Abstract
Background
Resectional techniques are the established method of posterior mitral valve leaflet repair for degenerative disease; however, use of neochordae in a robotically assisted approach is gaining acceptance because of its versatility for difficult multi-segment disease. The purposes of this study were to compare the versatility, safety, and effectiveness of neochordal vs. resectional techniques for robotic posterior mitral leaflet repair.
Methods
From 12/2007 to 7/2010, 334 patients underwent robotic posterior mitral leaflet repair for degenerative disease by a resectional (n=248) or neochordal (n=86) technique. Outcomes were compared unadjusted and after propensity score matching.
Results
Neochordae were more likely to be used than resection in patients with two (28% vs. 13%, P=.002) or three (3.7% vs. 0.87%, P=.08) diseased posterior leaflet segments. Three resection patients (0.98%) but no neochordal patient required reoperation for hemodynamically significant systolic anterior motion (SAM). Residual mitral regurgitation (MR) at hospital discharge was similar for matched neochordal vs. resection patients (P=.14) (MR 0+, 82% vs. 89%; MR 1+, 14% vs. 8.2%; MR 2+, 2.3% vs. 2.6%; one neochordal patient had 4+ MR and was reoperated). Among matched patients, postoperative mortality and morbidity were similarly low.
Conclusion
Compared with a resectional technique, robotic posterior mitral leaflet repair with neochordae is associated with shorter operative times and no occurrence of SAM. The versatility, effectiveness, and safety of this repair make it a good choice for patients with advanced multi-segment disease.
Keywords: mitral regurgitation, mitral systolic anterior motion, minimally invasive, surgery, outcomes
INTRODUCTION
Resectional techniques have been the established method of posterior mitral leaflet repair for degenerative disease; however, repair in patients with multi-segment disease and those with extensive prolapse and large scallops is more challenging because complete resection of all prolapsed tissue could compromise the integrity of repair and valve function. Therefore, neochordae that allow mitral valve (MV) repair without resection are an attractive alternative [1-4]. Both randomized and observational clinical studies have shown comparable results in repairs using neochordae [5,6], but concerns have been raised regarding technical complexity, operative time, reproducibility, and durability of the neochordal technique.
The purposes of this study were to compare the versatility, effectiveness, and safety of neochordal vs. resectional techniques for robotic posterior mitral leaflet repair in degenerative disease.
PATIENTS AND METHODS
Study Population
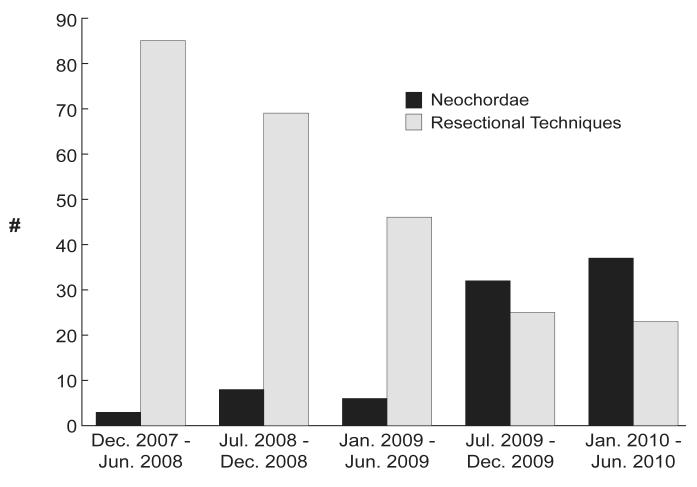
From December 2007 (which marks the month of the first robotic use of neochordae in our robotic practice) to July 2010, 443 patients underwent robotic MV repair for degenerative disease. Patients were excluded if neither neochordal nor resectional techniques were used (n=62), if an anterior leaflet procedure was performed (n=46), and if both neochordal and resectional techniques were used for the repair (n=1). Of the 334 remaining patients, 248 (74%) had intended repair with resectional techniques and 86 (26%) with neochordae. Over time, the proportion of repairs using neochordae has increased (Figure 1). All patients received flexible anuloplasty bands with their repairs.
Figure 1.
Temporal trend of neochordae use.
Surgical Technique
Neochordal
For neochordal implantation, we use No. 5-0 polytetrafluoroethylene (PTFE) monofilament suture. A dynamic left atrial retractor is advanced into the left ventricle to lift up the anterior leaflet of the MV, allowing excellent exposure of the subvalvar apparatus (Figure 2). One arm of the PTFE suture is passed through the fibrous tip of the papillary muscle two or three times, then twice through the free edge of the corresponding prolapsed segment of posterior leaflet [7]. The second arm is then passed twice through the free edge of the prolapsed leaflet. Length of the chordae is adjusted based on height of the nearest non-prolapsed posterior leaflet segment [1]. The suture is tied intracorporeally with robotic instrumentation. The repair is completed with an anuloplasty band sutured in a running fashion, as previously described [8].
Figure 2.
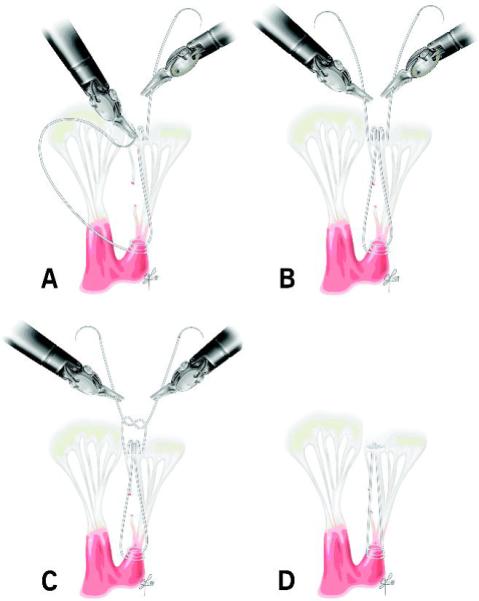
Posterior neochord implantation: One arm of suture is passed through the fibrous papillary muscle tip two to three times and twice through the free edge of the prolapsed segment. The second arm is passed twice through the free edge of the prolapsed leaflet. The neochord’s length is adjusted to the height of the nearest non-prolapsed posterior leaflet segment, and the suture is tied. A, B: Figure-of-eight neochordal implantation. C: Chordal height adjustment. D: Completed neochord.
Resectional
In resectional techniques, MV repair is performed using triangular or quadrangular resection of the prolapsed segment, as previously described [9,10].
Data
Clinical data were obtained from Cleveland Clinic’s Cardiovascular Information Registry, a prospective database updated concurrently with patient care. Echocardiography data were obtained from the Cleveland Clinic echocardiography database. Operative details (which segments were diseased, use of neochordal or resectional methods, number of intraoperative repair attempts—meaning that the patient was weaned from cardiopulmonary bypass [CPB] and found by intraoperative transesophageal echocardiography (TEE) to have important residual MR, necessitating return to CPB to address it—and procedures performed during these additional intraoperative repair attempts) were obtained by review of each patient’s medical record. Use of these data for research was approved by the Institutional Review Board, with patient consent waived.
Endpoints
Versatility was assessed by the frequency with which neochordae were used for multi-segment disease, number and outcomes of intraoperative repair attempts required to achieve satisfactory repair, conversion to sternotomy, and operative times.
Effectiveness was assessed by residual mitral regurgitation (MR), conversion of intended repair to valve replacement, mitral valve reoperation before hospital discharge, and presence of systolic anterior motion (SAM).
Safety was assessed by in-hospital morbidity and mortality as defined by the Society of Thoracic Surgeons (STS) National Database (http://www.ctsnet.org/file/rptDataSpecifications252_1_ForVendorsPGS.pdf)
Statistical Analysis
Although patient characteristics were generally similar between the two groups (Table 1), we used matching to adjust for remaining differences, revealed in Figures 3 and 4. Using multivariable logistic regression of preoperative variables (Table 1), we identified the preoperative factors associated with having repair using neochordae (parsimonious model). Variable selection used bagging [11]. For this, 1,000 bootstrap samples were analyzed by automated forward stepwise selection, with P≤.05 to retain variables. Variables appearing in 50% or more of the analyses were retained (aggregation step).
Table 1.
Demographics, Valvar Pathology, and Comorbidity
| Unmatched Group (total n=334) |
Matched Group (total n=172) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Resection (n=248) |
Neochordae (n=86) |
Resection (n=86) |
Neochordae (n=86) |
|||||||
| No. (%) or | No. (%) or | No. (%) or | No. (%) or | |||||||
| Variable | na | Mean ± SD | na | Mean ± SD | P | na | Mean ± SD | na | Mean ± SD | P |
| Demographics | ||||||||||
| Femaleb | 248 | 47 (19) | 86 | 21 (24) | .3 | 86 | 17 (20) | 86 | 21 (24) | .5 |
| Age (y)b | 248 | 56 ± 9.6 | 86 | 57 ± 10 | .4 | 86 | 56 ± 9.4 | 86 | 57 ± 10 | .7 |
| Body mass index (kg•m−2)b | 246 | 26 ± 4.4 | 86 | 26 ± 3.4 | .8 | 85 | 26 ± 4.5 | 86 | 26 ± 3.4 | .9 |
| Valvar pathology | ||||||||||
| Mitral regurgitation gradeb | 242 | 82 | .5 | 83 | 82 | .6 | ||||
| 2+ | 1 (0.41) | 1 (1.2) | 0 (0) | 1 (1.2) | ||||||
| 3+ | 42 (17) | 11 (13) | 12 (14) | 11 (13) | ||||||
| 4+ | 199 (82) | 70 (85) | 71 (86) | 70 (85) | ||||||
| Tricuspid regurgitation grade | 236 | 79 | .8 | 81 | 79 | .9 | ||||
| None | 132 (56) | 45 (57) | 41 (51) | 45 (57) | ||||||
| 1+ | 74 (31) | 22 (28) | 27 (33) | 22 (28) | ||||||
| 2+ | 25 (11) | 11 (14) | 12 (15) | 11 (14) | ||||||
| 3+ | 5 (2.1) | 1 (1.3) | 1 (1.2) | 1 (1.3) | ||||||
| Segment P1 diseasedb | 227 | 25 (11) | 81 | 9 (11) | >.9 | 79 | 17 (22) | 81 | 9 (11) | .07 |
| Segment P2 diseasedb | 230 | 217 (94) | 81 | 79 (98) | .2 | 81 | 74 (91) | 81 | 79 (98) | .09 |
| Segment P3 diseasedb | 228 | 22 (9.6) | 80 | 22 (28) | <.0001 | 80 | 13 (16) | 80 | 22 (28) | .08 |
| Number of P segments diseasedb | 230 | 81 | .001 | 81 | 81 | .2 | ||||
| 1 | 198 (86) | 55 (68) | 58 (72) | 55 (68) | ||||||
| 2 | 30 (13) | 23 (28) | 23 (28) | 23 (28) | ||||||
| 3 | 2 (0.87) | 3 (3.7) | 0 (0) | 3 (3.7) | ||||||
| Left atrial diameter (cm) | 242 | 4.6 ± 0.72 | 81 | 4.5 ± 0.84 | .4 | 82 | 4.6 ± 0.66 | 81 | 4.5 ± 0.84 | .5 |
| Cardiac comorbidity | ||||||||||
| Atrial fibrillation/flutterb | 243 | 12 (4.9) | 82 | 5 (6.1) | .7 | 85 | 6 (7.1) | 82 | 5 (6.1) | .8 |
| Heart failure | 248 | 13 (5.2) | 86 | 2 (2.3) | .3 | 86 | 6 (7.0) | 86 | 2 (2.3) | .15 |
| NYHA functional classb | 223 | 74 | .14 | 76 | 74 | .9 | ||||
| I | 111 (50) | 48 (65) | 47 (62) | 48 (65) | ||||||
| II | 90 (40) | 20 (27) | 21 (28) | 20 (27) | ||||||
| III | 21 (9.4) | 6 (8.1) | 8 (11) | 6 (8.1) | ||||||
| IV | 1 (0.45) | 0 (0) | — | — | ||||||
| Ejection fraction (%)b | 241 | 60 ± 4.1 | 82 | 59 ± 4.7 | .15 | 82 | 59 ± 4.2 | 82 | 59 ± 4.7 | >.9 |
| Noncardiac comorbidity | ||||||||||
| Peripheral arterial disease | 248 | 1 (0.4) | 86 | 0 (0) | .6 | 86 | 0 (0) | 86 | 0 (0) | — |
| Carotid disease | 248 | 14 (5.6) | 86 | 0 (0) | .02 | 86 | 4 (4.7) | 86 | 0 (0) | .04 |
| Stroke | 248 | 4 (1.6) | 86 | 0 (0) | .2 | 86 | 0 (0) | 86 | 0 (0) | — |
| Hypertensionb | 248 | 104 (42) | 86 | 33 (38) | .6 | 86 | 33 (38) | 86 | 33 (38) | >.9 |
| Pharmacologically treated diabetes |
248 | 1 (0.4) | 86 | 3 (3.5) | .02 | 86 | 1 (1.2) | 86 | 3 (3.5) | .3 |
| Smokingb | 248 | 90 (36) | 86 | 26 (30) | .3 | 86 | 25 (29) | 86 | 26 (30) | .9 |
| COPD | 248 | 9 (3.6) | 86 | 2 (2.3) | .6 | 86 | 2 (2.3) | 86 | 2 (2.3) | >.9 |
| Creatinine (mg•dL−1) | 248 | 0.98 ± 0.20 | 86 | 0.97 ± 0.19 | .4 | 86 | 0.96 ± 0.19 | 86 | 0.97 ± 0.19 | .9 |
| Blood urea nitrogen (mg•dL−1) | 248 | 17 ± 4.4 | 86 | 17 ± 4.4 | .7 | 86 | 18 ± 4.5 | 86 | 17 ± 4.4 | .9 |
Patients with data available.
Variables used in propensity model. In addition to these, we included preoperative hematocrit, triglycerides, and left ventricular end-diastolic volume and mass.
Key: COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association; SD, standard deviation.
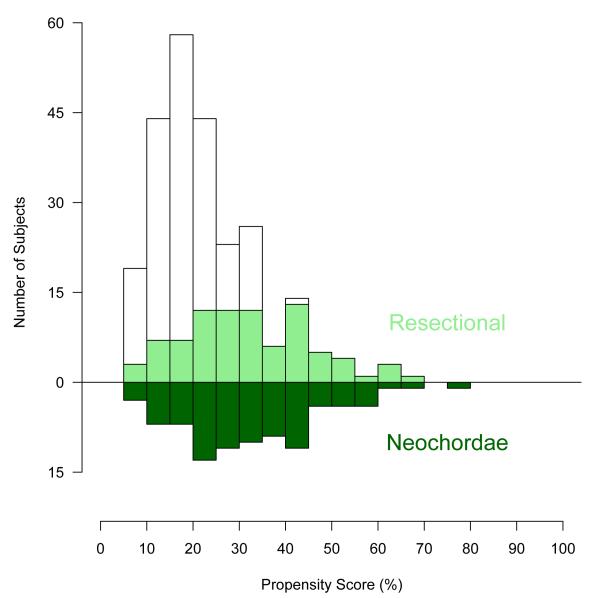
Figure 3.
Mirrored histogram of distribution of propensity scores for resectional (bars above zero line) and neochordal (bars below zero line) approaches. The darkened area represents matched patient pairs, showing that they cover the complete spectrum of cases.
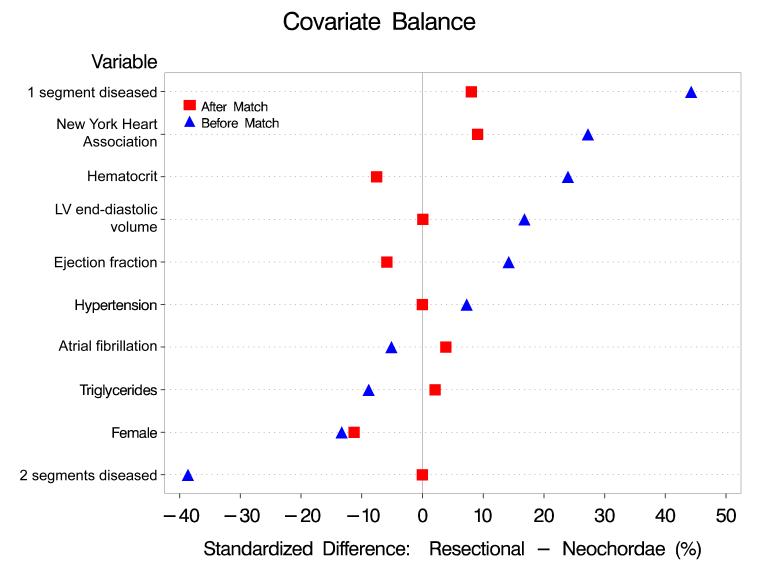
Figure 4.
Covariate balance for some selected variables before and after matching. Values on the horizontal axis represent the percent standardized differences between resectional and neochordal groups.
To this parsimonious model, we added variables representing groups of patient factors that might be related to unrecorded selection factors (semi-saturated propensity model) [12,13]. A propensity score was calculated for each patient by solving the saturated model for the probability of having a neochordal procedure. Using only the propensity score, all neochordal cases (100%) were matched to resectional cases using a greedy matching strategy [14]. This resulted in well-matched patients across the entire spectrum of propensity scores (see Figures 3 and 4).
Missing values
We used multiple imputation with a Markov Chain Monte Carlo technique to impute the missing covariate values where necessary [15]. Fivefold multiple imputation used PROC MI followed by PROC MI-ANALYZE (SAS v9.1; SAS, Inc., Cary, NC).
Presentation
Continuous variables are summarized by mean ± 1 standard deviation, and as 15th, 50th (median), and 85th percentiles when values are skewed. Comparisons were made using the Wilcoxon rank-sum test. Categorical data are summarized by frequencies and percentages. Comparisons were made using the chi-squared test or Fisher’s exact test when frequency was less than 5. All analyses were performed using SAS statistical software (SAS v9.1). Parametric estimates are accompanied by an asymmetric 68% confidence interval, comparable to ±1 standard error.
RESULTS
Versatility
Neochordae were used more frequently than resectional techniques in patients with two or three diseased posterior leaflet segments or a diseased P3 segment (Table 1). In matched and unmatched comparisons in both groups, a similar proportion of patients required multiple intraoperative attempts to achieve satisfactory repair (Table 2). One patient in the neochordae group required conversion to sternotomy. There were no conversions from repair to valve replacement. Anuloplasty band size was significantly larger in the neochordal group (Table 2).
Table 2.
Operative Details
| Unmatched Group (total n=334) |
Matched Group (total n=172) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Resection (n=248) |
Neochordae (n=86) |
Resection (n=86) |
Neochordae (n=86) |
|||||||
| No. (%) or | No. (%) or | No. (%) or | No. (%) or | |||||||
| Variable | na | Mean ± SD | na | Mean ± SD | P | na | Mean ± SD | na | Mean ± SD | P |
| Surgeon | ||||||||||
| Surgeon A | 248 | 119 (48) | 86 | 82 (95) | <.0001 | 86 | 48 (56) | 86 | 82 (95) | <.0001 |
| Neochordae use | ||||||||||
| Number of neochordae used | 248 | 80 | <.0001 | 86 | 80 | <.0001 | ||||
| None | 248 (100) | 0 (0) | 86 (100) | 0 (0) | ||||||
| 2 | 0 (0) | 41 (51) | 0 (0) | 41 (51) | ||||||
| 3 | 0 (0) | 33 (41) | 0 (0) | 33 (41) | ||||||
| 4 | 0 (0) | 4 (5) | 0 (0) | 4 (5) | ||||||
| 5 | 0 (0) | 2 (2.5) | 0 (0) | 2 (2.5) | ||||||
| Chordae to P1 | 248 | 0 (0) | 82 | 8 (9.8) | <.0001 | 86 | 0 (0) | 82 | 8 (9.8) | .003 |
| Chordae to P2 | 248 | 0 (0) | 82 | 80 (98) | <.0001 | 86 | 0 (0) | 82 | 80 (98) | <.0001 |
| Chordae to P3 | 248 | 0 (0) | 82 | 16 (20) | <.0001 | 86 | 0 (0) | 82 | 16 (20) | <.0001 |
| Additional components of repair | ||||||||||
| Anuloplasty band size (mm) | 246 | 86 | .0009 | 86 | 86 | .08 | ||||
| 28 | 0 (0) | 1 (1.2) | 0 (0) | 1 (1.2) | ||||||
| 30 | 3 (1.2) | 1 (1.2) | 1 (1.2) | 1 (1.2) | ||||||
| 32 | 3 (1.2) | 1 (1.2) | 0 (0) | 1 (1.2) | ||||||
| 33 | 0 (0) | 1 (1.2) | 0 (0) | 1 (1.2) | ||||||
| 34 | 47 (19) | 9 (10) | 16 (19) | 9 (10) | ||||||
| 35 | 0 (0) | 1 (1.2) | 0 (0) | 1 (1.2) | ||||||
| 36 | 120 (49) | 31 (36) | 40 (47) | 31 (36) | ||||||
| 38 | 73 (30) | 38 (44) | 28 (33) | 38 (44) | ||||||
| 40 | 0 (0) | 3 (3.5) | 0 (0) | 3 (3.5) | ||||||
| Commisuroplasty | 248 | 7 (2.8) | 86 | 8 (9.3) | .01 | 86 | 4 (4.7) | 86 | 8 (9.3) | .2 |
| Cleft closure | 248 | 33 (13) | 84 | 15 (18) | .3 | 86 | 13 (15) | 84 | 15 (18) | .6 |
| Folding valvuloplasty | 248 | 26 (10) | 86 | 0 (0) | .002 | 86 | 9 (10) | 86 | 0 (0) | .002 |
| Initial edge-to-edge repair | 248 | 2 (0.81) | 86 | 2 (2.3) | .3 | 86 | 2 (2.3) | 86 | 2 (2.3) | >.9 |
| Any atrial fibrillation procedure | 248 | 24 (9.7) | 86 | 7 (8.1) | .7 | 86 | 9 (10) | 86 | 7 (8.1) | .6 |
| ASD/PFO suture closure | 248 | 27 (11) | 86 | 13 (15) | .3 | 86 | 9 (10) | 86 | 13 (15) | .4 |
| Number of intraoperative repair attempts required to achieve satisfactory repair |
248 | 86 | .5 | 86 | >.9 | |||||
| 1 | 229 (92) | 78 (91) | 78 (91) | 78 (91) | ||||||
| 2 | 17 (6.8) | 8 (9.3) | 8 (9.3) | 8 (9.3) | ||||||
| 3 | 2 (0.8) | 0 (0) | 0 (0) | 0 (0) | ||||||
| Edge-to-edge repair for post-repair SAM (same operation) |
248 | 6 (2.4) | 86 | 1 (1.2) | .5 | 86 | 1 (1.2) | 86 | 1 (1.2) | >.9 |
| Edge-to-edge repair for post-repair remaining MR (same operation) |
248 | 1 (0.4) | 86 | 1 (1.2) | .4 | 86 | 1 (1.2) | 86 | 1 (1.2) | >.9 |
| Times | ||||||||||
| Myocardial ischemia (min) | 248 | 86 ± 28 | 86 | 79 ± 16 | .04 | 86 | 85 ± 31 | 86 | 79 ± 16 | .3 |
| Cardiopulmonary bypass (min) | 248 | 119 ± 34 | 86 | 106 ± 22 | .002 | 86 | 118 ± 35 | 86 | 106 ± 22 | .06 |
Patients with data available.
Key: ASD/PFO, atrial septal defect/patent foramen ovale; MR, mitral regurgitation; SAM, systolic anterior motion; SD, standard deviation.
Myocardial ischemic time was shorter for the neochordal group in unmatched comparison, and this difference remained but was statistically insignificant after matching, largely due to decreased population in the matched comparison (Table 2). Cardiopulmonary bypass time was shorter for the neochordal group in both unmatched and matched comparisons (see Table 2).
Effectiveness
All patients had successful repair, and none required MV replacement. Degree (0-4+) of residual MR preceding discharge or MV reoperation was similar after neochordal and resectional repair (Table 3). Postoperative SAM occurred in three patients undergoing resection in the unmatched population (Table 4). One patient in the neochordal group required robotically assisted reoperation for 4+ residual MR due to a linear tear in the anterior mitral leaflet, likely caused by the dynamic left atrial retractor. Four patients who underwent repair with resectional techniques required reoperation early after repair through a complete sternotomy: three for hemodynamically significant left ventricular outflow obstruction due to SAM and one for acute occlusion of the proximal left anterior descending coronary artery, apparently from embolic material in an otherwise normal artery, requiring emergency coronary artery bypass grafting.
Table 3.
Effectiveness
| Unmatched Group (total n=334) |
Matched Group (total n=172) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Resection (n=248) |
Neochordae (n=86) |
Resection (n=86) |
Neochordae (n=86) |
|||||||
| No. (%) or | No. (%) or | No. (%) or | No. (%) or | |||||||
| Variable | na | Mean ± SD | na | Mean ± SD | P | na | Mean ± SD | na | Mean ± SD | P |
| Residual MR | ||||||||||
| Residual MR degree: preceding discharge or MV reoperation |
219 | 76 | .14 | 72 | 76 | .5 | ||||
| 0 | 196 (89) | 62 (82) | 64 (89) | 62 (82) | ||||||
| 1+ | 18 (8.2) | 11 (14) | 6 (8.3) | 11 (14) | ||||||
| 2+ | 5 (2.3) | 2 (2.6) | 2 (2.8) | 2 (2.6) | ||||||
| 3+ | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||
| 4+ | 0 (0) | 1 (1.3) | 0 (0) | 1 (1.3) | ||||||
| Systolic Anterior Motion | ||||||||||
| SAM detected postoperatively after initial repair |
248 | 3 (1.2) | 86 | 0 (0) | .3 | 86 | 0 (0) | 86 | 0 (0) | __ |
| MV Reoperation | ||||||||||
| MV reoperation performed during same hospitalization |
248 | 4 (1.6) | 86 | 1 (1.2) | .8 | 86 | 1 (1.2) | 86 | 1 (1.2) | >.9 |
Patients with data available.
Key: MR, mitral regurgitation; MV, mitral valve; SAM, systolic anterior motion; SD, standard deviation.
Table 4.
Outcomes of Patients Requiring Multiple Intraoperative Repair Attempts Compared with Successful Initial Repair
| Unmatched Group (total n=334) |
|||||
|---|---|---|---|---|---|
| Multiple Intraoperative Repair Attempts (n=27) |
Successful Initial Repair (n=307) |
||||
| No. (%) or | No. (%) or | ||||
| Variable | na | Mean ± SD | na | Mean ± SD | P |
| Operative mortality | 27 | 0 (0) | 307 | 0 (0) | — |
| Permanent stroke | 27 | 1 (3.7) | 307 | 8 (2.6) | .5 |
| Perioperative myocardial infarction | 27 | 0 (0) | 307 | 3 (0.98) | >.9 |
| Renal failure requiring dialysis | 27 | 0 (0) | 307 | 0 (0) | — |
| Prolonged ventilation (>24 hours) | 27 | 1 (3.7) | 307 | 10 (3.3) | >.9 |
| Septicemia | 27 | 0 (0) | 307 | 0 (0) | — |
| Atrial fibrillation | 25 | 7 (30) | 284 | 49 (20) | .2 |
| MV reoperation performed during the same hospitalization | 27 | 0 (0) | 307 | 5 (1.6) | >.9 |
| Systolic anterior motion after initial repair | 27 | 0 (0) | 307 | 3 (0.98) | >.9 |
| MV regurgitation degree: preceding discharge or MV reoperation | 25 | 270 | .01 | ||
| 0 | 17 (68) | 241 (89) | |||
| 1+ | 6 (24) | 23 (8.5) | |||
| 2+ | 2 (8) | 5 (1.9) | |||
| 3+ | 0 (0) | 0 (0) | |||
| 4+ | 0 (0) | 1 (0.37) | |||
| Myocardial ischemic time (min) | 27 | 125 ± 34 | 307 | 80 ± 22 | <.0001 |
| Cardiopulmonary bypass time (min) | 27 | 164 ± 37.5 | 307 | 111 ± 28 | <.0001 |
Patients with data available.
Key: MV, mitral valve; SD, standard deviation.
Further examination of the 27 patients requiring multiple intraoperative repair attempts shows that they were more likely to have a slightly higher degree of residual MR after surgery than those whose repairs were completed on the first attempt (Table 4). Operations requiring multiple intraoperative repair attempts also had significantly longer myocardial ischemic and CPB times (Table 4). No MV reoperations occurred in the group requiring multiple intraoperative repair attempts.
Safety
There was no in-hospital mortality or occurrence of aortic dissection in either group. Occurrence of postoperative morbid events (as defined by the STS National Database) was similar between unmatched and matched groups (Table 5.
Table 5.
Safety
| Unmatched Group (total n=334) |
Matched Group (total n=172) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Resection (n=248) |
Neochordae (n=86) |
Resection (n=86) |
Neochordae (n=86) |
|||||||
| Variable | na | No. (%) or Mean ± SD |
na | No. (%) or Mean ± SD |
P | na | No. (%) or Mean ± SD |
na | No. (%) or Mean ± SD |
P |
| Hospital mortality | 248 | 0 (0) | 86 | 0 (0) | — | 86 | 0 (0) | 86 | 0 (0) | — |
| Permanent stroke | 248 | 8 (3.2) | 86 | 1 (1.2) | .3 | 86 | 1 (1.2) | 86 | 1 (1.2) | >.9 |
| Perioperative myocardial infarction | 248 | 2 (0.81) | 86 | 1 (1.2) | .8 | 86 | 2 (2.3) | 86 | 1 (1.2) | .6 |
| Renal failure requiring dialysis | 248 | 0 (0) | 86 | 0 (0) | — | 86 | 0 (0) | 86 | 0 (0) | — |
| Prolonged ventilation (>24 hours) | 248 | 9 (3.6) | 86 | 2 (2.3) | .6 | 86 | 1 (1.2) | 86 | 2 (2.3) | .6 |
| Septicemia | 248 | 0 (0) | 86 | 0 (0) | — | 86 | 0 (0) | 86 | 0 (0) | — |
| Atrial fibrillation (new onset)b | 232 | 37 (16) | 77 | 19 (25) | .08 | 79 | 13 (16) | 77 | 19 (25) | .2 |
Patients with data available.
Patients with preoperative atrial fibrillation are not at risk and hence are excluded from number of patients with data available (n=16).
Key: SD, standard deviation.
COMMENT
Principal Findings
We found that robotic posterior mitral leaflet repair using neochordae is a versatile technique, particularly for multi-segment disease for which resectional techniques have limited potential. The technique is as effective and safe as resectional methods.
The aim of MV repair is to restore the morphology and function of the native valve. The advantage of MV repair using neochordae is in restoring valve function without decreasing orifice size and creating a transvalvar gradient, as is the occasional case when resectional techniques are used [6]. Neochordal techniques decrease the potential for postoperative SAM by moving the leaflet coaptation point posteriorly, resulting in relative restriction of the posterior leaflet. This was substantiated by observing postoperative SAM only in the resectional group. All patients in the resected group had no or only mild SAM at the end of the operation, documented by intraoperative TEE. Delayed occurrence of SAM early postoperatively most likely reflects decreased left ventricular size and improved left ventricular contractility.
Although posterior MV repair with neochordae has been viewed as technically challenging, we found that operative times in neochordal procedures using the robotic approach were shorter. This can be explained by superior exposure and visualization of the subvalvar MV apparatus by robotic technology, as well as superior handling of robotic instruments. Furthermore, we simplified the chordal implantation technique by using a figure-of-eight suture anchoring into the tip of the papillary muscles, thereby reducing the time-consuming placement of pledget-reinforced anchoring sutures [1].
This study also addressed outcomes of patients requiring multiple intraoperative attempts to achieve MV repair. Nevertheless, these patients all left the hospital with a repair rather than replacement, although with somewhat more residual MR.
Limitations
This is a single-institution clinical study with analysis of outcomes limited to hospital course. Since the Cleveland Clinic first used the robotic approach in 2006, we have had a high volume of robotically assisted MV repairs for posterior leaflet disease. This type of MV operation was reflective of surgeon preference (primarily one surgeon), not standardized and not randomized. To mitigate these limitations, we have used propensity matching for fair comparison with a contemporaneous group of resectional patients. We have used standard Carpentier definitions [7,16] of mitral valve pathology, although we recognize the morphologic heterogeneity of degenerative MV disease [17].
Conclusions
Compared with a resectional technique, robotic posterior mitral leaflet repair with neochordae is associated with shorter operative times and no occurrence of SAM. The versatility, effectiveness, and safety of this repair make it a good choice for patients with advanced multi-segment disease.
ACKNOWLEDGMENTS
This study was supported in part by the Kenneth Gee and Paula Shaw, PhD, Chair in Heart Research, held by Dr. Blackstone; and the Peter Boyle Fund for Robotic Cardiac Surgery Research and Donna and Ken Lewis Endowed Chair held by Dr. Mihaljevic. Gregory Pattakos, MD, MS, is a National Heart, Lung and Blood Institute Clinical Research Scholar of the Cardiothoracic Surgical Trials Network, and his master of science in clinical research has been funded by NIH grant 1U01HL088955-01. No sponsor had direct involvement of any kind in this research study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.David TE, Bos J, Rakowski H. Mitral valve repair by replacement of chordae tendineae with polytetrafluoroethylene sutures. J Thorac Cardiovasc Surg. 1991;101:495–501. [PubMed] [Google Scholar]
- 2.von Oppell UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann Thorac Surg. 2000;70:2166–8. doi: 10.1016/s0003-4975(00)02047-6. [DOI] [PubMed] [Google Scholar]
- 3.Kuntze T, Borger MA, Falk V, et al. Early and mid-term results of mitral valve repair using premeasured Gore-Tex loops (‘loop technique’) Eur J Cardiothorac Surg. 2008;33:566–72. doi: 10.1016/j.ejcts.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Seeburger J, Kuntze T, Mohr FW. Gore-tex chordoplasty in degenerative mitral valve repair. Semin Thorac Cardiovasc Surg. 2007;19:111–5. doi: 10.1053/j.semtcvs.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Falk V, Seeburger J, Czesla M, et al. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Cardiovasc Surg. 2008;136:1205. doi: 10.1016/j.jtcvs.2008.07.028. discussion -6. [DOI] [PubMed] [Google Scholar]
- 6.Seeburger J, Falk V, Borger MA, et al. Chordae replacement versus resection for repair of isolated posterior mitral leaflet prolapse: a egalite. Ann Thorac Surg. 2009;87:1715–20. doi: 10.1016/j.athoracsur.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Filsoufi F, Carpentier A. Principles of reconstructive surgery in degenerative mitral valve disease. Semin Thorac Cardiovasc Surg. 2007;19:103–10. doi: 10.1053/j.semtcvs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Mihaljevic T, Jarrett CM, Gillinov AM, Blackstone EH. A novel running annuloplasty suture technique for robotically assisted mitral valve repair. J Thorac Cardiovasc Surg. 2010;139:1343–4. doi: 10.1016/j.jtcvs.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 9.George KM, Mihaljevic T, Gillinov AM. Triangular resection for posterior mitral prolapse: rationale for a simpler repair. J Heart Valve Dis. 2009;18:119–21. [PubMed] [Google Scholar]
- 10.Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg. 2011;141:72–80. e1–4. doi: 10.1016/j.jtcvs.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Breiman L. Bagging predictors. Machine Learning. 1996;24:123–40. [Google Scholar]
- 12.Blackstone EH. Comparing apples and oranges. J Thorac Cardiovasc Surg. 2002;123:8–15. doi: 10.1067/mtc.2002.120329. [DOI] [PubMed] [Google Scholar]
- 13.Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med. 2007;26:20–36. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 14.Bergstralh EJ, Konsanke JL. Technical report No. 56. Department of Health Science Research; Rochester, MN: Mayo Clinic: 1995. Computerized matching of cases to controls. [Google Scholar]
- 15.Rubin DB. Multiple imputation for non-response in surveys. Wiley; New York: 1987. [Google Scholar]
- 16.Carpentier AF, Lessana A, Relland JY, et al. The "physio-ring": an advanced concept in mitral valve annuloplasty. Ann Thorac Surg. 1995;60:1177–85. doi: 10.1016/0003-4975(95)00753-8. discussion 85-6. [DOI] [PubMed] [Google Scholar]
- 17.Adams DH, Rosenhek R, Falk V. Degenerative mitral valve regurgitation: best practice revolution. Eur Heart J. 2010;31:1958–66. doi: 10.1093/eurheartj/ehq222. [DOI] [PMC free article] [PubMed] [Google Scholar]