Abstract
Purpose
ARN-509 is a novel androgen receptor (AR) antagonist for the treatment of castration-resistant prostate cancer (CRPC). ARN-509 inhibits AR nuclear translocation and AR binding to androgen response elements and, unlike bicalutamide, does not exhibit agonist properties in the context of AR overexpression. This first-in-human phase I study assessed safety, tolerability, pharmacokinetics, pharmacodynamics, and antitumor activity of ARN-509 in men with metastatic CRPC.
Patients and Methods
Thirty patients with progressive CRPC received continuous daily oral ARN-509 at doses between 30 and 480 mg, preceded by administration of a single dose followed by a 1-week observation period with pharmacokinetic sampling. Positron emission tomography/computed tomography imaging was conducted to monitor [18F]fluoro-α-dihydrotestosterone (FDHT) binding to AR in tumors before and during treatment. Primary objective was to determine pharmacokinetics, safety, and recommended phase II dose.
Results
Pharmacokinetics were linear and dose proportional. Prostate-specific antigen declines at 12 weeks (≥ 50% reduction from baseline) were observed in 46.7% of patients. Reduction in FDHT uptake was observed at all doses, with a plateau in response at ≥ 120-mg dose, consistent with saturation of AR binding. The most frequently reported adverse event was grade 1/2 fatigue (47%). One dose-limiting toxicity event (grade 3 abdominal pain) occurred at the 300-mg dose. Dose escalation to 480 mg did not identify a maximum-tolerated dose.
Conclusion
ARN-509 was safe and well tolerated, displayed dose-proportional pharmacokinetics, and demonstrated pharmacodynamic and antitumor activity across all dose levels tested. A maximum efficacious dose of 240 mg daily was selected for phase II exploration based on integration of preclinical and clinical data.
INTRODUCTION
Prostate cancer is the second most frequently diagnosed cancer and sixth leading cause of cancer death in men, accounting for 14% (903,500) of total new cancer cases and 6% (258,400) of total cancer deaths in men worldwide.1 The approvals of the second-generation androgen receptor (AR) antagonist enzalutamide and the 7-α-hydroxylase/17,20-lyase (CYP17A) inhibitor abiraterone acetate as therapies for metastatic castration-resistant prostate cancer (CRPC) represent tremendous advances in the treatment of the lethal phase of the disease and underscore the critical and continued role of AR signaling in the castration-resistant state.2–4
Although drugs targeting AR signaling have provided dramatic benefit to many, not all patients respond, and among those who do, the durability of response can be limited. ARN-509 is a second-generation antiandrogen that emerged from a structure/activity relationship–guided medicinal chemistry program to design more potent antiandrogens with no significant agonistic activity in the setting of AR overexpression.5 The drug binds to the ligand-binding domain of AR with five-fold greater affinity than bicalutamide, and unlike bicalutamide, it does not induce robust AR nuclear translocation or DNA binding. In preclinical model systems, ARN-509 induced partial or complete regression in both castration-sensitive and -resistant human prostate cancer xenograft models and showed maximal antitumor efficacy in these models at a three-fold lower dose and approximately nine-fold lower plasma level than enzalutamide, suggestive of a higher therapeutic index.6
On the basis of these promising preclinical results, a first-in-human phase I study of ARN-509 was initiated for patients with progressive metastatic CRPC. The primary objectives were to assess pharmacokinetics (PKs), safety, and tolerability and to define a recommended phase II dose (RP2D) of ARN-509. Secondary objectives were to assess antitumor effects on the basis of prostate-specific antigen (PSA) kinetics, imaging, and circulating tumor cell (CTC) number. To optimize dose selection, preclinical xenograft data were incorporated into the analysis of the clinical response parameters to establish a maximum efficacious dose (MED) without risking undue toxicity by escalating to a maximum-tolerated dose (MTD).
PATIENTS AND METHODS
The phase I study was conducted at the Sidney Kimmel Center for Prostate and Urologic Cancers, Memorial Sloan-Kettering Cancer Center (MSKCC), between July 2010 and May 2012, in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. The protocol was approved by the MSKCC Institutional Review Board, and patients provided written informed consent before participation.
Study Population
Patients with progressive, metastatic CRPC were enrolled. Eligibility criteria included histologically confirmed adenocarcinoma of the prostate without neuroendocrine differentiation or small-cell features, and progressive disease based on a minimum of three rising PSA levels at least 1 week apart, with the last result being at least 2 ng/mL, or new or progressive soft tissue and/or bone disease confirmed on computed tomography (CT)/magnetic resonance imaging or bone scans, excluding brain metastases. Patients who had not undergone orchiectomy were required to maintain castrate levels of testosterone (< 50 ng/dL) with androgen-deprivation therapy. Other eligibility criteria included Eastern Cooperative Oncology Group performance status of 0 or 1, QTc interval ≤ 450 ms, and adequate cardiac, renal, hepatic, and bone marrow function. Patients were excluded if they had received prior treatment with ketoconazole or > two prior regimens of taxane-based chemotherapy in the metastatic setting. In March 2011, the protocol was amended to exclude patients previously treated with enzalutamide or abiraterone acetate. Patients with prior history of seizures or conditions that predispose to seizures or those requiring concurrent therapy with medications known to have seizure potential were excluded from participation.
Study Design
Patients were assigned sequentially to escalating dose levels of ARN-509 following a traditional 3 + 3 design. The objective was to determine the MTD and/or RP2D of ARN-509 leading to a dose-limiting toxicity (DLT) in ≤ 30% of patients. On day 1, a single dose of ARN-509 was administered, followed by daily blood sampling for PK assessments. At the end of the first week (ie, PK week), patients started continuous daily dosing and remained on study until disease progression or unacceptable toxicity. Each cycle of treatment was 28 days.
The starting dose was 30 mg, administered orally, once daily. At doses ≥ 300 mg (at least 10 capsules total), patients were allowed to switch to a twice-daily regimen, taking half of the capsules in the morning and half in the evening. At least three patients at each dose level were monitored for DLTs through day 28 of cycle one. The total evaluation period for each cycle was 4 weeks, not including the initial PK week. DLTs were defined as any grade 3/4 nonhematologic toxicity and/or grade 4 hematologic toxicity > 5 days in duration (GI toxicities must have persisted at grade 3 or 4 despite maximal medical therapy) according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE; version 4.0).7 A treatment-related seizure of any grade, occurring at any time during the study, was considered a DLT.
Dosing cohorts of three to six patients per cohort were added sequentially, based on the occurrence of one DLT in the first three patients in each cohort. If > two of six patients experienced a DLT at a given dose level, dose escalation was stopped, and the MTD was defined as the previous dose level. If no DLTs were observed, the RP2D was to be defined based on the overall PK and safety profile of ARN-509 and the optimal biologic dose (extrapolated from preclinical antitumor data and patient outcomes), not necessarily the MTD. If only three patients had been treated at a dose level under consideration as the MTD and/or RP2D, an additional three patients were added to confirm the MTD and/or RP2D. Patients in the dose-escalation cohorts receiving < 70% of the daily doses in cycle one (ie, < 20 of 28 daily doses) without DLTs were replaced. Intrapatient dose escalation was not permitted until after the RP2D was selected.
Safety Assessments
Clinical and laboratory assessments were performed weekly during the first cycle of treatment, monthly thereafter, and 28 days after discontinuation of study drug. Adverse events were graded using NCI CTCAE version 4.0. Laboratory assessments included routine hematology and chemistry panels, fasting lipid panel, coagulation parameters, thyroid function, and urinalysis. Twelve-lead single ECGs were obtained before treatment on PK day 1, at weeks 2 and 4 of cycle one, and at the last treatment visit to assess for potential QTc changes from baseline.
PK Assessments
Blood samples for PK analysis were obtained on PK day 1 at 0 (predose) and at 0.5, 1.5, 2, 3, 4, 6, 8, 24, 72, and 96 hours after a single dose of ARN-509 and just before the start of continuous dosing on day 1 of cycle one (ie, 7 days postdose). Pretreatment blood samples were collected weekly during cycle one and before each new cycle of treatment, starting with cycle two. On day 22 (cycle one, week 4), samples were collected at 0 (predose) and at 0.5, 1.5, 2, 3, 4, 6, 8, and 24 hours postdose. Samples were shipped frozen to Tandem Laboratories (Salt Lake City, UT) for analysis of ARN-509 concentrations (and its main metabolite, ARN-308) using a validated liquid chromatography–tandem mass spectrometry assay.
PK analysis for all parameters was performed using WINNonlin (Scientific Consultant, Apex, NC) Professional (version 5.3; Pharsight Corporation, St Louis, MO). Parameters analyzed included: maximum observed plasma concentration (Cmax) and minimum observed plasma concentration (Cmin) for the dosing interval, time of maximum observed plasma concentration (Tmax), terminal elimination half-life (T½), and area under the plasma concentration-time curve (AUC) for various time intervals.
Pharmacodynamic Biomarkers
All patients were offered participation in a study assessing 16β-[18F]fluoro-α-dihydrotestosterone (FDHT) –positron emission tomography (PET)/CT scans under a dedicated molecular imaging protocol approved by the MSKCC Institutional Review Board8–11; 16 patients elected to enroll. The FDHT radiopharmaceutical was administered under investigational new drug No. 66115. Standard operating procedures stipulated release criteria for each batch, which was manufactured in compliance with appropriate US Food and Drug Administration regulations in the Radiochemistry and Molecular Imaging Probe Core Facility at MSKCC. Release criteria include radionuclide purity > 95%, chemical purity > 95%, radiochemical identity based on FDHT standard comparison with radio high-performance liquid chromatography, residual solvent analyses, and endotoxin limit test < 2 EU/mL. Specific activity analysis from eight consecutive patient runs was obtained between February and April 2013 and ranged from 1.58 to 3.08 mCi/nmol (average, 2.12 mCi [18]F/nmol FDHT).
The uptake of FDHT, an analog of endogenous dihydrotestosterone, reflects AR expression and binding capacity. Reduction in FDHT uptake indicates effective targeting of the drug to the AR.12 FDHT-PET/CT scans were obtained before initiation of therapy and after 4 weeks of therapy. Patients were imaged from midskull to upper thighs on a Discovery STE PET/CT scanner (GE Healthcare, Waukesha, WI) approximately 30 to 40 minutes after injection of FDHT approximately 333 MBq. An experienced nuclear medicine physician evaluated the reconstructed PET, CT, and fused PET/CT images using a PET volume computer-assisted reading software package (GE Healthcare). The nuclear medicine physician was not blinded to dose. AR-positive lesions were determined by qualitative inspection of the FDHT-PET images.13 Any focus of activity visually higher than local background and not attributable to physiologic tracer distribution (eg, blood pool, excretion) was considered a true lesion. A volume of interest was semiautomatically placed around each lesion, and the calculated maximum standard uptake value (SUVmax) was recorded. SUVmax values were adjusted for the contribution of stromal signal by subtracting a previously published population-based background threshold. Adjusted SUV data were then binned and averaged (SUVmax-avg) according to therapeutic dose level (30 to 390 mg) and time point (baseline, 4 weeks). The percent change in SUVmax-avg between time points was calculated separately for each dose level.
CTCs
CTCs were isolated, analyzed, and enumerated using the CellSearch System (Veridex, Raritan, NJ) and reported as number of CTCs per 7.5 mL of blood as previously described.14 Samples were collected at baseline, 4 weeks, 12 weeks, and/or study termination and analyzed in the Clinical Laboratory Improvement Amendments–certified clinical chemistry laboratory at MSKCC.
Antitumor Activity
Antitumor activity was assessed based on changes in individual disease manifestations using the Prostate Cancer Clinical Trials Working Group 2 criteria.15 For PSA, percent change from baseline to 12 weeks, along with the maximum decline in PSA occurring at any point after treatment, was reported using waterfall plots. Imaging was performed at baseline, at 12-week intervals, and at the end of treatment. Changes in soft tissue disease were based on CT imaging using modified RECIST guidelines,16 and changes in osseous disease were reported as improved, stable, or progressive (worse) based on radionuclide bone scan per the working group criteria.
Statistical Analysis
All patients who received at least one dose of ARN-509 were included in the analyses. During dose escalation, the number of patients enrolled onto each dose-escalation cohort was dependent on the observed safety profile. MTD was defined as the highest dose with an observed incidence of DLT in ≤ one of six patients. For PK analysis, all available PK data from patients who received ARN-509 and had adequate concentration profiles were included. Descriptive statistics were used to summarize patient characteristics, safety, PK parameters, and antitumor activity.
RESULTS
Thirty patients were enrolled across nine dose levels: 30, 60, 90, 120, 180, 240, 300, 390, and 480 mg. Table 1 lists baseline demographic and clinical characteristics. At the time of data cutoff (May 2012), 17 patients (57%) had withdrawn from the study; 16 had disease progression (biochemical, radiographic, or clinical), and one withdrew consent. Median duration of study participation was 9.5 months. No patient discontinued the study because of toxicity.
Table 1.
Baseline Patient Demographic and Clinical Characteristics (N = 30)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 68 | |
| Range | 45 to 81 | |
| White race | 30 | 100 |
| ECOG PS | ||
| 0 | 20 | 67 |
| 1 | 10 | 33 |
| Gleason score | ||
| ≤ 7 | 10 | 33 |
| 8 to 10 | 20 | 67 |
| PSA, ng/mL | ||
| Median | 42.0 | |
| Range | 2.3 to 326.6 | |
| Disease | ||
| Bone | 28 | 87 |
| Soft tissue | 16 | 53 |
| Prior treatment of primary tumor | ||
| Surgery | 18 | 60 |
| Irradiation | 29 | 97 |
| None | 1 | 3 |
| No. of prior chemotherapy regimens | ||
| 0 | 25 | 83 |
| 1 | 4 | 13 |
| 2 | 1 | 3 |
| CTC count, cells/7.5 mL blood | ||
| < 5 | 23 | 77 |
| ≥ 5 | 7 | 23 |
Abbreviations: CTC, circulating tumor cell; ECOG PS, Eastern Cooperative Oncology Group performance status; PSA, prostate-specific antigen.
Safety
The most frequently reported adverse event (any cause) was fatigue (47%), which was restricted to grades 1 and 2 (Table 2). There were only four grade 3 adverse events, of which three were considered unrelated to study treatment. There was one DLT at the 300-mg dose level (grade 3 abdominal pain in patient with history of irritable bowel syndrome), which resolved with drug interruption and subsequent dose reduction to 240 mg (120 mg twice per day). Three additional patients were treated at the 300-mg dose level with no additional DLTs. Importantly, no seizures were reported at any dose level.
Table 2.
Most Commonly Reported (> 10%) Adverse Events (any cause)*
| Adverse Event | Grade 1 |
Grade 2 |
Grade 3 |
Grades 4 and 5 |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Fatigue | 12 | 40 | 2 | 7 | 0 | 0 | 0 | 0 | 14 | 47 |
| Back pain | 9 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 30 |
| Diarrhea | 6 | 20 | 3 | 10 | 0 | 0 | 0 | 0 | 9 | 30 |
| Dyspnea | 7 | 23 | 2 | 6 | 0 | 0 | 0 | 0 | 9 | 30 |
| Nausea | 7 | 23 | 1 | 3 | 1 | 3 | 0 | 0 | 9 | 30 |
| Abdominal pain | 6 | 20 | 1 | 3 | 1 | 3 | 0 | 0 | 8 | 27 |
| Arthralgia | 7 | 23 | 0 | 0 | 1 | 3 | 0 | 0 | 9 | 27 |
| Constipation | 7 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 23 |
| Headache | 6 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 20 |
| Peripheral sensory neuropathy | 5 | 17 | 1 | 3 | 0 | 0 | 0 | 0 | 6 | 20 |
| Musculoskeletal pain | 5 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 17 |
| Pain in extremity | 5 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 17 |
| Peripheral edema | 5 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 17 |
| Hot flush | 4 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 13 |
| Pain | 3 | 10 | 0 | 0 | 1 | 3 | 0 | 0 | 4 | 13 |
| Hemorrhage (urinary tract) | 3 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 10 |
By Common Terminology Criteria for Adverse Events (version 4.0).
PK Parameters
ARN-509 was rapidly absorbed, with measurable plasma concentrations within 30 minutes after ingestion of a single oral dose of 1 to 16 capsules (30-mg capsules; total ARN-509 doses, 30 to 480 mg). On average, peak plasma concentrations in each dose group occurred 2 to 3 hours after administration (Table 3).
Table 3.
Pharmacokinetic Parameters for ARN-509 at Steady-State
| Dose Group (mg) | Tmax (hours) | Cmax (μg/mL) | AUC0-24 h (h × μg/mL) | T1/2eff (hours) |
|---|---|---|---|---|
| 30 (n = 3) | ||||
| Mean | 0.7 | 0.841 | 14.7 | 104.0 |
| CV, % | 43.3 | 16.1 | 13.6 | 58.9 |
| 60 (n = 2 or 3) | ||||
| Mean | 2.0 | 1.60 | 29.7 | 96.0 |
| CV, % | 86.6 | 11.0 | 21.6 | 23.7 |
| 90 (n = 3) | ||||
| Mean | 1.2 | 2.53 | 38.3 | 86.8 |
| CV, % | 65.5 | 6.9 | 9.8 | 29.6 |
| 120 (n = 2 or 3) | ||||
| Mean | 9.3 | 3.30 | 59.4 | 108.0 |
| CV, % | 136.0 | 16.4 | 18.2 | 38.8 |
| 180 (n = 3) | ||||
| Mean | 1.0 | 5.98 | 93.7 | 72.0 |
| CV, % | 0.0 | 20.7 | 29.1 | 15.1 |
| 240 (n = 2 or 3) | ||||
| Mean | 3.5 | 7.55 | 127.0 | 86.2 |
| CV, % | 112.0 | 15.3 | 28.7 | 18.0 |
| 300 (n = 4 or 5) | ||||
| Mean | 6.1 | 7.08 | 134.0 | 72.6 |
| CV, % | 164.0 | 31.0 | 27.9 | 26.7 |
| 390 (n = 3) | ||||
| Mean | 1.0 | 8.91 | 140.0 | 74.8 |
| CV, % | 50.0 | 11.2 | 15.7 | 22.0 |
| 480 (n = 3) | ||||
| Mean | 1.2 | 11.2 | 202.0 | 86.1 |
| CV, % | 49.5 | 4.2 | 4.8 | 6.8 |
Abbreviations: AUC0-24 h, area under plasma concentration-time curve over 0 to 24 hours; Cmax, maximum observed plasma; CV, coefficient of variation; T1/2eff, effective half-life; Tmax, time of maximum observed plasma.
Plasma ARN-509 peak concentrations and AUCs were dose proportional. Plasma concentrations declined slowly, with mean half-life values at steady-state of 3 to 4 days. At the RP2D of 240 mg, mean steady-state Cmax and AUC0-24 hours were 7.6 and 127 μg.h/mL, respectively. Drug half-life and time to steady-state were independent of dose. Plasma trough concentrations of ARN-509 increased steadily with time and in proportion to dose across cohorts. On the basis of these trough levels, most patients reached steady-state exposure after 3 weeks of continuous administration (ie, by week 4).
FDHT-PET/CT Analysis
AR binding was assessed in 16 patients receiving doses ranging from 30 to 390 mg per day, with FDHT-PET/CT imaging before and during treatment with ARN-509 (Fig 1; Appendix Fig A1, online only). At each tested dose level and for all lesions, the percent decline in SUVmax-avg at 4 weeks increased in a dose-dependent fashion, reaching a plateau at ≥ 120 mg daily. At doses ≥ 120 mg, there was a > 90% decline in SUVmax-avg at 4 weeks, whereas doses ≤ 90 mg showed declines of approximately ≤ 50%.
Fig 1.
Percent change in 16β-[18F]fluoro-α-dihydrotestosterone standard uptake value (SUVmax-avg) after 4 weeks of treatment in 16 patients receiving ARN-509 30 to 390 mg daily. Total numbers of lesions and patients at each dose level are shown below each bar.
CTC Enumeration
Overall, only seven patients (23%) had unfavorable CTC counts (ie, ≥ five cells per 7.5 mL blood [mean ± standard deviation, 23 ± 32]) at baseline. Of these, four (57%) showed a conversion from unfavorable to favorable status (< five cells per 7.5 mL blood) after treatment with ARN-509.
Post-Therapy PSA Changes
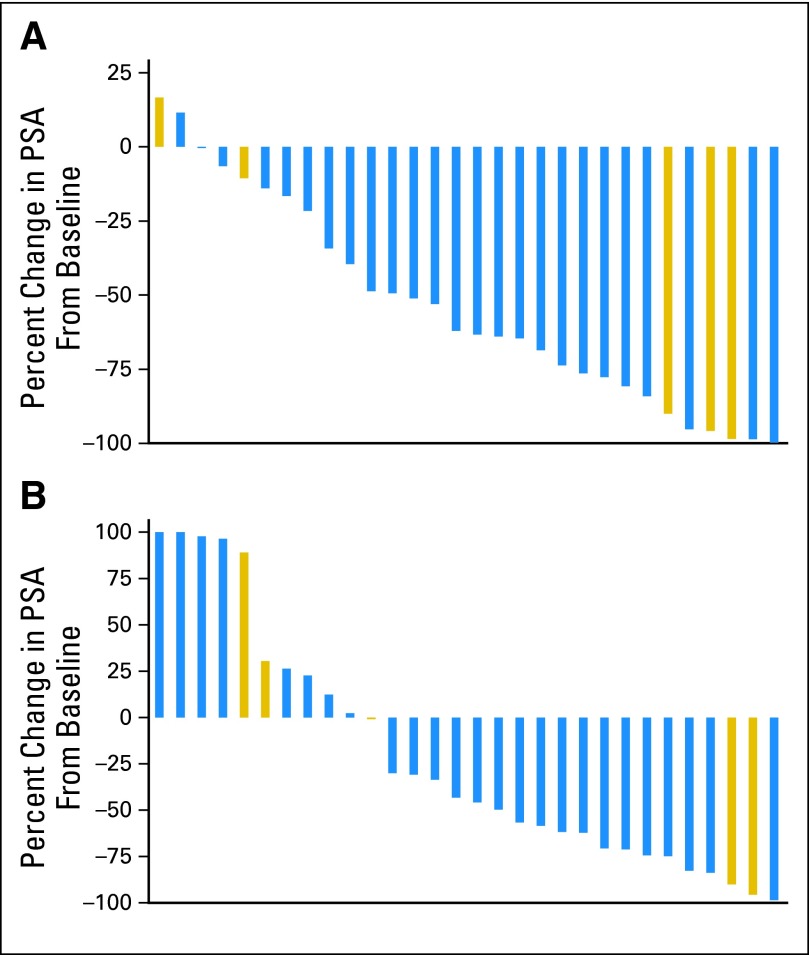
Figure 2A depicts the maximum decrease in PSA from baseline for each individual patient across all nine dose levels. Eighteen patients (60%) had a ≥ 50% decline in PSA as compared with baseline, and of those, six (20%) had a ≥ 90% decline.
Fig 2.
Prostate-specific antigen (PSA) kinetics (with prior chemotherapy in gold). (A) Maximum PSA decline during study (n = 30); (B) PSA levels at 12 weeks (n = 30).
At 12 weeks, 14 (46.7%) of 30 patients had a ≥ 50% decline in PSA as compared with baseline. The median PSA change from baseline at 12 weeks was −43.2% (range, −98.6% to 120.6%), and the maximum median decline on study was −62.7% (range, −99.8% to 16.7%; Fig 2B).
Objective Responses
Ten patients presented with measurable soft tissue disease at baseline. Of those, five (50%) maintained stable disease responses > 6 months, one (10%) experienced disease progression based on the appearance of new lesions, and four (40%) had indeterminate responses.
DISCUSSION
We report the results of the first-in-human phase I portion of an ongoing phase I/II study of ARN-509 in CRPC in which an RP2D was established using an integrative approach that compared preclinical xenograft data with real-time analysis of clinical PKs, pharmacodynamics, efficacy, and safety in humans. This integrated preclinical and clinical approach led to the establishment of an exposure/efficacy relationship that favored selection of a safe and fully efficacious dose without the need to risk the potential toxicity of escalating to an MTD. ARN-509 was safe and well tolerated, with linear PK consistent with preclinical studies. Across all doses tested, antitumor activity was evident as shown by PSA kinetics, radiographic responses, and CTC enumeration.
FDHT-PET/CT imaging was used to measure pharmacodynamic response to ARN-509 and capture the biologic diversity of multifocal metastatic disease through visualization of in situ AR binding. ARN-509 reduced FDHT uptake across dose levels, indicating an on-target effect of AR inhibition. The response reached a plateau level at doses ≥ 120 mg (with FDHT uptake near background), indicating saturation of AR binding and thus achievement of the optimal concentration of drug. Although 120 mg seemed to fully occupy available AR binding sites via FDHT uptake, the mean plasma trough levels associated with this dose in humans (2.5 μg/mL) were at the lower end of the range that produced tumor regressions in the LNCaP/AR murine model of CRPC. The steady-state plasma levels associated with 240 mg in humans (3 to 6 μg/mL) were more firmly in the range sufficient to elicit tumor regressions in the mouse xenograft (Fig 3). Hence, ARN-509 240 mg daily was chosen as the MED in patients with CRPC and was selected as the RP2D for further study.
Fig 3.
Steady-state ARN-509 plasma trough concentrations in patients and in murine model of castration-resistant prostate cancer (CRPC). Mean predose ARN-509 concentrations for patients in each dose cohort (error bars indicate standard deviation) and steady-state trough concentrations associated with efficacy in clinically validated LNCaP/androgen receptor model of CRPC.
After achieving the equivalent of the MED of 240 mg daily in preclinical models, two additional dose levels (300 and 480 mg) were tested to further establish the safety margin of ARN-509. The most frequent treatment-related adverse events were fatigue (47%; no grade 3/4 events) and GI symptoms such as nausea (30%) and abdominal pain (20%). Recent preclinical and clinical studies have established a risk of seizure with antiandrogens as a class, which is thought to be mediated through off-target binding of the CNS-based GABAA receptor.3,17,18 Because ARN-509 can also bind to the GABAA receptor, clinical efficacy data were integrated with detailed preclinical dose-response studies to select an MED that optimized AR inhibition and antitumor response while avoiding the need to dose escalate until unacceptable toxicity. In this phase I study, there were no reported seizures at any dose level or treatment discontinuations because of toxicity; therefore, MTD was not defined.
In summary, the selected RP2D of 240 mg exhibited an excellent safety profile and safety margin based on dose escalation to 480 mg (twice the MED). A daily dose of 240 mg achieved maximal AR inhibition (based on FDHT-PET/CT) accompanied by robust and durable declines in PSA; all of these activities were consistent with preclinical modeling. This integrative approach may be useful for development of future antiandrogens (ie, treating with MED rather than MTD may help to prevent unacceptable event such as seizure). The favorable safety profile and high therapeutic index of ARN-509 have the potential to allow for treatment across the entire spectrum of prostate cancer, as both monotherapy and in combination with other agents that target pathways critical to the malignant progression of the disease.
Acknowledgment
We thank all the patients, their families, and their caregivers for their participation in this study and the investigators and their staff at participating sites.
Glossary Term
- Circulating tumor cells (CTCs):
Demonstration of isolated tumor cell circulation/dissemination in the peripheral blood.
Appendix
Fig A1.
16β-[18F]fluoro-α-dihydrotestosterone (FDHT) response to ARN-509. Left hemipelvis at (A) baseline and (B) 4 weeks. Arrows indicate representative bone metastases that have increased uptake of FDHT prior to treatment, and decreased uptake after treatment due to displaced tracer by ARN-509.
Footnotes
Supported by National Cancer Institute Grant No. P50 CA092629, Department of Defense Grant No. PC081610, a Prostate Cancer Foundation Young Investigator Award (D.E.R.), a Prostate Cancer Foundation Challenge Award (H.I.S.), and Aragon Pharmaceuticals.
Presented in part at the 2012 Genitourinary Cancers Symposium, San Francisco, CA, February 2-4, 2012, and 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other granting agencies or institutions.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT01171898.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Jeffrey H. Hager, Aragon Pharmaceutical (C); Peter J. Rix, Aragon Pharmaceuticals (C); Edna Chow Maneval, Aragon Pharmaceutical (C); Isan Chen, Aragon Pharmaceuticals (C) Consultant or Advisory Role: Michael J. Morris, Millenium (C); Charles L. Sawyers, Aragon Pharmaceuticals (C); Howard I. Scher, Aragon Pharmaceuticals (U), Medivation (U), Orion (C), Millennium Pharmaceuticals (C), Ortho Biotech (C), Johnson & Johnson (U) Stock Ownership: Jeffrey H. Hager, Aragon Pharmaceutical; Peter J. Rix, Aragon Pharmaceuticals; Edna Chow Maneval, Aragon Pharmaceutical; Isan Chen, Aragon Pharmaceutical; Charles L. Sawyers, Aragon Pharmaceuticals Honoraria: None Research Funding: Dana E. Rathkopf, Aragon Pharmaceuticals, Medivation, Janssen Pharmaceuticals; Michael J. Morris, Medivation, Janssen Pharmaceuticals, Takeda Pharmaceuticals; Howard I. Scher, Aragon Pharmaceuticals, Medivation, Janssen Pharmaceuticals, Millennium Pharmaceuticals Expert Testimony: None Patents: None Other Remuneration: Charles L. Sawyers, co-inventor of ARN-509 (entitled to royalties)
AUTHOR CONTRIBUTIONS
Conception and design: Dana E. Rathkopf, Michael J. Morris, Edna Chow Maneval, Isan Chen, Steven M. Larson, Charles L. Sawyers, Howard I. Scher
Provision of study materials or patients: Michael J. Morris, Howard I. Scher
Collection and assembly of data: Dana E. Rathkopf, Michael J. Morris, Edna Chow Maneval, Isan Chen, Steven M. Larson
Data analysis and interpretation: Dana E. Rathkopf, Michael J. Morris, Josef J. Fox, Daniel C. Danila, Susan F. Slovin, Jeffrey H. Hager, Peter J. Rix, Edna Chow Maneval, Isan Chen, Mithat Gonen, Martin Fleisher, Steven M. Larson, Howard I. Scher
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 3.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung ME, Ouk S, Yoo D, et al. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC) J Med Chem. 2010;53:2779–2796. doi: 10.1021/jm901488g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Cancer Institute. Common Terminology Criteria for Adverse Events. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
- 8.Beattie BJ, Smith-Jones PM, Jhanwar YS, et al. Pharmacokinetic assessment of the uptake of 16beta-18F-fluoro-5alpha-dihydrotestosterone (FDHT) in prostate tumors as measured by PET. J Nucl Med. 2010;51:183–192. doi: 10.2967/jnumed.109.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanzonico PB, Finn R, Pentlow KS, et al. PET-based radiation dosimetry in man of 18F-fluorodihydrotestosterone, a new radiotracer for imaging prostate cancer. J Nucl Med. 2004;45:1966–1971. [PubMed] [Google Scholar]
- 10.Larson SM, Morris M, Gunther I, et al. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366–373. [PubMed] [Google Scholar]
- 11.Dehdashti F, Picus J, Michalski JM, et al. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur J Nucl Med Mol Imaging. 2005;32:344–350. doi: 10.1007/s00259-005-1764-5. [DOI] [PubMed] [Google Scholar]
- 12.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1-2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox JJ, Autran-Blanc E, Morris MJ, et al. Practical approach for comparative analysis of multilesion molecular imaging using a semiautomated program for PET/CT. J Nucl Med. 2011;52:1727–1732. doi: 10.2967/jnumed.111.089326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danila DC, Heller G, Gignac GA, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 15.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Rathkopf D, Liu G, Carducci MA, et al. Phase I dose-escalation study of the novel antiandrogen BMS-641988 in patients with castration-resistant prostate cancer. Clin Cancer Res. 2011;17:880–887. doi: 10.1158/1078-0432.CCR-10-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster WR, Car BD, Shi H, et al. Drug safety is a barrier to the discovery and development of new androgen receptor antagonists. Prostate. 2011;71:480–488. doi: 10.1002/pros.21263. [DOI] [PubMed] [Google Scholar]