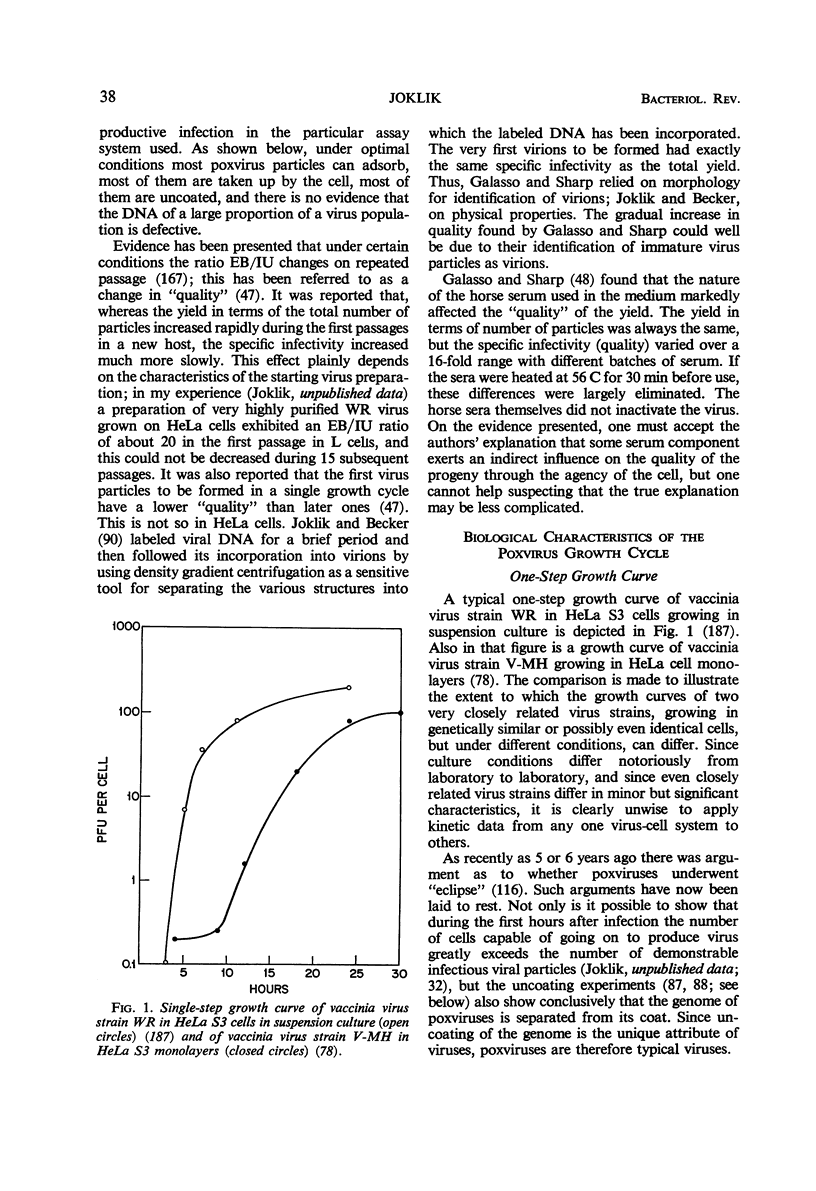
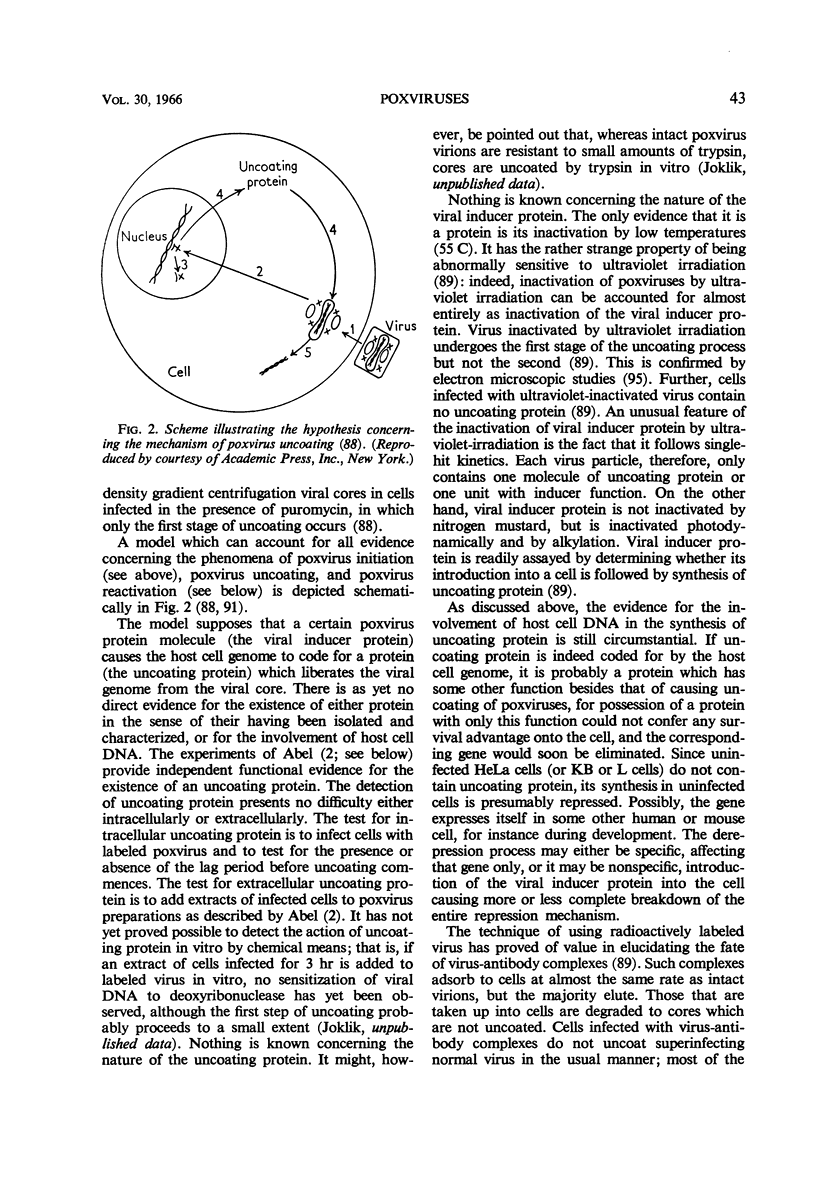
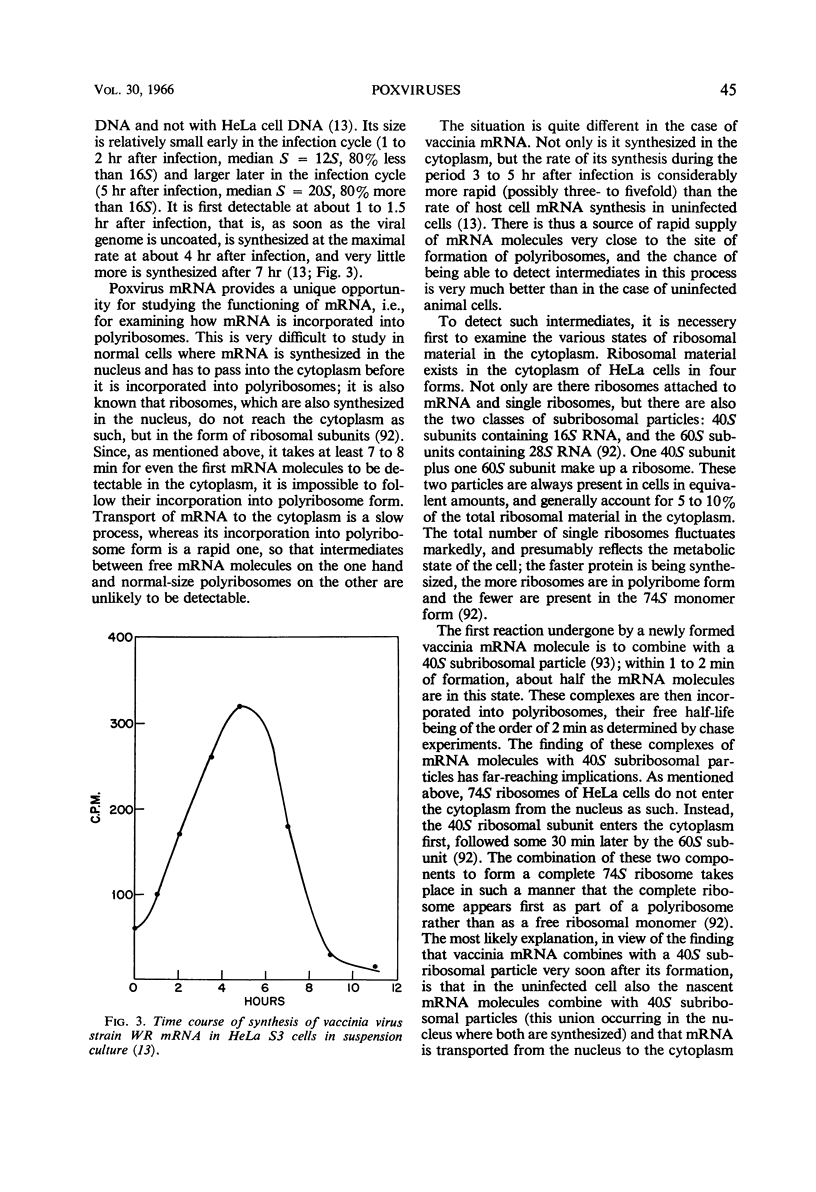
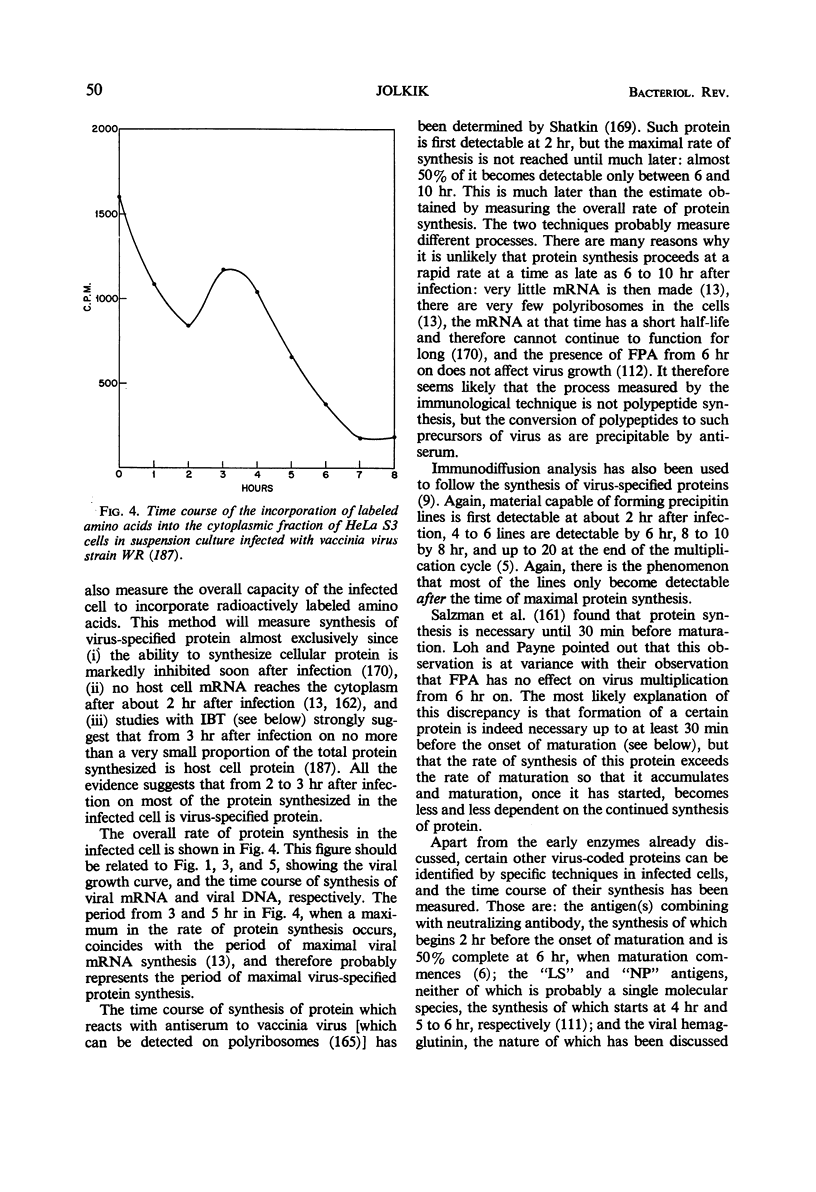
Full text
PDF




























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL P. Multiplicity reactivation and marker rescue with vaccinia virus. Virology. 1962 Aug;17:511–519. doi: 10.1016/0042-6822(62)90150-2. [DOI] [PubMed] [Google Scholar]
- ABEL P. REACTIVATION OF HEATED VACCINIA VIRUS IN VITRO. Z Vererbungsl. 1963 Nov 21;94:249–252. doi: 10.1007/BF00894768. [DOI] [PubMed] [Google Scholar]
- ALLISON A. C., BURKE D. C. The nucleic acid contents of viruses. J Gen Microbiol. 1962 Feb;27:181–194. doi: 10.1099/00221287-27-2-181. [DOI] [PubMed] [Google Scholar]
- APPLEYARD G., WESTWOOD J. C. A PROTECTIVE ANTIGEN FROM THE POX-VIRUSES. II. IMMUNISATION OF ANIMALS. Br J Exp Pathol. 1964 Apr;45:162–173. [PMC free article] [PubMed] [Google Scholar]
- APPLEYARD G., WESTWOOD J. C. THE GROWTH OF RABBITPOX VIRUS IN TISSUE CULTURE. J Gen Microbiol. 1964 Dec;37:391–401. doi: 10.1099/00221287-37-3-391. [DOI] [PubMed] [Google Scholar]
- APPLEYARD G., WESTWOOD J. C., ZWARTOUW H. T. The toxic effect of rabbitpox virus in tissue culture. Virology. 1962 Oct;18:159–169. doi: 10.1016/0042-6822(62)90001-6. [DOI] [PubMed] [Google Scholar]
- APPLEYARD G., ZWARTOUW H. T. THE EFFECT OF P-FLUOROPHENYLALANINE ON THE REPLICATION OF RABBITPOX VIRUS AND ITS NUCLEIC ACID. J Gen Microbiol. 1965 Mar;38:429–436. doi: 10.1099/00221287-38-3-429. [DOI] [PubMed] [Google Scholar]
- APPLEYARD G., ZWARTOUW H. T., WESTWOOD J. C. A PROTECTIVE ANTIGEN FROM THE POX-VIRUSES. I. REACTION WITH NEUTRALIZING ANTIBODY. Br J Exp Pathol. 1964 Apr;45:150–161. [PMC free article] [PubMed] [Google Scholar]
- Appleyard G., Hume V. B., Westwood J. C. The effect of thiosemicarbazones on the growth of rabbit pox virus in tissue culture. Ann N Y Acad Sci. 1965 Jul 30;130(1):92–104. doi: 10.1111/j.1749-6632.1965.tb12543.x. [DOI] [PubMed] [Google Scholar]
- BACH M. K., MAGEE W. E. Biochemical effects of isatin beta-thiosemicarbazone on development of vaccinia virus. Proc Soc Exp Biol Med. 1962 Jul;110:565–567. doi: 10.3181/00379727-110-27580. [DOI] [PubMed] [Google Scholar]
- BAUER D. J. The chemotherapy of ectromelia infection with isatin beta-dialkylthiosemicarbazones. Br J Exp Pathol. 1963 Apr;44:233–242. [PMC free article] [PubMed] [Google Scholar]
- BECKER Y., JOKLIK W. K. MESSENGER RNA IN CELLS INFECTED WITH VACCINIA VIRUS. Proc Natl Acad Sci U S A. 1964 Apr;51:577–585. doi: 10.1073/pnas.51.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEDSON H. S., DUMBELL K. R. The effect of temperature on the growth of pox viruses in the chick embryo. J Hyg (Lond) 1961 Dec;59:457–469. doi: 10.1017/s0022172400039152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOOM S., GOLDBERG B., GREEN H. THE LIFETIME OF MESSENGER RNA FOR COLLAGEN AND CELL PROTEIN SYNTHESIS IN AN ESTABLISHED MAMMALIAN CELL LINE. Biochem Biophys Res Commun. 1965 Apr 23;19:317–321. doi: 10.1016/0006-291x(65)90461-4. [DOI] [PubMed] [Google Scholar]
- BUETTNER D., GIESE H., MUELLER G., PETERS D. DIE FEINSTRUKTUR REIFER ELEMENTARKOERPER DES ECTHYMA CONTAGIOSUM UND DER STOMATITIS PAPULOSA. Arch Gesamte Virusforsch. 1964 Jun 17;14:657–673. [PubMed] [Google Scholar]
- Bauer D. J. Clinical experience with the antiviral drug marboran (1-methylisatin 3-thiosemicarbazone). Ann N Y Acad Sci. 1965 Jul 30;130(1):110–117. doi: 10.1111/j.1749-6632.1965.tb12545.x. [DOI] [PubMed] [Google Scholar]
- Bresnick E., Thompson U. B., Morris H. P., Liebelt A. G. Inhibition of thymidine kinase activity in liver and hepatomas by TTP and d-CTP. Biochem Biophys Res Commun. 1964 Jun 15;16(3):278–284. doi: 10.1016/0006-291x(64)90340-7. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The initiation of vaccinia infection. Virology. 1960 Jul;11:603–623. doi: 10.1016/0042-6822(60)90103-3. [DOI] [PubMed] [Google Scholar]
- CHAPPLE P. J., WESTWOOD J. C. ELECTRON MICROSCOPY OF MYXOMA VIRUS. Nature. 1963 Jul 13;199:199–200. doi: 10.1038/199199a0. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., MUNIER R. Effets des analogues structuraux d'aminoacides sur la croissance, la synthèse de protéines et la synthèse d'enzymes chez Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):347–356. doi: 10.1016/0006-3002(59)90007-1. [DOI] [PubMed] [Google Scholar]
- Cogniaux-Le Clerc J. Inhibitory effect of 8-azaguanine on the synthesis of vaccinia virus deoxyribonucleic acid. Biochim Biophys Acta. 1965 Sep 6;108(1):5–10. doi: 10.1016/0005-2787(65)90102-4. [DOI] [PubMed] [Google Scholar]
- DALES S. An electron microscope study of the early association between two mammalian viruses and their hosts. J Cell Biol. 1962 May;13:303–322. doi: 10.1083/jcb.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S., KAJIOKA R. THE CYCLE OF MULTIPLICATION OF VACCINIA VIRUS IN EARLE'S STRAIN L CELLS. I. UPTAKE AND PENETRATION. Virology. 1964 Nov;24:278–294. doi: 10.1016/0042-6822(64)90167-9. [DOI] [PubMed] [Google Scholar]
- DALES S., SIMINOVITCH L. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961 Aug;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON E. H., ALLFREY V. G., MIRSKY A. E. Gene expression in differentiated cells. Proc Natl Acad Sci U S A. 1963 Jan 15;49:53–60. doi: 10.1073/pnas.49.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. ISOLATION AND PROPERTIES OF VACCINIA MUTANTS DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Feb;22:214–225. doi: 10.1016/0042-6822(64)90006-6. [DOI] [PubMed] [Google Scholar]
- DUMBELL K. R., BEDSON H. S. THE USE OF CEILING TEMPERATURE AND REACTIVATION IN THE ISOLATION OF POX VIRUS HYBRIDS. J Hyg (Lond) 1964 Jun;62:133–140. doi: 10.1017/s0022172400039863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S. Pentration of animal viruses into cells. Prog Med Virol. 1965;7:1–43. [PubMed] [Google Scholar]
- Davison P. F. THE EFFECT OF HYDRODYNAMIC SHEAR ON THE DEOXYRIBONUCLEIC ACID FROM T(2) AND T(4) BACTERIOPHAGES. Proc Natl Acad Sci U S A. 1959 Nov;45(11):1560–1568. doi: 10.1073/pnas.45.11.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EASTERBROOK K. B. Analysis of the early stages of vaccinia virus infection in KB cells using sodium azide. Virology. 1961 Dec;15:417–427. doi: 10.1016/0042-6822(61)90109-x. [DOI] [PubMed] [Google Scholar]
- EASTERBROOK K. B. Interference with the maturation of vaccinia virus by isatin beta-thiosemicarbazone. Virology. 1962 Jun;17:245–251. doi: 10.1016/0042-6822(62)90114-9. [DOI] [PubMed] [Google Scholar]
- EASTERBROOK K. B. The multiplication of vaccinia virus in suspended KB cells. Virology. 1961 Dec;15:404–416. doi: 10.1016/0042-6822(61)90108-8. [DOI] [PubMed] [Google Scholar]
- FENNER F., BURNET F. M. A short description of the poxvirus group (vaccinia and related viruses). Virology. 1957 Oct;4(2):305–314. doi: 10.1016/0042-6822(57)90065-x. [DOI] [PubMed] [Google Scholar]
- FENNER F. Genetic studies with mammalian poxviruses. II. Recombination between two strains of vaccinia virus in single HeLa cells. Virology. 1959 Aug;8:499–507. doi: 10.1016/0042-6822(59)90051-0. [DOI] [PubMed] [Google Scholar]
- FENNER F., HOLMES I. H., JOKLIK W. K., WOODROOFE G. M. Reactivation of heat-inactivated poxviruses: a general phenomenon which includes the fibroma-myxoma virus transformation of Berry and Dedrick. Nature. 1959 May 9;183(4671):1340–1341. doi: 10.1038/1831340a0. [DOI] [PubMed] [Google Scholar]
- FENNER F., SAMBROOK J. F. THE GENETICS OF ANIMAL VIRUSES. Annu Rev Microbiol. 1964;18:47–94. doi: 10.1146/annurev.mi.18.100164.000403. [DOI] [PubMed] [Google Scholar]
- FENNER F. The biological characters of several strains of vaccinia, cowpox and rabbitpox viruses. Virology. 1958 Jun;5(3):502–529. doi: 10.1016/0042-6822(58)90042-4. [DOI] [PubMed] [Google Scholar]
- FENNER F., WOODROOFE G. M. The reactivation of poxviruses. II. The range of reactivating viruses. Virology. 1960 May;11:185–201. doi: 10.1016/0042-6822(60)90061-1. [DOI] [PubMed] [Google Scholar]
- FERRARI W., GESSA G. L., LODDO B., SCHIVO M. L. DECREASED PATHOGENICITY FOR RABBIT SKIN OF IDU-RESISTANT VACCINIA VIRUS. Virology. 1965 May;26:154–155. doi: 10.1016/0042-6822(65)90039-5. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN-KIEN A. E., ROWE W. P., BANFIELD W. G. Milker's nodules: isolation of a poxvirus from a human case. Science. 1963 Jun 21;140(3573):1335–1336. doi: 10.1126/science.140.3573.1335. [DOI] [PubMed] [Google Scholar]
- FUJIO Y. EFFECTS OF ACTINOMYCIN ON GROWTH AND HEMAGGLUTININ PRODUCTION OF VACCINIA VIRUS. Biken J. 1963 Oct;6:197–203. [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. EFFECTS OF HEAT ON THE INFECTING, ANTIBODY-ABSORBING, AND INTERFERING POWERS OF VACCINIA VIRUS. J Bacteriol. 1965 Mar;89:611–616. doi: 10.1128/jb.89.3.611-616.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. HOMOLOGOUS INHIBITION, TOXICITY, AND MULTIPLICITY REACTIVATION WITH ULTRAVIOLET-IRRADIATED VACCINIA VIRUS. J Bacteriol. 1963 Jun;85:1309–1314. doi: 10.1128/jb.85.6.1309-1314.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. Homologous inhibition with heated and ultraviolet-treated vaccinia virus in cultures of L cells. Virology. 1963 May;20:1–13. doi: 10.1016/0042-6822(63)90135-1. [DOI] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. Inhibitory effect of heated vaccinia virus on growth of vaccinia virus in Earle's L cells. Proc Soc Exp Biol Med. 1961 Aug-Sep;107:957–960. doi: 10.3181/00379727-107-26808. [DOI] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. Quality and yield of vaccinia virus from L cell cultures. Proc Soc Exp Biol Med. 1963 May;113:43–47. doi: 10.3181/00379727-113-28271. [DOI] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. RELATIVE PLAQUE-FORMING, CELL-INFECTING, AND INTERFERING QUALITIES OF VACCINIA VIRUS. J Bacteriol. 1964 Aug;88:433–439. doi: 10.1128/jb.88.2.433-439.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. THE EFFECT OF HORSE SERUM ON THE QUALITY OF VACCINIA VIRUS GROWN IN L CELL CULTURES. J Immunol. 1963 Apr;90:647–653. [PubMed] [Google Scholar]
- GAUSH C. R., YOUNGNER J. S. Studies on the lipids of virusinfected cells. I. Lipid analysis of a soluble hemagglutinin from chorioallantoic membranes infected with vaccinia virus. Virology. 1963 Apr;19:573–579. doi: 10.1016/0042-6822(63)90053-9. [DOI] [PubMed] [Google Scholar]
- GEMMELL A., CAIRNS J. Linkage in the genome of an animal virus. Virology. 1959 Jul;8(3):381–383. doi: 10.1016/0042-6822(59)90037-6. [DOI] [PubMed] [Google Scholar]
- GEMMELL A., FENNER F. Genetic studies with mammalian poxviruses. III. White (u) mutants of rabbitpox virus. Virology. 1960 May;11:219–235. doi: 10.1016/0042-6822(60)90063-5. [DOI] [PubMed] [Google Scholar]
- GEORGIEV G. P., SAMARINA O. P., LERMAN M. I., SMIRNOV M. N., SEVERTZOV A. N. BIOSYNTHESIS OF MESSENGER AND RIBOSOMAL RIBONUCLEIC ACIDS IN THE NUCLEOLOCHROMOSOMAL APPARATUS OF ANIMAL CELLS. Nature. 1963 Dec 28;200:1291–1294. doi: 10.1038/2001291a0. [DOI] [PubMed] [Google Scholar]
- GIRARD M., LATHAM H., PENMAN S., DARNELL J. E. ENTRANCE OF NEWLY FORMED MESSENGER RNA AND RIBOSOMES INTO HELA CELL CYTOPLASM. J Mol Biol. 1965 Feb;11:187–201. doi: 10.1016/s0022-2836(65)80050-x. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M., CHAGOYA V. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION. V. ENZYMES OF DEOXYRIBONUCLEIC ACID SYNTHESIS IN CELLS INFECTED BY ADENOVIRUS AND VACCINIA VIRUS. J Biol Chem. 1964 Apr;239:1188–1197. [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- Gerstl F. Ein Beitrag zur Diagnostik und Morphologie des Erregers des ansteckenden Lippengrindes (Ecthyma contagiosum) der Gemse. Zentralbl Bakteriol Orig. 1964 Dec;195(2):182–189. [PubMed] [Google Scholar]
- Grausgruber W. Lippengrind (Ecthyma contagiosum) bei Gemsen. Zentralbl Bakteriol Orig. 1964 Dec;195(2):175–181. [PubMed] [Google Scholar]
- HANAFUSA H. Factors involved in the initiation of multiplication of vaccinia virus. Cold Spring Harb Symp Quant Biol. 1962;27:209–217. doi: 10.1101/sqb.1962.027.001.021. [DOI] [PubMed] [Google Scholar]
- HANAFUSA T., KAMAHORA J., HANAFUSA H. Transformation of ectromelia into vaccinia virus in tissue culture. Virology. 1959 Aug;8:525–527. doi: 10.1016/0042-6822(59)90053-4. [DOI] [PubMed] [Google Scholar]
- HAREL L., HAREL J., BOER A., IMBENOTTE J., CARPENI N. PERSISTANCE D'UNE SYNTH'ESE DE D-RNA DANS LE FOIE DE RAT TRAIT'E PAR L'ACTINOMYCINE. Biochim Biophys Acta. 1964 Jun 22;87:212–218. [PubMed] [Google Scholar]
- HARRIS W. J., WESTWOOD J. C. PHOSPHOTUNGSTATE STAINING OF VACCINIA VIRUS. J Gen Microbiol. 1964 Mar;34:491–495. doi: 10.1099/00221287-34-3-491. [DOI] [PubMed] [Google Scholar]
- HINZE H. C., WALKER D. L. RESPONSE OF CULTURED RABBIT CELLS TO INFECTION WITH THE SHOPE FIBROMA VIRUS. I. PROLIFERATION AND MORPHOLOGICAL ALTERATION OF THE INFECTED CELLS. J Bacteriol. 1964 Oct;88:1185–1194. doi: 10.1128/jb.88.4.1185-1194.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYDE J. M., GAFFORD L. G., RANDALL C. C. FINE STRUCTURE OF THE COAT AND NUCLEOID MATERIAL OF FOWLPOX VIRUS. J Bacteriol. 1965 Jun;89:1557–1569. doi: 10.1128/jb.89.6.1557-1569.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRAELI E., SACHS L. CELL-VIRUS INTERACTIONS WITH THE SHOPE FIBROMA VIRUS ON CULTURES OF RABBIT AND RAT CELLS. Virology. 1964 Aug;23:473–485. doi: 10.1016/0042-6822(64)90231-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., ABEL P., HOLMES I. H. Reactivation of poxviruses by a non-genetic mechanism. Nature. 1960 Jun 18;186:992–993. doi: 10.1038/186992b0. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., HOLMES I. H., BRIGGS M. J. The reactivation of poxviruses. III. Properties of reactivable particles. Virology. 1960 May;11:202–218. doi: 10.1016/0042-6822(60)90062-3. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., RODRICK J. M. Biochemical studies on vaccinia virus in cultured cells. I. Incorporation of adenine-8-C14 into normal and infected cells. Virology. 1959 Nov;9:396–416. doi: 10.1016/0042-6822(59)90131-x. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR FATE OF RABBITPOX VIRUS RENDERED NONINFECTIOUS BY VARIOUS REAGENTS. Virology. 1964 Apr;22:620–633. doi: 10.1016/0042-6822(64)90084-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. I. THE FATE OF RADIOACTIVELY-LABELED RABBITPOX VIRUS. J Mol Biol. 1964 Feb;8:263–276. doi: 10.1016/s0022-2836(64)80136-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. II. THE MOLECULAR BASIS OF THE UNCOATING PROCESS. J Mol Biol. 1964 Feb;8:277–288. doi: 10.1016/s0022-2836(64)80137-6. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Joklik W. K., Becker Y. Studies on the genesis of polyribosomes. I. Origin and significance of the subribosomal particles. J Mol Biol. 1965 Sep;13(2):496–510. doi: 10.1016/s0022-2836(65)80112-7. [DOI] [PubMed] [Google Scholar]
- Joklik W. K., Becker Y. Studies on the genesis of polyribosomes. II. The association of nascent messenger RNA with the 40 S subribosomal particle. J Mol Biol. 1965 Sep;13(2):511–520. doi: 10.1016/s0022-2836(65)80113-9. [DOI] [PubMed] [Google Scholar]
- Joklik W. K. The molecular basis of the viral eclipse phase. Prog Med Virol. 1965;7:44–96. [PubMed] [Google Scholar]
- Jungwirth C., Joklik W. K. Studies on "early" enzymes in HeLa cells infected with vaccinia virus. Virology. 1965 Sep;27(1):80–93. doi: 10.1016/0042-6822(65)90145-5. [DOI] [PubMed] [Google Scholar]
- KAJIOKA R., SIMINOVITCH L., DALES S. THE CYCLE OF MULTIPLICATION OF VACCINIA VIRUS IN EARLE'S STRAIN L CELLS. II. INITIATION OF DNA SYNTHESIS AND MORPHOGENESIS. Virology. 1964 Nov;24:295–309. doi: 10.1016/0042-6822(64)90168-0. [DOI] [PubMed] [Google Scholar]
- KAKU H., KAMAHORA J. A STUDY ON THE GIANT CELL FORMATION OF L CELLS INFECTED WITH UV INACTIVATED VACCINIA VIRUS. Biken J. 1964 Apr;7:37–40. [PubMed] [Google Scholar]
- KAKU H., KAMAHORA J. GIANT CELL FORMATION IN L CELLS INFECTED WITH ACTIVE VACCINIA VIRUS. Biken J. 1964 Jan;6:299–315. [PubMed] [Google Scholar]
- KATES M., ALLISON A. C., TYRELL D. A., JAMES A. T. Origin of lipids in influenza virus. Cold Spring Harb Symp Quant Biol. 1962;27:293–301. doi: 10.1101/sqb.1962.027.001.027. [DOI] [PubMed] [Google Scholar]
- KATO S., OGAWA M., MIYAMOTO H. NUCLEOCYTOPLASMIC INTERACTION IN POXVIRUS-INFECTED CELLS. I. RELATIONSHIP BETWEEN INCLUSION FORMATION AND DNA METABOLISM OF THE CELLS. Biken J. 1964 Jul;7:45–56. [PubMed] [Google Scholar]
- KILHAM L. The fibroma-myxoma virus transformation. Adv Virus Res. 1960;7:103–129. doi: 10.1016/s0065-3527(08)60008-1. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. Biochemistry of vaccinia-infected mouse fibroblasts (strain L-M). I. Effects on nucleic acid and protein synthesis. Virology. 1962 Oct;18:274–285. doi: 10.1016/0042-6822(62)90014-4. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. Biochemistry of vaccinia-infected mouse fibroblasts (strain L-M). II. Properties of the chromosomal DNA of infected cells. Virology. 1962 Oct;18:286–293. doi: 10.1016/0042-6822(62)90015-6. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J. Enhanced thymidine phosphorylating activity of mouse fibroblasts (strain LM) following vaccinia infection. Biochem Biophys Res Commun. 1962 Jun 19;8:72–75. doi: 10.1016/0006-291x(62)90238-3. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J. Inhibitory effects of puromycin and fluorophenylalanine on induction of thymidine kinase by vaccinia infected L-cells. Biochem Biophys Res Commun. 1963 May 3;11:176–181. doi: 10.1016/0006-291x(63)90330-9. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. PROPERTIES OF DEOXYTHYMIDINE KINASE PARTIALLY PURIFIED FROM NONINFECTED MOUSE FIBROBLAST CELLS. Virology. 1965 May;26:16–27. doi: 10.1016/0042-6822(65)90021-8. [DOI] [PubMed] [Google Scholar]
- KIT S., VALLADARES Y., DUBBS D. R. EFFECTS OF AGE OF CULTURE AND VACCINIA INFECTION ON URIDINE KINASE ACTIVITY OF L-CELLS. Exp Cell Res. 1964 Apr;34:257–265. doi: 10.1016/0014-4827(64)90362-3. [DOI] [PubMed] [Google Scholar]
- LEVINTHAL C., KEYNAN A., HIGA A. Messenger RNA turnover and protein synthesis in B. subtilis inhibited by actinomycin D. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1631–1638. doi: 10.1073/pnas.48.9.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOH P. C., PAYNE F. E. EFFECT OF 5-FLUORO-2'-DEOXYURIDINE ON THE SYNTHESIS OF VACCINIA VIRUS. Virology. 1965 Apr;25:575–584. doi: 10.1016/0042-6822(65)90085-1. [DOI] [PubMed] [Google Scholar]
- LOH P. C., PAYNE F. E. EFFECT OF P-FLUOROPHENYLALANINE ON THE SYNTHESIS OF VACCINIA VIRUS. Virology. 1965 Apr;25:560–574. doi: 10.1016/0042-6822(65)90084-x. [DOI] [PubMed] [Google Scholar]
- LOH P. C., RIGGS J. L. Demonstration of the sequential development of vaccinial antigens and virus in infected cells: observations with cytochemical and differential fluorescent procedures. J Exp Med. 1961 Jul 1;114:149–160. doi: 10.1084/jem.114.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGEE W. E., SHEEK M. R., BURROUS M. J. The synthesis of vaccinial deoxyribonucleic acid. Virology. 1960 May;11:296–299. doi: 10.1016/0042-6822(60)90070-2. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- MARQUARDT J., HOLM S. E., LYCKE E. IMMUNOPRECIPITATING SOLUBLE VACCINIA VIRUS ANTIGENS. Arch Gesamte Virusforsch. 1965;15:178–187. doi: 10.1007/BF01257729. [DOI] [PubMed] [Google Scholar]
- MARQUARDT J., HOLM S. E., LYCKE E. PREPARATION OF A PURIFIED VACCINIA VIRUS FREED FROM SOLUBLE ANTIGENS. Proc Soc Exp Biol Med. 1964 May;116:112–116. doi: 10.3181/00379727-116-29175. [DOI] [PubMed] [Google Scholar]
- MCAUSLAN B. R. THE INDUCTION AND REPRESSION OF THYMIDINE KINASE IN THE POXVIRUS-INFECTED HELA CELL. Virology. 1963 Nov;21:383–389. doi: 10.1016/0042-6822(63)90199-5. [DOI] [PubMed] [Google Scholar]
- MCCLAIN M. E., GREENLAND R. M. RECOMBINATION BETWEEN RABBITPOX VIRUS MUTANTS IN PERMISSIVE AND NONPERMISSIVE CELLS. Virology. 1965 Apr;25:516–522. doi: 10.1016/0042-6822(65)90079-6. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- MOSCOVICI C., COHEN E. P., SANDERS J., DELONG S. S. ISOLATION OF A VIRAL AGENT FROM PSEUDOCOWPOX DISEASE. Science. 1963 Sep 6;141(3584):915–916. doi: 10.1126/science.141.3584.915. [DOI] [PubMed] [Google Scholar]
- MUELLER G., PETERS D. SUBSTRUKTUREN DES VACCINEVIRUS, DARGESTELLT DURCH NEGATIVKONTRASTIERUNG. Arch Gesamte Virusforsch. 1963 Aug 26;13:435–451. [PubMed] [Google Scholar]
- MUNYON W., KIT S. INHIBITION OF THYMIDINE KINASE FORMATION IN LM (TK-) CELLS SIMULTANEOUSLY INFECTED WITH VACCINIA AND A THYMIDINE KINASELESS VACCINIA MUTANT. Virology. 1965 Jun;26:374–377. doi: 10.1016/0042-6822(65)90287-4. [DOI] [PubMed] [Google Scholar]
- Magee W. E., Bach M. K. Biochemical studies on the antiviral activities of the isatin-beta-thiosemicarbazones. Ann N Y Acad Sci. 1965 Jul 30;130(1):80–91. doi: 10.1111/j.1749-6632.1965.tb12542.x. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R., Herde P., Pett D., Ross J. Nucleases of virus-infected animal cells. Biochem Biophys Res Commun. 1965 Sep 8;20(5):586–591. doi: 10.1016/0006-291x(65)90439-0. [DOI] [PubMed] [Google Scholar]
- NAGINGTON J., HORNE R. W. Morphological studies of orf and vaccinia viruses. Virology. 1962 Mar;16:248–260. doi: 10.1016/0042-6822(62)90245-3. [DOI] [PubMed] [Google Scholar]
- NAGINGTON J., NEWTON A. A., HORNE R. W. THE STRUCTURE OF ORF VIRUS. Virology. 1964 Aug;23:461–472. doi: 10.1016/0042-6822(64)90230-2. [DOI] [PubMed] [Google Scholar]
- NAGINGTON J., PLOWRIGHT W., HORNE R. W. The morphology of bovine papular stomatitis virus. Virology. 1962 Jun;17:361–364. doi: 10.1016/0042-6822(62)90129-0. [DOI] [PubMed] [Google Scholar]
- NEFF B. J., ACKERMANN W. W., PRESTON R. E. STUDIES OF VACCINIA HEMAGGLUTININ OBTAINED FROM VARIOUS VACCINIA INFECTED TISSUES. DENSITY GRADIENT CENTRIFUGATION AND ELECTRON MICROSCOPY. Proc Soc Exp Biol Med. 1965 Mar;118:664–671. doi: 10.3181/00379727-118-29934. [DOI] [PubMed] [Google Scholar]
- NISHMI M., KELLER R. Observations on the mode of spread of vaccinia virus in HeLa cell monolayer cultures. Virology. 1962 Jan;16:91–92. doi: 10.1016/0042-6822(62)90206-4. [DOI] [PubMed] [Google Scholar]
- NISHMI M., KELLER R. The microepidemiology of vaccinial infection as studied in HeLa cell stationary cultures. Virology. 1962 Sep;18:109–117. doi: 10.1016/0042-6822(62)90183-6. [DOI] [PubMed] [Google Scholar]
- NOYES W. F. Further studies on the structure of vaccinia virus. Virology. 1962 Dec;18:511–516. doi: 10.1016/0042-6822(62)90051-x. [DOI] [PubMed] [Google Scholar]
- NOYES W. F. OBSERVATIONS ON TWO POX-TUMOR VIRUSES. Virology. 1965 Apr;25:666–669. doi: 10.1016/0042-6822(65)90097-8. [DOI] [PubMed] [Google Scholar]
- Nagington J., Tee G. H., Smith J. S. Milker's nodule virus infections in Dorset and their similarity to orf. Nature. 1965 Oct 30;208(5009):505–507. doi: 10.1038/208505a0. [DOI] [PubMed] [Google Scholar]
- O'SULLIVAN D. G., SA DLER P. W. Agents with high activity against type 2 poliovirus. Nature. 1961 Oct 28;192:341–343. doi: 10.1038/192341a0. [DOI] [PubMed] [Google Scholar]
- ODA M. PLAQUE-HEMADSORPTION TECHNIQUE FOR THE DETECTION OF NONHEMAGGLUTINATING VACCINIA VIRUS. Virology. 1964 Jul;23:432–434. doi: 10.1016/0042-6822(64)90268-5. [DOI] [PubMed] [Google Scholar]
- ODA M. RESCUE OF DERMOVACCINIA ABORTIVE INFECTION BY NEUROVACCINIA VIRUS IN L CELLS. Virology. 1965 Apr;25:664–666. doi: 10.1016/0042-6822(65)90096-6. [DOI] [PubMed] [Google Scholar]
- ODA M. VACCINIA VIRUS-HELA CELL INTERACTION. II. THE EFFECT OF MITOMYCIN C ON THE PRODUCTION OF INFECTIVE VIRUS, COMPLEMENT-FIXING ANTIGEN, AND HEMAGGLUTININ. Virology. 1963 Dec;21:533–539. doi: 10.1016/0042-6822(63)90225-3. [DOI] [PubMed] [Google Scholar]
- PETERS D., MUELLER G., BUETTNER D. THE FINE STRUCTURE OF PARAVACCINIA VIRUSES. Virology. 1964 Aug;23:609–611. doi: 10.1016/0042-6822(64)90246-6. [DOI] [PubMed] [Google Scholar]
- PFAU C. J., MCCREA J. F. STUDIES ON THE DEOXYRIBONUCLEIC ACID OF VACCINIA VIRUS. III. CHARACTERIZATION OF DNA ISOLATED BY DIFFERENT METHODS AND ITS RELATION TO VIRUS STRUCTURE. Virology. 1963 Nov;21:425–435. doi: 10.1016/0042-6822(63)90204-6. [DOI] [PubMed] [Google Scholar]
- PFAU C. J., McCREA J. F. Release of deoxyribonucleic acid from vaccinia virus by 2-mercaptoethanol and pronase. Nature. 1962 Jun 2;194:894–895. doi: 10.1038/194894a0. [DOI] [PubMed] [Google Scholar]
- PLANTEROSE D. N., NISHIMURA C., SALZMAN N. P. The purification of vaccinia virus from cell cultures. Virology. 1962 Oct;18:294–301. doi: 10.1016/0042-6822(62)90016-8. [DOI] [PubMed] [Google Scholar]
- PLOWRIGHT W., FERRIS R. D. Ether sensitivity of some mammalian pox viruses. Virology. 1959 Mar;7(3):357–358. doi: 10.1016/0042-6822(59)90208-9. [DOI] [PubMed] [Google Scholar]
- PRESCOTT D. M., BENDER M. A. Synthesis of RNA and protein during mitosis in mammalian tissue culture cells. Exp Cell Res. 1962 Mar;26:260–268. doi: 10.1016/0014-4827(62)90176-3. [DOI] [PubMed] [Google Scholar]
- PRUSOFF W. H., BAKHLE Y. S., MCCREA J. F. INCORPORATION OF 5-IODO-2'-DEOXYURIDINE INTO THE DEOXYRIBONUCLEIC ACID OF VACCINIA VIRUS. Nature. 1963 Sep 28;199:1310–1311. doi: 10.1038/1991310a0. [DOI] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL C. C., GAFFORD L. G., ARHELGER R. B. Electron microscopic examination of isolated fowlpox inclusions. Virology. 1961 Jul;14:380–382. doi: 10.1016/0042-6822(61)90325-7. [DOI] [PubMed] [Google Scholar]
- RANDALL C. C., GAFFORD L. G., DARLINGTON R. W., HYDE J. COMPOSITION OF FOWLPOX VIRUS AND INCLUSION MATRIX. J Bacteriol. 1964 Apr;87:939–944. doi: 10.1128/jb.87.4.939-944.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISNER A. H., SOBEY W. R., CONOLLY D. DIFFERENCES AMONG THE SOLUBLE ANTIGENS OF MYXOMA VIRUSES ORIGINATING IN BRAZIL AND IN CALIFORNIA. Virology. 1963 Jul;20:539–541. doi: 10.1016/0042-6822(63)90106-5. [DOI] [PubMed] [Google Scholar]
- REVEL M., HIATT H. H. THE STABILITY OF LIVER MESSENGER RNA. Proc Natl Acad Sci U S A. 1964 May;51:810–818. doi: 10.1073/pnas.51.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RONDLE C. J., DUMBELL K. R. Antigens of cowpox virus. J Hyg (Lond) 1962 Mar;60:41–49. doi: 10.1017/s0022172400039292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D. THE SYNTHESIS OF A DNA-LIKE RNA IN THE CYTOPLASM OF HELA CELLS INFECTED WITH VACCINIA VIRUS. J Mol Biol. 1964 Mar;8:405–416. doi: 10.1016/s0022-2836(64)80204-7. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D. Viral protein and DNA synthesis in vaccinia virus-infected HeLacell cultures. Virology. 1963 Apr;19:542–550. doi: 10.1016/0042-6822(63)90049-7. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P. The rate of formation of vaccinia deoxyribonucleic acid and vaccinia virus. Virology. 1960 Jan;10:150–152. doi: 10.1016/0042-6822(60)90015-5. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., PREVIC E. P., GILLESPIE M. E. MESSENGER RNA AND POLYRIBOSOMES IN BACILLUS MEGATERIUM. J Mol Biol. 1965 May;12:119–129. doi: 10.1016/s0022-2836(65)80286-8. [DOI] [PubMed] [Google Scholar]
- SCHARFF M. D., SHATKIN A. J., LEVINTOW L. ASSOCIATION OF NEWLY FORMED VIRAL PROTEIN WITH SPECIFIC POLYRIBOSOMES. Proc Natl Acad Sci U S A. 1963 Oct;50:686–694. doi: 10.1073/pnas.50.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER K., LATHAM H., DARNELL J. E. Demonstration of an unstable RNA and of a precursor to ribosomal RNA in HeLa cells. Proc Natl Acad Sci U S A. 1963 Feb 15;49:240–248. doi: 10.1073/pnas.49.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP D. G., SADHUKHAN P., GALASSO G. J. QUALITY CHANGES IN VACCINIA VIRUS DURING ADAPTATION TO GROWTH IN CULTURES OF EARLE'S L CELLS. J Bacteriol. 1964 Aug;88:309–312. doi: 10.1128/jb.88.2.309-312.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHATKIN A. J. ACTINOMYCIN D AND VACCINIA VIRUS INFECTION OF HELA CELLS. Nature. 1963 Jul 27;199:357–358. doi: 10.1038/199357a0. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J., SALZMAN N. P. Deoxyribonucleic acid synthesis in vaccinia virus-infected HeLa cells. Virology. 1963 Apr;19:551–560. doi: 10.1016/0042-6822(63)90050-3. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J., SEBRING E. D., SALZMAN N. P. VACCINIA VIRUS DIRECTED RNA: ITS FATE IN THE PRESENCE OF ACTINOMYCIN. Science. 1965 Apr 2;148(3666):87–90. doi: 10.1126/science.148.3666.87. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J. The formation of vaccinia virus protein in the presence of 5-fluorodeoxyuridine. Virology. 1963 Jun;20:292–301. doi: 10.1016/0042-6822(63)90118-1. [DOI] [PubMed] [Google Scholar]
- STAEHELIN T., WETTSTEIN F. O., NOLL H. Breakdown of rat-liver ergosomes in vivo after actinomycin inhibition of messenger RNA synthesis. Science. 1963 Apr 12;140(3563):180–183. doi: 10.1126/science.140.3563.180. [DOI] [PubMed] [Google Scholar]
- SZYBALSKI W., ERIKSON R. L., GENTRY G. A., GAFFORD L. G., RANDALL C. C. Unusual properties of fowlpox virus DNA. Virology. 1963 Apr;19:586–589. doi: 10.1016/0042-6822(63)90056-4. [DOI] [PubMed] [Google Scholar]
- THOMPSON R. L., DAVIS J., RUSSELL P. B., HITCHINGS G. H. Effect of aliphatic oxime and isatin thisemicarbazones on vaccinia infection in the mouse and in the rabbit. Proc Soc Exp Biol Med. 1953 Nov;84(2):496–499. doi: 10.3181/00379727-84-20690. [DOI] [PubMed] [Google Scholar]
- VERNA J. E. CELL-CULTURE RESPONSE TO FIBROMA VIRUS. J Bacteriol. 1965 Feb;89:524–528. doi: 10.1128/jb.89.2.524-528.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLA-TREVINO S., FARBER E., STAEHELIN T., WETTSTEIN F. O., NOLL H. BREAKDOWN AND REASSEMBLY OF RAT LIVER ERGOSOMES AFTER ADMINISTRATION OF ETHIONINE OR PUROMYCIN. J Biol Chem. 1964 Nov;239:3826–3833. [PubMed] [Google Scholar]
- WAGNER R. R. INHIBITION OF INTERFERON BIOSYNTHESIS BY ACTINOMYCIN D. Nature. 1964 Oct 3;204:49–51. doi: 10.1038/204049a0. [DOI] [PubMed] [Google Scholar]
- WESTWOOD J. C., HARRIS W. J., ZWARTOUW H. T., TITMUSS D. H., APPLEYARD G. STUDIES ON THE STRUCTURE OF VACCINIA VIRUS. J Gen Microbiol. 1964 Jan;34:67–78. doi: 10.1099/00221287-34-1-67. [DOI] [PubMed] [Google Scholar]
- WESTWOOD J. C., ZWARTOUW H. T., APPLEYARD G., TITMUSS D. H. COMPARISON OF THE SOLUBLE ANTIGENS AND VIRUS PARTICLE ANTIGENS OF VACCINIA VIRUS. J Gen Microbiol. 1965 Jan;38:47–53. doi: 10.1099/00221287-38-1-47. [DOI] [PubMed] [Google Scholar]
- WOODROOFE G. M., FENNER F. Genetic studies with mammalian poxviruses. IV. Hybridization between several different poxviruses. Virology. 1960 Oct;12:272–282. doi: 10.1016/0042-6822(60)90200-2. [DOI] [PubMed] [Google Scholar]
- WOODROOFE G. M., FENNER F. Serological relationships within the poxvirus group: an antigen common to all members of the group. Virology. 1962 Mar;16:334–341. doi: 10.1016/0042-6822(62)90255-6. [DOI] [PubMed] [Google Scholar]
- WOODROOFE G. M., FENNER F. VIRUSES OF THE MYXOMA-FIBROMA SUBGROUP OF THE POXVIRUSES. I. PLAQUE PRODUCTION IN CULTURED CELLS, PLAQUE-REDUCTION TESTS, AND CROSS-PROTECTION TESTS IN RABBITS. Aust J Exp Biol Med Sci. 1965 Apr;43:123–142. [PubMed] [Google Scholar]
- Woodson B., Joklik W. K. The inhibition of vaccinia virus multiplication by isatin-beta-thiosemicarbazone. Proc Natl Acad Sci U S A. 1965 Sep;54(3):946–953. doi: 10.1073/pnas.54.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZWARTOUW H. T. THE CHEMICAL COMPOSITION OF VACCINIA VIRUS. J Gen Microbiol. 1964 Jan;34:115–123. doi: 10.1099/00221287-34-1-115. [DOI] [PubMed] [Google Scholar]
- ZWARTOUW H. T., WESTWOOD J. C., APPLEYARD G. Purification of pox viruses by density gradient centrifugation. J Gen Microbiol. 1962 Nov;29:523–529. doi: 10.1099/00221287-29-3-523. [DOI] [PubMed] [Google Scholar]
- ZWARTOUW H. T., WESTWOOD J. C., HARRIS W. J. ANTIGENS FROM VACCINIA VIRUS PARTICLES. J Gen Microbiol. 1965 Jan;38:39–45. doi: 10.1099/00221287-38-1-39. [DOI] [PubMed] [Google Scholar]


