Abstract
Despite living in an environment that promotes weight gain in many individuals, some individuals maintain a thin phenotype while self-reporting expending little or no effort to control their weight. When compared with obesity prone (OP) individuals, we wondered if obesity resistant (OR) individuals would have higher levels of spontaneous physical activity (SPA) or respond to short-term overfeeding by increasing their level of SPA in a manner that could potentially limit future weight gain. SPA was measured in 55 subjects (23 OP and 32 OR) using a novel physical activity monitoring system (PAMS) that measured body position and movement while subjects were awake for 6 days, either in a controlled eucaloric condition or during 3 days of overfeeding (1.4× basal energy) and for the subsequent 3 days (ad libitum recovery period). Pedometers were also used before and during use of the PAMS to provide an independent measure of SPA. SPA was quantified by the PAMS as fraction of recording time spent lying, sitting, or in an upright posture. Accelerometry, measured while subjects were in an upright posture, was used to categorize time spent in different levels of movement (standing, walking slowly, quickly, etc.). There were no differences in SPA between groups when examined across all study periods (P > 0.05). However, 3 days following overfeeding, OP subjects significantly decreased the amount of time they spent walking (−2.0% of time, P = 0.03), whereas OR subjects maintained their walking (+0.2%, P > 0.05). The principle findings of this study are that increased levels of SPA either during eucaloric feeding or following short term overfeeding likely do not significantly contribute to obesity resistance although a decrease in SPA following overfeeding may contribute to future weight gain in individuals prone to obesity.
INTRODUCTION
The increase in the prevalence of obesity over the last 20 years is widely thought to be because of changes in the typical environment in which most people live (1,2). Ready access to highly palatable, inexpensive food combined with a reduced need to engage in physical activity in daily life has likely promoted a state of positive energy balance that promotes weight gain in many individuals. Although weight gain may be a slow and gradual process, it may be that increases in fat mass occur episodically following brief periods of positive energy balance that are not adequately compensated for. Most people experience periods where food intake far exceeds energy expenditure (EE). These periods lasting from one meal to several days can occur on holidays, vacations, weekends, or times of celebration and may lead to clinically significant weight gain (3-5). Whether or not weight gain occurs continuously or episodically, it results from a failure of regulatory systems to appropriately compensate for periods of positive energy balance produced by episodes of excessive food intake.
On the other hand, there are clear differences in people’s susceptibility to weight gain. Some people maintain a normal body weight despite living in what is for most an “obesogenic environment” (6-8). How do these “obesity resistant” (OR) people remain thin in the current environment? In particular, how do they respond to brief periods of positive energy balance in a manner that prevents weight gain? The possibilities include increases in resting metabolic rate, increases in physical activity, reductions in food intake, or alterations in nutrient metabolism that ultimately favor oxidation of excess nutrients. Currently it is unclear which, if any, of these mechanisms play an important role.
The Energy Adaptations over Time Study was designed to measure a number of adaptive responses to short-term overfeeding that might protect from, or predispose to, weight gain in obesity prone (OP) and OR humans. With the measures reported here we sought to examine the potential role that differences in spontaneous physical activity (SPA) might play in resistance to weight gain following short-term overfeeding between OP and OR subjects. Energy can be expended in physical activity during bouts of exercise or during activities of daily living. The latter has been termed nonexercise activity thermogenesis (NEAT) by Levine (9). It has been hypothesized that NEAT increases in people as a homeostatic response to periods of over-nutrition in order to restore energy balance (10). Levine et al. showed that overfeeding lean adults by 1000 kcal/day for 8 weeks led to a change in NEAT (measured as the difference between resting EE and total EE (TEE)) which was inversely related to fat gain (9). This group employed the same overfeeding protocol in lean and obese individuals and measured NEAT using novel physical activity monitoring systems (PAMS) (11,12). In this study, overfeeding led to a modest decrease in walking in both lean and obese individuals, with the two groups reducing walking to a similar degree (11).
Although the previous studies showed that NEAT changed with 8 weeks of overfeeding, most people experience shorter periods of time where food intake far exceeds EE. The aim of the current study was to determine if a brief period of overfeeding (3 days) similar to what might occur in normal living would lead to a change in SPA. Given that obesity itself might reduce SPA levels, we were interested in determining if people who were not currently obese but were at risk for weight gain would have activity patterns that were different from individuals who seem to be resistant to obesity. We sought to determine if SPA responds differently to short-term overfeeding in these two groups. We predicted that overfeeding would result in an overall decrease in SPA but that OP subjects would have a greater decrease in physical activity as compared to OR subjects.
METHODS AND PROCEDURES
Subjects
Subjects were healthy men and women ages 25–35 years empirically classified as either OR or OP based on personal and family weight history. OR subjects had a BMI of 16.9–25.5 kg/m2, had no first degree relatives with a BMI >30 kg/m2 and defined themselves as constitutionally thin based on their perception of difficulty gaining weight despite expending any effort to maintain their current weight. These individuals responded to advertisements for “naturally thin people”, reported no history of ever being overweight and self-reported a sense that their weight regulation was “different” from other people. OP subjects in contrast had a BMI of 19.6–30.6 kg/m2 had at least one first degree relative with a BMI >30 kg/m2, reported having put effort into not gaining weight, reported previous attempts to lose weight, but were not actively attempting to lose weight and were weight stable for at least 3 months before being studied. At baseline, all subjects underwent a screening history, physical examination and biochemical testing to exclude significant medical illness. Subjects were excluded if they took medications known to affect weight or lipid metabolism. They also completed a number of questionnaires to exclude eating disorders (13-15) or significant psychological dysfunction (16,17). To assess habitual physical activity, subjects wore a pedometer (Digi- Walker, New-Lifestyles, Lee’s Summit, MO) for 1 week before beginning studies. Body composition was measured by dual-energy X-ray absorptiometry (Hologic Discovery-W, Bedford, MA), resting EE by hood indirect calorimetry (ParvoMedics Model: TrueOne 2400, Sandy, UT) and 24-h TEE by whole-room calorimetry (18). The energy requirements for free living eucaloric feeding were determined based on resting EE derived from the average of (i) direct measurement by hood indirect calorimetry and (ii) an estimation using the following equation: [(23.9 × fat-free mass in kg) + 372], where fat-free mass was measured by dual-energy X-ray absorptiometry (19). The resting EE determined from those two methods was then multiplied by an activity factor (1.4–1.65), which was based on subjects’ average steps taken during a week of baseline pedometer monitoring. To determine energy requirements for the chamber stays during the experimental interventions, TEE was directly measured during a “baseline” stay in the room calorimeter (independent of and before the study periods reported). This value for TEE in the chamber was compared with the “free living” TEE estimated by the method outlined above. TEE in the room calorimeter was found to be about 8% less than the TEE calculated free living TEE values. Therefore, subjects were fed 8% fewer calories on the day that they were in the calorimeter as compared with the free living periods.
Study design
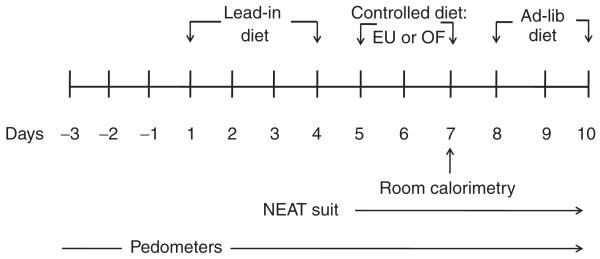
Subjects were studied on the Clinical and Translational Research Center at the University of Colorado, School of Medicine on two occasions separated by at least 1 month. For the first 4 days of each study period, subjects consumed a controlled eucaloric “run-in diet” (20% protein, 30% fat, and 50% carbohydrate) to ensure energy and nutrient balance (Figure 1). For the next 3 days, participants consumed in random order either a controlled eucaloric (EU) study diet or a controlled hypercaloric study diet (OF) containing 1.4× estimated basal energy needs. The macronutrient content of each study diet was the same as that of the lead in diet. On the third day of each study diet period, subjects spent 23 h in the whole- room calorimeter during which time a number of metabolic measurements were made. Given the limitations in movement in the room calorimeter, subjects consumed 8% fewer calories on this study day for both diet phases, which was based on a pre-study stay in the calorimeter to validate their energy requirments and familiarize them with the environment. For the next 3 days subjects consumed an ad libitum diet. During this period subjects were offered a diet with the same composition but containing 25% more calories than their eucaloric diet. Subjects were instructed to consume as much food as they wanted and to return all leftover food items to the Clinical and Translational Research Center kitchen for measurement. All food along with wrappers and containers were weighed before and after consumption by the Clinical and Translational Research Center nutrition staff to determine total energy intake. For the duration of each 4-day “run-in” period and the 10-day study period subjects consumed only food and beverages provided by the Clinical and Translational Research Center metabolic kitchen.
Figure 1.
Protocol schematic overview of the study design.
Physical activity monitoring
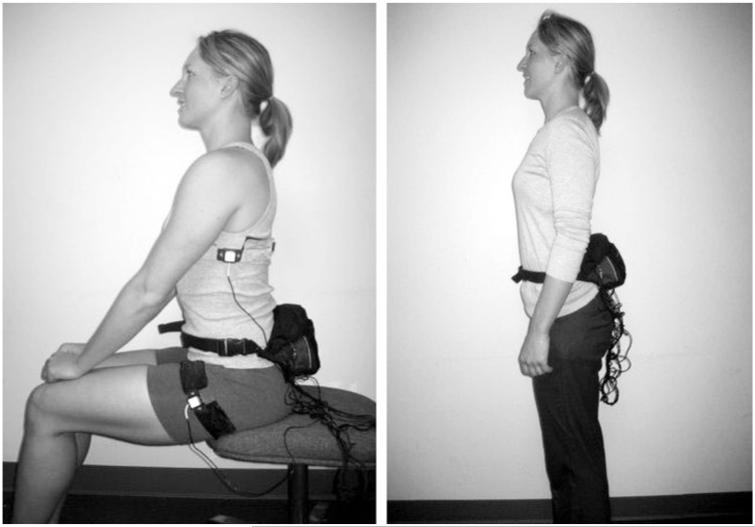
SPA was measured for six consecutive days (during the 3-day controlled diet period and the 3-day ad libitum diet period) in both the EU and OF study phases using the PAMS designed by Dr James Levine and colleagues (Mayo Clinic). PAMS are composed of paired inclinometers (20) and accelerometers (21) (ICSensors 3031–010, Druck Nederland, The Netherlands) worn on the left and right sides of the upper and lower body (Figure 2) that measure body position (lying, sitting, and standing) and movement. A cable connects the accelerometers and inclinometers to a portable data logger (Tattletale 5F, Onset Computer, 512 kB, 16-bit, 10 × 70 × 35 mm, 250 g; Bourne, MA).
Figure 2.
Physical activity measurement system. Attachment of inclinometers and accelerometers onto adult’s clothing for physical activity measurement.
Data from the PAMS were collected at two readings per second and averaged into 15-s blocks. Input data included interval (in seconds), and volts (0–5) for upper body, lower body, X-, Y-, and Z-axis, and time in hours and minutes. The voltages from upper and lower body inclinometers were converted into angles in order to estimate body position. The following formula was used to convert volts to angles, with an error of less than 0.01°:
where A is the gain, B is the phase, C is the period, and D is the offset. Table 1 shows how overall body position was determined using the calculated angles. We assumed that energy expended when subjects were in the lying or seated positions would be low and therefore did not use accelerometry data from these body positions in the final analyses. We examined accelerometry data more closely for the time subjects spent in an upright posture as this time likely represented activities with a wide range of EEs. If data from the inclinometers indicated that the subject was upright, accelerometry data were used to further subdivide recording time into five categories representing different levels of activity. These movement levels were based on the acceleration values from the X-, Y-, and Z-axes and were calculated using the following formula:
for both left and right sides. The values were then summed into 15-s blocks and averaged between the left and right sides. The different levels of movement in the standing position were then categorized as shown in Table 2.
Table 1. Body position based on inclinometers. Overall body position (lying, sitting, or standing) was determined using angles of inclinometers on the upper and lower body.
| Angles (in degrees) |
Upper body state |
Lower body state |
Overall body postition |
|---|---|---|---|
| 0–49 | Vertical | Vertical | Standing |
| 50–109 | Horizontal | Horizontal | Lying |
| 110–254 | Vertical | Horizontal | Sitting |
| 255–299 | Horizontal | Horizontal | Lying |
| 300–360 | Vertical | Vertical | Standing |
Table 2. Type of movement based on acceleration. Level of movement was determined by volts taken from accelerometers while subjects where in the standing position.
| Movement in standing postition | Accel (V) |
|---|---|
| Standing still | <2.5 |
| Walking slow pace | 2.5–5 |
| Walking normal pace | 5–8 |
| Walking quick pace | 8–10 |
| Vigorous activity | >10 |
Pilot data were obtained from PAMS recordings taken on normal volunteers who were instructed to stand, move in a manner mimicking daily activities such as putting dishes away and walk at defined speeds on a treadmill to validate this approach. These acceleration values were used to determine the fraction of the PAMS recording time spent standing still, walking slowly, quickly, etc. for each day of the study. Total acceleration in the upright position (summed for all levels of movement) is also reported as an estimate of overall movement in the upright position. Participants were instructed to remove the PAMS while showering, and were allowed to remove the system while sleeping. Data were excluded if subjects wore the device for less than 8 h a day, if both left and right sensors had large gaps in data, or if there were values outside of the valid range of 0–5 V over large time periods. Data were analyzed in a blinded manner for (i) percent of recording time spent in each position and level of movement and (ii) total acceleration while in an upright posture and for each level of movement.
Subjects wore pedometers to measure SPA during a 7-day baseline period to help determine basal energy needs. During this period, the PAMS devices were not worn. In addition, in an effort to acquire an independent measure of SPA during and following controlled diets, pedometers were also worn during the entire period that the PAMS devices were worn. This was done to obtain confirmation and corroboration of any differences seen with the PAMS and to see if the PAMS provided substantially more information than the pedometers alone.
Statistical analysis
Data were analyzed using SPSS version 17.0 (Cary, NC). Subject characteristics data are reported as means and standard deviations. A repeated measures ANCOVA was used to examine eucaloric and overfeeding effects on different sub-categories of movement (walking slowing, walking quickly, total steps, etc.), with P values identified for interactions and main effects of obesity (OP and OR) and study phase (eucaloric and hypercaloric). Percent body fat and sex were used as covariates when comparing OP to OR because of the difference in these variables between the groups. Pairwise comparisons were examined on adjusted means to determine if the study phase (EU vs. OF) significantly affected SPA in OP and OR groups. Values are reported as means and standard errors.
The controlled diet period consisted of 3 days in each study phase; however, the entire third day of this period was spent in a room calorimeter. Because overall movement is limited in this relatively small room, activity data from this day was excluded from the analysis.
RESULTS
Fifty-five subjects (23 OP and 32 OR) completed both EU and OF study periods. Subjects in the two groups were matched with regards to age and resting metabolic rate (Table 3). OP subjects had significantly higher BMI and percent body fat compared with OR subjects (P < 0.05) although the mean BMI in the OP group was well below the overweight level.
Table 3. Subject characteristics. Values are means ± s.d.
| OP | OR | |
|---|---|---|
| n | 23 | 32 |
| Men/women | 8/15 | 16/16 |
| Age | 28.5 ± 2.3 | 28.1 ± 2.9 |
| BMI (kg/m2) | 23.8 ± 2.7 | 20.6 ± 2.2* |
| Body fat (%) | 26.5 ± 7.9 | 19.4 ± 5.4* |
| Fat-free mass (kg) | 51.0 ± 9.8 | 51.6 ± 11.2 |
| RMR (kcal/day) | 1540 ± 212 | 1541 ± 288 |
| Calorie intake EU | 2324 ± 322 | 2352 ± 463 |
| Calorie intake OF | 3243 ± 425 | 3216 ± 342 |
EU, eucaloric diet period; OF, overfed diet period; OP, obesity prone; OR, obesity resistant; RMR, resting metabolic rate.
Significant difference by group, P < 0.05.
Physical activity as measured by the PAMS
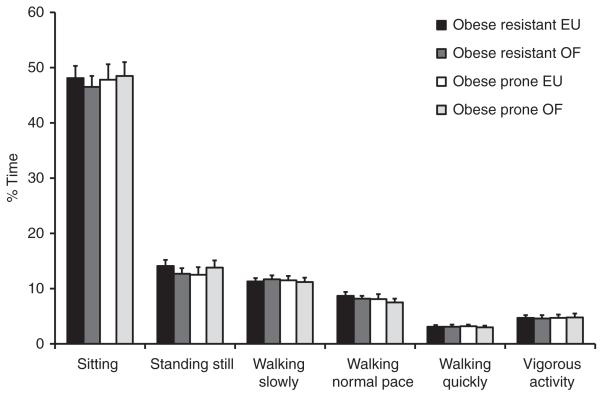
The PAMS was worn by all study participants for an average of 14.0 ± 3.0 h/day, and there was no difference in the amount of time that the devices were worn between OP and OR groups (13.98 h ± 3.4 and 14.03 h ± 2.8, respectively). Physical activity levels were described by the fraction of the monitoring time spent in a lying, sitting or upright posture and by the fraction of time spent in an upright posture with increasing levels of movement as categorized by the accelerometers. Data were analyzed over entire study periods (days 5–10 of the EU or OF conditions) and by specific days in each phase of the study. In general, all subjects spent the majority of their waking hours sitting (47.8 ± 9.8%) regardless of study phase or diet period (Figure 3). Neither movement while sitting nor percent of time spent sitting were different between OP and OR subjects in the eucaloric or overfed study conditions for any study day or grouped together over study phases (P > 0.05). When all study days (controlled diet and ad lib diet) were averaged together for each study phase (EU vs. OF), there were no differences in physical activity measured by the PAMS between OP and OR subjects for any posture category or level of activity in the upright posture (Figure 3). Neither OP nor OR subjects had differences in their level of SPA when examined across the entire OF phase compared with the entire EU phase in both the control (days 5 and 6) and ad lib (days 8–10) diet periods (P < 0.05); however, examining the data on individual study days revealed a number of significant differences in activity levels in the EU as compared with the OF study phases and between OP and OR subjects.
Figure 3.
Percent of time spent in various positions across all study days. Data presented is measured physical activity on days 5–10 of the EU and OF diet periods. Physical activity is presented as the percent of time that the physical activity measurement system were worn in the different body positions and levels of movement. EU, eucaloric diet period; OF, overfed diet period; OP, obesity prone; OR, obesity resistant.
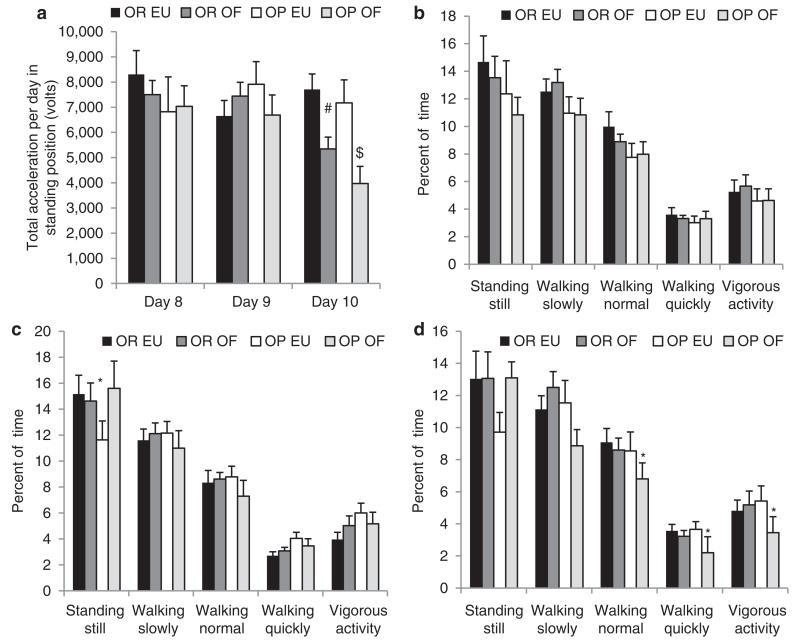
With overfeeding, OP subjects reduced their measured levels of SPA to a greater degree than OR subjects. This decrease in physical activity was most noticeable following overfeeding during the ad lib diet period, most dramatically on the third day following 40% overfeeding (Figure 4a). Overall acceleration measured during upright posture, a global assessment of movement, was lower in both the OP and OR groups (−3201 V; P < 0.001 and −2354 V; P = 0.02, respectively) following overfeeding only on the third day of consuming the ad libitum diet as compared with the same day of the eucaloric condition (Figure 4a). On days 8 and 9 of the overfeeding condition, all subjects maintained their level of movement (Figure 4b-c); however on day 10, OP subjects spent significantly less time walking at a normal pace (−1.74%; P = 0.04), walking quickly (−1.46%; P = 0.02), and engaging in vigorous activity (−1.97%; P = 0.04) as compared with day 10 of the eucaloric condition, whereas OR subjects maintained their level of activity at this time point in the two experimental conditions (P > 0.05) (Figure 4d).
Figure 4.
Physical activity measured with physical activity measurement system (PAMS) days 8–10. (a) Total acceleration in upright position on days 8–10 during the eucaloric (EU) and overfed (OF) study periods in obesity resistant and obesity prone subjects. Data are presented as volts as measured from the PAMS on each day. (b) Percent of time in upright position with increasing levels of movement on the first day of the ad lib diet (day 8). Physical activity is presented as the percent of time that the PAMS were worn in the different levels of movement. (c) Percent of time in upright position with increasing levels of movement on the second day of the ad lib diet (day 9). Physical activity is presented as the percent of time that the PAMS were worn in the different levels of movement. (d) Percent of time in upright position with increasing levels of movement on the third day of the ad lib diet (day 10). Physical activity is presented as the percent of time that the PAMS were worn in the different levels of movement. *P < 0.05 EU vs. OF within obese group; #P = 0.02 EU vs. OF within obese group; P < 0.001 EU vs. OF within obese group.
Physical activity as measured by pedometers
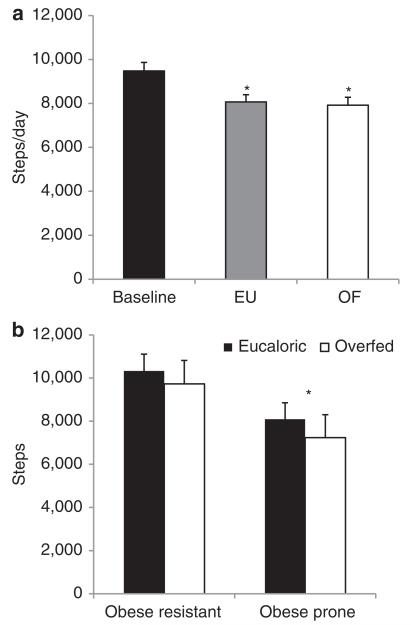
Pedometers were used to provide an independent measure of physical activity in addition to that measured with the PAMS. Pedometer data was available from the baseline and run-in periods (days −3−4), a period when the PAMS was not being worn. Pedometer data was also available during days 5–10 when PAMS data was simultaneously being collected. The pedometer data show that all subjects decreased the number of steps taken while they wore the PAMS (baseline steps without PAMS = 9477 ± 388; steps wearing PAMS during EU = 8068 ± 328; P = 0.002 steps wearing PAMS during OF = 7918 ± 364, P < 0.001), suggesting that wearing these devices reduced subjects’ level of SPA (Figure 5a). The pedometer data also confirmed the general finding from the PAMS data that there were no differences between OP and OR subjects in their average number of steps taken when measured over the entire duration of the study (OP = 7967 ± 560, OR = 8017 ± 441, P > 0.05). During the 1-week period of baseline pedometer monitoring, OP subjects had an average of 9984 ± 608 steps, and OR had an average of 9082 ± 501 steps, P = 0.26). However, as had been seen in the PAMS data, there was a significant main effect of obesity classification on the third day of consuming the ad lib diet, with OP subjects taking significantly fewer steps than OR following overfeeding (OP = 7622 ± 932, OR = 9240 ± 728, P < 0.05) (Figure 5b).
Figure 5.
Physical activity measured with pedometer. (a) Steps taken by all subjects during the baseline period (days −3−4) while physical activity measurement system (PAMS) were not worn and during the entire eucaloric (EU) and overfed (OF) diet periods (days 5–10) while the PAMS were worn. *P < 0.05 compared with baseline. (b) Steps taken on the third day of the ad lib diet (day 10). Data presented is the average number of steps taken on the third day of the ad lib diet in both the EU and OF diet periods for OP and OR subjects. *P < 0.05 for main effect of obese group.
Although examining sex-based differences was not a primary goal of this study, we did observe that women tended to have a greater amount of SPA as compared with men. Specifically, women spent a greater percent of time standing than men in the eucaloric study condition (15.8 ± 7.6% vs. 10.9 ± 5.2%, P = 0.05).
DISCUSSION
The main findings from this study are that nonobese individuals who are prone or resistant to obesity do not drastically differ in measured levels of SPA. Following a period of short-term overfeeding, all subjects moved significantly less on the third day following overfeeding. OP individuals were found to have a significant decrease in the amount of time that they spent moving at normal, quick, and vigorous paces on the third day after overfeeding as compared with what was observed following the controlled eucaloric diet; whereas, OR individuals maintained their usual level of physical activity on this day. Although OP subjects did have a statistically significant decrease in the percent of time spent in an upright posture moving at these levels, an estimate of the energetic value of this difference in activity was only ~160 kcal less than that expended by OR subjects on this day based on an estimate of the average EE of walking at these rates (22). A limitation to this study is that we did not translate the PAMS data into EE. This modest decline in estimated EE is quite small in comparison with the excess energy ingested during the overfeeding period which averaged 920 kcal. However, it is possible that the heavier OP subjects had greater EE during similar physical activities; therefore their estimated EE could be similar despite slightly lower PAMS-measured activity. The degree to which the decrease in physical activity following overfeeding could contribute to eventual weight gain in OP individuals then is not large but could perhaps be relevant over long periods of time and multiple episodes of overfeeding.
This study adds to a growing literature on the role of SPA in the development of obesity. Studies in OP (4) and OR animal models suggest that levels of NEAT are at least partially genetically determined and can promote or protect against obesity (23,24). Human studies have also observed NEAT to be a familial trait, which may account for large inter-individual differences in its contribution to TEE and may predict the propensity for weight gain (25,26). Obese individuals have been shown to walk significantly less than lean individuals (11). Given that obesity makes it difficult for individuals to be physically active because of joint pain (27), social stigma (28), and other factors, it is difficult to say from studies like this if moving less contributes to the development of obesity or if obesity itself leads to lower levels of SPA. A strength of the current study is that we chose to measure SPA in people who were not yet obese but who were selected for a propensity to gain weight or remain thin. On the other hand it is difficult to determine the accuracy of the method we used in categorizing OP and OR individuals. Much of the categorization relied on the subject’s perception of their tendency to gain weight or not. We are following these individuals prospectively to determine their weight trajectory over time. However, we have already seen individuals who were initially categorized as OP who decided to consciously lead a healthy lifestyle to minimize weight gain so that they would not develop the health problems that they had seen family members endure. Other OR subjects have undergone life events that resulted in substantial weight gain. This demonstrates to us that longitudinally determined weight gain may not be a better reflection of a biological predisposition to weight gain than subjects’ own sense of their predisposition to weight gain.
A number of previous studies have examined the effects of overfeeding on EE. Most of these studies have imposed hypercaloric diets for periods of time that do not routinely occur with normal living (29-31). These studies demonstrate that there is a good deal of heterogeneity in the degree of weight gain following overfeeding suggesting genetic variation in the adaptive responses to overfeeding that either predispose to or protect from weight gain (9,31). These studies did not directly measure SPA but have found either an increase in nonresting EE following overfeeding (9) or no change (32). A number of studies have examined short-term overfeeding and demonstrated increases in TEE especially associated with overfeeding of a high carbohydrate diet (33-35). One of these studies found that SPA measured by radar in a room calorimeter decreased following 3 days of overfeeding (36). Others found either no change in measured SPA (37,38) or increases in nonresting EE (39). This is to our knowledge the first study that examined the effects of short-term overfeeding on directly measured SPA in OP and OR men and women.
Another limitation of this study is the statistical approach employed. If we use a more rigorous approach accounting for multiple comparisons we do not find any significant differences. Although it may be that there are no differences between groups or conditions, we saw similar patterns in the pedometer data and this suggests to us that there are real, albeit small effects of phenotype and overfeeding. Why would there be differences in SPA 3 days following overfeeding though? Interestingly Bray and colleagues found evidence for corrective responses in food intake measured in free living human subjects that occurs with a lag time of 3–4 days (40). It may be that the body makes adaptive responses to alterations in energy balance not over hours but rather over days. This study supports this view of how the weight regulatory system functions. However, it may be that larger or different effects would have been seen if more than 3 days of overfeeding or a greater degree of overfeeding had been employed.
Another finding of the study is that as assessed by pedometers, subjects moved less while they were wearing the NEAT suits. Although these devices allow greater precision in the measurement of SPA in freely living human subjects, because of their cumbersome nature, they may in and of themselves lead to a decrease in physical activity. As shown in the photographs of the devices, the sensors were connected to data loggers that had to be worn in a pack outside of clothing. Many subjects reported that they were less inclined to go out for a run when wearing the devices because of their cumbersome nature. It may be that all devices used to measure physical activity exert some effect on the parameter being measured. It may be that there is a trade-off between the accuracy of the data and the influence the device has on the measured parameter.
Based on the findings from this study, it seems unlikely that nonobese individuals who are resistant to obesity differ substantially from OP individuals in habitual levels of physical activity. However, OP individuals may decrease their daily movement following short-term overeating to a greater degree than individuals who are resistant to obesity, possibly contributing to future weight gain.
ACKNOWLEDGMENTS
This study was funded by the National Institutes (NIHS) of Health through RO1 DK62874. D.H.B received support from the National Institutes of Health through K24DK002935. S.L.S. received support from T32DK007446. The study was further supported by the NIH/NCRR Colorado CTSI Grant: UL 1 RR025780 and the Colorado Nutrition Obesity Research Center: P30DK048520. Dr James Levine of the Mayo Clinic kindly provided training and support in the construction, use of and interpretation of data from the PAMS. The contents of this manuscript are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
See the online ICMJE Conflict of Interest Forms for this article.
REFERENCES
- 1.Hill JO, Wyatt HR, Melanson EL. Genetic and environmental contributions to obesity. Med Clin North Am. 2000;84:333–346. doi: 10.1016/s0025-7125(05)70224-8. [DOI] [PubMed] [Google Scholar]
- 2.Peters JC, Wyatt HR, Donahoo WT, Hill JO. From instinct to intellect: the challenge of maintaining healthy weight in the modern world. Obes Rev. 2002;3:69–74. doi: 10.1046/j.1467-789x.2002.00059.x. [DOI] [PubMed] [Google Scholar]
- 3.Yanovski JA, Yanovski SZ, Sovik KN, et al. A prospective study of holiday weight gain. N Engl J Med. 2000;342:861–867. doi: 10.1056/NEJM200003233421206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branscum P, Kaye G, Succop P, Sharma M. An Evaluation of Holiday Weight Gain Among Elementary-aged Children. J Clin Med Res. 2010;2:167–171. doi: 10.4021/jocmr414w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phelan S, Wing RR, Raynor HA, et al. Holiday weight management by successful weight losers and normal weight individuals. J Consult Clin Psychol. 2008;76:442–448. doi: 10.1037/0022-006X.76.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchard C. Current understanding of the etiology of obesity: genetic and nongenetic factors. Am J Clin Nutr. 1991;53:1561S–1565S. doi: 10.1093/ajcn/53.6.1561S. [DOI] [PubMed] [Google Scholar]
- 7.Costanzo PR, Schiffman SS. Thinness–not obesity–has a genetic component. Neurosci Biobehav Rev. 1989;13:55–58. doi: 10.1016/s0149-7634(89)80052-1. [DOI] [PubMed] [Google Scholar]
- 8.Bulik CM, Allison DB. The genetic epidemiology of thinness. Obes Rev. 2001;2:107–115. doi: 10.1046/j.1467-789x.2001.00030.x. [DOI] [PubMed] [Google Scholar]
- 9.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 10.Kotz CM. Integration of feeding and spontaneous physical activity: role for orexin. Physiol Behav. 2006;88:294–301. doi: 10.1016/j.physbeh.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Levine JA, McCrady SK, Lanningham-Foster LM, et al. The role of free-living daily walking in human weight gain and obesity. Diabetes. 2008;57:548–554. doi: 10.2337/db07-0815. [DOI] [PubMed] [Google Scholar]
- 12.Lanningham-Foster LM, Jensen TB, McCrady SK, et al. Laboratory measurement of posture allocation and physical activity in children. Med Sci Sports Exerc. 2005;37:1800–1805. doi: 10.1249/01.mss.0000175050.03506.bf. [DOI] [PubMed] [Google Scholar]
- 13.Lowe MR, Butryn ML, Didie ER, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53:114–118. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 15.Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: psychometric features and clinical correlates. Psychol Med. 1982;12:871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- 16.Collins ME. Body figure perceptions and preferences among preadolescent children. Int J Eating Disord. 1991;10:199–208. [Google Scholar]
- 17.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 18.Sun M, Reed GW, Hill JO. Modification of a whole room indirect calorimeter for measurement of rapid changes in energy expenditure. J Appl Physiol. 1994;76:2686–2691. doi: 10.1152/jappl.1994.76.6.2686. [DOI] [PubMed] [Google Scholar]
- 19.Grunwald GK, Melanson EL, Forster JE, et al. Comparison of methods for achieving 24-hour energy balance in a whole-room indirect calorimeter. Obes Res. 2003;11:752–759. doi: 10.1038/oby.2003.105. [DOI] [PubMed] [Google Scholar]
- 20.Levine JA, Lanningham-Foster LM, McCrady SK, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 21.Levine JA, Baukol PA, Westerterp KR. Validation of the Tracmor triaxial accelerometer system for walking. Med Sci Sports Exerc. 2001;33:1593–1597. doi: 10.1097/00005768-200109000-00024. [DOI] [PubMed] [Google Scholar]
- 22.Levine JA, Schleusner SJ, Jensen MD. Energy expenditure of nonexercise activity. Am J Clin Nutr. 2000;72:1451–1454. doi: 10.1093/ajcn/72.6.1451. [DOI] [PubMed] [Google Scholar]
- 23.Novak CM, Kotz CM, Levine JA. Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am J Physiol Endocrinol Metab. 2006;290:E396–E403. doi: 10.1152/ajpendo.00293.2005. [DOI] [PubMed] [Google Scholar]
- 24.Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R889–R899. doi: 10.1152/ajpregu.00536.2005. [DOI] [PubMed] [Google Scholar]
- 25.Zurlo F, Ferraro RT, Fontvielle AM, et al. Spontaneous physical activity and obesity: cross-sectional and longitudinal studies in Pima Indians. Am J Physiol. 1992;263:E296–E300. doi: 10.1152/ajpendo.1992.263.2.E296. [DOI] [PubMed] [Google Scholar]
- 26.Toubro S, Christensen NJ, Astrup A. Reproducibility of 24-h energy expenditure, substrate utilization and spontaneous physical activity in obesity measured in a respiration chamber. Int J Obes Relat Metab Disord. 1995;19:544–549. [PubMed] [Google Scholar]
- 27.Nevitt MC, Lane N. Body weight and osteoarthritis. Am J Med. 1999;107:632–633. doi: 10.1016/s0002-9343(99)00297-1. [DOI] [PubMed] [Google Scholar]
- 28.Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring) 2009;17:941–964. doi: 10.1038/oby.2008.636. [DOI] [PubMed] [Google Scholar]
- 29.Harris AM, Jensen MD, Levine JA. Weekly changes in basal metabolic rate with eight weeks of overfeeding. Obesity (Silver Spring) 2006;14:690–695. doi: 10.1038/oby.2006.78. [DOI] [PubMed] [Google Scholar]
- 30.Roberts SB, Young VR, Fuss P, et al. Energy expenditure and subsequent nutrient intakes in overfed young men. Am J Physiol. 1990;259:R461–R469. doi: 10.1152/ajpregu.1990.259.3.R461. [DOI] [PubMed] [Google Scholar]
- 31.Bouchard C, Tremblay A, Després JP, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 32.Siervo M, Frühbeck G, Dixon A, et al. Efficiency of autoregulatory homeostatic responses to imposed caloric excess in lean men. Am J Physiol Endocrinol Metab. 2008;294:E416–E424. doi: 10.1152/ajpendo.00573.2007. [DOI] [PubMed] [Google Scholar]
- 33.Weyer C, Vozarova B, Ravussin E, Tataranni PA. Changes in energy metabolism in response to 48 h of overfeeding and fasting in Caucasians and Pima Indians. Int J Obes Relat Metab Disord. 2001;25:593–600. doi: 10.1038/sj.ijo.0801610. [DOI] [PubMed] [Google Scholar]
- 34.Lammert O, Grunnet N, Faber P, et al. Effects of isoenergetic overfeeding of either carbohydrate or fat in young men. Br J Nutr. 2000;84:233–245. [PubMed] [Google Scholar]
- 35.Dirlewanger M, di Vetta V, Guenat E, et al. Effects of short-term carbohydrate or fat overfeeding on energy expenditure and plasma leptin concentrations in healthy female subjects. Int J Obes Relat Metab Disord. 2000;24:1413–1418. doi: 10.1038/sj.ijo.0801395. [DOI] [PubMed] [Google Scholar]
- 36.Wijers SL, Saris WH, van Marken Lichtenbelt WD. Individual thermogenic responses to mild cold and overfeeding are closely related. J Clin Endocrinol Metab. 2007;92:4299–4305. doi: 10.1210/jc.2007-1065. [DOI] [PubMed] [Google Scholar]
- 37.Joosen AM, Bakker AH, Westerterp KR. Metabolic efficiency and energy expenditure during short-term overfeeding. Physiol Behav. 2005;85:593–597. doi: 10.1016/j.physbeh.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Ravussin E, Schutz Y, Acheson KJ, et al. Short-term, mixed-diet overfeeding in man: no evidence for “luxuskonsumption”. Am J Physiol. 1985;249:E470–E477. doi: 10.1152/ajpendo.1985.249.5.E470. [DOI] [PubMed] [Google Scholar]
- 39.Klein S, Goran M. Energy metabolism in response to overfeeding in young adult men. Metab Clin Exp. 1993;42:1201–1205. doi: 10.1016/0026-0495(93)90281-r. [DOI] [PubMed] [Google Scholar]
- 40.Bray GA, Flatt JP, Volaufova J, Delany JP, Champagne CM. Corrective responses in human food intake identified from an analysis of 7-d food-intake records. Am J Clin Nutr. 2008;88:1504–1510. doi: 10.3945/ajcn.2008.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]