Abstract
A number of cytotoxic T-cell epitopes are cryptic epitopes generated from non-conventional sources. These include epitopes that are encoded by alternative open reading frames or in generally non-coding genomic regions, such as introns. We have previously observed a frequent recognition of cryptic epitopes by tumor infiltrating lymphocytes isolated from melanoma patients. Here, we show that such cryptic epitopes are more frequently recognized than antigens of the same class encoded by canonical reading frames. Furthermore, we report the presence of T cells specific for three cryptic epitopes encoded in intronic sequences, as a result of incomplete splicing, in the circulation of melanoma patients. One of these epitopes derives from antigen isolated from immunoselected melanoma 2 (AIM2), while the two others are encoded in an alternative open reading frame of an incompletely spliced form of N-acetylglucosaminyl-transferase V (GNT-V) known as NA17-A. We have detected frequent T-cell responses against AIM2 and NA17-A epitopes in the blood of melanoma patients, both prior and after one round of in vitro peptide stimulation, but not in the circulation of healthy individuals and patients with breast or renal carcinoma. In summary, our findings indicate that the T-cell reactivity against AIM2 and NA17-A in the blood of melanoma patients is extensive, suggesting that—similar to melan A (also known as MART1)—these antigens might be used for immunomonitoring or as model antigens in several clinical and preclinical settings.
Keywords: CD8 T cells, T-cell reactivity, antigens, cryptic T-cell epitopes, melanoma
Introduction
When dissecting the antigen specificity of tumor-infiltrating lymphocytes (TILs) in melanoma patients, we detected T-cell responses against 18 different epitopes, predominantly from differentiation antigens such as melan A (also known as MART1) and gp100.1 Surprisingly, we also observed frequent responses against tumor-associated antigens (TAAs) that contain cryptic T-cell epitopes. Two of these epitopes were encoded by antigen isolated from immunoselected melanoma 2 (AIM2)2 and by an alternative open reading frame of an incompletely spliced form of N-acetylglucosaminyl-transferase V (GNT-V) known as NA17-A.3
AIM2 was originally identified when T-cell clones established from a mixed lymphocyte-tumor cell culture were found to recognize a previously undescribed antigen.2 An autologous cDNA library was screened in the presence of a reactive T-cell clone to precisely identify the target of reactivity, leading to the discovery of AIM2. AIM2 seems to be composed of two short open reading frames (ORFs) and a retained intronic sequence. ORF2, which is overlapping with the intron, encodes an HLA-A1-restricted T-cell epitope, namely the decapeptide RSDSGQQARY. AIM2 is expressed by the vast majority of melanomas and glioblastomas, and—at comparatively lower levels—by several other neoplasms, including breast carcinomas, ovarian carcinomas, colon carcinomas, and neuroectodermal tumors. Only low expression levels of AIM2 have been detected in (a few) normal tissues.
NA17-A was identified in a similar manner as AIM2, but the primordial NA17-A-reactive T-cell clone was established from TILs.3 The cDNA clone encoding the target for T-cell reactivity was found to contain two exons in common with GNT-V. However, these exons were separated by an unspliced intron, which encodes two T-cell epitopes. A putative cryptic promoter region was identified in the intronic sequence, driving the transcription of an alternative ORF. This promoter is active in melanoma, but not in normal cells, causing the melanoma-specific expression of NA17-A in 50% of patients. NA17-A encodes two HLA-A2-restricted T-cell epitopes, the nonapeptide VLPDVFIRC, and the overlapping decapeptide VLPDVFIRCV.
Based on the notion that these AIM2- and NA17-A-derived epitopes are frequently recognized by TILs isolated from melanoma lesions, we decided to study the corresponding T-cell reactivity in the peripheral blood of healthy individuals and cancer patients, including patients with melanoma, breast carcinoma, and renal cell carcinoma. Furthermore, we decided to compare the T-cell recognition of cryptic epitopes to that of epitopes encoded by canonical reading frames, to better understand the impact of these antigens in antitumor immune responses.
Our findings indicate that there is an extensive T-cell reactivity against AIM2 and NA17-A in the circulation of melanoma patients, nearly as abundant as that against MART1.4 Thus, AIM2- and NA17-A-derived peptides stand out as ideal candidates for immunomonitoring and as model antigens in multiple clinical and preclinical settings.
Results and Discussion
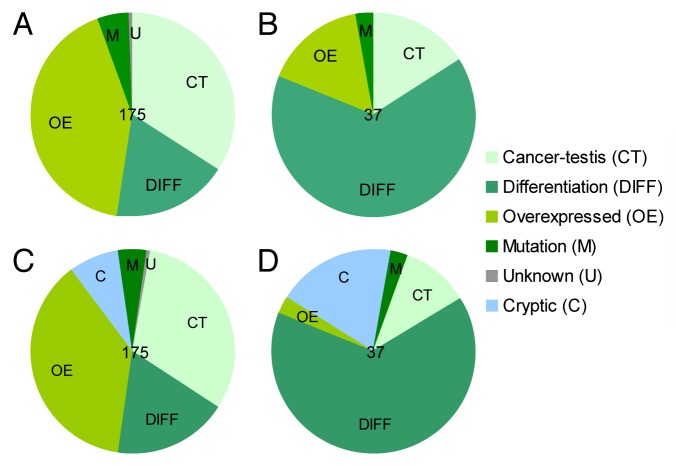
In a previous study, we screened the reactivity of TILs isolated from melanoma lesions against all known melanoma-associated T-cell epitopes (n = 175, selected for HLA-A1, -A2, -A3, -A11, and -B7) (Fig. 1A). For the most part, TILs recognized and reacted against differentiation antigens (Fig. 1B), but—surprisingly—we also detected frequent T-cell reactivity against various cryptic epitopes encoded in alternative ORFs or in generally non-coding genomic regions, such as introns.1 These cryptic epitopes were sorted according to the corresponding canonical ORF and therefore inaccurately classified as cancer-testis or overexpressed antigens. We have examined a peptide library including all known melanoma-associated T-cell epitopes for the occurrence of cryptic epitopes, and found that 14 of 175 peptides corresponded indeed to cryptic epitopes (Fig. 1C). We detected responses in melanoma-infiltrating lymphocytes against five of these epitopes, suggesting that this group of antigens significantly contributes to antigen recognition in this setting. In absolute terms, cryptic epitopes were the second most frequently recognized group of antigens, only outcompeted by differentiation antigens (Fig. 1D). However, when the number of cryptic (n = 14) vs. differentiation (n = 32) epitopes tested was taken into account, the fraction of responses against the former (36%) and the latter (35%) was comparable. Furthermore, we observed a tendency for cryptic epitopes to promote more T-cell responses (7 of 37) than what expected based on their relative abundance within the epitope library (14 of 175) (P = 0.06, Fisher’s exact test), as this was clearly the case for differentiation antigens (24 of 37 responses, 32 of 175 epitopes; P < 0.0001, Fisher’s exact test). The prominent contribution of cryptic epitopes to TIL reactivity against overexpressed TAAs is evident by the comparison of Figure 1B and D. Indeed, the majority of T-cell responses against overexpressed TAAs detected among melanoma-infiltrating lymphocytes was in reality specific for cryptic epitopes.
Figure 1. Contribution of tumor-associated antigen classes to T-cell reactivity in melanoma-infiltrating lymphocytes. (A and B) Pie charts show the classification of 175 previously described melanoma-associated T-cell epitopes (A) and the distribution of 37 T-cell responses against these tumor-associated antigens (TAAs) previously detected among tumor-infiltrating lymphocytes (TILs) (B) into classes. Please note that each response is only counted once in each patient.1 (C and D) Pie charts as in (A and B), respectively, with cryptic epitopes considered as a standalone class of TAAs.
These results not only indicate that cryptic epitopes are frequently recognized by T cells in melanoma patients, but also suggest that the identification and correct classification of cryptic epitopes may be relevant for understanding the differences in the immune recognition of different classes of antigens. It may be speculated, but remains to be experimentally verified, that the high response rate against this class of antigens as compared with conventional overexpressed TAAs might reflect differences in the induction of tolerance.
Cryptic T-cell epitopes may be highly relevant for cancer immunotherapy, since alternative ORFs are often selectively (or at least predominantly) expressed by malignant cells.2,3,5,6 Furthermore, frequent T-cell reactivity against AIM2 and NA17-A was indeed detected among TILs isolated from melanoma lesions, demonstrating that these epitopes may be relevant for both tumor targeting and monitoring in the course of immunotherapy.
To elucidate the occurrence of T-cell reactivity against AIM2 and NA17-A, we screened the peripheral blood of cancer patients and healthy individuals using MHC multimers and an interferon γ (IFNγ)-specific ELISPOT assay. Since the expression of both these antigens had previously been shown to be predominant among selected groups of cancer patients, we first screened peripheral blood lymphocytes (PBLs) from these patients for their reactivity against AIM2- and NA17-A-derived epitopes. We analyzed the PBLs directly ex-vivo, using MHC multimers, as well as after one week of culture upon peptide stimulation, using MHC multimers and IFNγ-specific ELISPOT assays. We detected AIM2-specific T cells in 6/13 (46%) melanoma patients tested, upon the culture of PBLs in the presence of peptide stimulation, by means of MHC multimers (Fig. 2A; Fig. S1). In one of these patients we were also able to detect the AIM2-specific T cells directly ex-vivo. In contrast, we were unable to detect AIM2-specific T cells by IFNγ-specific ELISPOT (Fig. 2B), which may indicate that these T cells exert cytotoxic effects via the secretion of cytokines other than IFNγ or have limited functionality.
Figure 2. T-cell reactivity against AIM2 and NA17-A in the circulation of melanoma patients. (A, C, and E) T-cell responses measured with MHC multimers. Responses detectable on freshly thawed peripheral blood lymphocytes (PBLs) are illustrated in gray, while responses detectable upon culture in the presence of relevant peptides are shown in black. Striped bars indicate responses that were below the detection limit of 10 MHC multimer+ events. The X-axis report patient ID (MM), while the Y-axis (logarithmic scale) depicts the frequency of multimer+ cells. Representative dot plots are shown in Figure S1. (B, D, and F) T-cell responses measured with interferon γ (IFNγ)-specific ELISPOT assays. Black bars indicate positive responses, with more than twice as many spots in experimental wells than in control wells, while white bars indicate responses that were below the detection limit. Data are reported as mean ± SD of 2 replicate measurements. The X-axis report patient ID while the Y-axis depict the number of spots per 105 cells recorded in IFNγ ELISPOT assays.
Two epitopes from NA17-A were screened, a nonapeptide and a decapeptide. We detected responses against the nonapeptide in freshly thawed PBLs from 3/16 melanoma patients tested. The PBLs from the same patients were reactive against the NA17-A-derived nonapeptide also upon culture in the presence of peptide stimulation (Fig. 2C). In this setting, we observed T-cell responses in 6/16 (38%) patients, although not all of them were detectable with both methods (Fig. 2C and D). A reactivity against the decapeptide was observed in the PBLs of 6/16 (38%) melanoma patients tested, upon culture in the presence of peptide stimulation. Surprisingly, these were more readily detected by IFNγ-specific ELISPOT than with MHC multimers (Fig. 2E and F). Furthermore, we were unable to document any of these responses on freshly thawed PBLs. It may therefore be speculated, but remains to be formally demonstrated, that T-cell responses that are only detectable by ELISPOT may involve T-cell receptors (TCRs) that exhibit a low avidity for peptide-MHC complexes, making them more difficult to be detected with MHC multimers than with ELISPOT assays. It has been reported that the half-life of some TCR-MHC multimers might be excessively short for an efficient staining at some conditions,7 perhaps also applying to TCRs specific for our NA17-A-derived decapeptide. To get further insights into this issue, we tested freshly thawed PBLs for their reactivity against the NA17-A-derived decapeptide by IFNγ-specific ELISPOT assays, but no responses were detected (data not shown).
After investigating AIM2 and NA17-A T-cell reactivity in melanoma patients, we focused on subjects affected by two other types of cancer as well as on healthy individuals. To this aim, we screened the PBLs from 7 (NA17-A) or 9 (AIM2) breast carcinoma patients and from 4 patients with renal cell carcinoma, as well as the peripheral blood mononuclear cells (PBMCs) of 15 (NA17-A) or 13 (AIM-2) healthy subjects, by the exact same approach that we had used earlier for melanoma patients. We detected no reactivity against AIM2 and NA17-A in healthy donors as well as in patients bearing neoplasms other than melanoma (Table 1). Statistical analyses revealed a significant difference in the T-reactivity of melanoma patients and healthy donors against AIM2- and NA17-A-derived epitopes, as well as between AIM2-specific responses in melanoma patients and subjects bearing breast carcinoma (Table 1). There was no statistically significant difference between melanoma patients and any other group of cancer patient relative to NA17-A reactivity. The lack of responses against AIM2- and NA17-A-derived cryptic epitopes in breast and renal cell carcinoma patients observed in this study does not preclude the existence of T cells targeting AIM2 and NA17-A in other types of cancer than melanoma. Since the expression of NA17-A by breast and renal cell carcinomas has not been demonstrated yet, and as AIM2 is expressed by a reduced fraction of non-melanoma cancers, these results were expected. To better elucidate the reactivity of T cells from breast carcinoma and renal cell carcinoma patients to AIM2- and NA17-A-derived cryptic epitopes, large patient cohorts should be analyzed.
Table 1. Overview of T-cell responses against three cryptic epitopes in healthy subjects and melanoma, breast carcinoma, and renal cell carcinoma patients.
| AIM2 | NA17-A nonamer | NA17-A decamer | |
|---|---|---|---|
| Melanoma |
6/13 (46%) |
6/16 (38%) |
6/16 (38%) |
| Breast cancer |
0/9 (0%)* |
0/7 (0%) |
0/7 (0%)> |
| Renal cell carcinoma |
0/4 (0%) |
0/4 (0%) |
0/4 (0%) |
| Healthy donors | 0/13 (0%)* | 0/15 (0%)* | 0/15 (0%)* |
P < 0.05 (Fisher’s exact test), as compared with melanoma patients.
Since we could not detect AIM2-reactive T cells by IFNγ ELISPOT assays, we further investigated the functionality of AIM2-specific T cells. To this aim, we isolated AIM2-specific cells from the PBLs of 5 melanoma patients by fluorescence-activated cell sorting (FACS) and expanded them using feeder cells, anti-CD3 antibodies, and interleukin-2 (IL-2). Three of the resulting T-cell cultures were tested for their ability to produce IFNγ, tumor necrosis factor α (TNFα) and IL-2. All of them were found to secrete IFNγ and TNFα, but not IL-2 (Fig. 3A), perhaps owing to the fact that T cells were cultured in the presence of exogenous IL-2. All three cultures were also able to lyse BM36.1 cells pulsed with the AIM2-derived peptide but not with an irrelevant HIV-1-derived peptide (Fig. 3B). In addition, we tested the cytotoxic capacities of five AIM2-specific T-cell cultures on two HLA-A1+AIM2+ melanoma cell lines. All these 5 cultures were indeed able to kill HLA-A1+AIM2+ melanoma cells (Fig. 3C; Fig. S2A). Conversely, HLA-A1−AIM2+ cancer cell lines were resistant to the cytotoxic activity of these T-cell cultures, pointing to an HLA-A1-restricted effector mechanism (Fig. 3C; Fig. S2B). HLA restriction as well as AIM2 specificity were further confirmed for one T-cell culture by blocking cytotoxicity with an anti-MHC Class I antibody (W6/32) and by increasing lytic functions with an exogenous AIM2-derived peptide, respectively (Fig. 3C). Furthermore, cold target inhibition using unlabeled BM36.1 cells pulsed with an AIM2-derived peptide decreased cytotoxicity, while unlabeled BM36.1 cells pulsed with an irrelevant HIV-1-derived peptide did not do so (Fig. 3C). Finally, we analyzed the ability of one T-cell culture to kill PBMCs from two healthy individuals, since low AIM2 expression levels have been reported for some types of normal cells. Healthy PBMCs were not killed by T cells, indicating a cancer-specific cytotoxic activity (Fig. S2B). Taken together, these data demonstrate that AIM2-specific T cells may indeed be functional, in terms of both cytokine secretion and cytotoxic activity.
Figure 3. Functionality of AIM2-specific T-cell cultures. Four FACS-sorted, expanded AIM2-specific T-cell cultures were established from patients MM5, MM7, MM8, and MM9. (A) Three of these cultures were stimulated with BM36.1 cells pulsed with the AIM2 decapeptide or HIV-A1 (control peptide) at a 10:1 effector:target ratio, and cytokine production was measured by intracellular cytokine staining. Background was <0.4% and was subtracted from positive samples. The frequency of AIM2-specific multimer+CD8+ T lymphocytes (among total CD8+ T cells) in each culture was: 19.6%, 14%, and 61.6% for MM7, MM8, and MM9, respectively. (B) T-cell cultures from patients MM7, MM8, and MM9 were stimulated with BM36.1 cells pulsed with the AIM2 decapeptide or HIV-A1 (control peptide) at the indicated effector:target ratio and cytotoxicity was measured by 51Cr-release assays. The frequency of AIM2 specific multimer+ T cells (among total live cells) was: 17.2%, 2.7%, and 31.6% for MM7, MM8, and MM9, respectively. (C) The T-cell culture established from patient MM5 was stimulated with the indicated melanoma cell lines at the indicated effector:target ratio and cytotoxicity was measured by 51Cr-release assays. FM28 cells are HLA-A1+ while FM48 and FM74 cells are HLA-A1−, yet all express AIM2 (as determined by PCR; data not shown). To test peptide-specificity and HLA-restriction, FM28 cells were pulsed with the AIM2-derived decapeptide or incubated with an anti-MHC Class I antibody (W6/32). Furthermore, cold target inhibition was performed using unlabeled BM36.1 cells pulsed with either HIV-A1 or the AIM2-derived decapeptide at an inhibitor:target ratio of 20:1. There were 68% AIM2 specific T cells in the culture. Data are reported as mean ± SD of 2 replicate measurements.
AIM2- and NA17-A-specific T cells are frequently detected in the circulation of melanoma patients, and thus far resembling MART1.4 Nonetheless, neither of these antigens has widely been used in experimental and clinical settings. AIM2 is poorly described, and after its discovery in 20012 only a few groups have worked with this antigen, and mostly in the context of glioma.8,9 A limited number of publications describe the use of NA17-A-derived epitopes as model antigens, but the majority of these publications come from the same research group.10-14 NA17-A-specific T cells have been studied in melanoma-bearing lymph nodes15 as well as in ascites from a melanoma patient,16 two settings in which a T-cell reactivity against NA17-A was reported. In a vaccination study in mice, strong and frequent T-cell responses against NA17-A (measured as reactivity against the nonapeptide VLPDVFIRC) were achieved.17 This nonapeptide has also been included in one clinical trial, reporting an increase in the frequency of NA17-A-specific T cells after the administration of peptide-loaded dendritic cells in 2/9 patients and the presence of NA17-A-specific TILs upon treatment in a third patient.18 No objective responses were observed in this study, but two patients achieved stable disease. Notably, both the subjects exhibited T-cell reactivity against NA17-A. Together with these results, our findings indicate that NA17-A may represent a highly relevant melanoma-associated antigen.
In conclusion, both AIM2 and NA17-A are frequently detected by T cells in melanoma patients, and hence may serve as relevant targets for immunotherapy or might be used for the immunomonitoring of clinical trials testing unspecific immunotherapies, such as the CTLA4-targeting antibody ipilimumab19 or the adoptive transfer of ex vivo expanded TILs.20
Materials and Methods
Peptides
Peptides were purchased from Pepscan (Pepscan Presto BV) or KJ Ross-Petersen ApS (Denmark) and dissolved in DMSO or H2O to stock concentrations of 10 or 2 mM, respectively. Sequences: AIM2-derived decapeptide, RSDSGQQARY; NA17-A-derived nonapeptide, VLPDVFIRC; NA17-A-derived decapeptide VLPDVFIRCV; HIV-1-derived negative control peptide HIV-A1, GSEELRSLY; and HIV-1-derived negative control peptide HIV-A2 (ILKEPVHGV).
Samples from cancer patients and healthy subjects
PBLs were obtained from leukapheresis products of late-stage melanoma, breast and renal cell carcinoma patients enrolled in immunotherapeutic protocols at the University Hospital Herlev, depleted of monocytes (by adherence) and cryopreserved at −150 °C in fetal bovine serum (FBS) containing 10% DMSO. Alternatively, PBLs were obtained from peripheral blood samples of breast cancer patients at primary diagnosis, purified by density centrifugation on Lymphoprep™ (Axis-Shield PoC) and cryopreserved at −150 °C in FBS containing 10% DMSO. No patients received therapy at the time of PBL collection, but late-stage patients had been subjected to several rounds of previous therapy. Blood from healthy individuals was obtained from the blood bank of the Copenhagen University Hospital. PBMCs were isolated by density centrifugation on Lymphoprep™ (Axis-Shield PoC) and cryopreserved at −150 °C in FBS and 10% DMSO. All the procedures were approved by the Scientific Ethics Committee for the Capital Region of Denmark. Written informed consent was obtained, according to the Declaration of Helsinki.
T-cell staining
MHC multimers were produced as described in Supplementary Materials and Methods (final concentration: 10 μg/mL). No more than 2 × 106 freshly thawed or cultured PBLs/PBMCs were stained with 2.5 μl phycoerythrin (PE)-conjugated MHC multimers and 2.5 μL allophycocyanin (APC)- or brilliant violet 421 (BV421)-conjugated MHC multimers in a final volume of 50 μL PBS supplemented with 2% (for most stainings) or 50% (for some AIM2 stainings, due to high background) FBS. Cells were incubated at 37 °C for 15 min and then placed on ice. An antibody mix was prepared with anti-CD3, anti-CD8, anti-CD4 antibodies (all from BD), and the dead cell marker NIR-ViD (LIVE/DEAD Fixable Near-IR; Invitrogen) in PBS supplemented with 2% FBS (for most stainings) or FBS-free PBS (for AIM2 stainings with a high background). Cells were stained with 50 μL of this antibody mix and incubated for 30 min on ice. Next, cells were washed twice with PBS supplemented with 2% FBS. Data acquisition was performed on LSR-II, FacsAria or FacsCanto flow cytometers (BD), and first line analyses were performed by means of the FacsDiva software (BD). We gated on single (FSC-A, FSC-W), live CD3+CD4−CD8+multimer+ lymphocytes. We always included HIV-specific multimers as negative controls. The detection limit was set at ≥10 MHC multimer+ events.
IFNγ-specific ELISPOT assays
ELISPOT assays to quantify the amount of peptide-specific IFNγ-releasing effector cells were performed as described previously21 and in Supplementary Materials and Methods. Before analysis, PBLs/PBMCs were stimulated once in vitro with 20 μM peptide and 40 U/ml IL-2 (PeproTech), to extend the sensitivity of the assay. Effector cells were added to the wells in duplicate instances, at different concentrations (1 × 105 and 3 × 105 cells per well), and in the presence of 5 μM HIV-1-derived or relevant peptides. In addition, 104 T2 (ATCC CRL-1992) or BM36.1 cells were added to each well, and plates were incubated overnight. The spots were counted using an ImmunoSpot® Analyzer plate reader and the software ImmunoCapture 6.0 and ImmunoSpot professional satellite 4.0.17 (C.T.L. Cellular Technology Ltd.). The frequency of peptide-specific CTLs was calculated as the average number of peptide-specific spots formed in ELISPOT assays, i.e., the number of spots formed in the wells containing AIM2- or NA17-A-derived peptides upon subtraction of the number of spots formed in the presence of HIV-1-derived peptides. A response was defined as positive if there were at least twice as many spots in experimental wells than in negative control wells.
Establishment of antigen-specific T-cell cultures
PBLs were stimulated once with 20 μM peptide and 40 U/ml IL-2 and cultured for one week. Cultured PBLs were stained in RPMI 1640 medium (Gibco) supplemented with 2% human serum (Sigma), as described above in the T-cell staining section (though no dead cell marker was used). Multimer+ cells were sorted on a FacsAria cell sorter into a 96-well plate containing 105 irradiated (25 Gy) allogenic feeder cells per well as well as 120 U/mL (for MM7, MM8, and MM9 patients) or 1000 U/mL (for MM3 and MM5 patients) IL-2 and 15 ng/mL anti-CD3 antibodies (OKT-3; from Ortho Biotech). A maximum of 500 cells were sorted per well. Cells were then cultured in X-vivo medium (Bio Whittaker) supplemented with 5% human serum and every 3–4 d 120 U/mL (for MM7, MM8, and MM9 patients) or 3000 U/mL (for MM3 and MM5 patients) IL-2 was added. After three weeks, MM7, MM8, and MM9 cultures were restimulated with irradiated, allogeneic feeder cells (in a 1:1 ratio), 15 ng/mL anti-CD3 antibodies, and 120 U/mL IL-2. Cultures from MM3 and MM5 patients were tested before re-stimulation. An MHC multimer staining was always performed in parallel with intracellular cytokine stainings and 51Cr-release assays to test the purity of the culture at the time of functional tests.
Chromium release assays
Conventional 4 h 51Cr-release assays to assess T-cell cytotoxicity were performed as described elsewhere.22 In brief, target cells were labeled with Na251CrO4 (Perkin Elmer) for 1 h, washed, and co-cultured with effector cells for 4 h. Next, the level of 51Cr in the supernatant was measured using a Perkin Elmer Wallac Wizard 1470 automatic γ counter. BM36.1 cells, FM48 cells (HLA-A1− melanoma cells, generated in house), FM74 (HLA-A1− melanoma cells, ESTDAB-022), and FM28 (HLA-A1+ melanoma cells, ESTDAB-006) were used as target cells. In one case, lysis was inhibited with an anti-MHC Class I antibody (W6/32; Dako). Alternatively, cold target inhibition was performed by means of unlabeled BM36.1 cells pulsed with 10 μM HIV-A1 or AIM2-derived decapeptide.
Intracellular cytokine staining
Intracellular cytokine staining was performed as previously described.1 Briefly, target cells (BM36.1) were pulsed with HIV-A1 or the AIM2-derived decapeptide for 1 h, washed, and then used to stimulate T-cell cultures at 37 °C in X-vivo medium supplemented with 5% human serum (effector to target ratio = 10:1). After 1 h, 1 μl/mL GolgiPlug reagent (BD) was added, and incubation was continued for additional 4 h. Cells were harvested, stained with anti-CD3 and anti-CD8 antibodies, fixed, permeabilized, and stained with anti-IFNγ, anti-TNFα, and anti-IL-2 antibodies (all from BD). Data acquisition was performed on an LSR-II flow cytometer.
Supplementary Material
Acknowledgments
We thank Tina Seremet for excellent technical assistance and Tobias Wirenfeldt Klausen for statistical assistance. This work was supported by The Danish Council for Strategic Research.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
Supplementary materials may be found here:
http://www.landesbioscience.com/journals/oncoimmunology/article/25374
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25374
References
- 1.Andersen RS, Thrue CA, Junker N, Lyngaa R, Donia M, Ellebæk E, et al. Dissection of T-cell antigen specificity in human melanoma. Cancer Res. 2012;72:1642–50. doi: 10.1158/0008-5472.CAN-11-2614. [DOI] [PubMed] [Google Scholar]
- 2.Harada M, Li YF, El-Gamil M, Ohnmacht GA, Rosenberg SA, Robbins PF. Melanoma-Reactive CD8+ T cells recognize a novel tumor antigen expressed in a wide variety of tumor types. J Immunother. 2001;24:323–33. doi: 10.1097/00002371-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Guilloux Y, Lucas S, Brichard VG, Van Pel A, Viret C, De Plaen E, et al. A peptide recognized by human cytolytic T lymphocytes on HLA-A2 melanomas is encoded by an intron sequence of the N-acetylglucosaminyltransferase V gene. J Exp Med. 1996;183:1173–83. doi: 10.1084/jem.183.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittet MJ, Valmori D, Dunbar PR, Speiser DE, Liénard D, Lejeune F, et al. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med. 1999;190:705–15. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Tong-On P, Li Y, Riley JP, El-Gamil M, Parkhurst MR, et al. Identification of BING-4 cancer antigen translated from an alternative open reading frame of a gene in the extended MHC class II region using lymphocytes from a patient with a durable complete regression following immunotherapy. J Immunol. 2002;168:2402–7. doi: 10.4049/jimmunol.168.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godet Y, Moreau-Aubry A, Guilloux Y, Vignard V, Khammari A, Dreno B, et al. MELOE-1 is a new antigen overexpressed in melanomas and involved in adoptive T cell transfer efficiency. J Exp Med. 2008;205:2673–82. doi: 10.1084/jem.20081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubio-Godoy V, Dutoit V, Rimoldi D, Lienard D, Lejeune F, Speiser D, et al. Discrepancy between ELISPOT IFN-gamma secretion and binding of A2/peptide multimers to TCR reveals interclonal dissociation of CTL effector function from TCR-peptide/MHC complexes half-life. Proc Natl Acad Sci U S A. 2001;98:10302–7. doi: 10.1073/pnas.181348898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu G, Yu JS, Zeng G, Yin D, Xie D, Black KL, et al. AIM-2: a novel tumor antigen is expressed and presented by human glioma cells. J Immunother. 2004;27:220–6. doi: 10.1097/00002371-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JG, Eguchi J, Kruse CA, Gomez GG, Fakhrai H, Schroter S, et al. Antigenic profiling of glioma cells to generate allogeneic vaccines or dendritic cell-based therapeutics. Clin Cancer Res. 2007;13:566–75. doi: 10.1158/1078-0432.CCR-06-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labarriere N, Diez E, Pandolfino MC, Viret C, Guilloux Y, Le Guiner S, et al. Optimal T cell activation by melanoma cells depends on a minimal level of antigen transcription. J Immunol. 1997;158:1238–45. [PubMed] [Google Scholar]
- 11.Gervois N, Guilloux Y, Diez E, Jotereau F. Suboptimal activation of melanoma infiltrating lymphocytes (TIL) due to low avidity of TCR/MHC-tumor peptide interactions. J Exp Med. 1996;183:2403–7. doi: 10.1084/jem.183.5.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouquié R, Bonnin A, Bernardeau K, Khammari A, Dréno B, Jotereau F, et al. A fast and efficient HLA multimer-based sorting procedure that induces little apoptosis to isolate clinical grade human tumor specific T lymphocytes. Cancer Immunol Immunother. 2009;58:553–66. doi: 10.1007/s00262-008-0578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labarrière N, Bretaudeau L, Gervois N, Bodinier M, Bougras G, Diez E, et al. Apoptotic body-loaded dendritic cells efficiently cross-prime cytotoxic T lymphocytes specific for NA17-A antigen but not for Melan-A/MART-1 antigen. Int J Cancer. 2002;101:280–6. doi: 10.1002/ijc.10605. [DOI] [PubMed] [Google Scholar]
- 14.Nolte A, Scheffold C, Slotty J, Huenefeld C, Schultze JL, Grabbe S, et al. Generation of melanoma-specific cytotoxic T lymphocytes for allogeneic immunotherapy. J Immunother. 2003;26:257–69. doi: 10.1097/00002371-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Labarriere N, Pandolfino MC, Raingeard D, Le Guiner S, Diez E, Le Dréan E, et al. Frequency and relative fraction of tumor antigen-specific T cells among lymphocytes from melanoma-invaded lymph nodes. Int J Cancer. 1998;78:209–15. doi: 10.1002/(SICI)1097-0215(19981005)78:2<209::AID-IJC15>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Harlin H, Kuna TV, Peterson AC, Meng Y, Gajewski TF. Tumor progression despite massive influx of activated CD8(+) T cells in a patient with malignant melanoma ascites. Cancer Immunol Immunother. 2006;55:1185–97. doi: 10.1007/s00262-005-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh MK, Li C-L, Fayolle C, Dadaglio G, Murphy A, Lemonnier FA, et al. Induction of HLA-A2-restricted CTL responses by a tubular structure carrying human melanoma epitopes. Vaccine. 2002;20:2463–73. doi: 10.1016/S0264-410X(02)00185-8. [DOI] [PubMed] [Google Scholar]
- 18.Lesimple T, Neidhard E-M, Vignard V, Lefeuvre C, Adamski H, Labarrière N, et al. Immunologic and clinical effects of injecting mature peptide-loaded dendritic cells by intralymphatic and intranodal routes in metastatic melanoma patients. Clin Cancer Res. 2006;12:7380–8. doi: 10.1158/1078-0432.CCR-06-1879. [DOI] [PubMed] [Google Scholar]
- 19.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen MH, Svane IM, Kvistborg P, Nielsen OJ, Balslev E, Reker S, et al. Immunogenicity of Bcl-2 in patients with cancer. Blood. 2005;105:728–34. doi: 10.1182/blood-2004-07-2548. [DOI] [PubMed] [Google Scholar]
- 22.Andersen MH, Bonfill JE, Neisig A, Arsequell G, Sondergaard I, Valencia G, et al. Phosphorylated peptides can be transported by TAP molecules, presented by class I MHC molecules, and recognized by phosphopeptide-specific CTL. J Immunol. 1999;163:3812–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.