Abstract
The role of nitric oxide (NO·) as a mediator of cancer phenotype has led researchers to investigate strategies for manipulating in vivo production and exogenous delivery of this molecule for therapeutic gain. Unfortunately, NO· serves multiple functions in cancer physiology. In some instances, NO· or nitric oxide synthase (NOS) levels correlate with tumor suppression and in other cases they are related to tumor progression and metastasis. Understanding this dichotomy has been a great challenge for researchers working in the field of NO· and cancer therapy. Due to the unique chemical and biochemical properties of NO·, it’s interactions with cellular targets and the subsequent downstream signaling events can be vastly different based upon tumor heterogeneity and microenvironment. Simple explanations for the vast range of NO-correlated behaviors will continue to produce conflicting information about the relevance of NO· and cancer. Paying considerable attention to the chemical properties of NO· and the methodologies being used will remove many of the discrepancies in the field and allow for in depth understanding of when NO-based chemotherapeutics will have beneficial outcomes.
Keywords: Nitric oxide, cancer, metastasis, nitric oxide synthase, chemotherapy
INTRODUCTION
Nitric Oxide (NO·) is a ubiquitous free radical signaling molecule that regulates many cellular processes including angiogenesis, smooth muscle tone, immune response, apoptosis, and synaptic communication [1]. In addition to the many normal physiologic functions of NO·, it has been implicated in the etiology and progression of many disease processes including cancer [1–3]. The role of NO· in cancer is complex and spans the range from cause to cure. Nitric oxide can be genotoxic under certain circumstances indicating it may be involved in the etiology of many cancers. At the same time, numerous studies suggest that NO· suppresses various cancer phenotypes. As a result, many experimental chemo-therapeutic agents have been designed to manipulate the bioavailability of NO·. Nitric oxide appears to be stimulatory or inhibitory on cancer depending on a variety of factors. Despite the general acceptance of this paradox, interpretation of research in this field continues to provide as many questions as answers.
Nitric oxide has been implicated in various aspects of metastasis, which causes 90% of cancer related deaths[4], leading researchers to actively search for ways to pharmacologically manipulate NO-mediated responses. Nitric oxide is not limited to classical receptor-ligand interactions. Once it is synthesized, NO· targets a wide variety of molecules within the cell. As a result, there are numerous examples taken from seemingly similar circumstances that implicate NO· in the progression of cancer and other examples demonstrating its inhibitory properties. While there are many proposed reasons for the contradictory behavior of cancer in response to NO·, the simplest and probably most overlooked explanation is that the phenotypic responses of cancer cells are determined by the chemical properties of NO·. The effect NO· has on signaling events can be dramatically different depending on the concentration and the duration of exposure [5]. Both of these properties are affected by the cellular milieu and redox environment of the cell. It is the cellular environment that determines the types of chemical reactions of NO· and these chemical reactions will influence the concentration of NO· and its interactions with cellular targets. Thus effective intervention of NO· signaling pathways involved in cancer progression will have to consider the type of cancer being targeted and where and when NO· is being released. Since NO· can have either stimulatory or inhibitory effects on cancer progression, effective treatments will have to either raise or lower the bioavailability of NO· at the correct time, duration, and location. Though conceptually simple, achieving this with a high level of precision presents a significant technical challenge.
In addition to the molecular biological reasons for differences in NO· signaling, the field is complicated further by the wide variety of methods used to analyze NO· cancer interactions. Nitric oxide synthase (NOS) knockouts, pharmacological inhibition of NOS, and a slew of different NO· donor compounds are all used to infer the role of NO· in cell culture and organisms. All of these strategies are valid means to address certain questions, but it is important not to overstate the results achieved by any one method. It has been shown, for example, that laparotomized nude mice injected with MDA-MB-231 breast cancer cells demonstrated significantly less bone metastasis if they were concomitantly treated with a NOS inhibitor (L-NAME) [6]. Conversely, when MDA-MB-231 cells in culture were treated with the NO-releasing com-pound JSK-1, it inhibited their ability to invade through matrigel and up-regulated tissue inhibitor of metalloprotease 2 (TIMP2) [7]. Others have shown that NO· donors can increase the in vitro migration and invasion of these cells (unpublished results). Thus, even when researchers use the same cell line, their conclusions concerning the impact of NO· on the metastatic properties of breast cancer differ greatly. Although excellent studies, when compared with one another, they also emphasize the complexity of these processes and not surprisingly indicate that there are multiple additional factors that must be considered before assigning the outcome solely to the presence or absence of NO.
In fact, it is generally true that the interaction of the tumor with the surrounding soma is highly varied and important in defining the metastatic fate of the tumor [8–11]. It is clear that in order to appreciate the influences of NO· on cancer progression a more extensive understanding of the entire molecular makeup of tumor cells and their localized environments is necessary. In addition to NO-producing tumors, the production of NO· in surrounding somatic tissue can also impact the metastatic progression of a tumor. The following review examines some of the discrepancies associated with the field of NO· and cancer with an emphasis on metastasis and therapeutic intervention and also points out various possible explanations for these dissimilarities.
NITRIC OXIDE SYNTHASE AND METASTASIS
Metastasis is a multistage process by which tumors colonize other sites of the body. The canonical order of events (local invasion, intravasation into the circulation, transport through the circulation, extravasation from the circulation, and finally colony formation in a distant tissue) are broadly true for most types of cancer [12–14]. Although these events are similar for most tumors, there are tissue and tumor specific distinctions which manifest in molecular and phenotypic differences. Understanding the role of NO· in metastatic progression will require a thorough mapping of the molecular events associated with these differences.
One of the defining features of metastasis is the specificity by which some cancers colonize specific tissues [15, 16]. Prostate cancer, for example, largely metastasizes to the bone [17] while ocular melanoma is almost always confined to the liver [18]. Breast cancers, on the other hand, colonize a range of tissues including bone, brain, liver and lung [19]. Interestingly, lung adenocarcinomas colonize the same tissues, but the time scale of metastasis is drastically different [20]. Lung adenocarcinomas tend to metastasize within months of detection [21, 22] while breast cancer metastasis can reoccur after years of remission [23, 24]. Numerous studies have attempted to find a correlation between the expression of various mRNA’s or proteins and the onset of metastatic behavior. Ideally, researchers are looking for proteins that are not only predictive of a metastatic phenotype but that are also fundamental in the malignant progression. The NOS proteins are potential markers that have received considerable attention in the past decade. Unfortunately, there does not seem to be an overall trend which conclusively shows that NOS expression is indeed leading to more aggressive disease phenotypes and poor patient outcome across the board. The following summaries of the studies examining NOS expression in tumors by no means cover the entire field (a comprehensive review already exists [3]). Rather, they highlight the ambiguity of the story at present.
iNOS
Nitric oxide is synthesized enzymatically from NOS. Although there are three isoforms of this enzyme, the inducible form (iNOS) has the most compelling relationship with cancer progression and metastasis. Expression of iNOS is correlated with poor outcomes in terms of patient survival in stage III malignant melanoma patients [25] and to a lesser extent breast cancer patients [26]. Patients with stage III ovarian cancer showed a better response to first line chemotherapy when iNOS was not present [27]. Increased iNOS expression in head and neck squamous cell carcinoma (HNSCC) was found in 41% of SCC samples examined but only in 9% of precancerous dysplasias [28]. Not only does iNOS appear to promote the growth of primary tumors in some cases, but it has been implicated in the metastatic process as well. One study examining HNSCC metastases in regional lymph nodes found that 22/27 cases showing an extracapsular spread phenotype stained positively for iNOS while only 8/21 of those that were still encapsulated expressed iNOS [29].
Studies using a p53−/− mouse model showed that treatment of these animals with C. Parvum increased iNOS expression and NO· metabolites in the blood of in these animals. They also demonstrated that C. Parvum treatment increased the tumorigenesis in these animals. The researchers argue that, “NO· production under inflammatory conditions can inhibit apoptosis, increases proliferation, and modulates the immune profile, giving rise to an internal milieu that is conductive to tumor growth [30].” While their data may support this conclusion, it is of interest that they also show that a double mouse knock out, p53−/− iNOS−/−, without C. Parvum treatment shows the same increase in tumorigenesis as the p53−/− iNOS+/+ animals treated with C. Parvum. Thus while iNOS correlates with tumorigenesis when a large inflammatory response is induced, it is also true that lack of this enzyme, either through development or early disease progression, is also detrimental to the animal.
There are even experimental examples examining the correlation between iNOS and cancer progression in which seemingly identical experiments have produced conflicting results. In one example, two different groups crossed female C57BL/6-ApcMin/+ mice with male C57BL/6-iNosTm1Lau (iNOS−/−, disruption of exons 12 and 13 which constitute the calmodulin binding domain) mice. Both groups obtained their animals from the same source. ApcMin/+ mice have a germ-line nonsense mutation at codon 850 of the adenomatous polyposis open reading frame (Apc) and spontaneously develop multiple polyps in the small and large intestines [31]. Interestingly, one group found that there were fewer polyps in the intestine [32] while the other found more in the double knockout animals [33]. The main experimental difference between these studies was that the group finding fewer polyps killed the animals at 15 weeks while the other killed the animals at ∼8 weeks. This indicates a possible age or time dependent interaction of iNOS or NO· with this genetic background. Related studies found that induction of aberrant crypt foci in the colons of rats by treatment with azoxymethane (AOM) could be suppressed by iNOS inhibition [34, 35]. Collectively, these studies emphasize the difficulty in correlating NOS expression with phenotypic outcome.
Many attempts to explore the correlation between iNOS expression and poor patient outcome in experimental models have found conflicting results. Several groups transfected iNOS into cancer cell lines and observed their behavior with various in vitro assays and subsequent injection into nude mice. The oral cancer cell line b88t transfected with iNOS, for example, showed less migration in Boyden chamber assays and formed smaller tumors in nude mouse xenographs [36]. Similarly, transformation of K-1735 murine melanoma cells with iNOS caused increased apoptosis in vitro and less metastasis in nude mouse injections [37]. Transfection of DLD-1 human colon adenocarcinoma with murine iNOS decreased in vitro proliferation of this cell line. When these transformed cells were injected into nude mice, however, the resultant tumors grew much faster and had increased vascularization [38]. This is by no means a comprehensive list of studies of this nature, but they are indicative of the general trend. Introduction of iNOS tends to decrease in vitro indicators of metastatic potential. The in vitro studies, however, are not on the whole predictive of metastatic behavior in animal models [39].
Perhaps most interestingly, a seminal cancer microarray study attempting to identify a molecular signature of metastasis analyzed the mRNA expression patterns of adenocarcinomas [40]. They examined 64 primary adenocarcinomas and 12 metastatic adenocarcinoma nodules and found that down-regulation of iNOS was one of the markers of a metastatic phenotype. All tissue samples in this study had diverse points of origin including: breast, lung, prostate, colorectal, uterus and ovary. Using hierarchical clustering, the researchers found 64 mRNAs that were up-regulated and 64 mRNAs that were down-regulated consistently among all of the metastatic nodules. Interestingly, a few of the primary tumors exhibited an expression profile of these 128 mRNAs that was strikingly similar to the metastatic profile. Those patients whose primary tumor carried this profile had a more rapid metastatic onset and decreased long term survival rate. iNOS was one of the marker mRNAs that showed a significant decrease in expression in the metastatic nodules and the primary tumors that were more likely to become metastatic. The expression of iNOS in the primary tumors not carrying the metastatic mRNA expression profile was highly varied with many of the primary tumors up-regulating this mRNA and others down-regulating it. Thus in these studies, the expression of iNOS alone was not predictive of phenotype. Another fascinating observation embedded in this study was that when the entire expression prolife, encompassing 9,248 highly varying mRNAs, of the metastatic adenocarcinoma nodules was used to find primary tumors with similar expression profiles, the patients with those primary tumors showed no differences in metastatic onset and survival using the Kaplan-Meyer survival analysis. In other words, much of the differential regulation of mRNAs has little or nothing to do with the metastatic phenotype. When all of this variation is analyzed together, no predictive value could be obtained. Only when the set of mRNAs was reduced to those mRNAs that varied in the same manner among all twelve metastatic nodules did the differential expression of the mRNAs become predictive of phenotype. All of the 128 mRNAs showed a random variation in the non metastatic primary tumors implying that using one indicator alone is not sufficient to accurately predict a metastatic outcome. This study went further to show that they could use as few as 17 of the 128 mRNAs to classify samples as metastatic with almost the same level of statistical significance. Thus the minimal number of indicators that were required to predict phenotypic outcome far surpasses what is ever attempted in any of the immunohistochemical (IHC) iNOS studies. It is possible that proteins may be more predictive than mRNAs, but trying to correlate phenotype with one molecular change is challenging. While other studies have found that iNOS expression appears to correlate with a more invasive phenotype in some cancers, it is possible that some of the positive correlations between iNOS expression and increased malignancy are random. Given the more than 20 thousand open reading frames (ORF) with an undefined number of splice variants (it is known, for example, that one Axon guidance receptor often studied in Drosophila melanogaster (Dscam) has more than 38,000 splice variants alone [41] and standard quantitative molecular techniques certainly do not account for this variation), it is inevitable that some mRNA and protein molecules show statistically significant correlative variation with some aspect of disease progression in a given study while nevertheless contributing nothing to the process itself and perhaps not even being predictive among the population as a whole.
eNOS
Studies exploring the roll of endothelial NOS (eNOS) are even more ambiguous. A recent study found that eNOS−/− mice injected with N-diethylnitrosamine, a known carcinogen, had significantly greater tumor formation in the liver compared to wild-type controls. They also found that injection of tumor cells over expressing eNOS on a plasmid into the hepatic circulation formed less micro-metastasis in the liver [42]. On the other hand, several studies have found that eNOS is associated with tumor growth, invasion and angiogenesis in general and breast cancer in particular [43, 44]. Other studies have found that HIF1α stabilization by NO· leads to the up regulation of vascular endothelial growth factor (VEGF) which in turn can phosphorylate eNOS [45, 46] strongly promoting angiogenesis.
nNOS
Information on the relationship between neuronal NOS (nNOS) and cancer is scarce. One study examined 29 patients with grades II – IV astrocytoma and performed IHC staining for nNOS on surgically removed tumors. They found an increase in both distribution and intensity of staining with increasing grade of the disease [47]. No functional information was pursued in these studies. An earlier study found an increase in IHC staining of nNOS in grade III and IV gliomas compared to grades I and II. When they attempted to perform NOS activity assays, however, they could not detect increased NOS activity despite the IHC results[48]. It is possible that nNOS expression correlates with increased metastasis in some cases, but overall the data on nNOS and cancer are thin and inconclusive.
NOS AND THE TUMOR MICRO ENVIRONMENT
The last decade has seen significant changes in how we think about molecular and cellular biology in general. Researchers have increasing moved away from one-gene one-function, linear pathway explanations for maintenance of cellular homeostasis and have also begun to analyze organs and tissues on a system-wide basis. In terms of cancer, there has been a steady growth in the appreciation of the role of the tumor microenvironment in disease progression[8]. In the past, it was typical to disregard the non tumorigenic cells of the tumor despite the fact that these cells can make up to 99% of the tumor in the extreme case of Hodgkin’s lymphoma [49]. It has become increasing clear that these cells engage in a dialog with the tumor cells and often release compounds that aid in the degradation of the basement membrane, further releasing tethered growth factors and often secreting chemokines that maintain the growth and health of the tumor cells [49]. In terms of NO·, it is interesting that in some cases NOS is expressed by the cells of the primary tumor and other times it is only found in tumor associated macrophages (TAMs)[3]. TAMs are often present in large numbers in ovarian, kidney, breast, thyroid, colon, and melanoma [50], and correlate strongly (>80%) with a poor prognosis [51]. On the other hand, macrophages are integral to the innate immune response that kills many tumors before they ever become malignant [52–54]. It is possible that in some scenarios the timing of NOS induction may be critical in determining its impact on the cancers progression. One very interesting study examined tumor formation in wild type and iNOS−/− host animals after they were injected with two EMT-6 breast cancer cell lines (either + or − for iNOS expression) [55]. The researchers found that while iNOS expression in the cancer cell line did not change the ability of these cells to localize to the lungs in wild type animals, it did decrease their ability to form tumors at later time points. They went further to show that injection of these same cells into an animal with an iNOS −/− background increased the formation of tumors from both cell types. In this model, NOS expression in the cancer cells did not affect their ability to migrate to the target tissue, but it inhibited their ability to form successful colonies. At the same time, both iNOS positive and iNOS negative EMT-6 cells formed fewer nodules in the lungs of iNOS−/− mice. Thus it seems that careful attention to the location of NOS expression will be critical when determining its impact on metastatic progression. A very recent study found that the pro-drug AQ4N, which is activated by LPS stimulated macrophages, induced standby death in HT1080 and HC116 cancer cell lines under hypoxic conditions but not under aerobic conditions [56]. The researchers of this study hypothesized that the reductase domain of iNOS, which shares a strong similarity to p450 reductase domains, was able to more effectively reduce the pro-drug to its active form when the enzyme was not bound with oxygen. Certainly, more work needs to be done to confirm that the conversion of AQ4N to the topoisomerase inhibitor AQ4 is specifically carried out by iNOS and not one of the other multitude of enzymes differentially regulated by LPS induction. Nevertheless, these studies present a possible novel function for iNOS that has nothing to do with NO· production, and show that even under circumstances where TAMs are driving the metastatic progression of tumors, exploitation of NOS chemistry is a therapeutic option. On the whole, exploration of the tumor microenvironment is in its infancy and is one of the many layers of complexity that must be addressed if we want to understand tumor physiology in general.
CHEMICAL BIOLOGY OF NO
In order to fully appreciate the complexity of NO· signaling in cancer and the immense challenges associated with studying this molecule, a brief discussion of the biochemical and biophysical parameters governing the actions of NO· is required. As stated above, NO· is synthesized by the three isoforms of nitric oxide synthase (eNOS, iNOS, nNOS). The substrates for this enzyme are the amino acid arginine and oxygen (O2). It also requires the cofactors NADPH, FMN, BH4, and FAD. Changes in any one of these substrates or cofactors can have dramatic effects on the production of NO·. Under circumstances of an abundance of cofactors, the rate of NO· synthesis will be a function of both the arginine and O2 concentrations. Arginine availability can vary based on cellular uptake and competing consumptive pathways (i.e. arginase and the Urea Cycle). Oxygen availability is a function of its delivery from the vasculature and the rate at which it is consumed locally via mitochondrial respiration. The Km’s for arginine and O2 are different for each NOS isoform indicating that changes in these substrates will alter the output of NO· differentially dependent upon the isoform. For example, the Km for O2 for eNOS is 23 µM, for iNOS is 135 µM, and for nNOS is 350 µM [57]. This indicates that the rate of NO· synthesis from nNOS will be dramatically affected by oxygen fluctuations while production from eNOS will remain comparatively constant. Nitric oxide production in a hypoxic tumor could be substantially lower than a tumor which is well oxygenated even if they express an equivalent amount and type of NOS. The presence of NOS protein in a tumor does not necessarily correlate to the rate of synthesis or amount of NO·. Additionally, at a given O2 and arginine concentration the amount and duration of NO· production is different for each isoform. Although outside the scope of this review, the regulation of NOS is also controlled at the transcriptional, translational, and posttranscriptional level all of which can affect the magnitude of NO-mediated responses.
The rate, amount, and duration of NO· synthesis are only a few of the multiple factors determining the interaction of NO· with target molecules. Once synthesized the impact of NO· on tumor cell phenotype is determined by a variety of fundamental parameters. The steady-state concentration of NO· is an important determinant of its biological actions. This will determine its diffusional distance and thus the number and types of target molecules it interacts with. Unlike most signaling molecules, the biological half-life of NO· is short, ranging from milliseconds to a few seconds [58]. The steady-state concentration of NO· is determined by its rate of production and its rate of disappearance (metabolism). Interestingly, both the synthesis and metabolism of NO· are O2-dependent processes. The metabolism of NO· is slower at low oxygen concentrations but its rate of synthesis also decreases. Many tumor-related proteins are regulated by NO· in a concentration-dependent manner emphasizing how oxygen gradients, which control the steady-state concentration of NO·, could dramatically affect the tumor phenotype. Some proteins, such as soluble guanylate cyclase (sGC), are fully activated at low concentration of NO· (1nM) [59], and others, like p53, require considerably higher NO· levels (>400 nM) [60] to be activated. Interestingly, In vivo concentrations of NO· are still not precisely known. Recent advances in modeling techniques and NO· detection methods suggest that concentrations might be much lower than previously thought. In an excellent review by Hall and Garthwaite, the authors argue that the in vivo NO· concentration generated by NOS is roughly 100 pM to 5 nM[61]. At these concentrations, both the diffusional distance of NO· and its interaction with concentration-dependent targets would be substantially different than at the nM-µM NO· concentrations previously thought to exist under normal physiological conditions.
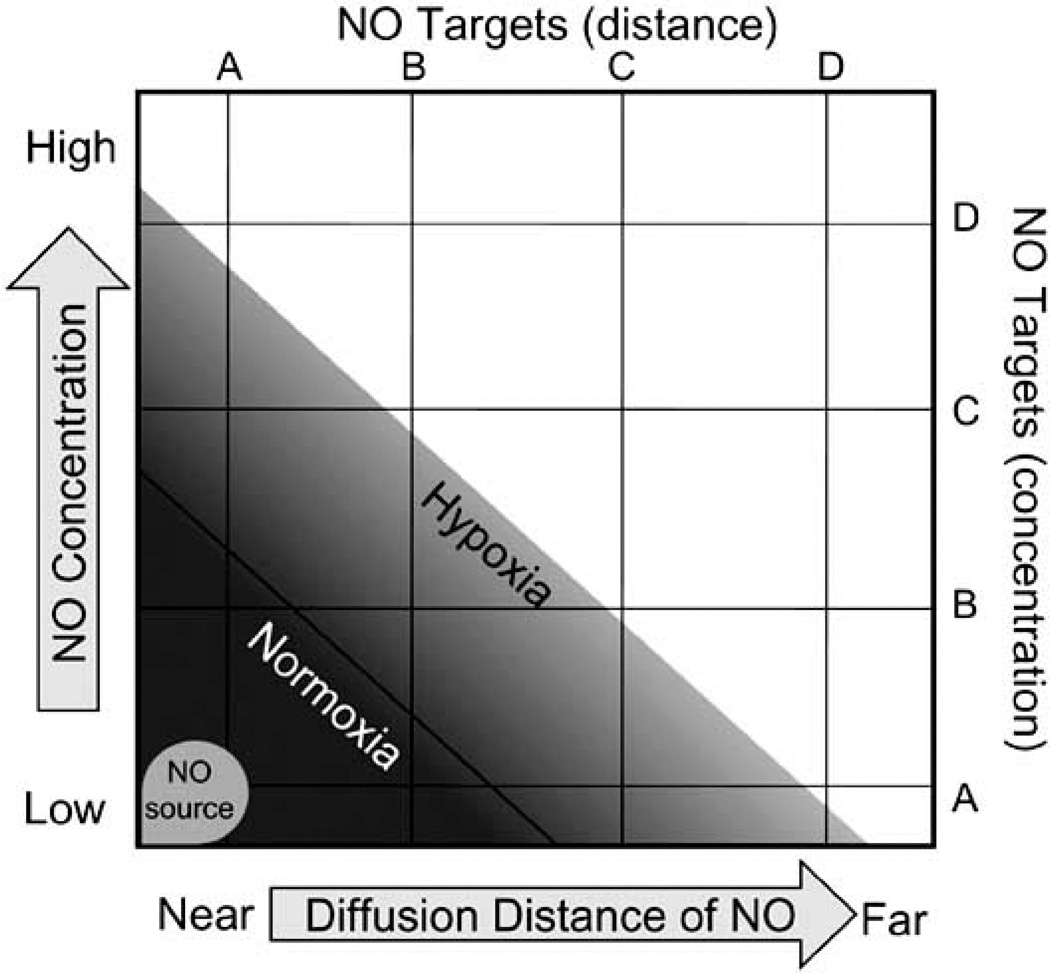
In addition to concentration dependent effects of NO·, there are also temporal parameters. Fig. (1) is a conceptual representation of how the tumor oxygen concentration could dramatically impact therapeutic NO· responses. If an NO-generating drug was administered, its influence on a hypoxic tumor could be considerably different than its effect on a well oxygenated one. Assuming drug delivery is not compromised by oxygen, substantially greater NO· concentrations will be achieved in hypoxic tumors as opposed to normoxic ones because the metabolism of NO· is much slower at low O2 concentrations. Since the concentration of NO· is a function of its rate of production (release from the drug) and its rate of disappearance (cellular metabolism), hypoxia will reduce its rate of disappearance increasing local NO· concentrations. Increasing the local concentration of NO· will allow it to interact with different cellular targets based solely on target-specific concentration-dependent differences in their sensitivity to NO·.
Fig. (1).
The effect of oxygen on nitric oxide releasing chemotherapeutics. Oxygen affects both the concentration of NO (y-axis) and the distance it diffuses (x-axis). The half-life of NO is a function of the oxygen concentration; low oxygen (hypoxia) increases the half-life of NO. The longer the half-life of NO, the greater its local concentration will be and the further distance it will diffuse from its point of origin. Various cellular targets (A, B, C, D) will be differentially affected by NO based simply on its local concentration. Hypoxia will extend the distance that NO diffuses and therefore allow it to interact with a greater number of targets that are a further distance away (x-axis, C and D). Hypoxia will also raise the local concentration of NO and increase the likelihood that it will activate proteins which are less sensitive to NO (y-axis, C and D). The impact of O2 on NO concentration is an important factor that greatly influences the effect of NO-based chemotherapeutics on tumor phenotype.
Some NO-regulated proteins respond immediately to the presence of NO· while others require hours. Depending on the response time, these proteins can be classified as “immediate responders” or “delayed responders” [5]. Taken together, these factors indicate that tumor cell phenotypes can be dramatically altered by changing the amount and/or duration of NO· exposure. The up or down-regulation of NOS protein levels does not necessarily produce equivalent changes in NO· concentration. Consequently, serious care must be taken when correlating NOS expression with beneficial or deleterious tumor phenotypes or even attributing these effects to NO· at all. Although in general the presence of NOS is a good indicator that NO· is being produced, without knowing some specifics about the tumor microenvironment it is extremely difficult to assess the amount of NO· it is producing.
The local redox environment of a tumor must also be considered. The presence of other radical species, such as superoxide (O2−), can substantially alter the concentration of NO·. Superoxide reacts with NO· in a diffusion controlled manner to produce peroxynitrite (ONOO−) which can then further react to form a variety of end products [62, 63]. Due to the rapid nature of this reaction, the generation of O2− can actually scavenge NO· thereby lowering the steady-state concentration of NO· and preventing its reaction with target molecules. Therefore, the presence of other radical species can alter NO-mediated cellular phenotypes by diminishing its steady-state concentration [64]. Since NO· moves away from its point of synthesis by random diffusion, its biological half-life will determine its diffusional distance: the longer the half-life of NO·, the greater distance it will diffuse [58]. Any process that increases or decreases the half-life of NO· and its steady-state concentration will have an influence on the amount and type of targets that it regulates. It can be seen from Fig. (1) how the NO· concentration (y-axis) will affect the diffusional distance (x-axis) of NO· and thus the number of potential targets it interacts with. Because oxygen (or radical scavengers of NO·) will reduce the local NO· concentration, NO· will not travel as far from its point of origin and therefore its influence on target molecules will be diminished.
These are a few important points to consider when assessing the impact of NO· production on tumor phenotype. It should be noted that many of the determinants of the actions of NO· are independent of its source (i.e. endogenously produced from NOS or exogenously generated from NO-releasing drugs). Like NOS, NO· generating anticancer drugs will be influenced by the local O2 concentration and the redox environment of a cell or tumor. Unlike NOS, however, most NO· donating compounds do not use oxygen as a substrate and will still generate NO· in anoxic conditions.
METHODOLOGICAL CONSIDERATIONS
While there is a wealth of literature attempting to explain the differential results found in numerous NO· cancer studies, little attention has been paid to the underlying methodological differences of the primary studies. Many literature reviews consider the numerous and diverse NO· donating compounds as equivalent sources of NO·. Others switch readily between endogenous vs. exogenous NO· sources and rarely consider differences that may be attributed to differing model systems. This broad approach is valid for developing a general idea of what NO· might do in the cancer setting, but it is increasingly clear that the impact of NO· under any given set of experimental conditions can be highly varied. Thus finding therapeutic targets that involve NO· biology will require paying close attention to the differing experimental designs and not simply attributing their outcomes to “NO· signaling”.
MODELS
Historically, cancer therapies have targeted the increased proliferation of cancer cells. Thus many late phase drug discovery programs rely heavily on fast growing xenograph models to determine the efficacy of their compounds. The ability of these models to predict the outcome of clinical trials, however, is limited [65, 66] partially because drug responsiveness is dependent upon the site and type of implantation [67]. It has been shown, for example, that implantation of tumor cell lines maintained in culture do not form the same structure and microenvironment as slices of implanted primary tumors. More extensive use of models that do not rely on subcutaneous injection and rather try to recreate complex tumor microenvironments will more accurately determine the impact a given compound might have on the metastatic progression of the disease. While this is generally true for the future of cancer research, it has particular relevance to a field that is so full of contradictory data. Further, finding that cancer cells have multiple modes of motility that respond differentially to pharmacological treatment implies that, while assays such as Boyden chambers are excellent tools, they may be limited in predicting the in vivo impact a compound has on the metastatic process [68, 69].
Microarray studies have found that acquisition of invasive abilities are an early and ongoing process in tumorigenesis [70]. Anti-invasive therapies might be most effective early on in the disease progression and could thus explain some of the failures of compounds used in late disease clinical trials. Therapies inhibiting NO· production or delivering increased levels of NO· need to be carried out on all stages of cancer as many of the properties of NO· suggest that it has both anti- and pro-developmental functions at early stages. Previously mentioned studies demonstrated that NOS expression had an opposite impact on tumor formation depending on whether it was expressed in the host animal or developing tumor [55]. One course of action that would help to resolve the role of NO· in cancer would be to digest both tumor and surrounding somatic tissue and examine mRNA expression of individual cells by microarray. Large amounts of molecular information are lost when tumor cells are analyzed as a lump average in terms of their molecular signatures. Although this is complex and expensive proposal, obtaining in depth knowledge of the molecular profiles of all cells involved with the disease process is likely the only way to resolve many of the seemingly contradictory results. These data could then be compiled with large scale IHC studies to generate enormous databases that would allow for more informed decisions when designing anti-cancer compounds. The unfortunate implication is that many of the types of studies needed to deconvolute this morass of data will be difficult to perform in small laboratories, and not profitable enough in the short run to be carried out by large companies. Metastatic progression of cancer, however, is a vastly complex process that will require equally complex, collaborative efforts to effectively combat.
NO· DONOR COMPOUNDS
Nitric oxide donating compounds, which release free NO·, or NO· mimetics, which have similar activity as free NO·, have potential as cancer therapeutic agents as well as being extremely useful tools to probe the chemistry, biochemistry, and cell signaling events brought about by NO· exposure. All NO· donor compounds, however, are not created equal. Some compounds spontaneously release free NO· while others require enzymatic activation or an oxidation/reduction event to generate NO· [71]. Other compounds don’t release free NO· at all but instead transfer a NO· equivalent to another molecule often in the form of a nitrosonium ion (NO+). These compounds have NO-like activity without generating any free NO·. The importance of understanding the chemistry of these compounds cannot be understated. Many of the discrepancies seen in the literature might be easily explained by methodological or chemical differences attributable to the choice of NO-donor compound used in a particular experiment. The most commonly used NO· donors fall into three broad categories: clinical nitrovasodilators [72], S-nitrosothiols [73], and NONOates (diazeniumdiolates) [71, 74]. In addition to these, other compounds are available to explore the redox chemistry of NO· (i.e., NO· metabolites and other nitrogen oxides) and can provide important insights into NO· biology. These include synthetic peroxynitrite (ONOO−) [75], SIN-1 (3-morpholinosydnonimine) which releases NO· and superoxide (O2−) [76], and Angeli's salt (Na2N2O3), which produces nitroxyl (HNO) [77]. All of these compounds have their experimental uses but cannot be considered equivalent.
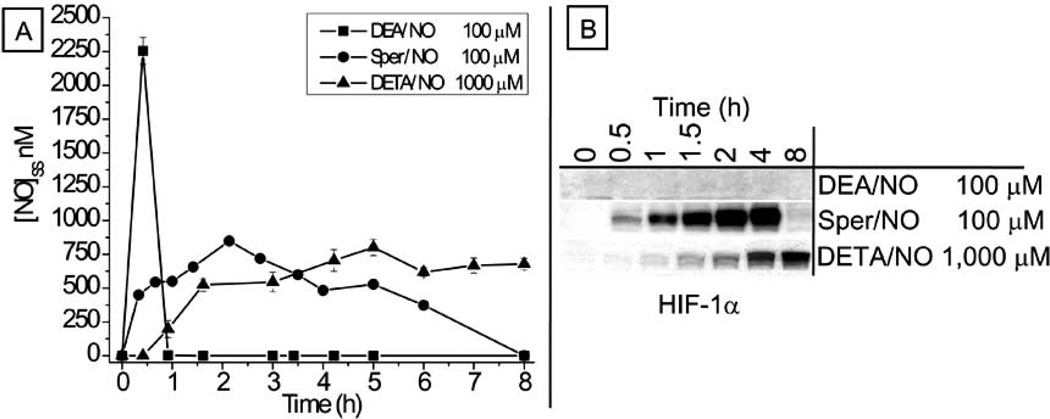
Another important and often overlooked point to consider when using any of these compounds is the differences in their release kinetics. This parameter can have a great influence on experimental outcome (in vivo and in vitro), as it largely determines the level of NO· present. Examining one class of NO-releasing compounds (NONOates), for example, demonstrates how seemingly similar experimental conditions can give dramatically different results. If an in vitro experiment was performed where three separate plates of cancer cells were exposed to NO· for the same amount of time, entirely different results would most likely be obtained depending upon the NO-donor that was used. Fig. (2) demonstrates the effect of treating three identical plates of cultured tumor cells with different NONOates (DEA/NO 100 µM, Sper/NO 100 µM, and DETA/NO 1,000 µM). Although all of these compounds spontaneously decay to release free NO·, their decomposition kinetics are drastically different. DEA/NO has a half-life of ≈2−4 min; Sper/NO has a half-life of about 39 min; DETA/NO has a half-life of >20h [71]. This complicates the experiment in several key ways. Although the starting NO-donor concentrations are the same for DEA/NO and Sper/NO (100 µM), both the duration of NO· exposure and the steady-state concentrations of NO· will be significantly diverse based solely on differences in their NO· release kinetics. One of the big misconceptions about using NO-donor compounds is that the starting donor concentration somehow directly translates to the actual concentration of NO· that the cells are experiencing. This is a problem for in vitro and in vivo experiments. A large starting concentration of a NO-donor does not necessarily indicate a large concentration of NO· will develop. This is exemplified by Fig. (2A) which shows differences in the measured steady-state NO· concentrations in the media of NONOate treated cells. It can be seen that cells treated with DEA/ NO will experience a very high concentration of NO· for only several minutes. On the other hand, cells treated with Sper/NO will be exposed to NO· for several hours and the DETA/NO treated cells will be exposed to a continuous steady-state NO· concentration for days. Since many NO-regulated proteins, like HIF-1α, respond in a concentration and duration dependent manner to NO· [60], it’s not surprising that these different treatment conditions would give drastically different results and lead to entirely different conclusions regarding the influence of NO· on this tumor-related pathway. Fig. (2B) demonstrates the regulation of HIF-1α by NO· using the identical NONOate treatment conditions of Fig. (2A). Looking at cells treated with DEA/NO, it appears that NO· does not regulate HIF-1α at all. When cells are treated with Sper/NO alone, NO· regulates HIF-1α at time points <4 hours and has no impact at time points >4 hours. The experiment with DETA/NO indicates that NO· regulates HIF-1α over a wide range of time points. Furthermore, although a concentration of 100 µM was sufficient to regulate HIF-1α when Sper/NO was use, this concentration of DETA/NO would not generate sufficiently high NO· concentrations to elicit this response. Even though it is already well established that at HIF-1α can be stabilized by NO· [60, 78, 79], the data presented here highlight the importance of donor compound release kinetics and emphasize how contradictory conclusions can be drawn based upon the time point, choice of donor, and donor concentration. Simple methodological differences can have a substantial impact on the interpretations of NO-regulated signaling pathways including those where a role of NO· has yet to be elucidated. These principals have to be kept in mind when analyzing both in vitro and in vivo data. Although a more detailed explanation of the differences between various NO-donor compounds is out of the scope of this review, many other factors should be considered before choosing a specific NO· donor for an experiment or clinical application. The choice will likely influence the outcome in ways that are not always immediately obvious. Some factors that can affect the release of NO· from NO-donating compounds or the concentration of NO· after it has been released include: light, pH, transition metals, media volume, +/− serum, culture vessel size, temperature, cell density, cell type, duration of exposure, scavengers, redox environment, pO2, etc. [71]. A greater appreciation of these parameters will improve experimental therapeutic design and allow researchers to determine what outcomes can be directly attributed to NO· exposure and what outcomes are the result of confounding variability introduced by experimental setup.
Fig. (2).
All donor compounds are not created equal. A) Measurements of NO in the media of NONOate treated cells. MCF-7 cells were treated with either DEA/NO, Sper/NO or DETA/NO, and NO concentrations were determined from 100µl aliquots of medium. Samples were analyzed by chemiluminescence at the indicated time points. B) Western blot demonstrating the temporal relationship between NO exposure and HIF-1α accumulation in MCF-7 cells exposed to the three different NONOates. Cells were serum-starved overnight, treated with NONOates and harvested at the indicated time points (modified from [60]).
Examination of microarray studies performed on the same cell line using different NO· donor compounds highlights the importance of paying careful attention to the chemical environment of the experiment when interpreting the results. Two different microarray studies using U937 cells provide an excellent example. In the first study, U937 cells, a non Hodgkin’s lymphoma isolated in 1974, were differentiated for 48 hrs with PMA then treated for 6 hrs with 400 µM of the nitrosothiol GSNO. RNA was then analyzed using Affimetrix HuGeneFL 6800 microarrays®[80]. The second study treated U937 cells 500 µM DPTA (3 h t1/2), a diazeniumdiolate. 15 min prior to treatment, cells were incubated with PTIO. Samples were collected at 4 and 14 hrs and RNA was analyzed using printed cDNA microarrays [81]. Examination of all of the genes listed as differentially regulated revealed that only 16% of the genes overlapped between the two studies. Some of the differential expression is undoubtedly caused by the use of different array platforms, but the vastly different treatment methods clearly are not targeting the same signaling pathways in the two experiments. This seems obvious when their methods are examined closely. Careful considerations of methodological differences, however, are largely overlooked in the review literature. The first study is investigating the effect of NO· on gene regulation using a nitrosothiol NO-donor. Although these results are interesting in their own right, caution must be taken not to overstate the role of NO·. It is well known that nitrosothiols can result in NO-like activity without releasing any free NO·. The methodology used in the second study favors the formation of higher nitrogen oxides (like N2O3) which again can have different and unique chemistry from that of pure NO·. The biological importance of nitrosothiols and various nitrogen oxides is an area of intense research and debate yet their significance is still a mystery. While these are both excellent and potentially important studies, they are fundamentally different and cannot be analyzed from the standpoint of NO· on gene regulation.
NOS INHIBITION
Nitric oxide inhibits the progression of cancer by decreasing tumor growth, angiogenesis, migration, metastasis, etc. Conversely, there are many instances where NO· augments these same tumorigenic properties. In these situations, from a therapeutic standpoint, it would be advantageous to down-regulate NOS expression or inhibit the endogenous production of NO·. Specific targeting of tumor NOS enzymes using NOS inhibitors is one approach that has been applied with mixed results. Many NOS inhibitors are structural analogs of arginine that inhibit substrate binding to the enzyme.
Several studies have demonstrated a reduction in tumor blood flow in response to oral administration of NG-nitro-L-arginine methyl ester (L-NAME), a non-specific NOS inhibitor. These effects could be completely reversed by supplementation with L-arginine, indicating changes in NO· production may be attributed to these effects [82]. Another study using a rat p22 carcinosarcoma model demonstrated that oral administration of a NOS inhibitor significantly decreased tumor volume compared to untreated controls over at 40 day period. Interestingly, cessation of NOS-inhibitor administration midway though treatment (day 15) had an immediate effect on tumor volume by accelerating the rate of tumor growth comparable to that of untreated controls [83].
Although NOS inhibition itself may be therapeutically beneficial for certain cancers, there is mounting evidence that selective NOS inhibitors may have even greater chemo-preventive properties when co-administered with other anticancer agents. One study demonstrated that the combined administration of SC-51, a selective iNOS inhibitor, with celecoxib, a COX-2 inhibitor, inhibited COX-2 activity to a greater extent than did either of these agents administered alone. Since COX-2 is known to play a key role in colon tumor development, it is thought that this might be a more efficacious treatment strategy [34]. Another group investigated the effects of iNOS inhibition in conjunction with IL-2 therapy in a C3-L5-mammary-adenocarcinoma-bearing mouse model. These mice were treated with one or two rounds of various doses of IL-2 with or without the NOS inhibitor L-NAME. They concluded that L-NAME had anti-tumor effects and reduced the severity of IL-2-induced capillary leakage which would make it a good candidate for IL-2-based immunotherapy of cancer [84]. In a rat mammary adenocarcinoma model, NOS inhibition prior to or immediately after a carcinogenic insult, protected against the development of cancer suggesting a possible chemo-preventive function. When rats were exposed to whole-body gamma-irradiation followed by a 3-day oral administration of NOS inhibitors, tumor incidence declined significantly (>25%) compared to untreated controls. Additionally, of the mammary tumors that did develop in the irradiated rats, all were estrogen receptor positive while the tumors that developed in the rats administered NOS inhibitors were estrogen receptor was negative. These results indicate that NO· may be involved in the development of estrogen-dependent mammary adenocarcinomas following radiation [85].
Although inhibition of NOS produces somewhat promising results when performed in conjunction with various chemotherapeutic approaches, there are several studies that suggest this might not be the case with radiotherapy. Part of the problem with solid tumors is that they tend to be hypoxic, and the degree of hypoxia correlates with resistance to chemo- and radio- therapies [86]. Nitric oxide, whether released from iNOS or exogenously administered via NO· donating drugs, has been shown to enhance radiosensitivity [87]. This is thought to happen partially through a reduction in hypoxic conditions by increasing tumor perfusion. These findings suggest that combining a NO· donor with conventional radiotherapy or chemo-radiotherapy may be a novel therapeutic strategy for cancer treatment. It also indicates that the use of NOS inhibitors in combination with radiotherapy might actually exacerbate the disease.
NO· RELEASING DRUGS
There is clear evidence that NO· plays a role in various aspects of the progression of certain cancers. The functions of NO· in tumor development, as we have seen, can be either stimulatory or inhibitory. Therefore researchers have investigated both NOS inhibitors and NO-donating compounds as potential anti-cancer agents. Many of the NO-donating compounds are the result of the combination of a NO-releasing moiety with an existing anti-cancer (or other) drug in an effort to increase the efficacy of the original drug. One class of these compounds is the nitric oxide-donating nonsteroidal antiinflammatories (NO-NSAIDs). These drugs consist of a conventional NSAID linked to a NO-releasing moiety and fall in into two main structural categories: organic nitrates and diazeniumdiolates. Compounds in both of these classes have shown promising anticancer properties. Recently, there has been some debate as to whether these compounds actually release free NO· and whether NO· is necessarily for their modes of action at all. The diazeniumdiolate compounds release NO· and have been shown in several models to have potential anti cancer properties. For example, the compound PABA/NO designed by Keefer’s group was shown to inhibit human leukemia cell proliferation and release free NO· upon activation by glutathione [88]. Another compound produced by this group is JS-K. It is a diazeniumdiolate prodrug designed to release free NO· when metabolized by the enzyme glutathione S-transferases. When it was tested in several human multiple myeloma models, it was capable of inhibiting tumor growth possibly through DNA damage [89].
Organic nitrates such as GT-094 synthesized by Thatcher’s group was designed as a potential chemo-preventive agent of colon cancer. This was the first nitrate reported to reduce aberrant crypt foci (by 45%) when administered after a carcinogen in a model of colorectal cancer. This compound shows promise for colorectal cancer chemoprevention due to its anti-inflammatory and cytoprotective activity [90]. Rigas’s group has done extensive research on NO-donating aspirin (NO-ASA), which also falls under the category of organic nitrates. They demonstrated that in human colon cancer models NO-ASA, like aspirin, has powerful antiproliferative effects, yet it is 1,000 more potent than aspirin [91].
Even though these organic nitrates show potential anticancer effects in a variety of different model systems, there is strong evidence that these effects may not be mediated by NO· at all. One proposed mechanism of action of a subset of NO-ASA involves the formation of a quinone methide with the NO-releasing group functioning as a leaving group [92–94]. The quinone methide is then thought to exert its toxicity through reactions with various cellular nucleophiles such as GSH. In fact when the NO-releasing moiety (− ONO2) in one NO-ASA was replaced with a chlorine (−Cl) it was just as potent, if not more potent, in inhibiting the growth of colon cancer cells [92]. In another study, replacement of (–ONO2) by bromine (–Br) in NO-ASA resulted in equal genotoxicity in cancer cells. Furthermore, several of these studies have demonstrated that these NO-NSAIDS actually down regulate iNOS expression [90]. Not only do some NO-NSAIDS not release free NO·, but they also potentially inhibit the endogenous production of NO·. Ironically then, NO-SAIDS might function by decreasing rather than increasing NO· levels and have nothing to do with cellular NO· chemistry.
DISCUSSION
Nitric oxide and cancer is an emerging, exciting, and potentially therapeutically viable field of research. Many important and revealing studies have been conducted that highlight the importance of NO· in the etiology, progression, and treatment of various cancers. Due to the unique chemical properties of NO·, researchers are faced with immense challenges in deciphering the specific contributions of this molecule to any one aspect of the disease. It is well established that the presence of NO· or NOS protein can be either stimulatory or inhibitory on the carcinogenic progress. The purpose of highlighting this dichotomy is not to diminish the potential impact of these studies or suggest that the apparent ambiguity of NO· and cancer has no clinical relevance. This area of investigation will produce promising therapeutic strategies when the underlying reasons for this bipolar behavior are resolved. Once many of the shortcomings in the methodological differences, protocol standardizations, and analytical technology are overcome, great strides can be made in this field.
The chemistry of NO· under biological conditions is exceedingly complicated. Trying to understand the biochemical reactions and cellular responses under controlled in vitro conditions is challenging. It becomes drastically more difficult when these studies are extended to in vivo animal models and of course more complicated still in a clinical setting. Very few fields of study investigate a molecule or drug without having a quantitative picture of its biological concentration. This is a problem with the field of NO· in general and not just in relation to cancer research. There are still no viable direct experimental or clinical means to measure the precise concentration of NO· in vivo. Although many electrochemical and imaging techniques allow for some qualitative measures of NO· in in vivo animals models, we still have very little idea of the actual concentration of NO· in a human tumor. It is difficult to attribute particular tumor phenotypes to the presence or absence of NO·, because its concentration can only be inferred by indirect means. Meticulous in vitro measurements have started to reveal the precise temporal and concentration-dependent parameters of NO· that result in specific and predictable molecular and phenotypic outcomes. Due to the difficulties in direct NO· measurements in live animals, the hope is that using these established in vitro “molecular signatures” of NO· will allow researchers to infer the contributions of NO· to observed in vivo phenotypes.
The predictive value of NOS mRNA and protein levels are problematic as well. Many excellent studies have pointed out that these levels may indeed be associated with a more or less metastatic tumor phonotype. Caution should be taken, however, in assigning the outcome to the presence or absence of NO·. The complexity of NOS regulation and the influence of substrate availability on NO· synthesis make it challenging to know the amount and duration of NO· production with any certainty. Oxygen availability can influence the amount of NO· produced from NOS. Lack of O2, such as in a hypoxic tumor, can abolish NO· production altogether, and several studies have demonstrated that hypoxia alone is enough to up-regulate NOS protein levels. Comparing NOS protein levels in a hypoxic tumor to normal or well oxygenated tissues of the same origin gives the erroneous impression that the hypoxic tumor is exposed to greater NO· levels even thought its synthesis would be halted or drastically reduced at low O2 concentrations. Nevertheless, NOS protein or mRNA levels alone may be sufficient and significant prognostic indicators even in the absence of any NO· production as predictive indicators can be present without contributing to disease etiology.
Given that NO· plays a potentially important role in the advancement or inhibition of certain tumors, therapeutic opportunities to exploit these pathway are being explored. These span the range from inhibition of endogenous NO· production, to exogenous delivery of NO-generating drugs. Both of these strategies have had treatment success in certain cancer models. Since each type of malignancy is drastically different from the next, cancer therapy must be specifically tailored to both the type and stage of the diseases. This is true for cancer chemotherapies in general, and not surprisingly, it is extended to those therapies that have a NO-mediated approach. Future research will determine the specific types of treatment and stage of disease progression that is most efficacious for NO· intervention. Advancements in drug delivery, specificity of tissue targeting, and accurate assessment of NO· pharmacokinetics will all potentially lead to viable NO· chemotherapeutics. Nitric oxide, or the nitrogen oxides that arise as intermediates from reactions of NO·, have many cellular targets. The list of possible direct NO· targets and NO-attributable protein, lipid, and DNA modifications are continually growing. For this reason, simple strategies of turning NO· production on or off in a tumor can have multiple ramifications. It is known, for instance, that high levels of NO· (>500 nM) induce p53 activation and that considerably less amount of NO· (≈100–200 nM) is required for HIF-1α stabilization. It should be theoretically possible, then, to design NO-releasing drugs that deliver cytotoxic or apoptotic levels of NO· that induce p53 activation. When these same drugs are administered under different circumstances, however, they might deliver less NO· potentially stimulating angiogenic and mitogenic pathways through HIF-1α activation. Because of tumor heterogeneity and kinetic constraints of NO-releasing drugs, it is impossible to deliver uniform NO· concentrations to a solid tumor. In addition to the numerous tumor-related proteins that could be beneficially targeted using NO·, there is always the risk of interfering with the numerous pathways necessary for normal physiologic function of the surrounding tissues. It’s difficult, for instance, to turn on p53 without affecting sGC, mitochondria respiration, and a host of other important, NO-driven responses. Although this example over simplifies the challenges of NO· chemotherapy, it emphasizes the potential pitfalls of using pure NO· donors alone to target solid tumors.
Regardless of the modes of action of NO-related anticancer agents, we must not lose site of the big picture: treating and curing cancer. That is to say, if an NO-designed drug reduces cancer morbidity or mortality and we determine it has no NO-relevant chemistry, it doesn’t matter. There is obviously great value in clinical observations independent of the precise mechanisms. Because of the extraordinary chemical properties of NO·, distinct challenges exist that are unique to this field. Nitric oxide as a cancer therapy has great potential and numerous possibilities. Continued investigations into this promising area will hopefully yield effective and selective treatments towards a host of cancer types.
ACKNOWLEDGMENTS
The project described was supported in part by Award Number K22CA113315 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Dr. Thomas also acknowledges ongoing support from the UIC Cancer Center.
ABBREVIATIONS
- AOM
Azoxymethane
- ApcMin/+
A germ-line nonsense mutation at codon 850 of the adenomatous polyposis open reading frame
- AQ4N
di-N-oxide of 1,4-bis[{2-(dimethylaminoethyl} -amino] 5,8-dihydroxy-anthracene-9,10-dione (AQ4), analkylaminoanthraquinone
- b88t
Oral cancer cell line
- BH4
Tetrahydrobiopterin
- C. Parvum
Cryptosporidium Parvum
- C57BL/6
"C57 black 6" or just "black 6" a common inbred strain of lab mouse
- cDNA
Complementary Deoxyribonucleic acid
- COX-2
Cyclooxygenase 2
- DEA/NO
2-(N,N-Diethylamino)-diazenolate-2-oxide.diethylammonium salt
- DETA/NO
1-[N-(2-Aminoethyl)-N-(2-ammonioethyl)amino]diazen-1 -ium-1,2-diolate
- DLD-1
Human colon adenocarcinoma
- Dscam
Down Syndrome Cell Adhesion Molecule
- EMT-6
Breast cancer cell line
- eNOS
Endothelial nitric oxide synthase
- FAD
Flavin adenine dinucleotide
- FMN
Flavin mononucleotide or riboflavin-5′-phosphate
- GSH
Reduced Glutathione
- GSNO
S-Nitrosoglutathione
- HC116
Human colon cancer cell line
- HIF-1α
Hypoxia-inducible factor alpha
- HNO
Nitroxyl
- HNSCC
Head and neck squamous cell carcinoma
- HT1080
Is a human fibrosarcoma cell line
- IHC
Immunohistochemistry
- IL-2
Interleukin 2
- JSK-1
O2-(2,4-dinitrophenyl) 1- [(4-ethoxy-carbonyl)piperazin-1 -yl]diazen-1-ium-1,2-diolate
- iNOS
Inducible nitric oxide synthase
- K-1735
Murine melanoma cell line
- L-NAME
L-NG-Nitroarginine methyl ester (hydrochloride)
- MDA-MB-231
Breast adenocarcinoma derived from a pleural effusion at MD Anderson on Oct 17,1973
- mRNA
Messenger ribonucleic acid
- Na2N203
Angeli’s salt
- NADPH
Reduced form of Nicotinamide adenine dinucleotide phosphate
- nNOS
Neuronal nitric oxide synthase
- NO·
Nitric Oxide
- NO-ASA
NO-donating aspirin
- NO-NSAID
Nitric oxide-donating nonsteroidal anti-inflammatories
- O2−
Superoxide
- ONOO−
Peroxynitrite
- ORF
Open reading frame
- P450
Cytochrome P450
- p53
Protein 53 or tumor protein 53
- PABA/NO
[O2-{2,4-dinitro-5-[4-(N-methylamino) benzoyloxy]phenyl} 1-(N,N-dimethylamino) diazen-1-ium-1,2-diolate
- SC-51
The prodrug of L-NIL, N6-(1-iminoethyl)- L-lysine 5-tetrazole amide
- sGC
Soluble Guanylyl/Guanylate Cyclase
- SIN-1
3-Morpholinosydnonimineases
- Sper/NO
(Z)-1-[N-(3-Ammoniopropyl)-N-[4-(3-aminopropylammonio) butyl]-amino]diazen-1-ium-1,2-diolate
- TAM
Tumor associated macrophages
- TIMP2
Tissue inhibitor of metalloprotease 2
- U937
Non Hodgkin’s lymphoma isolated in 1974
REFERENCES
- 1.Ignarro L. Nitric Oxide Biology and Pathobiology. New York: Academic Press; 2000. [Google Scholar]
- 2.Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149–156. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 3.Mocellin S, Bronte V, Nitti D. Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med Res Rev. 2007;27:317–352. doi: 10.1002/med.20092. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwasaki T, Higashiyama M, Kuriyama K, Sasaki A, Mukai M, Shinkai K, et al. NG-nitro-L-arginine methyl ester inhibits bone metastasis after modified intracardiac injection of human breast cancer cells in a nude mouse model. Jpn J Cancer Res. 1997;88:861–866. doi: 10.1111/j.1349-7006.1997.tb00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simeone AM, McMurtry V, Nieves-Alicea R, Saavedra JE, Keefer LK, Johnson MM, et al. TIMP-2 mediates the anti-invasive effects of the nitric oxide-releasing prodrug JS-K in breast cancer cells. Breast Cancer Res. 2008;10:R44. doi: 10.1186/bcr2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanna E, Quick J, Libutti SK. The tumour microenvironment: a novel target for cancer therapy. Oral Dis. 2009;15:8–17. doi: 10.1111/j.1601-0825.2008.01471.x. [DOI] [PubMed] [Google Scholar]
- 9.Blavier L, Lazaryev A, Dorey F, Shackleford GM, DeClerck YA. Matrix metalloproteinases play an active role in Wnt1-induced mammary tumorigenesis. Cancer Res. 2006;66:2691–2699. doi: 10.1158/0008-5472.CAN-05-2919. [DOI] [PubMed] [Google Scholar]
- 10.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 11.Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res. 2001;61:1320–1326. [PubMed] [Google Scholar]
- 12.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 13.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 15.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 17.Edlund M, Sung SY, Chung LW. Modulation of prostate cancer growth in bone microenvironments. J Cell Biochem. 2004;91:686–705. doi: 10.1002/jcb.10702. [DOI] [PubMed] [Google Scholar]
- 18.Triozzi PL, Eng C, Singh AD. Targeted therapy for uveal melanoma. Cancer Treat Rev. 2008;34:247–258. doi: 10.1016/j.ctrv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Patanaphan V, Salazar OM, Risco R. Breast cancer: metastatic patterns and their prognosis. South Med J. 1988;81:1109–1112. [PubMed] [Google Scholar]
- 20.Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355:479–485. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 22.Feld R, Rubinstein LV, Weisenberger TH. Sites of recurrence in resected stage I non-small-cell lung cancer: a guide for future studies. J Clin Oncol. 1984;2:1352–1358. doi: 10.1200/JCO.1984.2.12.1352. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt-Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, Blankenstein TJ, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci USA. 2003;100:7737–7742. doi: 10.1073/pnas.1331931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karrison TG, Ferguson DJ, Meier P. Dormancy of mammary carcinoma after mastectomy. J Natl Cancer Inst. 1999;91:80–85. doi: 10.1093/jnci/91.1.80. [DOI] [PubMed] [Google Scholar]
- 25.Ekmekcioglu S, Ellerhorst J, Smid CM, Prieto VG, Munsell M, Buzaid AC, et al. Inducible nitric oxide synthase and nitrotyrosine in human metastatic melanoma tumors correlate with poor survival. Clin Cancer Res. 2000;6:4768–4775. [PubMed] [Google Scholar]
- 26.Loibl S, Buck A, Strank C, von Minckwitz G, Roller M, Sinn HP, et al. The role of early expression of inducible nitric oxide synthase in human breast cancer. Eur J Cancer. 2005;41:265–271. doi: 10.1016/j.ejca.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Raspollini MR, Amunni G, Villanucci A, Boddi V, Baroni G, Taddei A, et al. Expression of inducible nitric oxide synthase and cyclooxygenase-2 in ovarian cancer: correlation with clinical outcome. Gynecol Oncol. 2004;92:806–812. doi: 10.1016/j.ygyno.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Connelly ST, Macabeo-Ong M, Dekker N, Jordan RC, Schmidt BL. Increased nitric oxide levels and iNOS over-expression in oral squamous cell carcinoma. Oral Oncol. 2005;41:261–267. doi: 10.1016/j.oraloncology.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Brennan PA, Dennis S, Poller D, Quintero M, Puxeddu R, Thomas GJ. Inducible nitric oxide synthase: correlation with extracapsular spread and enhancement of tumor cell invasion in head and neck squamous cell carcinoma. Head Neck. 2008;30:208–214. doi: 10.1002/hed.20675. [DOI] [PubMed] [Google Scholar]
- 30.Hussain SP, He P, Subleski J, Hofseth LJ, Trivers GE, Mechanic L, et al. Nitric oxide is a key component in inflammation-accelerated tumorigenesis. Cancer Res. 2008;68:7130–7136. doi: 10.1158/0008-5472.CAN-08-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 32.Ahn B, Ohshima H. Suppression of intestinal polyposis in Apc(Min/+) mice by inhibiting nitric oxide production. Cancer Res. 2001;61:8357–8360. [PubMed] [Google Scholar]
- 33.Scott DJ, Hull MA, Cartwright EJ, Lam WK, Tisbury A, Poulsom R, et al. Lack of inducible nitric oxide synthase promotes intestinal tumorigenesis in the Apc(Min/+) mouse. Gastroenterology. 2001;121:889–899. doi: 10.1053/gast.2001.27994. [DOI] [PubMed] [Google Scholar]
- 34.Rao CV, Indranie C, Simi B, Manning PT, Connor JR, Reddy BS. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165–170. [PubMed] [Google Scholar]
- 35.Kawamori T, Takahashi M, Watanabe K, Ohta T, Nakatsugi S, Sugimura T, et al. Suppression of azoxymethane-induced colonic aberrant crypt foci by a nitric oxide synthase inhibitor. Cancer Lett. 2000;148:33–37. doi: 10.1016/s0304-3835(99)00310-9. [DOI] [PubMed] [Google Scholar]
- 36.Harada K, Supriatno Kawaguchi S, Tomitaro O, Yoshida H, Sato M. Overexpression of iNOS gene suppresses the tumorigenicity and metastasis of oral cancer cells. In Vivo. 2004;18:449–455. [PubMed] [Google Scholar]
- 37.Xie K, Huang S, Dong Z, Juang SH, Gutman M, Xie QW, et al. Transfection with the inducible nitric oxide synthase gene suppresses tumorigenicity and abrogates metastasis by K-1735 murine melanoma cells. J Exp Med. 1995;181:1333–1343. doi: 10.1084/jem.181.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, et al. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wink DA, Ridnour LA, Hussain SP, Harris CC. The reemergence of nitric oxide and cancer. Nitric Oxide. 2008;19:65–67. doi: 10.1016/j.niox.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 41.Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, et al. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101:671–684. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- 42.Decker NK, Abdelmoneim SS, Yaqoob U, Hendrickson H, Hormes J, Bentley M, et al. Nitric oxide regulates tumor cell cross-talk with stromal cells in the tumor microenvironment of the liver. Am J Pathol. 2008;173:1002–1012. doi: 10.2353/ajpath.2008.080158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jadeski LC, Hum KO, Chakraborty C, Lala PK. Nitric oxide promotes murine mammary tumour growth and metastasis by stimulating tumour cell migration, invasiveness and angiogenesis. Int J Cancer. 2000;86:30–39. doi: 10.1002/(sici)1097-0215(20000401)86:1<30::aid-ijc5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 44.Jadeski LC, Lala PK. Nitric oxide synthase inhibition by N(G)-nitro-L-arginine methyl ester inhibits tumor-induced angiogenesis in mammary tumors. Am J Pathol. 1999;155:1381–1390. doi: 10.1016/S0002-9440(10)65240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J, Schmid T, Brune B. HIF-1α and p53 as targets of NO in affecting cell proliferation, death and adaptation. Curr Mol Med. 2004;4:741–751. doi: 10.2174/1566524043359926. [DOI] [PubMed] [Google Scholar]
- 46.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanriover N, Ulu MO, Isler C, Durak H, Oz B, Uzan M, et al. Neuronal nitric oxide synthase expression in glial tumors: correlation with malignancy and tumor proliferation. Neurol Res. 2008;30:940–944. doi: 10.1179/174313208X319099. [DOI] [PubMed] [Google Scholar]
- 48.Broholm H, Rubin I, Kruse A, Braendstrup O, Schmidt K, Skriver EB, et al. Nitric oxide synthase expression and enzymatic activity in human brain tumors. Clin Neuropathol. 2003;22:273–281. [PubMed] [Google Scholar]
- 49.Weinberg RA. The Biology of Cancer. USA: Garland Science; 2007. [Google Scholar]
- 50.van Ravenswaay Claasen HH, Kluin PM, Fleuren GJ. Tumor infiltrating cells in human cancer. On the possible role of CD 16+ macrophages in antitumor cytotoxicity. Lab Invest. 1992;67:166–174. [PubMed] [Google Scholar]
- 51.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 52.Klimp AH, de Vries EG, Scherphof GL, Daemen T. A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol Hematol. 2002;44:143–161. doi: 10.1016/s1040-8428(01)00203-7. [DOI] [PubMed] [Google Scholar]
- 53.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 54.Wink DA, Vodovotz Y, Laval J, Laval F, Dewhirst MW, Mitchell JB. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 55.Gauthier N, Lohm S, Touzery C, Chantome A, Perette B, Reveneau S, et al. Tumour-derived and host-derived nitric oxide differentially regulate breast carcinoma metastasis to the lungs. Carcinogenesis. 2004;25:1559–1565. doi: 10.1093/carcin/bgh158. [DOI] [PubMed] [Google Scholar]
- 56.Mehibel M, Singh S, Chinje EC, Cowen RL, Stratford IJ. Effects of cytokine-induced macrophages on the response of tumor cells to banoxantrone (AQ4N) Mol Cancer Ther. 2009 doi: 10.1158/1535-7163.MCT-08-0927. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 58.Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci USA. 2001;98:355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellamy TC, Wood J, Garthwaite J. On the activation of soluble guanylyl cyclase by nitric oxide. Proc Natl Acad Sci USA. 2002;99:507–510. doi: 10.1073/pnas.012368499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, et al. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci USA. 2004;101:8894–8899. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall CN, Garthwaite J. What is the real physiological NO· concentration in vivo? Nitric Oxide. 2009;21:92–103. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lymar SV, Khairutdinov RF, Hurst JK. Hydroxyl radical formation by O-O bond homolysis in peroxynitrous acid. Inorg Chem. 2003;42:5259–5266. doi: 10.1021/ic030104l. [DOI] [PubMed] [Google Scholar]
- 64.Thomas DD, Ridnour LA, Espey MG, Donzelli S, Ambs S, Hussain SP, et al. Superoxide fluxes limit nitric oxide-induced signaling. J Biol Chem. 2006;281:25984–25993. doi: 10.1074/jbc.M602242200. [DOI] [PubMed] [Google Scholar]
- 65.Elvin P, Garner AP. Tumour invasion and metastasis: challenges facing drug discovery. Curr Opin Pharmacol. 2005;5:374–381. doi: 10.1016/j.coph.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 66.Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1998;17:279–284. doi: 10.1023/a:1006140513233. [DOI] [PubMed] [Google Scholar]
- 68.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 69.Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weigelt B, Glas AM, Wessels LF, Witteveen AT, Peterse JL, van't Veer LJ. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc Natl Acad Sci USA. 2003;100:15901–15905. doi: 10.1073/pnas.2634067100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomas DD, Miranda KM, Espey MG, Citrin D, Jourd'heuil D, Paolocci N, et al. Guide for the use of nitric oxide (NO·) donors as probes of the chemistry of NO· and related redox species in biological systems. Methods Enzymol. 2002;359:84–105. doi: 10.1016/s0076-6879(02)59174-6. [DOI] [PubMed] [Google Scholar]
- 72.Thatcher GR, Nicolescu AC, Bennett BM, Toader V. Nitrates and NO· release: contemporary aspects in biological and medicinal chemistry. Free Radic Biol Med. 2004;37:1122–1143. doi: 10.1016/j.freeradbiomed.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 73.Hogg N. Biological chemistry and clinical potential of S-nitrosothiols. Free Radic Biol Med. 2000;28:1478–1486. doi: 10.1016/s0891-5849(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 74.Keefer LK. Nitric oxide (NO·)- and nitroxyl (HNO)-generating diazeniumdiolates (NONOates): emerging commercial opportunities. Curr Top Med Chem. 2005;5:625–636. doi: 10.2174/1568026054679380. [DOI] [PubMed] [Google Scholar]
- 75.Robinson KM, Beckman JS. Synthesis of peroxynitrite from nitrite and hydrogen peroxide. Methods Enzymol. 2005;396:207–214. doi: 10.1016/S0076-6879(05)96019-9. [DOI] [PubMed] [Google Scholar]
- 76.Feelisch M, Ostrowski J, Noack E. On the mechanism of NO release from sydnonimines. J Cardiovasc Pharmacol. 1989;14(Suppl 11):S13–S22. [PubMed] [Google Scholar]
- 77.Miranda KM, Dutton AS, Ridnour LA, Foreman CA, Ford E, Paolocci N, et al. Mechanism of aerobic decomposition of Angeli's salt (sodium trioxodinitrate) at physiological pH. J Am Chem Soc. 2005;127:722–731. doi: 10.1021/ja045480z. [DOI] [PubMed] [Google Scholar]
- 78.Sandau KB, Faus HG, Brune B. Induction of hypoxia-inducible-factor 1 by nitric oxide is mediated via the PI 3K pathway. Biochem Biophys Res Commun. 2000;278:263–267. doi: 10.1006/bbrc.2000.3789. [DOI] [PubMed] [Google Scholar]
- 79.Sandau KB, Fandrey J, Brune B. Accumulation of HIF-1α under the influence of nitric oxide. Blood. 2001;97:1009–1015. doi: 10.1182/blood.v97.4.1009. [DOI] [PubMed] [Google Scholar]
- 80.Cui X, Zhang J, Ma P, Myers DE, Goldberg IG, Sittler KJ, et al. cGMP-independent nitric oxide signaling and regulation of the cell cycle. BMC Genomics. 2005;6:151. doi: 10.1186/1471-2164-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turpaev K, Bouton C, Diet A, Glatigny A, Drapier JC. Analysis of differentially expressed genes in nitric oxide-exposed human monocytic cells. Free Radic Biol Med. 2005;38:1392–1400. doi: 10.1016/j.freeradbiomed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Andrade SP, Hart IR, Piper PJ. Inhibitors of nitric oxide synthase selectively reduce flow in tumor-associated neovasculature. Br J Pharmacol. 1992;107:1092–1095. doi: 10.1111/j.1476-5381.1992.tb13412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coulter JA, McCarthy HO, Xiang J, Roedl W, Wagner E, Robson T, et al. Nitric oxide--a novel therapeutic for cancer. Nitric Oxide. 2008;19:192–198. doi: 10.1016/j.niox.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 84.Orucevic A, Lala PK. Effects of N(g)-methyl-L-arginine, an inhibitor of nitric oxide synthesis, on interleukin-2-induced capillary leakage and antitumor responses in healthy and tumor-bearing mice. Cancer Immunol Immunother. 1996;42:38–46. doi: 10.1007/s002620050249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Inano H, Onoda M. Nitric oxide produced by inducible nitric oxide synthase is associated with mammary tumorigenesis in irradiated rats. Nitric Oxide. 2005;12:15–20. doi: 10.1016/j.niox.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 86.Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu VT. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med. 2009;9:442–458. doi: 10.2174/156652409788167087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yasuda H. Solid tumor physiology and hypoxia-induced chemo/radio-resistance: novel strategy for cancer therapy: nitric oxide donor as a therapeutic enhancer. Nitric Oxide. 2008;19:205–216. doi: 10.1016/j.niox.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 88.Chakrapani H, Wilde TC, Citro ML, Goodblatt MM, Keefer LK, Saavedra JE. Synthesis, nitric oxide release, and anti-leukemic activity of glutathione-activated nitric oxide prodrugs: Structural analogues of PABA/NO, an anti-cancer lead compound. Bioorg Med Chem. 2008;16:2657–2664. doi: 10.1016/j.bmc.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiziltepe T, Hideshima T, Ishitsuka K, Ocio EM, Raje N, Catley L, et al. JS-K, a GST-activated nitric oxide generator, induces DNA double-strand breaks, activates DNA damage response pathways, and induces apoptosis in vitro and in vivo in human multiple myeloma cells. Blood. 2007;110:709–718. doi: 10.1182/blood-2006-10-052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hagos GK, Carroll RE, Kouznetsova T, Li Q, Toader V, Fernandez PA, et al. Colon cancer chemoprevention by a novel NO· chimera that shows anti-inflammatory and antiproliferative activity in vitro and in vivo. Mol Cancer Ther. 2007;6:2230–2239. doi: 10.1158/1535-7163.MCT-07-0069. [DOI] [PubMed] [Google Scholar]
- 91.Gao J, Liu X, Rigas B. Nitric oxide-donating aspirin induces apoptosis in human colon cancer cells through induction of oxidative stress. Proc Natl Acad Sci USA. 2005;102:17207–17212. doi: 10.1073/pnas.0506893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hulsman N, Medema JP, Bos C, Jongejan A, Leurs R, Smit MJ, et al. Chemical insights in the concept of hybrid drugs: the antitumor effect of nitric oxide-donating aspirin involves a quinone methide but not nitric oxide nor aspirin. J Med Chem. 2007;50:2424–2431. doi: 10.1021/jm061371e. [DOI] [PubMed] [Google Scholar]
- 93.Dunlap T, Chandrasena RE, Wang Z, Sinha V, Thatcher GR. Quinone formation as a chemoprevention strategy for hybrid drugs: balancing cytotoxicity and cytoprotection. Chem Res Toxicol. 2007;20:1903–1912. doi: 10.1021/tx7002257. [DOI] [PubMed] [Google Scholar]
- 94.Dunlap T, Abdul-Hay SO, Chandrasena RE, Hagos GK, Sinha V, Wang Z, et al. Nitrates and NO-NSAIDs in cancer chemoprevention and therapy: in vitro evidence querying the NO· donor functionality. Nitric Oxide. 2008;19:115–124. doi: 10.1016/j.niox.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Varella EA, Nicolaides DN. Recent developments in the chemistry and in the biological applications of amidoximes. Curr Pharm Des. 2008;14(10):1001–1047. doi: 10.2174/138161208784139675. [DOI] [PubMed] [Google Scholar]
- 96.Motilva V, Talero E, Calvo JR, Villegas I, Alarcon-de-la-Lastra C, Sanchez-Fidalgo S. Intestinal immunomodulation. Role of regulative peptides and promising pharmacological activities. Curr Pharm Des. 2008;14(1):71–95. doi: 10.2174/138161208783330745. [DOI] [PubMed] [Google Scholar]
- 97.Sullivan R, Graham CH. Chemosensitization of cancer by nitric oxide. Curr Pharm Des. 2008;14(11):1113–1123. doi: 10.2174/138161208784246225. [DOI] [PubMed] [Google Scholar]