Abstract
Purpose
Racial disparities in cancer treatment and outcomes are a national problem. The nationwide Veterans Affairs (VA) health system seeks to provide equal access to quality care. However, the relationship between race and care quality for veterans with colorectal cancer (CRC) treated within the VA is poorly understood. We examined the association between race and receipt of National Comprehensive Cancer Network guideline–concordant CRC care.
Patients and Methods
This was an observational, retrospective medical record abstraction of patients with CRC treated in the VA. Two thousand twenty-two patients (white, n = 1,712; African American, n = 310) diagnosed with incident CRC between October 1, 2003, and March 31, 2006, from 128 VA medical centers, were included. We used multivariable logistic regression to examine associations between race and receipt of guideline-concordant care (computed tomography scan, preoperative carcinoembryonic antigen, clear surgical margins, medical oncology referral for stages II and III, fluorouracil-based adjuvant chemotherapy for stage III, and surveillance colonoscopy for stages I to III). Explanatory variables included demographic and disease characteristics.
Results
There were no significant racial differences for receipt of guideline-concordant CRC care. Older age at diagnosis was associated with reduced odds of medical oncology referral and surveillance colonoscopy. Presence of cardiovascular comorbid conditions was associated with reduced odds of medical oncology referral (odds ratio, 0.65; 95% CI, 0.50 to 0.89).
Conclusion
In these data, we observed no evidence of racial disparities in CRC care quality. Future studies could examine causal pathways for the VA's equal, quality care and ways to translate the VA's success into other hospital systems.
INTRODUCTION
The Veterans Affairs (VA) health care system is the largest provider of integrated cancer care in the United States, treating approximately 3% of newly diagnosed patients with cancer nationwide.1 Among VA patients, colorectal cancer (CRC) is a common disease.1 In fact, CRC is the third most commonly diagnosed cancer and third most common cause of cancer-related death nationwide.2 In nonfederal US health care systems, racial differences in CRC diagnosis, treatment, and mortality have been documented.3–13 For example, fewer African Americans receive adjuvant chemotherapy for CRC compared with whites.5,7,14 Compared with white patients, cancer surveillance is lower and cancer-related mortality is higher among African American patients.5,9,10 Unequal access to health care services has been attributed as a contributing factor for racial disparities in care.3,15,16 The VA health care system provides a good context in which to study racial disparities because of standard VA eligibility criteria and narrow distribution of family income among VA patients, making access issues among individuals of different races narrower than for non-VA systems.17 Although racial health care disparities have been noted for some conditions, the degree of racial disparities identified in the quality of VA health care is often less than what is found in other health care systems.18,19
There is paucity of information regarding racial disparities in VA CRC care quality for patients of diverse race. Previous studies suggest that racial differences in CRC treatment may be diminished in the VA.16,20,21 However, these studies were based on a limited number of quality measures that can be evaluated based solely on administrative data. We extend previous research by including additional data sources (ie, a comprehensive review of electronic health record data, supplemented with administrative data) to examine racial differences for receipt of guideline-concordant CRC care.
PATIENTS AND METHODS
Data Sources
Data were from the External Peer Review Program (EPRP), the national program for tracking quality of VA health care.22 Between July and August 2007, medical record abstraction was conducted under the guidance of the VA Office of Quality and Performance to assess CRC care quality. Abstractors accessed the electronic medical record (EMR) remotely, collecting data on disease characteristics and health care delivery provided to individuals across the VA nationwide. We supplemented EPRP with clinical comorbidity and demographic information from the VA Central Cancer Registry (VACCR) and administrative data. The institutional review boards at the Durham VA Medical Center and at the University of North Carolina at Chapel Hill approved this project.
Patient Sample
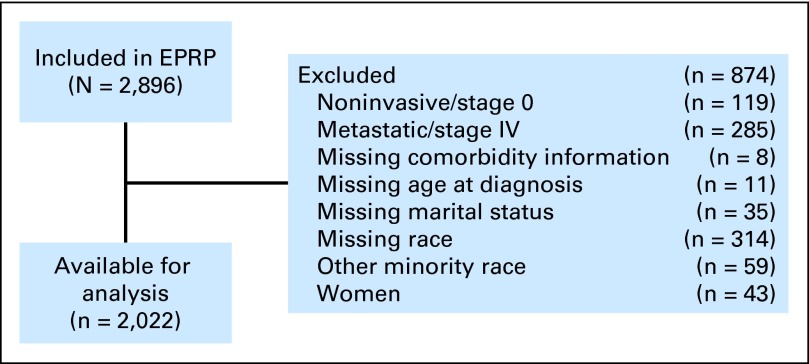
The sample has previously been described in detail.23 Briefly, patients were identified for EPRP inclusion based on a search algorithm defining a representative sample of VA patients diagnosed with CRC between October 1, 2003, and March 31, 2006, using administrative diagnosis, procedure, and encounter data.23,24 Eligible patients had an International Classification of Diseases code for colon and/or rectal cancer within 3 months of the study diagnosis time period.23,25 Eligible patients must have had a clinic visit, surgical procedure, or pathology report in the VA that corresponded with a specific visit or receipt of medical services within the aforementioned time frame. To be in the final analytic data set, patients also must have had nonmetastatic CRC (stages I to III), had an incident occurrence (first diagnosis of CRC occurred during the study time period), and received definitive CRC surgical resection. Because their recommended patterns of health care and life expectancy are different than patients with earlier stage disease, those with metastatic disease (stage IV) were excluded. Furthermore, to be included in this analysis, patients must have successfully linked with the VA administrative data sources. In other words, patients must have had matching records in both the EPRP quality tracking data source as well as the VA administrative data. The administrative data are generated when a patient interacts with the VA health care system, either in an inpatient or outpatient setting, to receive health care. Because of the small number of non–African American minorities and women, we restricted the study to white and African American male patients (Fig 1).
Fig 1.
Colorectal cancer cohort assembly. EPRP, External Peer Review Program.
Dependent Variables
We used six distinct CRC quality indicators based on 2003 National Comprehensive Cancer Network guidelines26,27 that have scientific evidence and/or National Comprehensive Cancer Network panel consensus.23,26,27 Each indicator applied to a subset of patients as determined by stage and other factors. The specific quality indicators, by stage, were as follows. For stages II and III CRC, the quality indicators were preoperative computed tomography (CT) scan of the abdomen and pelvis before definitive surgical resection; preoperative carcinoembryonic antigen determination before definitive surgical resection; documented radial margins free of tumor at the time of definitive surgical resection; and referral to a medical oncologist. Because our intent was to evaluate equity in access to quality care, patients with documentation of a refusal (eg, refused CT scan) in the EMR were included in the quality indicator as having received guideline-concordant care. For stage III CRC, the quality indicator was receipt of adjuvant fluorouracil- (FU) or capecitabine-based chemotherapy administered after definitive surgical resection. If the EMR contained a documented reason why FU was not administered, that was included in the quality indicator calculation as having received guideline-concordant care. For patients with stage I to III CRC who did not have documentation of an obstructing preoperative lesion, the quality indicator was receipt of surveillance colonoscopy within 7 to 18 months after definitive surgical resection. Consistent with previous analyses,23 7 months was used as a minimum because colonoscopies performed earlier might not be intended for surveillance, and 18 months was chosen because surveillance colonoscopies may not occur exactly within 1 year. To be included in the surveillance colonoscopy measure, patients must have survived at least 1 year after surgical resection.
Independent Variables
The primary independent variable of interest was race. We used a hierarchy of sources to determine the most accurate race measure. Because data were obtained through EMR review by trained cancer registrars, the VACCR was considered the most valid source. If race was not reported in the VACCR, then race was extracted from the inpatient medical record, followed by outpatient medical record information.
Other covariates included marital status (married or not), age at diagnosis (< 55, 55 to 64, 65 to 74, or ≥ 75 years), geographic region (west, south, east, or central), comorbid conditions, and, when applicable, stage of disease (I, II, or III). The included comorbid conditions were based on the National Cancer Institute Combined Comorbidity Index, which has been validated in patients with CRC.28 We identified diagnoses for comorbid conditions with International Classification of Diseases codes from medical inpatient and outpatient administrative data files.25 To be included, comorbid conditions must have been diagnosed within the year before the CRC diagnosis, excluding those comorbidities occurring in the 30 days leading up to diagnosis. This is because the 30 days before a cancer diagnosis often involve multiple interactions with the health care system, and patients may be diagnosed, sometimes erroneously, with comorbidities that are actually cancer symptoms or sequelae. To ensure that analyses had adequate statistical power to address the study question, we aggregated conditions based on consultation with a medical oncologist and statistical examination to ensure no valuable information was lost (eg, collapsing a positively correlated condition with a negatively correlated condition).29 Individual comorbid conditions included the following: liver disease; rheumatoid disease/AIDS; renal disease; dementia/paralysis; congestive heart failure, acute myocardial infarction, cardiovascular disease, or chronic obstructive pulmonary disease; and diabetes. This approach enabled examination of the effect of specific conditions on receipt of guideline-concordant care, an advantage over an aggregate comorbidity score.
Data Analysis
Multivariable logistic regression was used to determine the association between race and receipt of guideline-concordant CRC care. Pearson's χ2 tests were used to determine whether there were significant differences between groups for background demographic and clinical characteristics. Because this analysis examines multiple comparisons (eg, race, age at diagnosis), we used the Bonferroni adjustment to control for family-wise error. Therefore, associations between independent variables and quality indicators were considered statistically significant if P < .01. Stata 11 (StataCorp LP, College Station, TX) and SAS version 9.2 (SAS Institute, Cary, NC) were used for data management and analyses.
RESULTS
The final sample consisted of 2,022 men with incident CRC (Fig 1). The mean age at diagnosis was 68 years (range, 34 to 94 years). Reflecting the overall VA patient population, the sample was predominately white (85%) and married (52%) and lived in the south (38%). Stage was approximately evenly distributed. The most commonly diagnosed comorbid conditions were diabetes (27%) and cardiovascular-related diseases (24%; Table 1).
Table 1.
Key Variables
| Variable | No. of Patients/Total No. | % |
|---|---|---|
| Dependent variables | ||
| CT scan | 1,022/1,410 | 72.48 |
| Preoperative CEA | 1,175/1,410 | 83.33 |
| Clear surgical margins | 1,155/1,393 | 82.91 |
| Referral to a medical oncologist | 1,103/1,410 | 78.23 |
| Adjuvant FU chemotherapy | 487/653 | 74.58 |
| Surveillance colonoscopy | 869/2,006 | 43.32 |
| Independent variable | ||
| White race | 1,712/2,022 | 84.67 |
| Other control variables | ||
| Age at diagnosis, years | ||
| < 55 | 180/2,022 | 8.90 |
| 55-64 | 615/2,022 | 30.42 |
| 65-74 | 576/2,022 | 28.49 |
| 75+ | 651/2,022 | 32.20 |
| Married | 1,045/2,022 | 51.68 |
| Region | ||
| South | 760/2,022 | 37.59 |
| North | 386/2,022 | 19.09 |
| Central | 451/2,022 | 22.30 |
| West | 425/2,022 | 21.02 |
| Stage at diagnosis | ||
| I | 612/2,022 | 30.27 |
| II | 757/2,022 | 37.44 |
| III | 653/2,022 | 32.29 |
| Individual comorbid conditions | ||
| Liver disease | 11/2,022 | 0.54 |
| Rheumatoid disease or AIDS | 23/2,022 | 1.14 |
| Renal disease | 53/2,022 | 2.62 |
| Dementia or paralysis | 5/2,022 | 0.25 |
| CHF, acute MI, CVD, or COPD | 485/2,022 | 23.99 |
| Diabetes | 541/2,022 | 26.76 |
Abbreviations: CEA, carcinoembryonic antigen; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CT, computed tomography; CVD, cardiovascular disease; FU, fluorouracil; MI, myocardial infarction.
There were no significant racial differences in receipt of quality CRC care in the VA (Tables 2 and 3). In these data, race was not associated with receipt of guideline-concordant care for the examined quality indicators.
Table 2.
Multivariable Logistic Regression Results for Measures Involving Patients With Stage II or III CRC
| Variable | Preoperative CT Scan (n = 1,410) |
Preoperative CEA (n = 1,410) |
Clear Surgical Margins (n = 1,393) |
Medical Oncologist Referral (n = 1,410) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Demographic | ||||||||||||
| White | 1.22 | 0.88 to 1.70 | .23 | 1.17 | 0.80 to 1.72 | .41 | 0.71 | 0.43 to 1.19 | .19 | 1.46 | 1.00 to 2.13 | .05 |
| Married | 0.82 | 0.64 to 1.04 | .11 | 1.13 | 0.84 to 1.50 | .42 | 1.02 | 0.73 to 1.44 | .90 | 0.85 | 0.64 to 1.11 | .23 |
| Age at diagnosis, years | ||||||||||||
| < 55 | 1.03 | 0.67 to 1.58 | .88 | 1.04 | 0.62 to 1.80 | .87 | 0.70 | 0.39 to 1.25 | .23 | 2.45 | 1.42 to 4.23 | < .01* |
| 55-64 | 1.51 | 1.11 to 2.05 | .01 | 1.33 | 0.91 to 1.93 | .14 | 0.90 | 0.58 to 1.38 | .62 | 1.89 | 1.34 to 2.65 | < .01* |
| 65-74 | 1.20 | 0.59 to 1.63 | .24 | 0.87 | 0.61 to 1.24 | .44 | 1.00 | 0.63 to 1.60 | 1.00 | 1.66 | 1.18 to 2.35 | < .01* |
| 75+ (referent) | ||||||||||||
| Region | ||||||||||||
| North | 1.71 | 1.20 to 2.43 | < .01* | 1.32 | 0.88 to 1.98 | .18 | 2.25 | 1.22 to 4.14 | .01 | 3.22 | 1.96 to 5.30 | < .01* |
| Central | 1.91 | 1.37 to 2.67 | < .01* | 0.98 | 0.69 to 1.41 | .93 | 0.93 | 0.60 to 1.43 | .73 | 1.25 | 0.86 to 1.82 | .23 |
| West | 1.21 | 0.88 to 1.66 | .24 | 2.88 | 1.79 to 4.64 | < .01* | 0.85 | 0.55 to 1.31 | .46 | 0.42 | 0.30 to 0.58 | < .01* |
| South (referent) | ||||||||||||
| Comorbidity | ||||||||||||
| Liver disease | 1.15 | 0.23 to 5.82 | .86 | 0.37 | 0.08 to 1.61 | .18 | 0.47 | 0.09 to 2.48 | .38 | 0.78 | 0.14 to 4.34 | .78 |
| Rheumatoid disease or AIDS | 0.99 | 0.34 to 2.91 | .99 | 0.63 | 0.20 to 1.99 | .43 | —† | 1.53 | 0.42 to 5.59 | .52 | ||
| Renal disease | 0.60 | 0.30 to 1.24 | .17 | 3.43 | 0.80 to 14.65 | .10 | 1.80 | 0.41 to 7.85 | .43 | 1.08 | 0.46 to 2.54 | .85 |
| Dementia or paralysis | 0.67 | 0.11 to 4.20 | .67 | 0.13 | 0.02 to 0.83 | .03 | 0.18 | 0.03 to 1.15 | .07 | 1.27 | 0.13 to 12.15 | .84 |
| CHF, acute MI, CVD, or COPD | 0.82 | 0.63 to 1.09 | .19 | 0.96 | 0.69 to 1.35 | .82 | 0.86 | 0.58 to 1.29 | .47 | 0.65 | 0.50 to 0.89 | .01 |
| Diabetes | 0.93 | 0.71 to 1.23 | .62 | 1.03 | 0.73 to 1.43 | .88 | 1.20 | 0.80 to 1.80 | .38 | 1.02 | 0.74 to 1.40 | .90 |
| Stage at diagnosis | ||||||||||||
| III | 1.06 | 0.83 to 1.35 | .63 | 1.04 | 0.78 to 1.38 | .81 | 0.44 | 0.31 to 0.62 | < .01* | 2.70 | 2.03 to 3.60 | < .01* |
| II (referent) | ||||||||||||
Abbreviations: CEA, carcinoembryonic antigen; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CRC, colorectal cancer; CT, computed tomography; CVD, cardiovascular disease; MI, myocardial infarction; OR, odds ratio.
Indicates statistical significance of P < .01.
Because of perfect prediction between rheumatoid disease and AIDS, 17 observations were excluded.
Table 3.
Multivariate Logistic Regression Results for Receipt of Adjuvant FU-Based Chemotherapy and Surveillance Colonoscopy
| Variable | Adjuvant FU Chemotherapy* (n = 653) |
Surveillance Colonoscopy (n = 2006) |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Demographic | ||||||
| White | 1.23 | 0.77 to 1.97 | .40 | 1.32 | 1.01 to 1.73 | .04 |
| Married | 1.30 | 0.90 to 1.88 | .16 | 1.00 | 0.82 to 1.22 | 1.00 |
| Age at diagnosis, years | ||||||
| < 55 | 1.70 | 0.86 to 3.38 | .13 | 1.12 | 0.77 to 1.62 | .55 |
| 55-64 | 1.03 | 0.65 to 1.62 | .91 | 1.20 | 0.93 to 1.53 | .16 |
| 65-74 | 1.15 | 0.71 to 1.87 | .57 | 1.47 | 1.14 to 1.89 | < .01† |
| 75+ (referent) | ||||||
| Region | ||||||
| North | 1.75 | 1.00 to 3.07 | .05 | 1.28 | 0.96 to 1.71 | .09 |
| Central | 1.52 | 0.95 to 2.42 | .08 | 0.84 | 0.65 to 1.08 | .18 |
| West | 1.30 | 0.79 to 2.14 | .31 | 1.01 | 0.77 to 1.33 | .93 |
| South (referent) | ||||||
| Comorbidity | ||||||
| Liver disease | 0.28 | 0.04 to 2.11 | .22 | —‡ | ||
| Rheumatoid disease or AIDS | 1.11 | 0.20 to 6.17 | .90 | 1.00 | 0.40 to 2.46 | .99 |
| Renal disease | 0.68 | 0.22 to 2.14 | .51 | 0.87 | 0.49 to 1.56 | .65 |
| Dementia or paralysis | 0.17 | 0.01 to 2.03 | .16 | —§ | ||
| CHF, acute MI, CVD, or COPD | 0.82 | 0.53 to 1.25 | .35 | 0.84 | 0.67 to 1.06 | .14 |
| Diabetes | 0.84 | 0.56 to 1.26 | .40 | 0.86 | 0.69 to 1.08 | .20 |
| Stage at diagnosis | ||||||
| I | 0.71 | 0.56 to 0.90 | < .01† | |||
| II (referent) | ||||||
| III | 0.97 | 0.77 to 1.24 | .83 | |||
Abbreviations: CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; FU, fluorouracil; MI, myocardial infarction; OR, odds ratio.
Stage is not included because only patients with stage III colorectal cancer were included per National Comprehensive Cancer Network guidelines.
Indicates statistical significance of P < .01.
Because of issues of perfect prediction with liver disease, 11 observations were excluded.
Because of perfect prediction with dementia/paralysis, five observations were excluded.
Compared with patients age 75 years or older at diagnosis, patients age 55 to 64 years at diagnosis had marginally greater odds of having a preoperative CT scan (odds ratio [OR], 1.51; 95% CI, 1.11 to 2.05). Similarly, compared with patients age 75 years or older, younger patients had greater odds of a medical oncology referral (age < 55 years: OR, 2.45; 95% CI, 1.42 to 4.23; age 55 to 64 years: OR, 1.89; 95% CI, 1.34 to 2.65; age 65 to 74 years: OR, 1.66; 95% CI, 1.18 to 2.35; Table 2). Patients who were age 65 to 74 years old had greater odds of receiving surveillance colonoscopy than patients who were age 75 years or older (OR, 1.47; 95% CI, 1.14 to 1.89; Table 3).
For the majority of quality indicators, there was no association between individual comorbid conditions and receipt of guideline-concordant care. Patients with cardiovascular-related comorbidities had lower odds of referral to a medical oncologist (OR, 0.65; 95% CI, 0.50 to 0.89) than patients without cardiovascular conditions (Table 2).
Limited associations between stage and receipt of guideline-concordant care may reflect disease severity. Patients with stage III disease had lower odds of clear surgical margins (OR, 0.44; 95% CI, 0.31 to 0.62) than those with stage II disease (Table 2). Patients with stage III disease had increased odds of referral to medical oncology (OR, 2.70; 95% CI, 2.03 to 3.60). Odds of receiving surveillance colonoscopy were lower among patients with stage I disease (OR, 0.71; 95% CI, 0.56 to 0.90) compared with patients with stage II disease (Table 3).
Bivariate analyses indicated that patient refusals and/or physician documentation of a clinical contraindication for therapy were not masking racial disparities in care. One white patient refused preoperative CT scan. The association between race and CT refusal was not statistically significant (P = .36). Regarding receipt of adjuvant FU-based chemotherapy for patients with stage III disease, 11 patients (one African American and 10 white patients) refused treatment and 32 patients (five African American and 27 white patients) had physician documentation of a clinical contraindication precluding receipt of chemotherapy. This represented 3% of the sample for the FU-based chemotherapy measure (43 of 1,410 patients), and the association with race was not significant (P = .37).
DISCUSSION
Historically, the VA was criticized for its overall organization and management and providing poor care quality.30,31 In 1995, the VA began a system-wide transformational redesign with emphasis on using information technology, reporting quality performance, and integrating medical services.32–35 Recent reports suggest that the VA is now a leader in information technology and delivery of high-quality care.23,35–37 The question that we address is whether this overall quality transformation has also reduced racial disparities in guideline-concordant CRC care.
We found a lack of evidence of racial disparities in receipt of guideline-concordant CRC care. This contrasts with prior studies in nonfederal hospitals that observed racial differences in receipt of CRC care, for example, in receipt of screening colonoscopies.3,38,39 However, there is a dearth of information about racial differences in surveillance colonoscopy receipt after surgery. We identified no racial differences for surveillance colonoscopy.
Colonoscopy is not without risks; differences in receipt of surveillance colonoscopy based on stage and age at diagnosis may be appropriate. There is evidence that intensive postsurgery surveillance can increase survival,40–42 but a direct clinical benefit for surveillance colonoscopy alone has not been established. Despite this, clinical guidelines assert that surveillance colonoscopy is an important component of guideline-concordant CRC surveillance.26,27,43
Studies from the Surveillance, Epidemiology, and End Results–Medicare registry found differences in oncologist evaluation rates between white and African American patients, but the gap decreased substantially over time. Contingent on consulting with a medical oncologist, there were no racial differences in treatment receipt.44 However, there are differences based on age at diagnosis. Clinical trials generally do not enroll elderly people; the clinical benefit of adjuvant chemotherapy on older people has not been tested extensively in a trial setting, and older patients often do not receive FU-based chemotherapy in the private health care setting.45 Regarding race, there is evidence that the clinical benefit of FU may be lower for African American patients than for white patients.14,46
In our analysis, age and stage at diagnosis were associated with medical oncology referral. The difference in stage, where patients with stage III disease have greater odds of referral than patients with stage II disease, is likely clinically appropriate. Our study sample was diagnosed in 2003 to 2006. At the time these patients received treatment, chemotherapy for patients with stage II disease was controversial. This association may reflect clinicians' knowledge of clinical practice guidelines. However, differences in referral patterns based on stage, age, and cancer type require further examination. We found no association between race and medical oncology referral. Medical oncology referral typically precedes chemotherapy. In this VA population, we also found no association between race or age and FU-based chemotherapy receipt.
We found differences in receipt of care based on the presence of specific comorbid conditions—patients with cardiovascular conditions were less likely to be referred to a medical oncologist. This may be a result of contraindications for chemotherapy. Clinical appropriateness of referral was not addressed in our analysis.
There are several limitations to this study. First, we were unable to control for potentially confounding sociodemographic status, which may mediate associations between race and care quality. This potential omission bias is likely mitigated because VA patients receive care on a sliding fee scale based on ability to pay and by service-connected status.47 Nearly one half of VA patients have family incomes of less than $20,000.17 Second, we did not have information regarding Hispanic ethnicity. Third, data were abstracted from the VA EMR and administrative data; we lacked data on care received outside of the VA health care system or care not documented in the available data sources. We could not address possible reasons for the VA's lack of disparities. The VA strives to be an equal-access system, which may be one reason for the success in this area. Other reports have hypothesized that this is a potential reason for reduced levels of health care disparities in the VA.21,48,49 Generalizability of findings has been questioned in studies of VA populations; however, it is notable that our sample population is of similar age (eg, 68 years in our sample, 69 years nationally) and stage distribution to that of the US population of male patients with colon cancer. Additionally, we examined the data for specific situations, such as patients' refusal of care or physicians' documentation of clinical contraindications precluding care, that might impact our findings and mask potential racial disparities. Although these data suggest that this is not an issue, it remains possible that there may be unmeasured racial discrimination.50,51
In summary, we observed no evidence of racial disparities in receipt of quality CRC care provided by the VA health care system. As other clinical programs within the VA seek to strengthen their approach to quality management, they may evaluate the lessons that can be learned from successful CRC programs and specific changes such as electronic reminder systems, multidisciplinary collaborative improvement groups, and electronic tools for quality monitoring. Given that the VA treats 3% of newly diagnosed patients with cancer annually,1 it is plausible that the benefit of widespread quality improvement within the VA would influence the landscape of cancer care and outcomes nationally. Other health care systems could also consider aspects of VA care that could be translatable to reduce racial disparities in cancer care. Future studies with larger sample sizes are needed to examine causal pathways for the VA's equal, quality care and ways to translate the VA's success into other hospital systems.
Footnotes
Supported by the Durham Veterans Affairs (VA) Health Services Research and Development (HSR&D) Center of Excellence; development of the data set was funded by funds transfer from the Veterans Health Administration Office of Quality and Performance to the HSR&D Center of Excellence at the Durham VA Medical Center. Also supported by National Cancer Institute Grant No. 5R25CA116339 (L.L.Z.). M.W. is a VA HSR&D Senior Research Career Scientist (Grant No. RCS 91-408).
Presented in part at the American Society of Clinical Oncology Quality Care Symposium, November 30-December 1, 2012, San Diego, CA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Leah L. Zullig, Durham Veterans Affairs Medical Center (employee) Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Leah L. Zullig
Administrative support: Leah L. Zullig, William R. Carpenter
Provision of study materials or patients: Dawn Provenzale, George L. Jackson
Collection and assembly of data: Leah L. Zullig, Dawn Provenzale, George L. Jackson
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177:693–701. doi: 10.7205/milmed-d-11-00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Benarroch-Gampel J, Sheffield KM, Lin YL, et al. Colonoscopist and primary care physician supply and disparities in colorectal cancer screening. Health Serv Res. 2012;47:1137–1157. doi: 10.1111/j.1475-6773.2011.01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford ND, Jones CP, Richardson LC. Understanding racial and ethnic disparities in colorectal cancer screening: Behavioral Risk Factor Surveillance System, 2002 and 2004. Ethn Dis. 2010;20:359–365. [PubMed] [Google Scholar]
- 5.Dimou A, Syrigos KN, Saif MW. Disparities in colorectal cancer in African-Americans vs whites: Before and after diagnosis. World J Gastroenterol. 2009;15:3734–3743. doi: 10.3748/wjg.15.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Artinyan A, Mailey B, et al. An interaction of race and ethnicity with socioeconomic status in rectal cancer outcomes. Ann Surg. 2011;253:647–654. doi: 10.1097/SLA.0b013e3182111102. [DOI] [PubMed] [Google Scholar]
- 7.Obeidat NA, Pradel FG, Zuckerman IH, et al. Racial/ethnic and age disparities in chemotherapy selection for colorectal cancer. Am J Manag Care. 2010;16:515–522. [PubMed] [Google Scholar]
- 8.Rhoads KF, Cullen J, Ngo JV, et al. Racial and ethnic differences in lymph node examination after colon cancer resection do not completely explain disparities in mortality. Cancer. 2012;118:469–477. doi: 10.1002/cncr.26316. [DOI] [PubMed] [Google Scholar]
- 9.Singh GK, Williams SD, Siahpush M, et al. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: Part I—All cancers and lung cancer and Part II—Colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi: 10.1155/2011/107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White A, Vernon SW, Franzini L, et al. Racial disparities in colorectal cancer survival: To what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer. 2010;116:4622–4631. doi: 10.1002/cncr.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du XL, Lin CC, Johnson NJ, et al. Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: Findings from the National Longitudinal Mortality Study, 1979-2003. Cancer. 2011;117:3242–3251. doi: 10.1002/cncr.25854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry J, Bumpers K, Ogunlade V, et al. Examining racial disparities in colorectal cancer care. J Psychosoc Oncol. 2009;27:59–83. doi: 10.1080/07347330802614840. [DOI] [PubMed] [Google Scholar]
- 13.Berry J, Caplan L, Davis S, et al. A black-white comparison of the quality of stage-specific colon cancer treatment. Cancer. 2010;116:713–722. doi: 10.1002/cncr.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jessup JM, Stewart A, Greene FL, et al. Adjuvant chemotherapy for stage III colon cancer: Implications of race/ethnicity, age, and differentiation. JAMA. 2005;294:2703–2711. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 15.Laiyemo AO, Doubeni C, Pinsky PF, et al. Race and colorectal cancer disparities: Health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010;102:538–546. doi: 10.1093/jnci/djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landrum MB, Keating NL, Lamont EB, et al. Reasons for underuse of recommended therapies for colorectal and lung cancer in the Veterans Health Administration. Cancer. 2012;118:3345–3355. doi: 10.1002/cncr.26628. [DOI] [PubMed] [Google Scholar]
- 17.Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- 18.Rabeneck L, Souchek J, El-Serag HB. Survival of colorectal cancer patients hospitalized in the Veterans Affairs Health Care System. Am J Gastroenterol. 2003;98:1186–1192. doi: 10.1111/j.1572-0241.2003.07448.x. [DOI] [PubMed] [Google Scholar]
- 19.Robinson CN, Balentine CJ, Marshall CL, et al. Ethnic disparities are reduced in VA colon cancer patients. Am J Surg. 2010;200:636–639. doi: 10.1016/j.amjsurg.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Alexander DD, Waterbor J, Hughes T, et al. African-American and Caucasian disparities in colorectal cancer mortality and survival by data source: An epidemiologic review. Cancer Biomark. 2007;3:301–313. doi: 10.3233/cbm-2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominitz JA, Samsa GP, Landsman P, et al. Race, treatment, and survival among colorectal carcinoma patients in an equal-access medical system. Cancer. 1998;82:2312–2320. doi: 10.1002/(sici)1097-0142(19980615)82:12<2312::aid-cncr3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Kussman MJ. External Peer Review Program (EPRP) http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=1708.
- 23.Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28:3176–3181. doi: 10.1200/JCO.2009.26.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hynes DM, Perrin RA, Rappaport S, et al. Informatics resources to support health care quality improvement in the veterans health administration. J Am Med Inform Assoc. 2004;11:344–350. doi: 10.1197/jamia.M1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agency for Healthcare Research and Quality. Clinical Classifications Software (CCS) for ICD-9-CM. Rockville, MD: Agency for Healthcare Research and Quality; 2012. [Google Scholar]
- 26.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology 2003: Colon Cancer. Fort Washington, PA: National Comprehensive Cancer Network; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology 2003: Rectal Cancer. Fort Washington, PA: National Comprehensive Cancer Network; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter WR, Meyer AM, Wu Y, et al. Translating research into practice: The role of provider based research networks in the diffusion of an evidence-based colon cancer treatment innovation. Med Care. 2012;50:737–748. doi: 10.1097/MLR.0b013e31824ebe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner J. VA on the spot: Care quality, oversight to be probed by Congress. Mod Healthc. 1998;28:39. [PubMed] [Google Scholar]
- 31.Perlin JB. Transformation of the US Veterans Health Administration. Health Econ Policy Law. 2006;1:99–105. doi: 10.1017/S1744133105001222. [DOI] [PubMed] [Google Scholar]
- 32.Anderson M. Lessons learned from the Veterans Health Administration. Healthc Pap. 2005;5:30–37. doi: 10.12927/hcpap..17380. [DOI] [PubMed] [Google Scholar]
- 33.Eisen S, Francis J. Transformation of VHA health data into clinically useful information to provide quality veteran care. J Rehabil Res Dev. 2010;47:xiii–xv. doi: 10.1682/jrrd.2010.08.0150. [DOI] [PubMed] [Google Scholar]
- 34.Jackson GL, Weinberger M. A decade with the chronic care model: Some progress and opportunity for more. Med Care. 2009;47:929–931. doi: 10.1097/MLR.0b013e3181b63537. [DOI] [PubMed] [Google Scholar]
- 35.Jha AK, Perlin JB, Kizer KW, et al. Effect of the transformation of the Veterans Affairs Health Care System on the quality of care. N Engl J Med. 2003;348:2218–2227. doi: 10.1056/NEJMsa021899. [DOI] [PubMed] [Google Scholar]
- 36.Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Ann Intern Med. 2004;141:938–945. doi: 10.7326/0003-4819-141-12-200412210-00010. [DOI] [PubMed] [Google Scholar]
- 37.Keating NL, Landrum MB, Lamont EB, et al. Quality of care for older patients with cancer in the Veterans Health Administration versus the private sector: A cohort study. Ann Intern Med. 2011;154:727–736. doi: 10.7326/0003-4819-154-11-201106070-00004. [DOI] [PubMed] [Google Scholar]
- 38.Rich SE, Kuyateh FM, Dwyer DM, et al. Trends in self-reported health care provider recommendations for colorectal cancer screening by race. Prev Med. 2011;53:70–75. doi: 10.1016/j.ypmed.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White A, Vernon SW, Franzini L, et al. Racial and ethnic disparities in colorectal cancer screening persisted despite expansion of Medicare's screening reimbursement. Cancer Epidemiol Biomarkers Prev. 2011;20:811–817. doi: 10.1158/1055-9965.EPI-09-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Figueredo A, Rumble RB, Maroun J, et al. Follow-up of patients with curatively resected colorectal cancer: A practice guideline. BMC Cancer. 2003;3:26. doi: 10.1186/1471-2407-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeffery GM, Hickey BE, Hider P. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2002;1:CD002200. doi: 10.1002/14651858.CD002200. [DOI] [PubMed] [Google Scholar]
- 42.Renehan AG, Egger M, Saunders MP, et al. Impact on survival of intensive follow up after curative resection for colorectal cancer: Systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813. doi: 10.1136/bmj.324.7341.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desch CE, Benson AB, 3rd, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–8519. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 44.Davidoff AJ, Rapp T, Onukwugha E, et al. Trends in disparities in receipt of adjuvant therapy for elderly stage III colon cancer patients: The role of the medical oncologist evaluation. Med Care. 2009;47:1229–1236. doi: 10.1097/MLR.0b013e3181b58a85. [DOI] [PubMed] [Google Scholar]
- 45.Ades S. Adjuvant chemotherapy for colon cancer in the elderly: Moving from evidence to practice. Oncology (Williston Park) 2009;23:162–167. [PubMed] [Google Scholar]
- 46.Yothers G, Sargent DJ, Wolmark N, et al. Outcomes among black patients with stage II and III colon cancer receiving chemotherapy: An analysis of ACCENT adjuvant trials. J Natl Cancer Inst. 2011;103:1498–1506. doi: 10.1093/jnci/djr310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.US Department of Veterans Affairs. Ratings and Evaluations; Service Connection 3.303-1. Washington, DC: US Department of Veterans Affairs; 2011. [Google Scholar]
- 48.Page WF, Kuntz AJ. Racial and socioeconomic factors in cancer survival. A comparison of Veterans Administration results with selected studies. Cancer. 1980;45:1029–1040. doi: 10.1002/1097-0142(19800301)45:5<1029::aid-cncr2820450533>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 49.Jackson GL, Powell AA, Ordin DL, et al. Developing and sustaining quality improvement partnerships in the VA: The Colorectal Cancer Care Collaborative. J Gen Intern Med. 2010;25(suppl 1):38–43. doi: 10.1007/s11606-009-1155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kressin NR, Chang BH, Whittle J, et al. Racial differences in cardiac catheterization as a function of patients' beliefs. Am J Public Health. 2004;94:2091–2097. doi: 10.2105/ajph.94.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benjamins MR. Race/ethnic discrimination and preventive service utilization in a sample of whites, blacks, Mexicans, and Puerto Ricans. Med Care. 2012;50:870–876. doi: 10.1097/MLR.0b013e31825a8c63. [DOI] [PubMed] [Google Scholar]