intracellular IL-17 production in αβ (D) and γδ T cells (E). Data are pooled results from two to four independent experiments. P-values were determined using one-way ANOVA; *, P < 0.01; **, P < 0.001; ***, P < 0.0001.
As for effector cytokines, the mRNA levels of IL-15 and TNF, as well as IL-23p19, IL-17, and IL-22, were 3–6-fold higher in Il15ra−/− animals compared with WT mice upon IMQ application for 3 d (Fig. 5 C). Conversely, levels of these cytokines were at least twofold lower in Il15−/− mice compared with WT (Fig. 5 C).
In line with these expression data, IL-17–producing αβ and γδ T cells were increased in Il15ra−/− mice in comparison to WT, whereas Il15−/− animals had significantly lower counts of IL-17+ skin T cells (Fig. 5, D and E).
Administration of sIL-15Rα inhibits human and murine psoriasiform disease
Our data showing that IL-15Rα derived from stromal cells, most notably from keratinocytes, dampens IMQ-induced skin disease suggested that administration of IL-15Rα molecules might improve psoriasiform inflammation. As murine skin lacks distinct features of human skin, and thus cannot entirely mimic psoriasis, we expanded our findings in the IMQ mouse model by taking advantage of our well-established human xenograft psoriasis model (Boyman et al., 2004). As expected, transplantation of symptomless prepsoriatic skin grafts from a psoriasis patient onto immunodeficient AGR129 mice induced the skin grafts to spontaneously develop a fully-fledged psoriatic phenotype within 6–8 wk. Thus, human xenografts of PBS-treated control animals showed characteristic features of psoriasis, including acanthosis, papillomatosis, loss of granular cell layer, and dense infiltrates of mononuclear cells in the dermis and epidermis representing immune cells (Fig. 6, A and B). In sharp contrast to PBS controls, xenografts of AGR129 mice treated with sIL-15Rα did not develop any typical morphological signs of psoriasis, and, accordingly, the acanthosis and papillomatosis indices remained low (Fig. 6, A and B).
Figure 6.
Administration of sIL-15Rα inhibits human and murine psoriasiform disease. (A and B) Development of a psoriatic phenotype in symptomless prepsoriatic human skin transplantated onto AGR129 mice is prevented by injection of sIL-15Rα. Shown is representative hematoxylin-eosin staining at low- (left) and and high-power (right) magnification (A), and acanthosis (B, top), and papillomatosis index (B, bottom) of the human skin grafts 6 wk after transplantation onto AGR129 mice receiving either PBS or sIL-15Rα. Bars, 50 µm. Data are representative of four mice per group. (C and D) WT, WT receiving sIL-15Rα, and Il15ra−/− mice injected with sIL-15Rα were treated for 6 consecutive days with IMQ cream on their right ear. Mice were assessed as in Fig. 1 (B and C). (E) Primary human keratinocytes were stimulated with either PBS, IL-15, IL-15 plus sIL-15Rα, or sIL-15Rα, followed by analysis of IL-1β, IL-6, and TNF by ELISA. (F and G) Epidermal skin cells from WT mice were stimulated with either PBS, IL-15, IL-15 plus sIL-15Rα, or sIL-15Rα, followed by analysis using flow cytometry. Shown are percentages of IL-17–producing CD3+ αβ (F) and γδ T cells (G) as mean ± SD. Data are representative of two independent experiments. P-values were determined using one-way ANOVA; *, P < 0.01; **, P < 0.001; ***, P < 0.0001.
Similar to AGR129 mice, treatment with sIL-15Rα was also efficacious in the IMQ model of psoriasiform disease. Thus, WT animals receiving sIL-15Rα resembled Il15−/− mice in that development of cutaneous inflammation and thickening upon IMQ application was significantly blunted, corresponding to only 35% of the increase in thickness compared with WT controls (Fig. 6, C and D). Interestingly, administration of sIL-15Rα to Il15ra−/− animals controlled the aberrant skin inflammation in these mice (Fig. 6, C and D).
To gain insight into the target cells of IL-15 and to rule out the possibility that sIL-15Rα associates with soluble IL-15 to form agonistic complexes that stimulate T cells and keratinocytes (Rückert et al., 2000; Rubinstein et al., 2006; Stoklasek et al., 2006), we stimulated primary keratinocytes and T cells using IL-15 without or with addition of sIL-15Rα. Paralleling the aforementioned results, production of IL-1β, IL-6, and TNF by IL-15–stimulated primary human and murine keratinocytes was significantly reduced when sIL-15Rα was added to the cultures (Fig. 6 E; and not depicted). Moreover, culturing of total skin cells with IL-15 led to an increase of IL-17–producing αβ and γδ T cells, which was significantly reduced when sIL-15Rα was co-administered (Fig. 6, F and G). Notably, skin-resident T cells expressed IL-15 receptors, and levels of IL-15Rβ (CD122) and γc on these cells did not change upon application of IMQ (unpublished data).
Opposing regulation of IL-15 and sIL-15Rα in psoriasis patients influences T cell proliferation
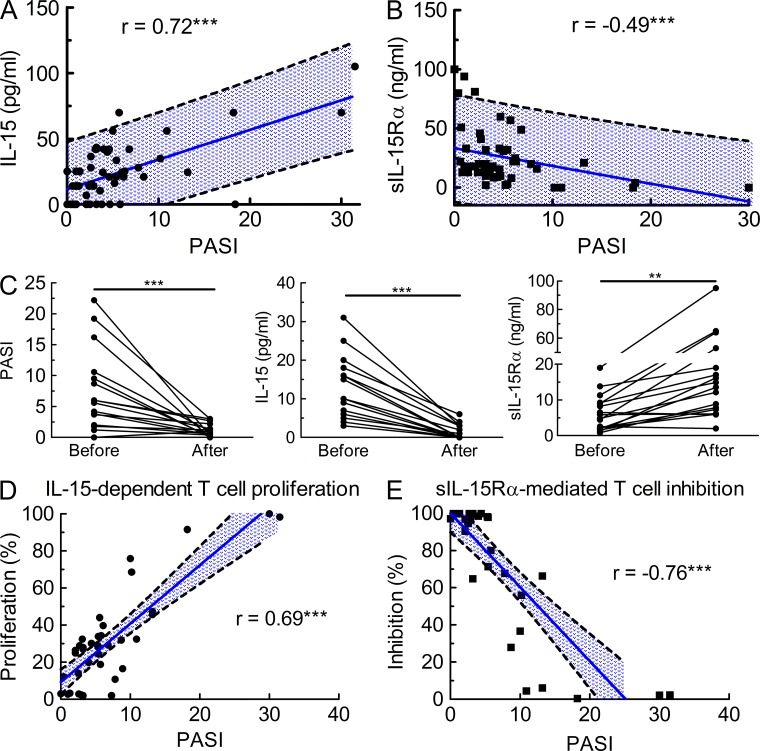
Our data on IL-15Rα expression on human keratinocytes and the role of sIL-15Rα in dampening psoriasiform disease in two relevant animal models suggested that the IL-15−IL-15Rα system might also play an important role in psoriasis patients. To this end, we assessed IL-15 and sIL-15Rα levels in serum of psoriasis patients (n = 52) using ultrasensitive radio-immunoassays. IL-15 levels steadily rose with increasing disease severity, as reflected by the psoriasis area and severity index, following a linear correlation (Fig. 7 A; r = 0.72; Spearman test, P < 0.0001). Strikingly, however, sIL-15Rα showed a completely opposite trend. Thus, serum sIL-15Rα levels decreased with higher PASI scores, leading to a negative linear correlation (Fig. 7 B; r = −0.49; Spearman test, P < 0.0001).
Figure 7.
Opposing regulation of IL-15 and sIL-15Rα in psoriasis patients influences T cell proliferation. (A and B) IL-15 and sIL-15Rα were assessed in the serum of psoriasis patients (n = 52) using specific radio-immunoassays. Shown are serum levels of IL-15 (A) and sIL-15Rα (B) versus the PASI, with each dot representing a patient, the blue line corresponding to the Pearson correlation and the dashed lines to the 95% confidence interval; r (IL-15) = 0.72; Spearman test, P < 0.0001; r (IL-15Rα) = −0.49; Spearman test, P < 0.0001. (C) Individual psoriasis patients (n = 17) were seen before and after antipsoriatic treatment (see Materials and methods), at which time-points PASI (left) and serum IL-15 (middle) and sIL-15Rα levels (right) were determined. Statistic comparison using a Mann-Whitney U test of the groups before and after resulted in P-values of 0.0034 (**) and 0.0001 (***). (D and E) Proliferation of CTLL-2 T cells incubated with either serum of psoriasis patients (D) or with a fixed saturating concentration of recombinant human IL-15 along with serum of psoriasis patients (E) was assessed by incorporation of [3H]thymidine. Shown is the Pearson correlation (blue line) with 95% confidence interval (dashed lines) of PASI versus proliferation (D; r = 0.69; Spearman test, P < 0.0001) and inhibition (E; r = −0.76; Spearman test, P < 0.0001), respectively. Data are pooled results of two independent experiments in triplicates.
The opposing regulation of IL-15 and sIL-15Rα could indicate interindividual variability. Thus, we measured these parameters in individual psoriasis patients before and after antipsoriatic treatment (n = 17). As expected, antipsoriatic therapy led to a marked reduction of disease severity in most of the patients as measured by PASI, which was paralleled by a significant decrease of serum IL-15 to background levels in the patients (Fig. 7 C, left and middle). In sharp contrast to IL-15, serum levels of sIL-15Rα rose 2–12-fold after treatment (Fig. 7 C, right).
To assess the biological activity of serum IL-15 and sIL-15Rα, we performed direct ex vivo proliferation assays using the IL-15–sensitive T cell line CTLL-2. Direct culturing of CTLL-2 cells with sera led to proliferation of the cells, which was most prominent when using sera from patients with higher PASI scores (Fig. 7 D). The biological property of sIL-15Rα was assessed by culturing CTLL-2 cells with a fixed concentration of recombinant human IL-15, followed by addition of sera. Samples of psoriasis patients with PASI scores of 5 and lower almost completely inhibited IL-15–mediated proliferation of CTLL-2 cells, whereas serum specimens of patients with increasing PASI scores progressively lost the ability to block IL-15–mediated T cell stimulation (Fig. 7 E).
These data establish in psoriasis patients that disease severity and IL-15 correlate directly with each other, whereas concomitant regulation of biologically active sIL-15Rα follows an opposite trend.
DISCUSSION
Our data demonstrate that during inflammation availability of IL-15 is regulated by proteolytic cleavage and shedding of IL-15Rα from epidermal stromal cells, unveiling an unexpected role of tissue-derived sIL-15Rα in psoriasis. Although the crucial function of IL-15Rα in the IL-15 system is well known, the role of endogenous sIL-15Rα in IL-15 homeostasis has remained elusive. Hence, Il15−/− and Il15ra−/− mice both lack memory CD8+ T cells and NK cells (Lodolce et al., 1998; Kennedy et al., 2000; Boyman et al., 2012), implying that IL-15 depends on IL-15Rα for signaling. This is explained by the fact that IL-15 binds cells expressing surface IL-15Rα together with CD122 and γc. In addition to this classical cis interaction, DCs can produce and present IL-15 in trans via membrane-bound IL-15Rα to neighboring CD8+ and NK cells expressing dimeric IL-15Rβγ, consisting of CD122 and γc (Dubois et al., 2002; Schluns et al., 2004; Burkett et al., 2004). Thus, binding and presentation of IL-15 by membrane-bound IL-15Rα delivers a stimulatory signal.
Moreover, IL-15Rα also exists in a soluble form and, unlike membrane-bound IL-15Rα, sIL-15Rα can act either as an agonist or antagonist of IL-15 in vivo. Thus, recombinant sIL-15Rα combined with recombinant IL-15, thereby forming IL-15−sIL-15Rα complexes, can cause massive expansion of memory CD8+ and NK cells in mice (Rubinstein et al., 2006; Stoklasek et al., 2006). Conversely, injection of recombinant sIL-15Rα is able to reduce collagen-induced arthritis (Ruchatz et al., 1998), and to inhibit homeostasis of memory CD8+ and NK cells in vivo (Khan et al., 2002; Nguyen et al., 2002). The implications from these data are that sIL-15Rα is a potent regulator of IL-15 activity, acting as an agonist or antagonist. However, there is no evidence as to whether endogenous, natural (i.e., not recombinant) sIL-15Rα is able to modulate IL-15 signaling in vivo. The data presented in this study demonstrate for the first time that endogenous sIL-15Rα is implicated in reducing cutaneous inflammation in psoriasis, both in mice and men.
We show that during psoriasiform inflammation, abundant production of IL-15 by DCs causes the activation of αβ and γδ effector T cells. These T cells need only to express dimeric IL-15Rβγ to efficiently bind IL-15 and signal, as demonstrated by experiments using Il15ra−/−–WT mixed BM chimeras lacking membrane-bound IL-15Rα on both T cells and DCs. Hence, Il15ra−/−–WT chimeras show identical inflammatory responses as WT–WT chimeras. Notably, the dimeric IL-15Rβγ possesses an affinity for IL-15 of ∼10−9 M, and thus is able to avidly bind IL-15, even in the absence of IL-15Rα in cis or in trans (Waldmann and Tagaya, 1999). However, sIL-15Rα has an affinity for IL-15 of ∼10−11 M, which is 100-fold higher than the dimeric IL-15Rβγ, thus, even small concentrations of sIL-15Rα are able to compete with IL-15 binding to the dimeric IL-15Rβγ. This notion fits well with our herein presented data. Moreover, as demonstrated by using WT-Il15ra−/− mixed BM chimeras, in the absence of stromal sIL-15Rα, the stimulatory action of IL-15 on αβ and γδ effector T cells becomes unopposed, thus leading to an inflammatory response that is even more pronounced than in WT mice.
These data also highlight the role of IL-15 in driving an (auto-) inflammatory pathology. IL-15 boosts the production of proinflammatory cytokines, including TNF, by keratinocytes and T cells, as well as IL-17, IL-22, and IL-23 by murine αβ and γδ T cells, as shown here, and IL-17 by human T cells (Hoeve et al., 2006). In turn, TNF is a well-known driver of the inflammatory response in psoriasis (Mease et al., 2000; Chaudhari et al., 2001; Boyman et al., 2004) and also enhances the production of other proinflammatory cytokines, including IL-15, thereby fueling a positive feedback loop. Moreover, IL-15 has been suggested to inhibit Fas-induced apoptosis of keratinocytes (Rückert et al., 2000).
In line with other studies showing that psoriasiform inflammation can be inhibited by blocking IL-15 signals using exogenous administration of an anti–IL-15 mAb (Villadsen et al., 2003), we demonstrate that this pathological inflammation can be dampened both in human and murine skin by injecting recombinant sIL-15Rα in the AGR mouse model of psoriasis or to WT and Il15ra−/− animals in the IMQ model. Remarkably, endogenous sIL-15Rα is able to exert a similar function as recombinant sIL-15Rα by depriving pathogenic T cells from contact with IL-15. In line with these data in mice, we also demonstrate that levels of sIL-15Rα negatively correlate with disease severity in psoriasis patients and that serum concentrations of sIL-15Rα rise upon successful therapy. Using direct ex vivo testing of patients’ sera containing high titers of sIL-15Rα, we found that sIL-15Rα inhibited IL-15–driven T cell proliferation. Likewise, treatment of patients with Crohn’s disease using the anti-TNF mAb infliximab has been shown to increase serum levels of sIL-15Rα, presumably via reverse signaling of infliximab through TNF receptors (Bouchaud et al., 2010), suggesting that sIL-15Rα might serve as an IL-15 antagonist also in inflammatory conditions other than psoriasis. It will be interesting to perform subgroup analyses of larger cohorts of psoriasis patients before and during antipsoriatic treatment to determine whether topical treatment is also able to correct the serum levels of sIL-15Rα and whether some systemic therapeutics are more efficient than others in restoring serum sIL-15Rα levels.
An important point of the present study worth emphasizing is that sIL-15Rα is produced and shed by stromal cells, thus contributing to the cross-talk between stroma and immune system. In our hands, IL-15Rα shedding was induced in human and murine keratinocytes upon incubation with different proinflammatory cytokines and was dependent on proteolytic cleavage by MMPs, as demonstrated by using a specific MMP inhibitor. Conversely, alternative splicing of IL-15Rα and production of IL-15Rα molecules lacking the transmembrane domain, was not increased during psoriasiform inflammation. These findings show that inflammation-mediated expression of MMPs provides a negative feedback via the release of sIL-15Rα to dampen IL-15–driven pathology. Moreover, the data illustrate that stromal cells, including keratinocytes, are not only able to amplify inflammation via the production of proinflammatory cytokines and antimicrobial peptides but they also possess the capacity to control an inflammatory response, at least to a certain degree. Thus, in psoriasis patients, serum sIL-15Rα is presumably able to buffer systemic IL-15 levels in mild disease. However, with increasing severity of psoriasis, the rise of IL-15 is not accompanied by a release of sIL-15Rα. This failure to further increase the levels of sIL-15Rα could result from limited bioavailability of stroma-derived sIL-15Rα, perhaps due to exhaustion or from a defect in shedding.
Our study highlights the importance of tissue-specific mechanisms in regulating availability of cytokines by showing the crucial role of the IL-15−IL-15Rα system in chronic inflammation. Manipulating IL-15 signaling, by provision of recombinant sIL-15Rα or use of neutralizing antibodies specific for IL-15, holds the promise to interfere with the vicious cycle driving chronic inflammatory disorders, including psoriasis, inflammatory bowel disease, and rheumatoid arthritis (Baslund et al., 2005).
MATERIALS AND METHODS
Mice and human subjects.
C57BL/6 (WT) and Il15ra−/− mice were purchased from The Jackson Laboratory, and IL-15−/− animals were from Taconic. Mice expressing DT receptor (DTR) under control of the CD11c (Cd11cDtr) or the Langerin promoter (LangDtr) have been published previously (Jung et al., 2002; Kissenpfennig et al., 2005) and were purchased from The Jackson Laboratory or provided by B. Malissen (Centre d’Immunologie de Marseille-Luminy, Marseille, France), respectively. All transgenic mice were on a C57BL/6 background. For depletion of CD11c+ cells in Cd11cDtr or Langerin+ cells in LangDtr mice, animals received intraperitoneal injections of 200 ng DT (Sigma-Aldrich) in PBS every other day, starting 1 d before IMQ treatment. AGR129 mice deficient in type I and II IFN receptors in addition to being Rag2−/− have been previously published (Boyman et al., 2004). Mice were maintained under specific pathogen–free conditions and used at 6–8 wk of age. Animal experiments were performed in accordance with Swiss Federal Veterinary Office guidelines and approved by the Cantonal Veterinary Office in Zurich (Veterinäramt, Gesundheitsdirektion Kanton Zürich). Use of human samples was approved by the local ethical committee and informed consent was obtained from patients. 17 patients were recruited and seen before and after antipsoriatic treatment. The patients’ mean age was 44.2 (±12.6) years; and the male/female ratio was 14:3; mean disease duration was 9.8 yr; 4 out of 17 patients had evidence of psoriatic arthritis; and on average patients had previously received 1–2 systemic treatments (range, 1 to 5). During this study, patients examined before and on treatment were administered systemic fumaric acid ester, systemic methotrexate, systemic TNF-blocking agents, or systemic p40 IL-12/-23–blocking agents.
IMQ-induced psoriasiform skin inflammation model.
For induction of skin inflammation, mouse ears or shaved backs were treated for 6 consecutive days using 60–80 mg Aldara cream containing 5% (3–4 mg) IMQ (3M Pharmaceuticals) or Vaseline (Vifor SA). Mice were evaluated daily by measuring ear thickness using a digital micrometer (Mitutoyo). For treatment using sIL-15Rα, mice received five consecutive intraperitoneal injections of 3 µg soluble human IL-15Rα-Fc (R&D Systems). The MMP inhibitor GM6001 (Merck) was given by intraperitoneal injections at 250 µg every day for 6 d.
AGR129 human xenograft psoriasis model.
Transplantation of symptomless prepsoriatic skin grafts from psoriasis patients onto immunodeficient AGR129 mice was performed as previously described (Boyman et al., 2004), leading to the spontaneous development of a fully-fledged psoriatic phenotype within 6–8 wk.
Histopathological and immunohistochemical analysis.
Murine and human skin samples were snap frozen and stained using hematoxylin-eosin or immunohistochemistry, as previously reported (Boyman et al., 2004). Epidermal thickness (termed acanthosis for human skin samples) and the ratio of maximal/minimal epidermal thickness (termed papillomatosis for human skin samples) were evaluated for at least 4 mice, as previously reported (Boyman et al., 2004). In murine skin samples, epidermal thickness was determined by measuring the thickness of the interfollicular epidermis at 10 representative sites of the histological slide. In human skin samples, acanthosis was obtained by measuring the thickness of the viable epidermis at 10 representative sites of the histological slide showing an uninterrupted epidermis. Sections were analyzed using ImageScope for image acquisition (Aperio Technologies, Inc.).
Quantification of cytokine levels.
Quantification of sIL-15Rα and IL-15 was determined, as previously established (Bouchaud et al., 2010). Sandwich radio-immunoassays were set up using goat anti–human IL-15Rα or mouse anti–human IL-15 mAbs (R&D Systems) as capture and radio-iodinated anti–human IL-15Rα (R&D Systems) or anti–human IL-15 mAbs (Diaclone) as tracer. Recombinant human sIL-15Rα (R&D Systems) and recombinant human IL-15 (eBioscience) served as standards. Supernatants of each well were collected, and the wells were washed twice with PBS. The radioactivity associated with the wells (bound fraction) or contained in the supernatants and washes (unbound fraction) was determined. The quantitative measurement of IL-1β, IL-6, and TNF was performed using commercially available solid-phase ELISA kits (R&D Systems). All samples were assayed in triplicates.
Keratinocyte cultures.
Primary human keratinocytes isolated from foreskin were cultured in 6-well plates using Keratinocyte-SFM media (Gibco) plus epidermal growth factor (5 ng/ml) and bovine pituitary extract (50 µg/ml) at 60–70% confluence for 24 h at 37°C, with the addition of either PBS, IL-1β (20 ng/ml), IFN-α (100 ng/ml), IFN-β (100 ng/ml), IFN-γ (100 ng/ml), or TNF (200 ng/ml), with or without GM6001 (0.2 nM). Subsequently, supernatants were collected and centrifuged before analysis. Alternatively, murine primary keratinocytes or the human keratinocyte cell line HaCaT were used by culturing these cells in DMEM supplemented with 2 mM L-glutamine and 10% FCS using the aforementioned stimulation conditions.
T cell proliferation.
The proliferative response to serum IL-15 and sIL-15Rα was measured by [3H]thymidine incorporation using the CTLL-2 cell line (ATCC-TIB-214) as previously described (Bouchaud et al., 2008). CTLL-2 cells were cultured in RPMI-1640 medium containing 8% heat-inactivated FCS, 2 mM glutamine, and 10 ng/ml rhIL-2. Cells were maintained in culture medium for 3 d, washed twice, and starved for 4 h in the same medium without cytokine, followed by plating at 104 cells in 100 µl and culturing for 48 h in medium supplemented with patient serum. Cells were pulsed for the last 16 h with 0.5 µCi/well of [3H]thymidine, before harvesting onto glass fiber filters and measuring of cell-associated radioactivity using a β-counter (Perkin-Elmer).
Generation of BM chimeras and BMDCs.
For the generation of BM chimeras, recipient mice were irradiated with 950 rad, followed by intravenous injection of 2–5 × 106 BM cells. 8–12 wk after BM reconstitution, mice were used for experiments.
BMDCs were generated by obtaining single-cell suspensions from BM of murine tibias and femurs, followed by culturing the cells in DC media containing RPMI supplemented with 5% FCS and nonessential amino acids, 2 mM L-glutamine, and 100 U/ml penicillin/streptomycin for 1 d. Subsequently, nonadherent cells were cultured with DC media plus 20 ng/ml GM-CSF (R&D Systems) for another 5 d, followed by maturation of cells (using LPS overnight), harvesting, and intradermal injection into the ear of 1 × 106 cells.
Flow cytometry.
For obtaining single-cell suspensions, mouse or human skin was incubated in dispase (5 mg/ml) to separate epidermis from dermis. Epidermal cell suspensions were prepared by treating epidermis with trypsin-EDTA. Collagenase IV, DNase I, and trypsin-EDTA were used to obtain dermal suspensions. Single-cell suspensions were stained according to standard protocols using PBS containing 1% FCS and 2 mM EDTA (Krieg et al., 2010), with the following fluorochrome-conjugated mAbs (BD, unless otherwise stated): anti–mouse αβ TCR, γδ TCR, CD3, CD4, CD8, CD11b, CD11c, CD45.2, CD207, (cyto-) keratin-19 (Dako), IL-15Rα, IL-17, and MHC class II; anti–human CD3, CD11b, CD11c, CD207, MHC class II, and anti–human IL-15Rα and (cyto-) keratin-19 (both from eBioscience). For intracellular IL-17 staining, mice received IMQ for 3 d, followed by intravenous injection of 250 µg of Brefeldin A (Sigma-Aldrich) 4 h before euthanization and analysis by flow cytometry. Alternatively, mouse cells were isolated and stimulated in vitro with 100 ng/ml recombinant human IL-15 without or with sIL-15Rα-Fc for 24 h in the presence of Golgi stop (BD). After cell surface staining, cells were fixed, permeabilized, and stained intracellularly for IL-17. Samples were harvested using a FACSCanto II (BD) and analyzed using FlowJo software (Tree Star).
RT-PCR.
Ears were immersed in mRNAlater solution (Applied Biosystems) and kept at −80°C, and then homogenized using Tissue Lyser II (QIAGEN) and total mRNA was purified using the Fibrous Mini kit (QIAGEN). After reverse transcription into cDNA using the Reverse Transcription kit (QIAGEN), PCR was performed on a Viia7 RT-PCR detection system (Applied Biosystems). Full-length versus Δtm Il15ra mRNA was assessed using RT-PCR, whereas levels of all other cytokines were determined via quantitative RT-PCR using SYBR Green supermix (QIAGEN). The sequences of specific primers (from Microsynth) were as follows: Il15 forward, 5′-CATCCATCTCGTGCTACTTGTG-3′; Il15 reverse, 5′-GCCTCTGTTTTAGGGAGACCT-3′; full-length Il15ra forward, 5′-ATGGCCTCGCCGCAG-3′; full-length Il15ra reverse, 5′-TTAGGCTCCTGTGTCTTCATC-3′; Δtm Il15ra forward, 5′-ATGGCCTCGCCGCAG-3′; Δtm Il15ra reverse, 5′-GGACTTGTGACTGCCTGTCC-3′; Il17 forward, 5′-TTGGAGGCAGACACCCACC-3′; Il17 reverse, 5′-GATAGCGGTCCTCATCCGTG-3′; Il22 forward, 5′-ATACATCGTCAACCGCACCTTT-3′; Il22 reverse, 5′-AGCCGGACATCTGTGTTGTTAT-3′; Il23p19 forward, 5′-TATCCAGTGTGAAGATGGTTGTG-3′; Il23p19 reverse, 5′-CACTAAGGGCTCAGTCAGAGTTG-3′; Tnfa forward, 5′-TGTAGCCCACGTCGTAGCAAA-3′; Tnfa reverse, 5′-CTGGCACCACTAGTTGGTTGT-3′. Gene expression was normalized using the ribosomal protein L27 (RPL27) housekeeping gene and data are represented as fold differences by the 2−ΔΔCt method, with ΔCt = Cttarget gene − CtRpl27, and ΔΔCt = ΔCtinduced − ΔCtreference, as previously published (Cai et al., 2011).
Statistical analysis.
Statistical analyses were done using one-way ANOVA with Bonferroni’s multiple comparison test, a Mann-Whitney U test, or a Spearman test, as indicated.
Acknowledgments
We thank Bernard Malissen for generously providing LangDtr mice, Emerita Ammann Meier and Christa Dudli-Furrer for excellent technical assistance, and Daniela Impellizzieri for help with histological slides.
This work was supported by Swiss National Science Foundation grants PP00P3-128421 (to O. Boyman) and 31003A-135391 (to A.A. Navarini and O. Boyman), a grant of the National Psoriasis Foundation (to O. Boyman), a grant of University of Zurich (to O. Boyman), and a grant of Fondation pour la Recherche Médical (to G. Bouchaud).
The authors have no competing financial interests.
Footnotes
Abbreviations used:
- BMDC
- BM-derived DC
- DT
- diphtheria toxin
- DTR
- DT receptor
- γc
- common γ chain
- IMQ
- imiquimod
- MMP
- matrix metalloproteinase
- PASI
- psoriasis area and severity index
- sIL-15Rα
- soluble IL-15Rα
References
- Barker J.N., Mitra R.S., Griffiths C.E., Dixit V.M., Nickoloff B.J. 1991. Keratinocytes as initiators of inflammation. Lancet. 337:211–214 10.1016/0140-6736(91)92168-2 [DOI] [PubMed] [Google Scholar]
- Baslund B., Tvede N., Danneskiold-Samsoe B., Larsson P., Panayi G., Petersen J., Petersen L.J., Beurskens F.J., Schuurman J., van de Winkel J.G., et al. 2005. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 52:2686–2692 10.1002/art.21249 [DOI] [PubMed] [Google Scholar]
- Bouchaud G., Garrigue-Antar L., Solé V., Quéméner A., Boublik Y., Mortier E., Perdreau H., Jacques Y., Plet A. 2008. The exon-3-encoded domain of IL-15ralpha contributes to IL-15 high-affinity binding and is crucial for the IL-15 antagonistic effect of soluble IL-15Ralpha. J. Mol. Biol. 382:1–12 10.1016/j.jmb.2008.07.019 [DOI] [PubMed] [Google Scholar]
- Bouchaud G., Mortier E., Flamant M., Barbieux I., Plet A., Galmiche J.P., Jacques Y., Bourreille A. 2010. Interleukin-15 and its soluble receptor mediate the response to infliximab in patients with Crohn’s disease. Gastroenterology. 138:2378–2387 10.1053/j.gastro.2010.02.044 [DOI] [PubMed] [Google Scholar]
- Boyman O., Hefti H.P., Conrad C., Nickoloff B.J., Suter M., Nestle F.O. 2004. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J. Exp. Med. 199:731–736 10.1084/jem.20031482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O., Conrad C., Tonel G., Gilliet M., Nestle F.O. 2007. The pathogenic role of tissue-resident immune cells in psoriasis. Trends Immunol. 28:51–57 10.1016/j.it.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Boyman O., Krieg C., Homann D., Sprent J. 2012. Homeostatic maintenance of T cells and natural killer cells. Cell. Mol. Life Sci. 69:1597–1608 10.1007/s00018-012-0968-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett P.R., Koka R., Chien M., Chai S., Boone D.L., Ma A. 2004. Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J. Exp. Med. 200:825–834 10.1084/jem.20041389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Shen X., Ding C., Qi C., Li K., Li X., Jala V.R., Zhang H.G., Wang T., Zheng J., Yan J. 2011. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 35:596–610 10.1016/j.immuni.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari U., Romano P., Mulcahy L.D., Dooley L.T., Baker D.G., Gottlieb A.B. 2001. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 357:1842–1847 10.1016/S0140-6736(00)04954-0 [DOI] [PubMed] [Google Scholar]
- Conrad C., Boyman O., Tonel G., Tun-Kyi A., Laggner U., de Fougerolles A., Kotelianski V., Gardner H., Nestle F.O. 2007. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat. Med. 13:836–842 10.1038/nm1605 [DOI] [PubMed] [Google Scholar]
- Di Meglio P., Perera G.K., Nestle F.O. 2011. The multitasking organ: recent insights into skin immune function. Immunity. 35:857–869 10.1016/j.immuni.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Dubois S., Magrangeas F., Lehours P., Raher S., Bernard J., Boisteau O., Leroy S., Minvielle S., Godard A., Jacques Y. 1999. Natural splicing of exon 2 of human interleukin-15 receptor alpha-chain mRNA results in a shortened form with a distinct pattern of expression. J. Biol. Chem. 274:26978–26984 10.1074/jbc.274.38.26978 [DOI] [PubMed] [Google Scholar]
- Dubois S., Mariner J., Waldmann T.A., Tagaya Y. 2002. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 17:537–547 10.1016/S1074-7613(02)00429-6 [DOI] [PubMed] [Google Scholar]
- Gilliet M., Conrad C., Geiges M., Cozzio A., Thürlimann W., Burg G., Nestle F.O., Dummer R. 2004. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch. Dermatol. 140:1490–1495 10.1001/archderm.140.12.1490 [DOI] [PubMed] [Google Scholar]
- Hoeve M.A., Savage N.D., de Boer T., Langenberg D.M., de Waal Malefyt R., Ottenhoff T.H., Verreck F.A. 2006. Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T cells. Eur. J. Immunol. 36:661–670 10.1002/eji.200535239 [DOI] [PubMed] [Google Scholar]
- Jung S., Unutmaz D., Wong P., Sano G., De los Santos K., Sparwasser T., Wu S., Vuthoori S., Ko K., Zavala F., et al. 2002. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 17:211–220 10.1016/S1074-7613(02)00365-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M.K., Glaccum M., Brown S.N., Butz E.A., Viney J.L., Embers M., Matsuki N., Charrier K., Sedger L., Willis C.R., et al. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15–deficient mice. J. Exp. Med. 191:771–780 10.1084/jem.191.5.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I.A., Moretto M., Wei X.Q., Williams M., Schwartzman J.D., Liew F.Y. 2002. Treatment with soluble interleukin-15Rα exacerbates intracellular parasitic infection by blocking the development of memory CD8+ T cell response. J. Exp. Med. 195:1463–1470 10.1084/jem.20011915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissenpfennig A., Henri S., Dubois B., Laplace-Builhé C., Perrin P., Romani N., Tripp C.H., Douillard P., Leserman L., Kaiserlian D., et al. 2005. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 22:643–654 10.1016/j.immuni.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Krieg C., Létourneau S., Pantaleo G., Boyman O. 2010. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc. Natl. Acad. Sci. USA. 107:11906–11911 10.1073/pnas.1002569107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J.G. 2012. Hiding under the skin: A welcome surprise in psoriasis. Nat. Med. 18:1750–1751 10.1038/nm.3025 [DOI] [PubMed] [Google Scholar]
- Laggner U., Di Meglio P., Perera G.K., Hundhausen C., Lacy K.E., Ali N., Smith C.H., Hayday A.C., Nickoloff B.J., Nestle F.O. 2011. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J. Immunol. 187:2783–2793 10.4049/jimmunol.1100804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R., Gregorio J., Facchinetti V., Chatterjee B., Wang Y.H., Homey B., Cao W., Wang Y.H., Su B., Nestle F.O., et al. 2007. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 449:564–569 10.1038/nature06116 [DOI] [PubMed] [Google Scholar]
- Leonardi C., Matheson R., Zachariae C., Cameron G., Li L., Edson-Heredia E., Braun D., Banerjee S. 2012. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N. Engl. J. Med. 366:1190–1199 10.1056/NEJMoa1109997 [DOI] [PubMed] [Google Scholar]
- Lodolce J.P., Boone D.L., Chai S., Swain R.E., Dassopoulos T., Trettin S., Ma A. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 9:669–676 10.1016/S1074-7613(00)80664-0 [DOI] [PubMed] [Google Scholar]
- Lowes M.A., Bowcock A.M., Krueger J.G. 2007. Pathogenesis and therapy of psoriasis. Nature. 445:866–873 10.1038/nature05663 [DOI] [PubMed] [Google Scholar]
- McInnes I.B., Liew F.Y. 1998. Interleukin 15: a proinflammatory role in rheumatoid arthritis synovitis. Immunol. Today. 19:75–79 10.1016/S0167-5699(97)01205-X [DOI] [PubMed] [Google Scholar]
- Mease P.J., Goffe B.S., Metz J., VanderStoep A., Finck B., Burge D.J. 2000. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 356:385–390 10.1016/S0140-6736(00)02530-7 [DOI] [PubMed] [Google Scholar]
- Merad M., Manz M.G., Karsunky H., Wagers A., Peters W., Charo I., Weissman I.L., Cyster J.G., Engleman E.G. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135–1141 10.1038/ni852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle F.O., Conrad C., Tun-Kyi A., Homey B., Gombert M., Boyman O., Burg G., Liu Y.J., Gilliet M. 2005. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 202:135–143 10.1084/jem.20050500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle F.O., Kaplan D.H., Barker J. 2009. Psoriasis. N. Engl. J. Med. 361:496–509 10.1056/NEJMra0804595 [DOI] [PubMed] [Google Scholar]
- Nguyen K.B., Salazar-Mather T.P., Dalod M.Y., Van Deusen J.B., Wei X.Q., Liew F.Y., Caligiuri M.A., Durbin J.E., Biron C.A. 2002. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 169:4279–4287 [DOI] [PubMed] [Google Scholar]
- Pantelyushin S., Haak S., Ingold B., Kulig P., Heppner F.L., Navarini A.A., Becher B. 2012. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 122:2252–2256 10.1172/JCI61862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp K.A., Leonardi C., Menter A., Ortonne J.P., Krueger J.G., Kricorian G., Aras G., Li J., Russell C.B., Thompson E.H., Baumgartner S. 2012. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N. Engl. J. Med. 366:1181–1189 10.1056/NEJMoa1109017 [DOI] [PubMed] [Google Scholar]
- Rubinstein M.P., Kovar M., Purton J.F., Cho J.H., Boyman O., Surh C.D., Sprent J. 2006. Converting IL-15 to a superagonist by binding to soluble IL-15Ralpha. Proc. Natl. Acad. Sci. USA. 103:9166–9171 10.1073/pnas.0600240103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchatz H., Leung B.P., Wei X.Q., McInnes I.B., Liew F.Y. 1998. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J. Immunol. 160:5654–5660 [PubMed] [Google Scholar]
- Rückert R., Asadullah K., Seifert M., Budagian V.M., Arnold R., Trombotto C., Paus R., Bulfone-Paus S. 2000. Inhibition of keratinocyte apoptosis by IL-15: a new parameter in the pathogenesis of psoriasis? J. Immunol. 165:2240–2250 [DOI] [PubMed] [Google Scholar]
- Schluns K.S., Klonowski K.D., Lefrançois L. 2004. Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells. Blood. 103:988–994 10.1182/blood-2003-08-2814 [DOI] [PubMed] [Google Scholar]
- Stoklasek T.A., Schluns K.S., Lefrançois L. 2006. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 177:6072–6080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortola L., Rosenwald E., Abel B., Blumberg H., Schäfer M., Coyle A.J., Renauld J.C., Werner S., Kisielow J., Kopf M. 2012. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J. Clin. Invest. 122:3965–3976 10.1172/JCI63451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L., Mourits S., Voerman J.S., Kant M., Boon L., Laman J.D., Cornelissen F., Mus A.M., Florencia E., Prens E.P., Lubberts E. 2009. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182:5836–5845 10.4049/jimmunol.0802999 [DOI] [PubMed] [Google Scholar]
- Villadsen L.S., Schuurman J., Beurskens F., Dam T.N., Dagnaes-Hansen F., Skov L., Rygaard J., Voorhorst-Ogink M.M., Gerritsen A.F., van Dijk M.A., et al. 2003. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J. Clin. Invest. 112:1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T.A., Tagaya Y. 1999. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 17:19–49 10.1146/annurev.immunol.17.1.19 [DOI] [PubMed] [Google Scholar]