Abstract
Aims
To evaluate the effect of iron deficiency (ID) and/or anaemia on health-related quality of life (HRQoL) in patients with chronic heart failure (CHF).
Methods and results
We undertook a post-hoc analysis of a cohort of CHF patients in a single-centre study evaluating cognitive function. At recruitment, patients provided baseline information and completed the Minnesota Living with Heart Failure questionnaire (MLHFQ) for HRQoL (higher scores reflect worse HRQoL). At the same time, blood samples were taken for serological evaluation. ID was defined as serum ferritin levels <100 ng/mL or serum ferritin <800 ng/mL with transferrin saturation <20%. Anaemia was defined as haemoglobin ≤12 g/dL. A total of 552 CHF patients were eligible for inclusion, with an average age of 72 years and 40% in NYHA class III or IV. The MLHFQ overall summary scores were 41.0 ± 24.7 among those with ID, vs. 34.4 ± 26.4 for non-ID patients (P = 0.003), indicating worse HRQoL. When adjusted for other factors associated with HRQoL, ID was significantly associated with worse MLHFQ overall summary (P = 0.008) and physical dimension scores (P = 0.002), whereas anaemia was not (both P > 0.05). Increased levels of soluble transferrin receptor were also associated with impaired HRQoL (P ≤ 0.001). Adjusting for haemoglobin and C-reactive protein, ID was more pronounced in patients with anaemia compared with those without (P < 0.001).
Conclusion
In patients with CHF, ID but not anaemia was associated with reduced HRQoL, mostly due to physical factors.
Keywords: Iron deficiency, Anaemia, Health-related quality of life
Introduction
Health-related quality of life (HRQoL) is impaired in chronic heart failure (CHF), resulting in considerable impact on patients' daily activities.1 The impact of CHF on HRQoL is at least comparable with that of other chronic diseases.2 Not surprisingly, HRQoL has become an important consideration in the evaluation and management of CHF patients.3
The functional limitations imposed by CHF have traditionally been measured using clinical tools such as the NYHA functional classification and the 6 min walk test (6MWT), but these measures may correlate only weakly or moderately with patient perceptions.4,5 In contrast, HRQoL instruments provide a means of exploring patient perceptions of the effects of CHF on daily living and thus provide additional information that cannot be directly extrapolated from clinical measures.4,5
Recent evidence suggests that iron deficiency (ID) in CHF patients may be associated with impaired exercise capacity, more severe disease (higher NYHA class), and poorer outcomes, although this latter observation remains controversial.6–8 ID is also associated with fatigue and impaired exercise capacity in otherwise healthy populations.9 Several trials of CHF patients with ID have revealed that correction of ID with i.v. iron can rapidly improve HRQoL, exercise capacity, NYHA class, and other parameters of disease severity,10–12 and these improvements appear to be independent of anaemia status.10 Although data from interventional studies with i.v. iron suggest that ID has a determining role in the HRQoL of patients with CHF, no study to date has directly evaluated patients with and without this co-morbidity in terms of patient-centred outcomes. Thus, given the limited data, further study is required into ID as a possible independent co-morbidity and as a cause of anaemia in typical CHF patients. The aim of the present study was to evaluate the influence of ID on HRQoL in CHF and to explore its influence according to anaemia status.
Methods
Study population and recruitment
The data presented are from a post-hoc analysis of a prospective single-centre study to evaluate the prevalence of cognitive impairment and the determinants of cognitive function in CHF patients (J. Comin-Colet et al., unpublished results), and the study population consisted of 805 consecutive CHF patients followed in a multidisciplinary heart failure programme. The study was conducted in accordance with the Declaration of Helsinki, the study protocol was approved by the local committee of ethics for clinical research, and all patients gave written informed consent after recruitment. For inclusion in the study, patients had to be in a stable condition and diagnosed with CHF with either reduced or preserved (≥45%) EF, according to the European Society of Cardiology diagnostic criteria. Exclusion criteria for the study were: significant primary valvular disease, haemoglobin (Hb) levels <8.5 g/dL, clinical signs of fluid overload, pericardial disease, restrictive cardiomyopathy, hypertrophic cardiomyopathy, active malignancy, and chronic liver disease. Patients without iron status or HRQoL evaluation available at screening were also excluded. According to these criteria, the final cohort consisted of 552 patients.
At recruitment, all patients provided peripheral blood samples and relevant clinical and demographic information, including NYHA functional class, current medical therapy, and the most recent LVEF evaluation. Medical and nursing personnel involved in recruitment and data collection were blinded to patients' ID and anaemia status.
Iron status and anaemia
Iron deficiency was defined using the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines criteria: ferritin level <100 ng/mL or the percentage of transferrin saturation (TSAT) <20% when ferritin is <800 ng/mL.13 Serum iron (μg/dL) was measured using spectrophotometry; serum ferritin (ng/mL) and transferrin (mg/dL) were measured using immunoturbidimetry. TSAT was estimated using the formula: TSAT = serum iron (μg/dL)/[serum transferrin (mg/dL) × 1.25].14 Iron status was also assessed by measuring serum soluble transferrin receptor (sTfR) levels using an enzyme immunoassay. Anaemia was defined as Hb ≤ 12 g/dL.10 Hb was measured used impedance laser colorimetry.
Evaluation of health-related quality of life
The Minnesota Living with Heart Failure Questionnaire (MLHFQ) is a self-administered questionnaire consisting of 21 individual items. In addition, it provides an overall measure of health [overall summary score (OSS)] and summary scores of the physical and emotional dimensions of health, based on eight and five items, respectively. The MLHFQ has been validated for use in Spain and was self-administered by all patients at inclusion in the study.15 This heart failure-specific questionnaire has shown similar metric properties compared with other specific questionnaires widely used in clinical research such as the Kansas City Cardiomyopathy Questionnaire.4
For each item in the MLHFQ, responses were scored from 0 (no impact on HRQoL) to 5 (maximum impact on HRQoL). Summary scores were obtained by summing responses to each item, giving an OSS of 0–105, a physical dimension score of 0–40, and an emotional dimension score of 0–25.
Statistical analysis
Demographic and other background data were summarized with basic descriptive statistics in the total cohort and for ID and non-ID groups. For quantitative variables, the arithmetic mean (± standard deviation) or median (interquartile range) were calculated as appropriate, and P-values were derived from a two-sample t-test (Mann–Whitney U-tests were used for skewed data). For qualitative variables, percentages within specified groups were calculated and P-values were derived using χ2 tests.
The preliminary comparisons for HRQoL between levels of background variables were performed using the descriptive methods stated above. For χ2 tests, HRQoL scores were expressed as less than, or greater than or equal to, the median, and unadjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated.
Simple (univariate) linear regression analyses were undertaken to explore the influence of key demographic and clinical variables, including measures of iron and anaemia status, on HRQoL (summary and composite scores). To evaluate the effect of ID and anaemia together on HRQoL, multivariable linear regression models were constructed. For each of the summary scores of the MLHFQ, two models were developed. The first model included ID according to the K/DOQI definition13 and anaemia (Hb ≤ 12 g/dL), whereas the second model included sTfR (iron status) and Hb as continuous variables. Multivariate models were adjusted for all covariates that showed an association with the relevant HRQoL score (P < 0.1) in univariate linear regression analyses, as well as age, gender, and LVEF. Given the differences between patients with and without ID, these models were also adjusted by covariates associated with iron status.
A general linear model (GLM) was used to explore interactions between ID and anaemia in their effects on HRQoL (adjusted marginal means). Further GLMs were used to investigate the relationship between HRQoL and Hb or sTfR levels expressed as quintiles (adjusted for sTfR or Hb, respectively). Similar models were constructed for ferritin, TSAT, and serum iron. All GLMs were adjusted for factors associated with HRQoL in univariate linear regression analyses as well as age, gender, and LVEF. In all models, skewed variables were transformed to fit the normal distribution.
All statistical tests and CIs were constructed with a type I error (alpha) level of 5% with no adjustments for multiplicity, and P-values ≤ 0.05 were considered statistically significant. SPSS® version 18.0 (IBM, Armonk, NY, USA) was used for statistical analyses.
Results
Between January 2005 and December 2010, 805 patients with CHF were screened. A total of 253 patients were excluded from the analysis because of unavailability of iron status measures (n = 177), HRQoL evaluation (n = 35), or both (n = 41). Thus, 552 patients met the inclusion criteria and were enrolled in the study (Table 1). Excluded patients, compared with those included, tended to be younger (P = 0.06) and had worse LVEF and NYHA class (both P < 0.05). Patients meeting the K/DOQI definition of ID13 represented 63% of the studied cohort and had lower levels of ferritin, TSAT, and serum iron, and higher levels of sTfR compared with non-ID patients (P < 0.001).
Table 1.
Demographics and baseline characteristics of the total population and according to iron status
| Variables | Overall (n = 552) | Patients with ID (n = 349) | Patients without ID (n = 203) |
|---|---|---|---|
| Age, years | 72 ± 11 | 73 ± 10** | 70 ± 12 |
| Female gender, n (%) | 239 (43) | 162 (46) | 77 (38) |
| BMI, kg/m2 | 29 ± 6 | 29 ± 6 | 29 ± 6 |
| Systolic BP, mmHg | 127 ± 22 | 127 ± 22 | 126 ± 22 |
| Heart rate, b.p.m. | 73 ± 14 | 73 ± 13 | 7214 |
| NYHA class (I/II/III/IV), n (%) | 98/236/187/31 (18/43/34/6) | 51/135/141/22 (15/39/40/6)* | 47/101/56/9 (23/50/23/4) |
| LVEF (%) | 45 ± 16 | 47 ± 17** | 43 ± 15 |
| LVEF ≥45%, n (%) | 263 (48) | 175 (50) | 88 (43) |
| Ischaemic aetiology of CHF, n (%) | 220 (40) | 145 (41) | 75 (37) |
| Co-morbidities | |||
| Hypertension, n (%) | 430 (78) | 279 (80) | 151 (74) |
| Diabetes mellitus, n (%) | 238 (43) | 163 (47)§ | 75 (37) |
| CKD (eGFR <60 mL/min/1.73 m2), n (%) | 302 (55) | 197 (56) | 105 (52) |
| Anaemia, n (%) | 206 (37) | 153 (44)* | 53 (26) |
| Dependency (Barthel index <90) | 108 (20) | 80 (23)§ | 28 (14) |
| Medications | |||
| ACEIs or ARBs, n (%) | 433 (80) | 267 (78) | 166 (83) |
| Beta-blockers, n (%) | 506 (92) | 318 (91) | 188 (93) |
| MRAs, n (%) | 197 (36) | 116 (33) | 81 (40) |
| Digoxin, n (%) | 60 (11) | 33 (9) | 27 (13) |
| Loop diuretics, n (%) | 479 (87) | 299 (86) | 180 (89) |
| Laboratory values | |||
| Haemoglobin, g/dL | 12.6 ± 1.8 | 12.3 ± 1.8* | 13.1 ± 1.9 |
| eGFR, mL/min/1.73 m2 | 60 ± 24 | 59 ± 23 | 62 ± 26 |
| Ferritin, ng/mL, median (IQR) | 132 (178) | 82 (112)*a | 232 (162) |
| Transferrin, mg/dL | 249 ± 47 | 257 ± 49* | 236 ± 41 |
| Serum iron, μg/dL | 64 ± 29 | 51 ± 23* | 86 ± 27 |
| % TSAT | 21 ± 10 | 16 ± 7* | 29 ± 8 |
| sTfR, mg/L | 1.87 ± 1.0 | 2.08 ± 1.09* | 1.50 ± 0.68 |
| NT-proBNP, pg/mL, median (IQR) | 1619 (2814) | 1778 (2813)§a | 1178 (2520) |
| C-reactive protein, mg/dL, median (IQR) | 0.8 (1.8) | 1.1 (2.2)*a | 0.7 (1.3) |
Data for continuous variables are presented as mean ±SD unless stated otherwise.
ACEIs, ACE inhibitors; BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ID, iron deficiency; IQR, interquartile range; MRAs, mineralocorticoid receptor antagonists; sTfR, soluble transferrin receptor; TSAT, transferrin saturation.
*P < 0.001; **P < 0.01; §P < 0.05.
aFerritin, NT-proBNP, and C-reactive protein are presented as median (IQR) and compared using Mann–Whitney tests.
Unadjusted HRQoL was significantly worse (i.e. higher scores) among the patients with ID compared with those without ID (P = 0.003), mostly due to differences in the physical dimension (Table 2). Unadjusted OSS was also associated with significantly reduced HRQoL among anaemic patients (44.2 ± 23.1) compared with non-anaemic patients (35.2 ± 26.3; P < 0.0001, respectively).
Table 2.
Health-related quality of life, according to iron status, assessed using the Minnesota Living with Heart Failure questionnaire overall summary, composite, and individual item scores (data presented as mean ± SD)
| ID (n = 349) | Non-ID (n = 203) | |
|---|---|---|
| Overall summary score (minimum: 0, maximum, 105) | 41.0 ± 24.7** | 34.4 ± 26.4 |
| Composite scores | ||
| Physical dimension (minimum: 0, maximum, 40) (sum of items 2, 3, 4, 5, 6, 7, 12, 13) | 23.3 ± 13.5* | 20.0 ± 14.3 |
| Emotional dimension (minimum: 0, maximum, 25) (sum of items 17, 18, 19, 20, 21) | 6.7 ± 5.8 | 6.0 ± 6.1 |
| Individual items of the MLHFQ (minimum: 0, maximum, 5) | ||
| 1. Swelling of legs | 3 (5) | 2 (5) |
| 2. Having a rest during the day | 4 (4)** | 3 (5) |
| 3. Walking or climbing stairs | 4 (3)** | 3 (5) |
| 4. Housekeeping or gardening | 4 (5)** | 2 (5) |
| 5. Going away | 4 (4)** | 3 (5) |
| 6. Sleep | 2 (5)§ | 1 (4) |
| 7. Friends family | 1 (3)§ | 0 (3) |
| 8. Earning profession | 0 (1) | 0 (1) |
| 9. Hobbies | 2 (3) | 1 (3) |
| 10. Sexual activity | 0 (3) | 0 (2) |
| 11. Appetite | 2 (4)** | 1 (3) |
| 12. Feeling short of breath | 4 (4)** | 3 (5) |
| 13. Feeling fatigued | 4 (3)* | 4 (5) |
| 14. Stay in hospital | 3 (5)§ | 1 (4) |
| 15. Cost of medical care | 0 (0) | 0 (0) |
| 16. Side effects drugs | 0 (0) | 0 (0) |
| 17. Burden for relatives | 0 (2) | 0 (2) |
| 18. Loss of control | 0 (2) | 0 (2) |
| 19. Worried | 3 (4)§ | 2 (3) |
| 20. Concentration | 0 (2) | 0 (2) |
| 21. Depression | 1 (3) | 0 (2) |
Individual item scores are presented as median (interquartile range) and were compared between groups using Mann–Whitney U-tests.
Sixty-three per cent of ID patients scored ≥4 on question 13 compared with 51% of non-ID patients (P < 0.01).
ID, iron deficiency; MLHFQ, Minnesota Living with Heart Failure questionnaire.
*P < 0.001; **P < 0.01; §P < 0.05.
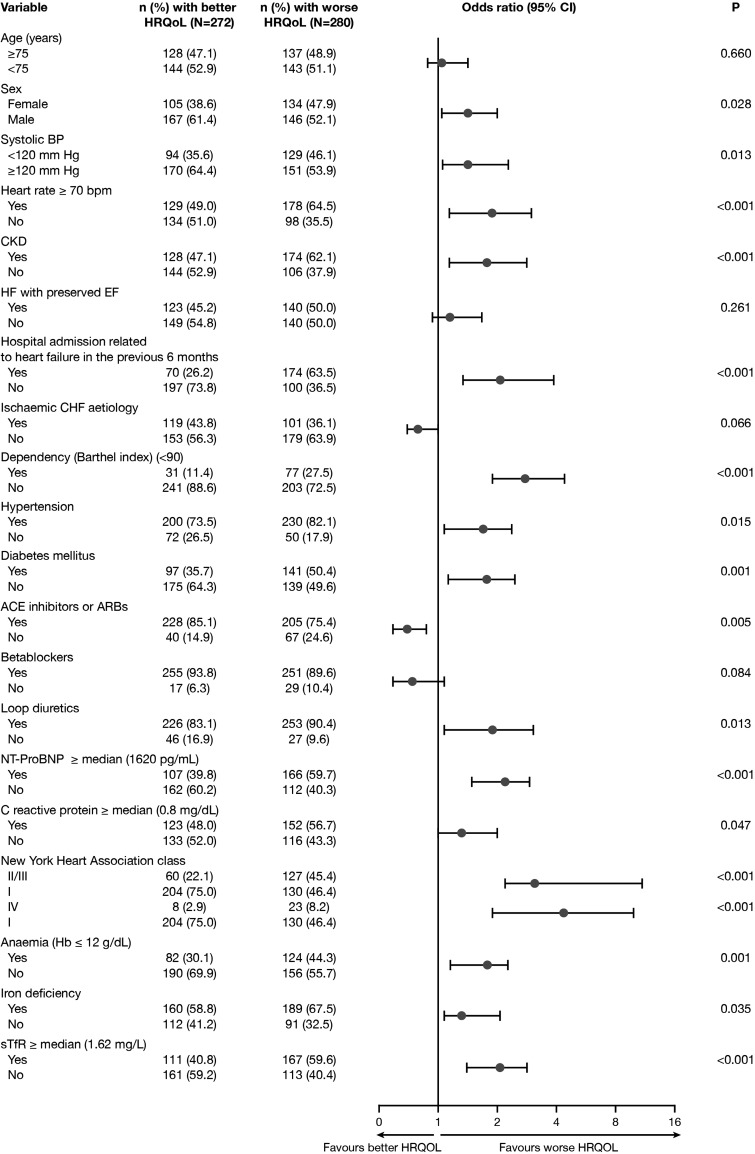
In the univariate binary logistic regression analysis, a range of demographic and clinical factors were associated with worse overall HRQoL (Figure 1). Body mass index did not show an association with HRQoL (P > 0.1). For the physical dimension score, ID (OR 1.7, 95% CI 1.2–2.4; P = 0.004), anaemia (OR 1.8, 95% CI 1.3–2.6; P = 0.001), and sTfR ≥ 1.62 mg/L (OR 2.0, 95% CI 1.4–2.8; P < 0.001) were all significantly associated with worse unadjusted HRQoL. Similar results were obtained using univariate linear regression analyses.
Figure 1.
Clinical factors associated with worse health-related quality of life (HRQoL) defined as an overall summary score ≥ the median score (42 points) in the univariate analyses. BP, blood pressure; CHF, chronic heart failure; CI, confidence interval; CKD, chronic kidney disease; Hb, haemoglobin; HF, heart failure; sTfR, soluble transferrin receptor.
Adjusting for the significant factors identified in the univariate analyses, we performed multivariate linear regression models using either the K/DOQI ID definition and anaemia defined as Hb ≤ 12 g/dL (model 1) or sTfR and Hb as continuous variables (model 2). In both multivariable models, measures of ID but not anaemia were associated with impaired overall and physical HRQoL (Table 3). Both models also showed that the association of ID or sTfR levels with the OSS was independent of LVEF. In a separate multivariate linear regression analysis of patients according to LVEF, we found that elevated sTfR levels were associated with worse overall HRQoL in patients with both reduced (standardized β coefficient = 0.21, P < 0.001) and preserved LVEF (standardized β coefficient = 0.15, P = 0.007). Hb levels were not associated with the OSS in either LVEF group (P > 0.1). In further sensitivity analyses, substitution of the K/DOQI ID definition by the ID definition used in the Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial (ferritin <100 ng/mL or ferritin 100–300 ng/mL and TSAT <20%)10 did not alter the OSS results (standardized β coefficient = 0.098, P < 0.012) or the physical dimension score result (standardized β coefficient = 0.116, P = 0.003) in model 1.
Table 3.
Multivariate linear regression evaluation of the association between of clinical variables (including markers of iron deficiency and anaemia status) and health-related quality of life
| Variable | Model 1: ID and anaemia* |
Model 2: sTfR and Hb** |
||||
|---|---|---|---|---|---|---|
| MLHFQ |
MLHFQ |
|||||
| Overall summary score | Physical dimension score | Emotional dimension score | Overall summary score | Physical dimension score | Emotional dimension score | |
| Sβc | Sβc | Sβc | Sβc | Sβc | Sβc | |
| Age, 1 year | –0.18* | –0.12** | –0.13§ | –0.17* | –0.11§ | –0.15** |
| Gender, female vs. male | –0.02 | 0.01 | 0.08 | –0.01 | 0.01 | 0.09§ |
| Systolic BP, 1 mmHg | –0.22* | –0.21* | –0.19* | –0.21* | –0.20* | –0.18* |
| Heart rate, 1 b.p.m. | 0.05 | 0.07 | 0.05 | 0.03 | 0.06 | 0.02 |
| NYHA class, I/II vs. III/IV | 0.20* | 0.20* | 0.17* | 0.20* | 0.20* | 0.15** |
| LVEF (%) | 0.05 | 0.05 | 0.02 | 0.06 | 0.06 | –0.01 |
| Hypertension, yes vs. no | 0.08 | 0.09§ | 0.05 | 0.08 | 0.09§ | 0.05 |
| Diabetes mellitus, yes vs. no | 0.15* | 0.09§ | 0.17* | 0.14* | 0.09§ | 0.16* |
| eGFR, 1 mL/min/1.73 m2 | –0.21* | –0.18* | –0.21* | –0.19* | –0.17* | –0.18* |
| Barthel index, 1 point | 0.01 | 0.03 | –0.07 | 0.01 | 0.03 | –0.06 |
| Time since last HF admission, 1 daya | –0.29* | –0.30* | –0.18* | –0.30* | –0.31* | –0.19* |
| ACEIs or ARBs, yes vs. no | –0.05 | –0.07 | –0.02 | –0.07 | –0.08 | 0.04 |
| Beta-blockers, yes vs. no | –0.05 | –0.05 | –0.02 | –0.06 | –0.05 | –0.02 |
| Loop diuretics, yes vs. no | 0.19* | 0.15* | 0.15** | 0.17* | 0.14* | 0.14** |
| Antiplatelet agents, yes vs. no | – | – | 0.02 | – | – | 0.05 |
| Iron deficiency, yes vs. no | 0.11** | 0.12** | – | – | – | – |
| Haemoglobin, g/dL | – | – | – | 0.01 | –0.01 | 0.07 |
| Anaemia, yes vs. no | 0.01 | 0.04 | –0.04 | – | – | – |
| sTfR, mg/L | – | – | – | 0.16* | 0.13** | 0.14** |
| NT-proBNP, 1 pg/mLa | 0.10 | 0.10§ | 0.02 | 0.10§ | 0.10§ | 0.02 |
| C-reactive protein, 1 mg/dLa | 0.07** | 0.09§ | –0.01 | 0.06 | 0.09 | – |
| Adjusted r2 for each model | 0.38 | 0.39 | 0.229 | 0.39 | 0.39 | 0.25 |
ACEIs, ACE inhibitors; BP, blood pressure; eGFR, estimated glomerular filtration rate; Hb, haemoglobin; ID, iron deficiency; MLHFQ, Minnesota Living with Heart Failure questionnaire; MRAs, mineralocorticoid receptor antagonists; Sβc, standardized β coefficient; sTfR, soluble transferrin receptor.
*P < 0.001; **P < 0.01; §P < 0.05.
aVariables were log-transformed to fit the normal distribution.
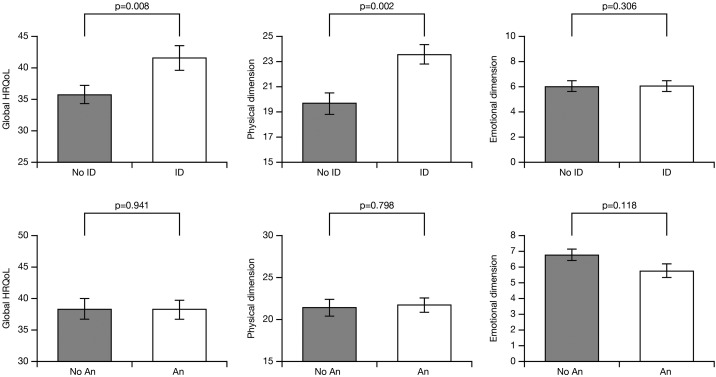
In multivariable general linear models, ID was significantly associated with worse adjusted OSS (P = 0.008) and the adjusted physical dimension score (P = 0.002; Figure 2), whereas anaemia was not (P = 0.941 and P = 0.798, respectively; Figure 2). The interaction between ID and anaemia was not significant for OSS, physical dimension score, or emotional dimension score (P = 0.274, 0.206, and 0.442, respectively).
Figure 2.
Iron deficiency (ID) or anaemia (An) status and their effect on overall, physical, and emotional health-related quality of life (HRQoL) in a multivariate analysis using general linear models. Bars represent the adjusted mean values (±SE) of the Minnesota Living with Heart Failure questionnaire (MLHFQ) overall summary score, physical dimension score, and emotional dimension score. HRQoL scores were adjusted for the presence of ID, anaemia, and other covariates associated with HRQoL in the univariate analyses.
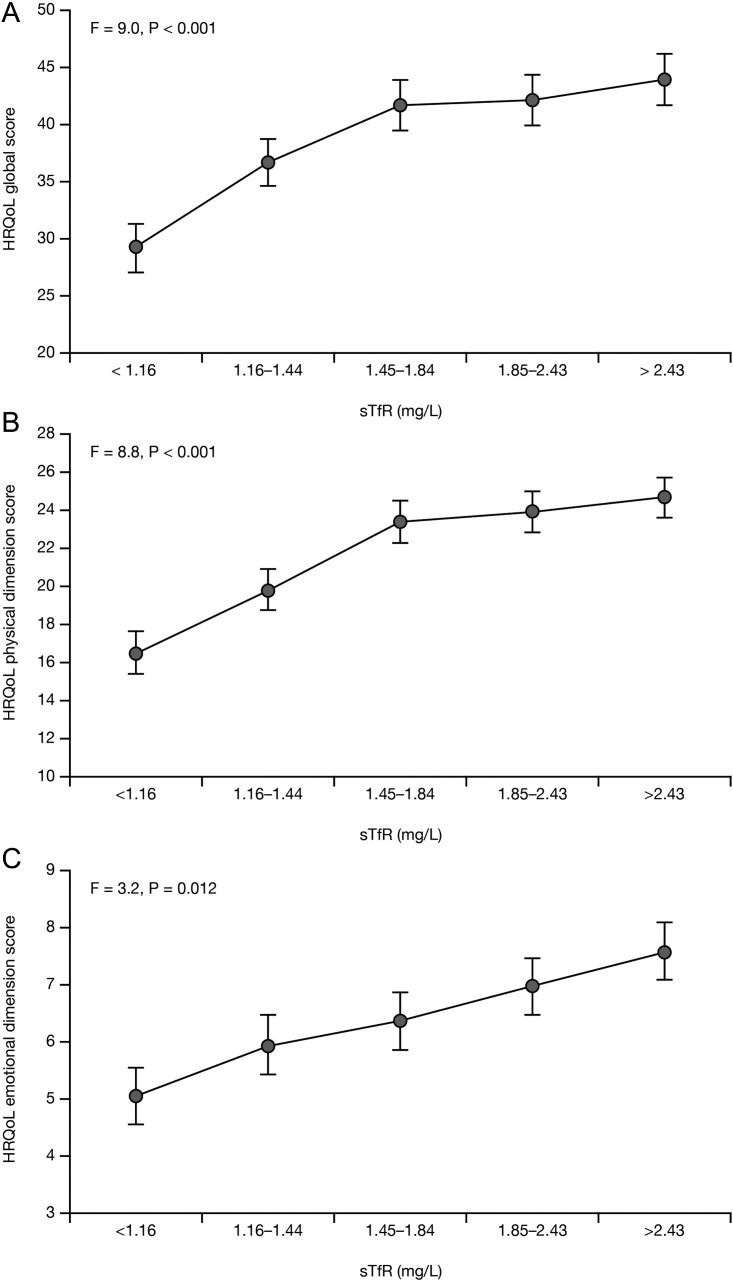
As represented in Figure 3A–C, sTfR divided into quintiles was associated with impaired overall HRQoL (P < 0.001) as well as the physical (P < 0.001) and emotional (P = 0.012) dimensions, and these relationships were linear. There was also a significant trend to worse HRQoL represented by overall summary and physical dimensions scores with low TSAT (P = 0.002 and P = 0.001, respectively), serum iron (both P = 0.03), and ferritin (P = 0.03 and P = 0.02, respectively). Hb divided into quintiles revealed no association with any adjusted MLHFQ scores (all P > 0.1; Supplementary material, Figure S1a–c).
Figure 3.
Association of soluble transferrin receptor (sTfR) with health-related quality of life (HRQoL) adjusted for covariates and haemoglobin. Minnesota Living with Heart Failure questionnaire (MLHFQ) overall summary (A), physical dimension (B), and emotional dimension (C) adjusted scores per quintiles of sTfR. Data are represented as the adjusted mean ± SE. P-values are for the association of sTfR with HRQoL. There was a significant linear trend (polynomial contrast) between sTfR quintiles and the overall summary, physical dimension, and emotional dimension adjusted scores (all P < 0.01).
Interestingly, we noticed that sTfR (adjusted for Hb and C-reactive protein) was higher in the patients with ID and anaemia than in those with ID without anaemia (2.0 ± 0.1 and 1.6 ± 0.1, respectively; P < 0.001). This may suggest that iron depletion was more pronounced in the former cohort compared with the latter regardless of Hb level or inflammation.
Discussion
In this study, we have shown that HRQoL reported by CHF patients in a multidisciplinary heart failure programme was adversely affected by ID (ferritin/TSAT or sTfR levels) independent of anaemia, and that the impairment was mostly due to elements of the MLHFQ physical domain rather than the emotional domain. Furthermore, although anaemia and Hb levels were associated with HRQoL in univariate analyses (Figure 1), in multivariable models (Figure 2, Table 3) they had no statistically significant effect on HRQoL when the effects of ID and other variables were taken into account. Interestingly, anaemia in the presence of ID was associated with greater iron depletion than in ID alone.
Iron is physiologically important for erythropoiesis and oxygen transport, and, as such, ID is one of the main causes of anaemia.16 It also plays a vital role in muscle function and exercise capacity that is independent of anaemia.9,16 Specifically in the context of CHF, there is accumulating evidence that ID may contribute to myocardial alterations, to fatigue and reduced exercise capacity, and to increased risk of mortality.6,8,17–19 In clinical trials, i.v. iron treatment can improve CHF symptoms, increase exercise capacity, and improve HRQoL in patients with ID with or without anaemia.10–12 These aspects have been confirmed in a recent meta-analysis.20 We observed that in patients with ID, sTfR concentrations were higher (indicating more pronounced ID) in anaemic compared with non-anaemic patients and that ID but not anaemia was independently associated with HRQoL. From this, we could hypothesize that ID is the key factor affecting self-perceived health status, and anaemia would merely be a marker of more pronounced iron depletion. Hypothetically, ID would impair submaximal exercise capacity, which in turn affects the performance of usual activities, a crucial determining factor in HRQoL. Further studies are needed to confirm this hypothesis.
Chronic heart failure severity itself is known to correlate with HRQoL.2 In multivariate analyses, we observed that a number of clinical variables were independently associated with impaired HRQoL, including CHF-related factors (NYHA class, time since last hospital admission for CHF, and administration of loop diuretics), as well as systolic blood pressure, presence of diabetes, renal function, and level of C-reactive protein (Table 3). However, neither LVEF, anaemia (Hb ≤ 12 g/dL), nor Hb as a continuous variable were independent predictors of impaired HRQoL, although anaemia might be associated with increased iron depletion in patients with ID. Interestingly, the effect of ID on HRQoL was also independent of the level of functional limitation determined with the NYHA class. This underscores the multidimensional nature of HRQoL since other factors besides dyspnoea, such as fatigue or pain, may entail a limitation of submaximal exercise capacity which in turn would interfere with usual activities, affecting the self-perceived health status of chronic patients.5 Regarding this, data coming from the FAIR-HF study show that i.v. iron repletion improved the domain of the EQ5D measuring the impact on pain and discomfort of patients with ID and CHF compared with placebo.12 Further study is required to understand the implications of these associations and their role in CHF management.
Despite the negative impact of ID on CHF, there is no currently accepted definition of ID in this area. Therefore, we took the definition of the nephrology K/DOQI guidelines (ferritin levels <100 ng/mL, or < 800 ng/mL with TSAT < 20%) as a starting point, but we acknowledge that the choice of markers and cut-offs in nephrology might not be suitable in CHF. For example, the presence of inflammation may disturb iron metabolism and complicate the interpretation of traditional markers such as ferritin or TSAT.21 Therefore, we considered measurement of sTfR, the truncated fragment of the membrane receptor of transferrin, which is unaffected by inflammation or gender. Several studies using bone marrow examination as a gold standard for iron status evaluation have shown that the sTfR level is an accurate measure of iron demand in patients with or without anaemia.22,23 It also offers the opportunity to evaluate iron levels as a continuum from normal through to absolute depletion, which might accurately reflect the pathophysiological mechanisms involved in the development of abnormal iron status in patients with chronic diseases such as CHF.21–23 Some authors suggest that the ferritin index (sTfR/log10[ferritin]) would also be an ideal marker for evaluating iron metabolism in patients with chronic conditions. This index combines information about iron demand (sTfR) and availability of iron (log10[ferritin]) for erythropoiesis and for other functional proteins.21–23 In our study, we focused on sTfR and, thus, further studies of the role of the ferritin index in the evaluation of iron metabolism in CHF are warranted.
We found that impaired HRQoL, adjusted for the effects of other variables identified in univariate analyses, correlated strongly with elevated sTfR when expressed in quintiles (P < 0.001). On the other hand, a similar analysis for Hb levels revealed no statistically significant relationship with HRQoL. This was expected given the absence of an association between anaemia and HRQoL in this cohort. We therefore recommend further study to assess the suitability of sTfR as a marker of ID in CHF, given the potential of this biomarker to express iron status as a spectrum of severity of iron depletion rather than a dichotomous state as in the K/DOQI definition.
These results add to the emerging understanding of the role of ID in CHF, and may help explain the results of clinical trials showing the benefits of i.v. iron treatment in CHF patients. However, the study is subject to some limitations. This was a post-hoc analysis of data from a single centre, so it is unclear how applicable the findings are to other countries and ethnicities, although a study in Poland has also shown the negative impact of ID on CHF independently of anaemia.6 This was a cross-sectional study, thus, assumptions about a causative role of ID in impaired HRQoL can only be hypothesized. However, interventional studies showing improvement of HRQoL with i.v iron in patients with ID and CHF support this hypothesis. Patients excluded from this analysis differ from the analysed cohort in terms of age, LVEF, and NYHA class. We cannot ascertain the effect of including the 253 excluded patients on the final results of this analysis. However, in our multivariable analyses, the influence of ID on HRQoL was independent of these factors and thus we may hypothesize that the results would not significantly differ from those presented. Given these limitations, and the lack of power to detect differences between groups, prospective, appropriately powered, multicentre studies are necessary to confirm the results. Although specific questionnaires, such as the MLHFQ, are superior to generic questionnaires to describe self-perceived health status in CHF patients, generic questionnaires allow comparisons between chronic conditions and provide additional information about important aspects in CHF that may be missed using specific tools.15 Thus, it would be desirable to include these generic tools in future research. Despite the limitations mentioned here, we have studied typical CHF patients, who are not always represented in clinical trials. Therefore, our observations may more closely reflect the real world than a controlled trial with rigorous inclusion and exclusion criteria. Finally, the analysis represents a single time point in the lives of these patients, and as such does not capture dynamic effects of the disease or the impact of treatment, including i.v. iron. Future studies would be desirable to analyse the changes of indices of iron status over time and the impact of these changes on measures of HRQoL.
In conclusion, we have shown that CHF patients with ID suffer impaired HRQoL and that the effects are not due to the role of iron in erythropoiesis. In patients with ID, anaemia itself had no effect on HRQoL, though reduced Hb levels could be associated with increased levels of iron depletion. Further study is required to deepen our understanding of the role iron plays in CHF aetiology and severity.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
Editorial support was funded by Vifor Pharma Ltd, Switzerland.
Acknowledgements
Joan Vila helped with the statistical analyses, and Dr David Floyd provided editorial support.
Conflict of interest: J.C.C. was a member of the FAIR-HF steering committee (sponsored by Vifor Pharma Ltd), has received honoraria for speaking from Vifor Pharma Ltd, and currently sits on the steering committee of the CONFIRM-HF trial sponsored by Vifor Pharma Ltd. All other authors have no conflict to declare.
References
- 1.Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7:243–251. doi: 10.1016/j.ejheart.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, Haass M. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87:235–241. doi: 10.1136/heart.87.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaarsma T, Beattie JM, Ryder M, Rutten FH, McDonagh T, Mohacsi P, Murray SA, Grodzicki T, Bergh I, Metra M, Ekman I, Angermann C, Leventhal M, Pitsis A, Anker SD, Gavazzi A, Ponikowski P, Dickstein K, Delacretaz E, Blue L, Strasser F, McMurray J. Palliative care in heart failure: a position statement from the palliative care workshop of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009;11:433–443. doi: 10.1093/eurjhf/hfp041. [DOI] [PubMed] [Google Scholar]
- 4.Comin-Colet J, Garin O, Lupon J, Manito N, Crespo-Leiro MG, Gomez-Bueno M, Ferrer M, Artigas R, Zapata A, Elosua R. Validation of the Spanish version of the Kansas City Cardiomyopathy Questionnaire. Rev Esp Cardiol. 2011;64:51–58. doi: 10.1016/j.recesp.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Myers J, Zaheer N, Quaglietti S, Madhavan R, Froelicher V, Heidenreich P. Association of functional and health status measures in heart failure. J Card Fail. 2006;12:439–445. doi: 10.1016/j.cardfail.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872–1880. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- 7.Parikh A, Natarajan S, Lipsitz SR, Katz SD. Iron deficiency in community-dwelling US adults with self-reported heart failure in the National Health and Nutrition Examination Survey III: prevalence and associations with anemia and inflammation. Circ Heart Fail. 2011;4:599–606. doi: 10.1161/CIRCHEARTFAILURE.111.960906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, von Haehling S, Doehner W, Banasiak W, Polonski L, Filippatos G, Anker SD, Ponikowski P. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. 2011;17:899–906. doi: 10.1016/j.cardfail.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Brownlie TT, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr. 2004;79:437–443. doi: 10.1093/ajcn/79.3.437. [DOI] [PubMed] [Google Scholar]
- 10.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 11.Toblli JE, Lombrana A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50:1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Comin-Colet J, Lainscak M, Dickstein K, Filippatos GS, Johnson P, Luscher TF, Mori C, Willenheimer R, Ponikowski P, Anker SD. The effect of intravenous ferric carboxymaltose on health-related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR-HF study. Eur Heart J. 2013;34:30–38. doi: 10.1093/eurheartj/ehr504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.KDOQI. KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471–530. doi: 10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Wu A. Tietz Clinical Guide to Laboratory Tests. 4th. St Louis, MO: Saunders Elsevier; 2006. pp. p56–61. [Google Scholar]
- 15.Garin O, Soriano N, Ribera A, Ferrer M, Pont A, Alonso J, Permanyer G. [Validation of the Spanish version of the Minnesota Living with Heart Failure Questionnaire] Rev Esp Cardiol. 2008;61:251–259. [PubMed] [Google Scholar]
- 16.Haas JD, Brownlie T., 4th Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–688S. doi: 10.1093/jn/131.2.676S. discussion 688S–690S. [DOI] [PubMed] [Google Scholar]
- 17.Jankowska EA, Ponikowski P. Molecular changes in myocardium in the course of anemia or iron deficiency. Heart Fail Clin. 2010;6:295–304. doi: 10.1016/j.hfc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Maeder MT, Khammy O, dos Remedios C, Kaye DM. Myocardial and systemic iron depletion in heart failure implications for anemia accompanying heart failure. J Am Coll Cardiol. 2011;58:474–480. doi: 10.1016/j.jacc.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 19.Macdougall IC, Canaud B, de Francisco AL, Filippatos G, Ponikowski P, Silverberg D, van Veldhuisen DJ, Anker SD. Beyond the cardiorenal anaemia syndrome: recognizing the role of iron deficiency. Eur J Heart Fail. 2012;14:882–886. doi: 10.1093/eurjhf/hfs056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avni T, Leibovici L, Gafter-Gvili A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta-analysis. Eur J Heart Fail. 2012;14:423–429. doi: 10.1093/eurjhf/hfs017. [DOI] [PubMed] [Google Scholar]
- 21.Cullis JO. Diagnosis and management of anaemia of chronic disease: current status. Br J Haematol. 2011;154:289–300. doi: 10.1111/j.1365-2141.2011.08741.x. [DOI] [PubMed] [Google Scholar]
- 22.Suominen P, Mottonen T, Rajamaki A, Irjala K. Single values of serum transferrin receptor and transferrin receptor ferritin index can be used to detect true and functional iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum. 2000;43:1016–1020. doi: 10.1002/1529-0131(200005)43:5<1016::AID-ANR9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Suominen P, Punnonen K, Rajamaki A, Irjala K. Serum transferrin receptor and transferrin receptor–ferritin index identify healthy subjects with subclinical iron deficits. Blood. 1998;92:2934–2939. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.