Abstract
Dendritic cell (DC)-based vaccines require the cells to relocate to lymph nodes (LNs). Unfortunately, however, DC migration rates are typically very poor. We investigated strategies to increase the migration efficacy of DC-based vaccines. Surprisingly, a reduction in DC number, but not the conditioning of the injection site, improved LN targeting.
Keywords: dendritic cell, imaging, migration, immunotherapy, 19F MRI
Anticancer vaccines based on dendritic cells (DC) loaded ex vivo with tumor-associated antigens require these cells to relocate from the injection site to immunoreactive sites for triggering an effective immune response. The migration rates of DCs to lymph nodes (LNs), however, are generally low and highly dependent on the route of administration.1 The intradermal injection of DCs is the strategy most frequently employed in clinical studies, but the number of cells that reach a single LN has never reproducibly exceeded 4% of the total amount of cells injected.1 Several reasons have been suggested to account for the poor migration rate of mature DCs, including the lack of a proper inflammatory microenvironment that would promote the emigration of immune cells to afferent lymphatic vessels. In mice, the pretreatment of the skin with pro-inflammatory cytokines has been shown to provoke a 5–10-fold increase in the number of DCs that reach the draining LNs, resulting in a similar improvement in T-cell activation.2 The frequency of DC delivery, the conditions of the vascular and lymphatic networks at site of inoculation and the local availability of oxygen and nutrients have also been suggested to play an important role in this setting.3 Thus, conditioning the injection site, and perhaps indirectly draining LNs, may significantly improve the clinical efficacy of DC-based immunotherapy.
We have recently investigated DC migration upon the intradermal delivery of radioactively (111In)-labeled DCs to metastatic melanoma patients participating in an ongoing clinical study.4 Scintigraphic imaging demonstrated that the migration of DCs to LNs mainly occurs within the first 24 h after intradermal vaccination. The establishment of local inflammation by pre-treating the injection site with activated DCs, tumor necrosis factor α (TNFα) or the synthetic Toll-like receptor (TLR)7/8 agonist Imiquimod slightly enhanced the migration rate of injected DC. However, migration did not significantly increase in conditioned vs. unconditioned sites of the same patient, and the amount of cells reaching LNs did not exceed 4% of total injected cells. We have previously shown that a large part of injected DCs die at the inoculation site and are cleared by freshly recruited macrophages.4,5 However, the co-injection of granulocyte macrophage colony-stimulating factor (GM-CSF) to enhance DC survival also did not significantly improve DC migration rates. Of note, the induration of injection sites was markedly larger upon the co-injection of DCs and GM-CSF than after the administration of DCs alone, suggesting that GM-CSF stimulates the random migration of DCs into the surrounding dermis.
Patient availability and ethical considerations hamper large in vivo migration studies in humans. In vitro models overcome these issues and provide a way to optimize multiple parameters that may influence migration rates. Some drawbacks of commonly exploited cell migration in vitro assays, which are often microscopy- or microtiter plate-based, limit their translational relevance. These techniques typically work only with small numbers of cells and/or non-opaque samples. Furthermore, most of these methods assess cell migration in a 2-dimensional setting, whereas in vivo migration entails 3D motility. To test the hypothesis that local cell density would constitute the key factor limiting DC migration upon intradermal delivery, we modified an in vitro assay that closely reflects in vivo vaccination conditions to measure human DC migration in a standardized manner in tissue samples.6 Thanks to this model, we were able to quantify the CCL21-directed migration of 19F-labeled DC-based vaccines over a prolonged period using 19F magnetic resonance imaging (MRI), which allows for the direct quantification of cell numbers from imaging data.7 Of note, the 19F particles used to label DCs are not toxic and do not affect their migration.8
Using this assay, we demonstrated that increasing the cell density indeed suppresses the 3D migration of DCs toward a source of CCL21 in vitro.4 We obtained similar results in patients receiving a DC-based vaccine, a setting in which the average percentage of migratory DCs increased significantly when the number of DCs per inoculation was reduced by using multiple injection sites. When we compared the migration data that we obtained in vitro using our 19F MRI-based assay with the clinical data obtained by means of scintigraphy on 111In-labeled DCs, we found that a comparable percentage of migratory DCs in vivo and in vitro, when low number of cells were used. However, due to the sensitivity limits of clinical scintigraphy, very small numbers of migratory DCs cannot be detected with current clinical imaging techniques.
In the past decades, various parameters of DC-based vaccination have been optimized. At this point, the paradigm is shifting from small proof-of-principle studies to large, randomized, and controlled clinical trials. Accordingly, the feasibility and efficacy of cellular immunotherapy on a large scale is now the true focus of attention. Although the intradermal route of administration is generally the easiest approach, and therefore preferred in most clinical trials, limited numbers of DCs reach draining LNs in this setting. Of note, the optimal amount of DCs per LN for the induction of adequate antitumor immune responses in humans has not yet been established. Some studies report a dose-dependent relationship between the amounts of intranodal DCs and immune responses in humans, while others suggest that small numbers of antigen-loaded DCs are sufficient to elicit robust immune responses.5,9,10 Given the elevated immunostimulatory potential of DCs in vitro, this latter hypothesis appears as the most plausible. We have previously shown that the intradermal—as compared with the intranodal—administration of DCs induce superior tumor-specific immune responses in melanoma patients.10 Hypothetically, unfavorable conditions at the site of delivery may allow only the fittest DCs, exhibiting high immunostimulatory activity, to migrate to LNs and induce immune responses (Fig. 1). Thus, we suggest that multiple intradermal injections with small amounts of DCs, de facto targeting multiple lymph nodes, would increase the clinical efficacy of DC-based immunotherapy.
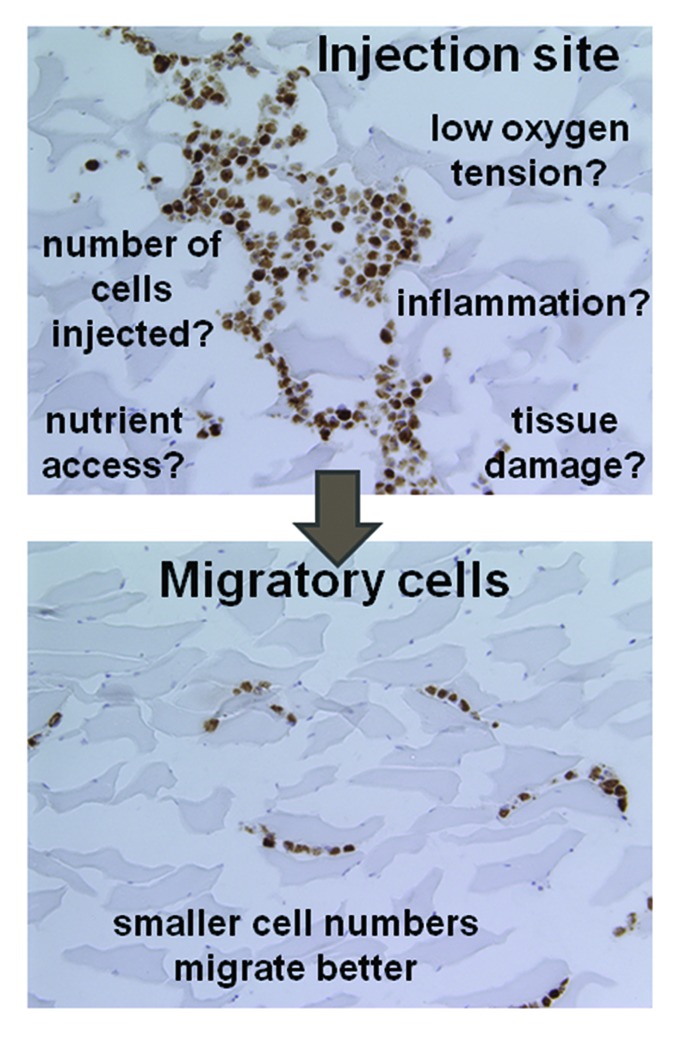
Figure 1. Factors influencing the migration of dendritic cells from the injection site to lymph nodes. Several factors can affect dendritic cell (DC) migration from the injection site to draining lymph nodes (LNs). These histological sections show a tissue injected ex vivo with DCs stained with a marker of hypoxia (brown, upper panel) and groups of migratory cells (lower panel). The inoculation site is particularly prone to problems such as those listed in the figure. Our findings indicate that the injection of low numbers of DCs can alleviate these issues and favor DC migration to LNs.
Acknowledgments
This work was supported by grants KUN 2008/035 from the Dutch Cancer Society, Netherlands Organization for Scientific Research (NWO), grants 920-03-250 and NWO-Veni 700.10.409, NWO-Vidi-917.76.363, AGIKO-92003250, The Radboud University Nijmegen Medical Centre AGIKO-2008-2-4, the ATK foundation, ENCITE, NWO Spinoza award, and a European Research Council (ERC) grant ERC-2010-AdG-269019-PATHFINDER and KWF2009-4402.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24661
References
- 1.Srinivas M, Aarntzen EH, Bulte JW, Oyen WJ, Heerschap A, de Vries IJ, et al. Imaging of cellular therapies. Adv Drug Deliv Rev. 2010;62:1080–93. doi: 10.1016/j.addr.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Fontecha A, Sebastiani S, Höpken UE, Uguccioni M, Lipp M, Lanzavecchia A, et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–21. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verdijk P, Aarntzen EH, Punt CJ, de Vries IJ, Figdor CG. Maximizing dendritic cell migration in cancer immunotherapy. Expert Opin Biol Ther. 2008;8:865–74. doi: 10.1517/14712598.8.7.865. [DOI] [PubMed] [Google Scholar]
- 4.Aarntzen EH, Srinivas M, Bonetto F, Cruz LJ, Verdijk P, Schreibelt G, et al. Targeting of 111In-labeled dendritic cell human vaccines improved by reducing number of cells. Clin Cancer Res. 2013;19:1525–33. doi: 10.1158/1078-0432.CCR-12-1879. [DOI] [PubMed] [Google Scholar]
- 5.Verdijk P, Aarntzen EH, Lesterhuis WJ, Boullart AC, Kok E, van Rossum MM, et al. Limited amounts of dendritic cells migrate into the T-cell area of lymph nodes but have high immune activating potential in melanoma patients. Clin Cancer Res. 2009;15:2531–40. doi: 10.1158/1078-0432.CCR-08-2729. [DOI] [PubMed] [Google Scholar]
- 6.Bonetto F, Srinivas M, Weigelin B, Cruz LJ, Heerschap A, Friedl P, et al. A large-scale (19)F MRI-based cell migration assay to optimize cell therapy. NMR Biomed. 2012;25:1095–103. doi: 10.1002/nbm.2774. [DOI] [PubMed] [Google Scholar]
- 7.Srinivas M, Heerschap A, Ahrens ET, Figdor CG, de Vries IJ. (19)F MRI for quantitative in vivo cell tracking. Trends Biotechnol. 2010;28:363–70. doi: 10.1016/j.tibtech.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivas M, Cruz LJ, Bonetto F, Heerschap A, Figdor CG, de Vries IJ. Customizable, multi-functional fluorocarbon nanoparticles for quantitative in vivo imaging using 19F MRI and optical imaging. Biomaterials. 2010;31:7070–7. doi: 10.1016/j.biomaterials.2010.05.069. [DOI] [PubMed] [Google Scholar]
- 9.Celli S, Day M, Müller AJ, Molina-Paris C, Lythe G, Bousso P. How many dendritic cells are required to initiate a T-cell response? Blood. 2012;120:3945–8. doi: 10.1182/blood-2012-01-408260. [DOI] [PubMed] [Google Scholar]
- 10.Lesterhuis WJ, de Vries IJ, Schreibelt G, Lambeck AJ, Aarntzen EH, Jacobs JF, et al. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res. 2011;17:5725–35. doi: 10.1158/1078-0432.CCR-11-1261. [DOI] [PubMed] [Google Scholar]


