Abstract
Purpose
To investigate the gliding ability and mechanical properties of decellularized intrasynovial tendons without and with surface modification designed to reduce gliding resistance.
Methods
Thirty-three canine flexor digitorum profundus tendons were randomly assigned to one of 3 groups: untreated fresh tendons, to serve as a control; tendons decellularized with trypsin and Triton X-100; and tendons decellularized as in group 2 with surface modification using carbodiimide-derivatized hyaluronic acid and gelatin (cd- HA-gelatin). Tendons were subjected to cyclic friction testing for 1000 cycles with subsequent tensile stiffness testing. The surface roughness after 1000 cycles was qualitatively evaluated using scanning electron microscopy.
Results
The gliding resistance of the decellularized group was significantly higher than that of both the control and cd-HA-gelatin tendons (0.20N, 0.09N and 0.11N after the first cycle, 0.41N, 0.09N and 0.14N after 1000 cycles, respectively).Gliding resistance between the control and cd-HA-gelatin groups was not significantly different. The Young modulus was not significantly different between the 3 groups. The surfaces of the control and cd-HA-gelatin treated tendons appeared smooth after 1000 cycles, while those of the decellularized tendons appeared rougher under scanning electron microscopy observation.
Conclusions
Decellularization with trypsin and Triton X-100 did not change tendon stiffness. However, this treatment, while effective in removing cells, adversely altered the tendon surface, both in appearance and gliding resistance. Surface modification with cd- HA-gelatin improved the tendon surface smoothness and significantly decreased the gliding resistance.
Clinical Relevance
The combination of decellularization and surface modification may improve the function of tendon allografts when used clinically.
Keywords: Allograft, Decellularization, Flexor Tendon, Gliding Resistance
INTRODUCTION
Repair of intrasynovial flexor tendon injury in the fingers (zone II) remains a challenge for hand surgeons[1, 2]. New suture materials[3, 4], suture techniques[5, 6], and postoperative rehabilitation protocols[7] have improved clinical outcomes after primary repair.
Unfortunately, primary repair is not always possible, and, when performed, not always successful. In complex injuries, in situations where the primary repair cannot be performed, or if the primary repair fails, tendon grafts may be indicated to restore digit function. Currently, extrasynovial tendons, such as the palmaris longus, plantaris, and toe extensor tendons, are most commonly used as sources of tendon autografts because of their relative ease of harvest and availability[8, 9]. However, these donor tendons do not match the functional and anatomic characteristics of the intrasynovial flexor tendons that they are intended to replace[10]. Extrasynovial tendon grafts do not have specially adapted gliding surfaces, and grafting of extrasynovial tendons results in more adhesions and scarring than is typical of intrasynovial tendon grafts[11–13]. Additionally, while intrasynovial tendon autografts of suitable size and shape are readily available in animal models, they are rarely available clinically. Toe flexors have a much shorter intrasynovial segment than does a finger flexor, and the intrasynovial portion of a finger extensor at the wrist is not only shorter but not positioned at the tendon end. In addition, harvest of intrasynovial autografts often results in donor-site morbidity and prolonged operative time[14]. Finally, in cases of multiple tendon injuries, the need for intrasynovial tendon grafts might exceed the limited supply[15].
Intrasynovial allografts may prove to be valuable substitutes. Intrasynovial tendon allografts can be matched for size and shape to donor site needs, and, if harvested from the same anatomic location as the recipient site, can be assumed to have mechanical properties similar to those of the tendon being replaced. However, allografts may induce an immunologic reaction. To reduce immunogenicity, allografts are commonly processed to reduce the presence of donor cells within the transplanted tissue[16]. These processes may, however, adversely affect mechanical function[17].
Less is known about the effect of these treatments on gliding resistance, but previous studies have shown that treatment with trypsin does increase tendon gliding resistance[18]. Concern exists that allograft processing, which also uses trypsin, might have a similar effect. Fortunately, chemical modification of the tendon surface with carbodiimide-derivatized hyaluronic acid combined with gelatin (cd-HA gelatin) has been shown to reduce tendon gliding resistance in vitro[19, 20]. Such treatment can also reduce adhesion formation in animal models of tendon reconstruction in vivo[21].
This study investigated the gliding ability of decellularized intrasynovial tendons with and without surface modification with cd-HA gelatin. We had 2 hypotheses. First, decellularization of the intrasynovial flexor tendon with trypsin and Triton X-100 would not change the Young modulus of the tendon but would increase gliding resistance compared with normal intrasynovial flexor tendon. Second, surface modification with cd- HA-gelatin would improve the gliding resistance of decellularized intrasynovial flexor tendon.
MATERIALS AND METHODS
Flexor digitorum profundus (FDP) tendons were harvested post mortem from mixed-breed dogs killed for other Institutional Animal Care and Use Committee approved studies. These studies involved surgery on 1 forepaw only. The dogs were otherwise healthy, and no systemic drugs were being used at the time of death.
A total of 37 FDP tendons from the hindpaw digits of 5 dogs were randomly assigned to one of 3 groups untreated, unprocessed fresh tendons, to serve as a control group; tendons decellularized with trypsin and Triton X-100; and tendons decellularized as in group 2and then treated with carbodiimide-derivatized hyaluronic acid and gelatin (cd-HA (hyaluronic acid)-gelatin) surface modification to improve gliding resistance. In each group, 8 tendons were used for mechanical testing, and 3 tendons were used for scanning electron microscopy (SEM) evaluation. In addition, 4 tendons (3 decellularized tendons and 1 normal tendon) were studied histologically to assess cellularity after the decellularization treatment.
Flexor Tendon Harvest
Tendons were divided distally at the bony insertion and proximally just distal to the common FDP tendon, at which level the 4 digital FDP tendons fuse together. Respective digits were disarticulated and prepared for friction testing, with each tendon sliding against its own pulley. In order to compare the findings in fresh tendons as compared to processed allografts, in which freezing and thawing is part of the preparation, tendons in the normal group were assessed immediately after harvest. The remaining tendons were frozen at −80° C until further use.
Decellularization of Tendon
Frozen tendons were thawed at room temperature. They were then immersed in trypsin 0.05% / 0.53mM ethylenediaminetetraacetic acid for 24 hours at 37° C followed by Triton X-100 0.5% for 24 hours at room temperature[22, 23]. Decellularized tendon scaffolds were washed in phosphate buffered saline for 1 hour and stored at −80° C until biomechanical testing.
Treatment of Tendons with cd-HA-Gelatin
For the cd-HA-gelatin treatment group, the decellularized tendons were immersed in a solution of 1% sodium hyaluronate (95%; Acros, Geel, Belgium), 1% 1-ethyl 1–3-(3- dimethylaminopropyl) carbodiimide hydrochloride (EDC; Sigma Chemical, St. Louis, Missouri), 1% N-hydroxysuccinimide (NHS; Pierce Biotechnology, Rockford, Illinois), and 10% gelatin (from porcine skin; Sigma Chemical) in 0.1 M Mes 2- [morpholino]ethanesulfonic acid buffered saline solution (Sigma Chemical), pH 6.0, for 1 minute. After surface treatment, tendons were wrapped in aluminum foil for 10 minutes at 37° C for gelation and to maintain hydration until friction testing[20]. Excess reagent was removed with a saline wash, and frictional testing was then performed.
Measurement of Gliding Resistance
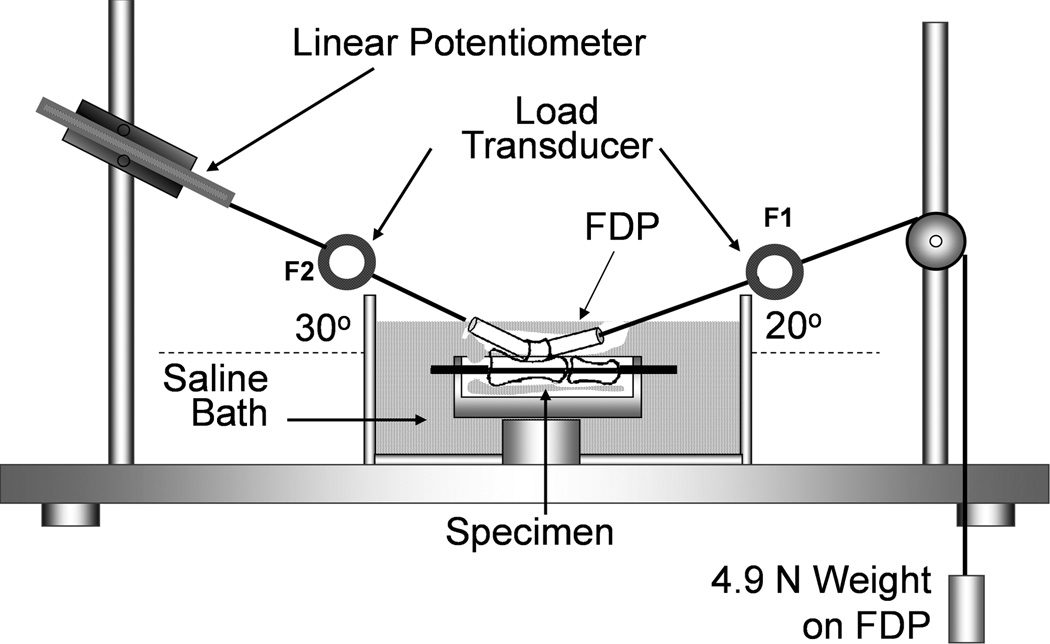
Gliding resistance between each FDP and its middle and proximal phalanx with intact proximal pulley was measured using previously described methods[24, 25]. The gliding resistance measurement system consisted of 1 mechanical actuator with a linear potentiometer, 2 tensile load transducers, and a mechanical pulley[26] (Fig. 1).
Figure 1.
Illustration of experimental set-up and testing apparatus for measurement of gliding resistance between the FDP tendon and A2 pulley. Permission will be requested from the publisher to reuse this figure. [26]
The tendon was pulled by the actuator against the weight at a rate of 2 mm/second. The excursion distance was set at 14 mm, which is the normal canine FDP excursion[27]. The gliding resistance was calculated as previously described[28]. The first 2 cycles of testing was used for preconditioning. The data were then recorded after every 50 cycles up to 500 cycles and then every 100 cycles up to 1000 cycles[29].
Biomechanical Assessment
After the gliding resistance testing was completed, the Young modulus was assessed. The tendon was mounted on a servo-hydraulic testing machine (MTS 858 Mini Bionix II, Eden Prairie, MN). The tendon region ranging between a point just proximal to the proximal pulley and 2 cm distal to that point was included in the gage length, with tissue adjacent to the region gripped in the clamps. Before testing, cross-section dimensions of each tendon were measured with a digital caliper (RS 232, Brantford, Canada). This caliper has a rated accuracy of 0.02mm and resolution of 0.01mm. Measurements were obtained at 3 different levels (proximal end, distal end, and midpoint). Area was calculated based on the assumption that the tendon cross-section was elliptical in shape. The cross-sectional area of the tendon was considered to be the average of the 3 levels. At the start of each test, the tendon was preconditioned with 10 cycles of loading from 10 to 50 N at a rate of 20 mm/min. Following the tenth cycle, the tendon was distracted until failure at a rate of 20 mm/min. Throughout testing, the tendon was kept moist with a saline mist. Force and displacement data were recorded at a sample rate of 20 Hz. The Young modulus was calculated from the slope of the linear region of the stress-strain curve.
Histology
To evaluate cellularity after our processing method, 3 full-length tendons were decellularized and examined histologically. One normal tendon was used as a control. These tendons were divided into thirds. Two cm segments of the distal, middle, and proximal regions of the tendon from each group were embedded in optical cutting temperature compound (Tissue-Tec, Sakura Finetek, Japan) and cut longitudinally at a thickness of 7 µm with a cryostat (Leica CM 1850, Wetzlar, Germany). The sections were stained with hematoxylin and eosin. The cellularity and gross appearance were noted at 200× in 8 randomly selected sections from each segment.
Scanning Electron Microscopy
Following 1000 cycles of gliding motion, 3 tendons per group were prepared for scanning electron microscopy (SEM). The selected tendons were first fixed using Trump fixative, a solution of buffered glutaraldehyde and formaldehyde, and then washed in phosphate-buffered saline solution. After dehydration in a graded ethanol series, the samples were critical-point dried and coated with gold-palladium alloy. They were then examined by SEM (Hitachi S-4700 FE-SEM; Japan) using secondary electron mode at 3 kV. The surface of the tendon subjected to friction contact was qualitatively evaluated for smoothness.
Statistical Analysis
The mean and standard deviation of the gliding resistance, cross-sectional area. and Young modulus were determined for each group. Gliding resistance, cross-sectional area and Young modulus were compared among the 3 groups with 1-way factorial analysis of variance. The Tukey-Kramer post hoc test for each pairwise comparison was performed if a significant difference was detected. The significance level was set at P<0.05 in all cases. The sample size of 8, used for our mechanical testing, was sufficient to detect large differences (effect size of 1 or more) with a power (1-β) of 0.80.
RESULTS
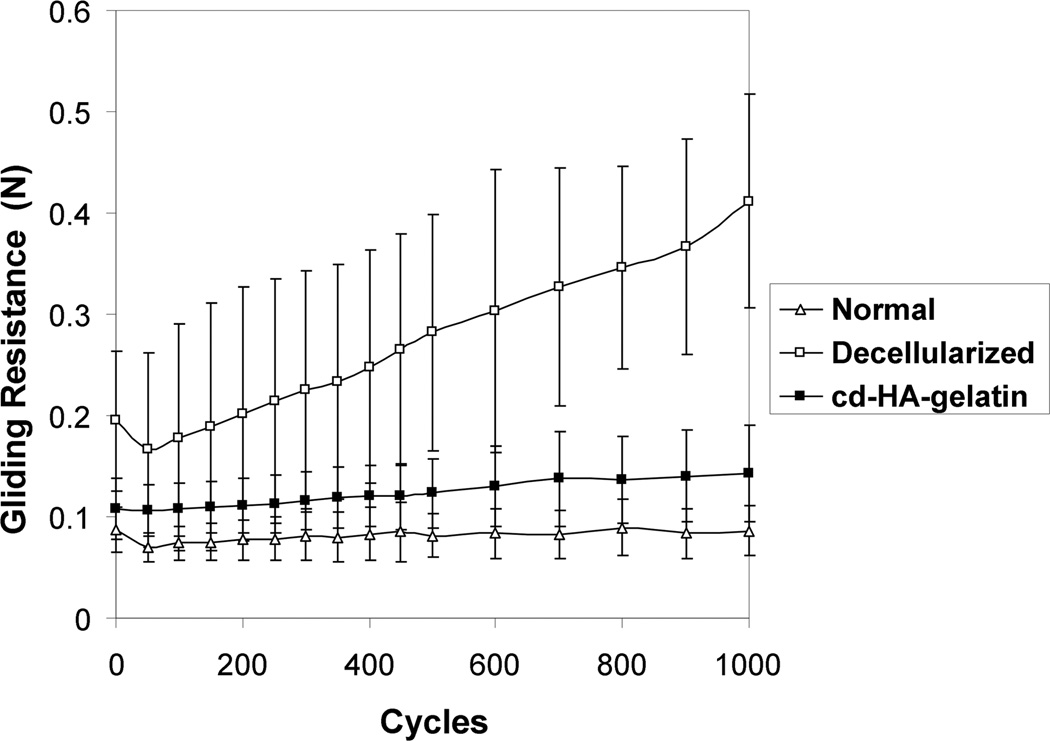
The gliding resistance of the decellularized tendon group gradually increased with cycle number. There was a significant difference in gliding resistance between the first and 1000th cycle. The gliding resistance of the normal tendon group and the cd-HA-gelatin group did not change significantly over 1000 cycles (Fig. 2 and 3).
Figure 2.
Mean gliding resistance of normal tendon, decellularized tendon without treatment and decellularized tendon treated with cd-HA-gelatin over 1000 cycles of tendon motion. Error bars represent standard deviation.
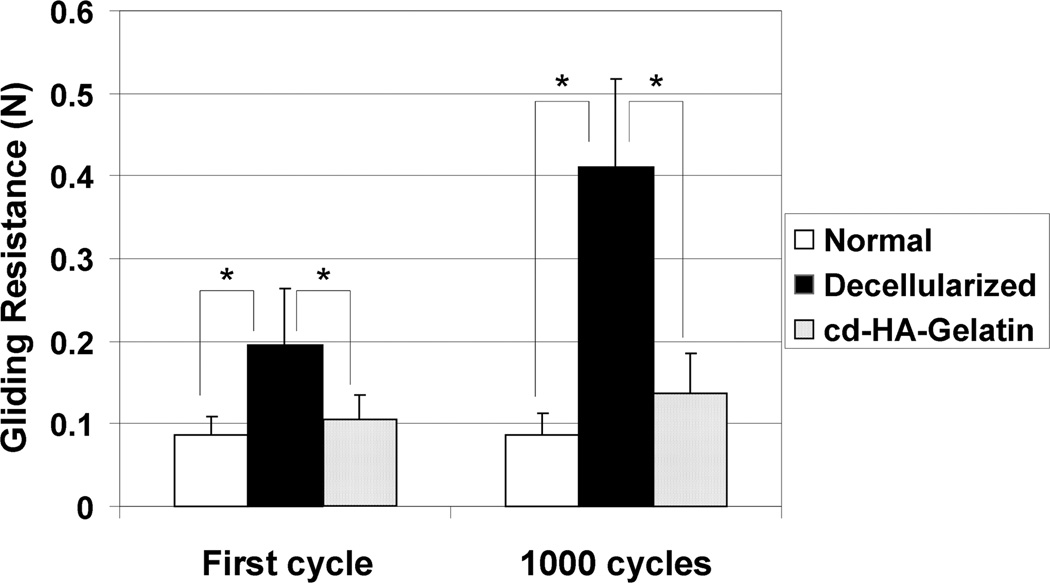
Figure 3.
Mean gliding resistance of first cycle and 1000th cycle for normal tendon, decellularized tendon without treatment and decellularized tendon treated with cd-HA-gelatin treatment. Error bars represent standard deviation. An asterisk indicates a significant difference (P<0.05).
The gliding resistance of the decellularized tendon group was significantly higher than that of the normal tendon group and of the cd-HA-gelatin group at each measurement point, (all P<0.05). In contrast, there was no significant difference between the gliding resistance of the normal tendons and decellularized tendons treated with cd- HA-gelatin at any measurement point.
The cross-sectional area of the decellularized tendon group was 3.98 (SD 0.65 mm2. This was significantly different than both the normal tendon group and the cd-HA-gelatin group (P<0.05) (3.27 mm2 (SD 0.45) and 2.91 mm2 (SD 0.22), respectively). All failures occurred at the clamp. Slippage of the tendon at the grip site occurred in 2 tendons in each group. All other tendons failed by breakage at the clamp.
The Young modulus was not significantly different between the 3 groups (P=0.39).
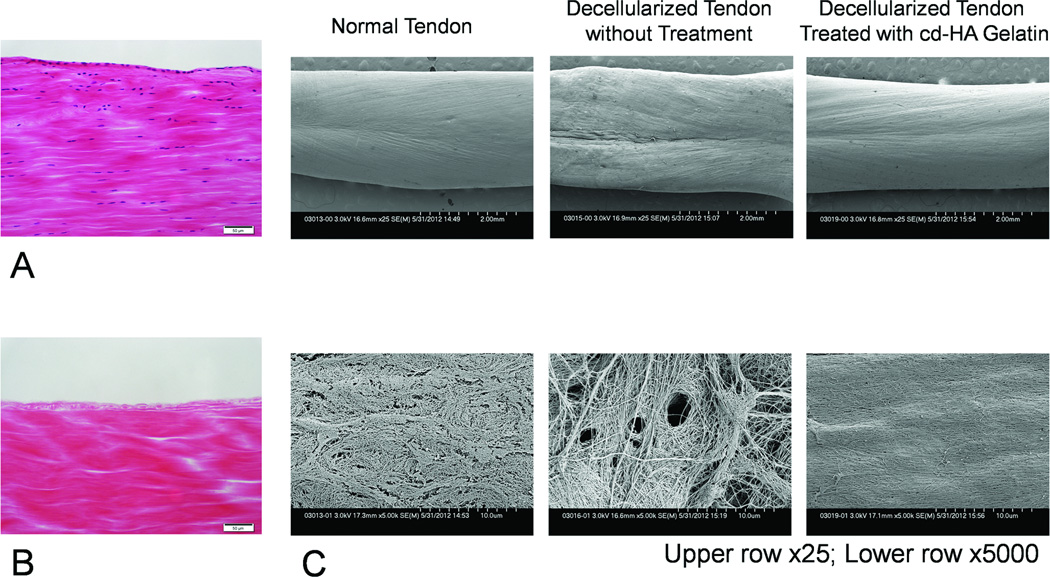
On histology at 200× magnification no cells were seen in any segment of the specimens treated with trypsin and Triton X-100 (Fig. 4, A and B), so quantitative cell counting was not done. Scanning electron microscopy showed that the surface of the normal tendon and cd-HA-gelatin modified tendon had a similar appearance after 1000 cycles of repetitive movement, but the surface of the decellularized tendon was more irregular in appearance (Fig. 4C).
Figure 4.
Histology of longitudinal sections of normal tendon (A) and decellularized tendon (B). No cells are visible in the decellularized tendon (hematoxylin and eosin staining 200×, scale bar: 50µm). (C) Scanning electron microscopic images of the tendon after 1000 cycles of repetitive motion. Untreated, decellularized tendon surfaces appeared rough, while decellularized tendon surfaces treated with cd-HA-gelatin appeared to be smoother, and similar to normal tendon.
DISCUSSION
The ideal intrasynovial tendon graft would be readily acquired, biocompatible, and would have mechanical properties resembling native intrasynovial tendons. The intrasynovial tendon allograft approaches this ideal, as it can be readily acquired, and, since it is itself an intrasynovial tendon, would have a similar size, shape, and mechanical properties as a native tendon; but its biocompatibility is compromised by its allogeneic nature. Processing techniques to reduce immunogenicity of allograft tissue includes freeze-thaw repetition[30], treatment with tri(n-butyl) phosphate [31], treatment with sodium dodecyl sulfate [32] or treatment with trypsin and Triton X-100[33]. We chose trypsin and Triton X for 2 reasons. Others have shown that Triton X results in less depletion of glycosaminoglycans than other treatment[16], and, in preliminary work for this project, we tried SDS and found that it did not fully remove cellular and nuclear debris.
In this study, treatment with trypsin and Triton X-100 did not change the Young modulus, consistent with the findings of Chong et al[33]. Retention of mechanical properties after decellularization is important for suture retention and load transfer.
Treatment with trypsin and Triton X-100 did increase the gliding resistance. Previous studies have shown that removal of lubricants on the tendon surface by trypsin treatment increase tendon gliding resistance[18]. This increase in gliding resistance is a potential clinical disadvantage, since higher gliding resistance is associated with increased adhesions in vivo animal studies[34–36]. Our data also showed that surface modification with cd-HA gelatin resulted in a smoother appearing surface and a significantly lower gliding resistance than with decellularized but untreated tendons.
There are several limitations to this study. First, this was not an in vivo study. In the in vivo environment, inflammation and cell repopulation might affect the surface morphology and tendon gliding ability differently. It is possible, for example, that the surface modification might inhibit recellularization, cell attachment, and proliferation. However, Chong et al. successfully reseeded tenocytes on decellularized tendon after a similar treatment[33], and others have shown that tenocytes in the host tendon migrate into surface-modified allografts with cd-HA–gelatin[35]. Therefore, this decellularization and surface modification should not inhibit graft incorporation or healing in vivo at the junction of the graft and host. Second, the accuracy of the manual caliper measurement of cross-sectional area was based on the assumption that the tendon had a relatively uniform elliptical shape. However, since we applied the same method to all tendons, we considered that the comparison among the groups was reliable. Third, we did not measure the maximum failure strength of the tendons. All testing was limited by failure occurring at the gripping site by either slipping or rupture. However, the Young modulus was reliably calculated despite this limitation. Fourth, we measured the tendon size after decellularization, so we do not know if the tendons were different or similar size before decellularization. If the decellularization process alters tendon diameter, this might affect factors such as fit beneath pulleys or the tensile properties of the tendon. We did not measure these factors in the current study. Finally, we did not perform immunohistochemical analysis to assess the presence of residual HA on the tendon surface after cyclic testing. However, a previous study showed that a thin layer of cd-HA remained on the surface of lyophilized tendons treated with cd-HA-gelatin after 1000 cycles of repetitive movement[20].
Further study will be required to investigate the efficacy of this surface modification technique in the treatment of decellularized tendon allografts in vivo.
Acknowledgement
This study was supported by grants from NIH/NIAMS (AR57745).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Amadio PC. What's new in hand surgery. J Bone Joint Surg Am. 2007;89(2):460–465. doi: 10.2106/JBJS.F.01448. [DOI] [PubMed] [Google Scholar]
- 2.Tang JB. Clinical outcomes associated with flexor tendon repair. Hand Clin. 2005;21(2):199–210. doi: 10.1016/j.hcl.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 3.McDonald E, Gordon JA, Buckley JM, Gordon L. Comparison of a new multifilament stainless steel suture with frequently used sutures for flexor tendon repair. J Hand Surg Am. 2011;36(6):1028–1034. doi: 10.1016/j.jhsa.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Miller B, Dodds SD, deMars A, Zagoreas N, Waitayawinyu T, Trumble TE. Flexor tendon repairs: the impact of fiberwire on grasping and locking core sutures. J Hand Surg Am. 2007;32(5):591–596. doi: 10.1016/j.jhsa.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Al-Qattan MM, Al-Rakan MA, Al-Hassan TS. A biomechanical study of flexor tendon repair in zone II: Comparing a combined grasping and locking core suture technique to its grasping and locking components. Injury. 2011;42(11):1300–1302. doi: 10.1016/j.injury.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Peltz TS, Haddad R, Scougall PJ, Nicklin S, Gianoutsos MP, Walsh WR. Influence of locking stitch size in a four-strand cross-locked cruciate flexor tendon repair. J Hand Surg Am. 2011;36(3):450–455. doi: 10.1016/j.jhsa.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Chesney A, Chauhan A, Kattan A, Farrokhyar F, Thoma A. Systematic review of flexor tendon rehabilitation protocols in zone II of the hand. Plast Reconstr Surg. 2011;127(4):1583–1592. doi: 10.1097/PRS.0b013e318208d28e. [DOI] [PubMed] [Google Scholar]
- 8.Bertelli JA, Santos MA, Kechele PR, Rost JR, Tacca CP. Flexor tendon grafting using a plantaris tendon with a fragment of attached bone for fixation to the distal phalanx: a preliminary cohort study. J Hand Surg Am. 2007;32(10):1543–1548. doi: 10.1016/j.jhsa.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Sun S, Ding Y, Ma B, Zhou Y. Two-stage flexor tendon reconstruction in zone II using Hunter's technique. Orthopedics. 2010;33(12):880. doi: 10.3928/01477447-20101021-10. [DOI] [PubMed] [Google Scholar]
- 10.Shin RH, Zhao C, Zobitz ME, Amadio PC, An KN. Mechanical properties of intrasynovial and extrasynovial tendon fascicles. Clin Biomech (Bristol, Avon) 2008;23(2):236–241. doi: 10.1016/j.clinbiomech.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamsson SO, Gelberman RH, Amiel D, Winterton P, Harwood F. Autogenous flexor tendon grafts: fibroblast activity and matrix remodeling in dogs. J Orthop Res. 1995;13(1):58–66. doi: 10.1002/jor.1100130110. [DOI] [PubMed] [Google Scholar]
- 12.Hasslund S, Jacobson JA, Dadali T, Basile P, Ulrich-Vinther M, Soballe K, et al. Adhesions in a murine flexor tendon graft model: autograft versus allograft reconstruction. J Orthop Res. 2008;26(6):824–833. doi: 10.1002/jor.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seiler JG, 3rd, Chu CR, Amiel D, Woo SL, Gelberman RH. The Marshall R. Urist Young Investigator Award. Autogenous flexor tendon grafts. Biologic mechanisms for incorporation. Clin Orthop Relat Res. 1997;(345):239–247. [PubMed] [Google Scholar]
- 14.LaSalle WB, Strickland JW. An evaluation of the two-stage flexor tendon reconstruction technique. J Hand Surg Am. 1983;8(3):263–267. doi: 10.1016/s0363-5023(83)80155-5. [DOI] [PubMed] [Google Scholar]
- 15.Hashizume H, Nishida K, Fujiwara K, Inoue H. Spontaneous "spaghetti" flexor tendon ruptures in the rheumatoid wrist. Mod Rheumatol. 2004;14(3):257–259. doi: 10.1007/s10165-004-0303-8. [DOI] [PubMed] [Google Scholar]
- 16.Vavken P, Joshi S, Murray MM. TRITON-X is most effective among three decellularization agents for ACL tissue engineering. J Orthop Res. 2009;27(12):1612–1618. doi: 10.1002/jor.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyland J, Larsen N, Burden R, Chang H, Caborn DN. Biomechanical and tissue handling property comparison of decellularized and cryopreserved tibialis anterior 15 tendons following extreme incubation and rehydration. Knee Surg Sports Traumatol Arthrosc. 2009;17(1):83–91. doi: 10.1007/s00167-008-0610-2. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Chen MY, Zhao C, An KN, Amadio PC. The effect of hyaluronidase, phospholipase, lipid solvent and trypsin on the lubrication of canine flexor digitorum profundus tendon. J Orthop Res. 2008;26(9):1225–1229. doi: 10.1002/jor.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun YL, Yang C, Amadio PC, Zhao C, Zobitz ME, An KN. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res. 2004;22(5):984–989. doi: 10.1016/j.orthres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda J, Zhao C, Sun YL, An KN, Amadio PC. Carbodiimide-derivatized hyaluronic acid surface modification of lyophilized flexor tendon: a biomechanical study in a canine in vitro model. J Bone Joint Surg Am. 2010;92(2):388–395. doi: 10.2106/JBJS.H.01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao C, Sun YL, Amadio PC, Tanaka T, Ettema AM, An KN. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic Acid. An in vivo canine model. J Bone Joint Surg Am. 2006;88(10):2181–2191. doi: 10.2106/JBJS.E.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saber S, Zhang AY, Ki SH, Lindsey DP, Smith RL, Riboh J, et al. Flexor tendon tissue engineering: bioreactor cyclic strain increases construct strength. Tissue Eng Part A. 2010;16(6):2085–2090. doi: 10.1089/ten.TEA.2010.0032. [DOI] [PubMed] [Google Scholar]
- 23.Zhang AY, Bates SJ, Morrow E, Pham H, Pham B, Chang J. Tissue-engineered intrasynovial tendons: optimization of acellularization and seeding. J Rehabil Res Dev. 2009;46(4):489–498. doi: 10.1682/jrrd.2008.07.0086. [DOI] [PubMed] [Google Scholar]
- 24.Uchiyama S, Coert JH, Berglund L, Amadio PC, An KN. Method for the measurement of friction between tendon and pulley. J Orthop Res. 1995;13(1):83–89. doi: 10.1002/jor.1100130113. [DOI] [PubMed] [Google Scholar]
- 25.Kolodzinskyi MN, Zhao C, Sun YL, An KN, Thoreson AR, Amadio PC, et al. The effects of hylan g-f 20 surface modification on gliding of extrasynovial canine tendon grafts in vitro. J Hand Surg Am. 2013;38(2):231–236. doi: 10.1016/j.jhsa.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva JM, Zhao C, An KN, Zobitz ME, Amadio PC. Gliding resistance and strength of composite sutures in human flexor digitorum profundus tendon repair: an in vitro biomechanical study. J Hand Surg Am. 2009;34(1):87–92. doi: 10.1016/j.jhsa.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao C, Amadio PC, Zobitz ME, Momose T, Couvreur P, An KN. Effect of synergistic motion on flexor digitorum profundus tendon excursion. Clin Orthop Relat Res. 2002;(396):223–230. doi: 10.1097/00003086-200203000-00033. [DOI] [PubMed] [Google Scholar]
- 28.Zhao C, Amadio PC, Zobitz ME, An KN. Gliding characteristics of tendon repair in canine flexor digitorum profundus tendons. J Orthop Res. 2001;19(4):580–586. doi: 10.1016/S0736-0266(00)00055-3. [DOI] [PubMed] [Google Scholar]
- 29.Zhao C, Amadio PC, Momose T, Zobitz ME, Couvreur P, An KN. Remodeling of the gliding surface after flexor tendon repair in a canine model in vivo. J Orthop Res. 2002;20(4):857–862. doi: 10.1016/S0736-0266(01)00168-1. [DOI] [PubMed] [Google Scholar]
- 30.Lee GH, Kumar A, Berkson E, Verma N, Bach BR, Jr, Hallab N. A biomechanical analysis of bone-patellar tendon-bone grafts after repeat freeze-thaw cycles in a cyclic loading model. J Knee Surg. 2009;22(2):111–113. doi: 10.1055/s-0030-1247734. [DOI] [PubMed] [Google Scholar]
- 31.Deeken CR, White AK, Bachman SL, Ramshaw BJ, Cleveland DS, Loy TS, et al. Method of preparing a decellularized porcine tendon using tributyl phosphate. J Biomed Mater Res B Appl Biomater. 2010 doi: 10.1002/jbm.b.31753. [DOI] [PubMed] [Google Scholar]
- 32.Pridgen BC, Woon CY, Kim M, Thorfinn J, Lindsey D, Pham H, et al. Flexor tendon tissue engineering: acellularization of human flexor tendons with preservation of biomechanical properties and biocompatibility. Tissue Eng Part C Methods. 2011;17(8):819–828. doi: 10.1089/ten.tec.2010.0457. [DOI] [PubMed] [Google Scholar]
- 33.Chong AK, Riboh J, Smith RL, Lindsey DP, Pham HM, Chang J. Flexor tendon tissue engineering: acellularized and reseeded tendon constructs. Plast Reconstr Surg. 2009;123(6):1759–1766. doi: 10.1097/PRS.0b013e3181a65ae7. [DOI] [PubMed] [Google Scholar]
- 34.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma. 2001;51(5):917–921. doi: 10.1097/00005373-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Zhao C, Sun YL, Ikeda J, Kirk RL, Thoreson AR, Moran SL, et al. Improvement of flexor tendon reconstruction with carbodiimide-derivatized hyaluronic acid and gelatin-modified intrasynovial allografts: study of a primary repair failure model. J Bone Joint Surg Am. 2010;92(17):2817–2828. doi: 10.2106/JBJS.I.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao C, Sun YL, Kirk RL, Thoreson AR, Jay GD, Moran SL, et al. Effects of a lubricin-containing compound on the results of flexor tendon repair in a canine model in vivo. J Bone Joint Surg Am. 2010;92(6):1453–1461. doi: 10.2106/JBJS.I.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]