Abstract
The role of B cells and antibodies in cancer is insufficiently understood but is receiving increasing attention. We have recently identified IgG4 as an antibody subclass elicited by melanoma-associated interleukin-10-driven inflammation. In this setting, IgG4 exhibit inefficient immunostimulatory capacity and block the cytotoxic activities of other antibodies. These previously unappreciated mechanisms of immune escape may constitute promising targets for the development of novel anticancer immunotherapies.
Keywords: B cells, FcγRI, IL-10, IgG4, VEGF, antibody effector functions, cancer inflammation, immune escape, melanoma
Insights into the mechanisms exploited by melanoma cells to escape immunosurveillance, especially in relation to cytotoxic T cells, regulatory T cells (Tregs) and negative immune regulators (e.g., CTLA-4, PD-1/PD-L1) informed the design of checkpoint blockade-targeting therapies and demonstrated that antibodies-based treatments in particular and immunotherapeutic approaches in general can provide great benefit to cancer patients.1,2
Despite constituting important components of adaptive immunity, B-cell responses, their cross-talk with cancer, antineoplastic functions, and contributions to tumor progression have until recently remained largely unexplored. The first clues on the importance of B cells for oncogenesis and tumor progression came from studies reporting the presence of antibodies specific for melanoma-associated antigens (e.g., NY-ESO-1, MAGE-3, Melan-A) in patients’ sera.3 More recently, we have reported the existence of memory B-cell responses against human melanoma. Thus, tumor-reactive IgG antibodies secreted by the B cells of melanoma patients can recognize allogeneic melanoma cells and mediate cytotoxic functions.4 Similar findings have been reported for other cancers. Moreover, B cells have been shown to undergo somatic hypermutation and class switch recombination within melanoma-associated lymphoid structures.5 Together with the positive prognostic relevance of tumor infiltration by B cells, these observations indicate that humoral immunity is not completely oblivious to tumors. Nevertheless, a fraction of melanoma patients has a poor prognosis, suggesting that tumors evolve mechanisms to evade immune responses. Indeed, the frequency of circulating tumor-reactive memory B cells is reduced with melanoma progression.4 Moreover, interleukin (IL)-21-secreting tumor-associated Tregs favor the accumulation of immature GrB+ regulatory B cells (Bregs), which exert immunosuppressive functions.6 B cells may thus mediate both tumor-stimulatory and tumor-inhibitory effects.
Three decades ago, Daveau and colleagues reported altered levels of IgG4 antibodies in the serum of melanoma patients.7 Although this indicated that B cells in melanoma patients undergo antibody class/subclass switching, the underlying mechanisms and significance remained unexplored. Most subsequent studies dissected the reactivity of antibody variable regions to tumor cells and antigens. Conversely, we recently sought to re-focus on the constant regions of antibodies, in particular IgG subclasses, for 3 reasons.8
First, the Fc region determines the antibody affinity for Fc receptors expressed on the surface of effector cells, its biodistribution, biological function, and potency, with profound implications on the inherent capacity of antibodies to activate effector cells.8 IgG4 are considered as the weakest IgGs in activating Fcγ receptors (FcγRs) on effector cells and fixing complement, and their upregulation in cancer patients could suppress tumor-specific immune responses. Second, the IgG4 class switching and the proliferation of IgG4-expressing B cells are promoted by the local expression of IL-10 and IL-4. Melanomas are characterized by high levels of TH2 cytokines like IL-10, which may hence alter antibody subclass production. Third, chronic inflammatory conditions termed “IgG4-related diseases” are characterized by the infiltration of some organs by IgG4-expressing cells. IgG4s normally accompany chronic antigen exposure, as documented in individuals exposed for years to occupational antigens as well as in allergic patients receiving allergen-based immunotherapy. These conditions de facto divert humoral immunity away from conventional IgE-dominated responses. Tumor microenvironments featuring both IL-10-driven inflammation and chronic antigen exposure may hence promote the production of IgG4s.
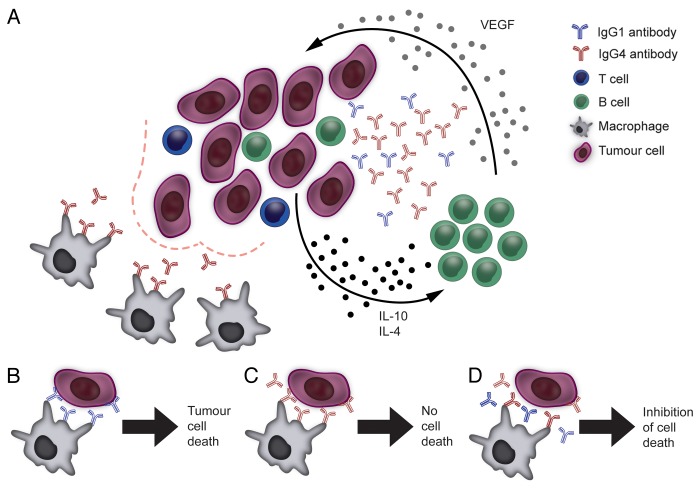
We have recently reported the presence of IgG4-expressing mature B-cell (CD22+IgG4+) infiltrates in melanoma lesions, alongside with the expression of TH2 cytokines (IL-4, IL-10) favoring IgG4 secretion.8 Melanoma-derived B cells were polarized to produce IgG4 antibodies, which are generally rare, confirming an IgG subclass expression bias in the tumor microenvironment. Tumor-associated IgG4+ B cells were antigen-experienced, since they produced melanoma-reactive IgG4 antibodies. In allogeneic stimulation experiments, melanoma cells could directly influence IgG4 polarization by releasing IL-10 and by stimulating B cells to secrete vascular endothelial growth factor (VEGF). These TH2-biased conditions are consistent with melanoma-associated inflammation (Fig. 1).
Figure 1. Mechanisms underpinning the IgG4 bias of the tumor microenvironment and the suppression of immune effector cells by IgG4s. (A) Malignant cells, aided by immune and stromal cells of the tumor microenvironment, can polarize B cells to secrete IgG4 antibodies by releasing TH2 cytokines such as interleukin (IL)-10 and IL-4 as well as by stimulating B cells to produce vascular endothelial growth factor (VEGF). This may be part of a feedback circuitry delivering constant class-switching and activation signals to tumor-infiltrating B cells. IgG4 antibodies are poor activators of antitumor effector cell functions (bottom left). (B) Tumor-specific IgG1 antibodies can mediate effective antibody-dependent cellular cytotoxicity by immune effector cells (e.g., monocytes/macrophages) through the activation of FcγRI (CD64) signaling. (C) IgG4 antibodies do not activate effector cells to kill malignant cells as they are inefficient at inducing FcγRI signaling upon binding to the receptor. (D) IgG4 antibodies can impair IgG1-dependent tumor cell killing by competing for the binding of FcγRI receptors on immune effector cells.
Importantly, by engineering and functional analyses of IgG1 and IgG4 antibodies specific for a tumor-associated antigen, we identified 2 mechanisms by which IgG4 may protect malignant cells from immune attacks. First, in line with its limited effector potency, IgG4s, unlike IgG1s, failed to activate human monocytes against melanoma xenografts, despite successfully localizing to neoplastic lesions. This suggests that tumors infiltrated by inert IgG4 antibodies can escape effector cell clearance. Second, IgG4s impeded the tumoricidal activity of otherwise cytotoxic IgG1s in vitro and in vivo, and these effects were independent of IgG4 antigen specificity. Clues on the immunosuppressive effects of IgG4s came from competition and antibody-mediated tumor killing assays. Tumor killing by human monocytes in the presence of IgG1 was impaired by the blockade of FcγRI (CD64), confirming that the IgG1-dependent cytotoxic activity of monocytes is mediated by this receptor.9 In our experiments, IgG4s failed to activate the inhibitory receptor FcγRIIb but prevented the phosphorylation of FcγR-related activatory signal transducers (i.e., SRC, MEK, AKT) and competed with IgG1s for binding to FcγRI, preventing IgG1-FcγR binding and hence the activation of antitumor effector functions.
These previously unidentified aspects of the tumor-induced reeducation of humoral immunity may have important therapeutic implications. Tumors may divert host B cells to express antibodies of low cytotoxic potential such as IgG4s, which can compete with both host-derived and therapeutic antibodies for FcγR binding, hence impairing their antitumor potential. These mechanisms may protect tumors from active immunosurveillance and contribute to cancer progression.
We also observed an inverse correlation between the relative abundance of IgG4 (over total IgG) in the serum of 33 melanoma patients and overall survival. This paves the way for the evaluation of IgG4 relative levels as a potential circulating biomarker of melanoma and possibly other cancers. Future studies in larger patient cohorts harbor translational promise toward an ever broader vision of personalized medicine and improved patient benefits.
In summary, we demonstrated that IgG4 antibodies, promoted by tumor-induced TH2-biased inflammatory conditions, impair antitumor immunity by inhibiting immune effector cell activation. Our findings are supported by a recent report demonstrating the existence of IgG4-positive infiltrates in extrahepatic cholangiocarcinoma,10 and not only reveal a previously unappreciated aspect of B cell-cancer cell interactions but also provide novel insights into the mechanisms contributing to tumor immune escape. Opportunities for translating this knowledge into therapeutic interventions include the design of agents that are not influenced by the inhibitory effects of IgG4 antibodies. In a wider context, we and others provide a strong rationale for dissecting the largely unexplored biology of the cross-talk between B cells and malignant cells. Similarly to understanding the role of T cells in cancer-associated inflammation, dissecting B-cell responses can yield important therapeutic benefits.
Acknowledgments
The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The authors acknowledge support by Cancer Research UK (C30122/A11527) (SNK); (C16736/A8371); (FON, SNK) Mary Dunhill Trust (FON); CR UK//NIHR in England/DoH for Scotland, Wales and Northern Ireland Experimental Cancer Medicine Centre (FON); CR UK/EPSRC/MRC/NIHR KCL/UCL Comprehensive Cancer Imaging Centre (C1519/A10331) (SNK); and the Overseas Research Students Award Scheme (AEG). The authors thank Mr. Philippe Dufour-Feronce for his expert assistance with the illustration. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Glossary
Abbreviations:
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- MAGE
melanoma-associated antigen
- PD1
programmed cell death 1
- PDL1
programmed cell death 1 ligand 1
- VEGF
vascular endothelial growth factor
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24889
References
- 1.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–91. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stockert E, Jäger E, Chen YT, Scanlan MJ, Gout I, Karbach J, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349–54. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert AE, Karagiannis P, Dodev T, Koers A, Lacy K, Josephs DH, et al. Monitoring the systemic human memory B cell compartment of melanoma patients for anti-tumor IgG antibodies. PLoS One. 2011;6:e19330. doi: 10.1371/journal.pone.0019330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cipponi A, Mercier M, Seremet T, Baurain JF, Théate I, van den Oord J, et al. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 6.Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TF, et al. Interleukin 21-Induced Granzyme B-Expressing B Cells Infiltrate Tumors and Regulate T Cells. Cancer Res. 2013;73:2468–79. doi: 10.1158/0008-5472.CAN-12-3450. [DOI] [PubMed] [Google Scholar]
- 7.Daveau M, Pavie-Fischer J, Rivat L, Rivat C, Ropartz C, Peter HH, et al. IgG4 subclass in malignant melanoma. J Natl Cancer Inst. 1977;58:189–92. doi: 10.1093/jnci/58.2.189. [DOI] [PubMed] [Google Scholar]
- 8.Karagiannis P, Gilbert AE, Josephs DH, Ali N, Dodev T, Saul L, et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest. 2013;123:1457–74. doi: 10.1172/JCI65579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancardi DA, Albanesi M, Jönsson F, Iannascoli B, Van Rooijen N, Kang X, et al. The high-affinity human IgG receptor FcγRI (CD64) promotes IgG-mediated inflammation, anaphylaxis, and antitumor immunotherapy. Blood. 2013;121:1563–73. doi: 10.1182/blood-2012-07-442541. [DOI] [PubMed] [Google Scholar]
- 10.Harada K, Shimoda S, Kimura Y, Sato Y, Ikeda H, Igarashi S, et al. Significance of immunoglobulin G4 (IgG4)-positive cells in extrahepatic cholangiocarcinoma: molecular mechanism of IgG4 reaction in cancer tissue. Hepatology. 2012;56:157–64. doi: 10.1002/hep.25627. [DOI] [PubMed] [Google Scholar]