Abstract
AIM: To investigate neural and behavioral correlates of emotional experiences as potential vulnerability markers in remitted depression.
METHODS: Fourteen remitted participants with a history of major depression and fourteen closely matched healthy control participants took part in the study. We used two psychiatric interviews (Hamilton Depression Rating Scale, Montgomery-Asberg Depression Rating Scale) and one self-report scale (Beck Depression Inventory) to assess remission. Healthy control participants were interviewed by an experienced psychiatrist to exclude those who showed any current or lifetime psychiatric or neurological disorders. To explore psychosocial and cognitive-interpersonal underpinnings of potential vulnerability markers of depression, early life stress, coping styles and alexithymia were also assessed. We induced pleasant and unpleasant emotional states using congruent combinations of music and human emotional faces to investigate neural and behavioral correlates of emotional experiences; neutral stimuli were used as a control condition. Brain responses were recorded using functional magnetic resonance imaging. Behavioral responses of pleasantness, arousal, joy and fear were measured via button-press inside the resonance imaging scanner.
RESULTS: The mean age of the sample was 54.9 (± 11.3) years. There were no differences between remitted depressed (RD) (n = 14; 9 females and 5 males) and healthy participants (n = 14; 8 females and 6 males) regarding age, current degree of depression, early life stress, coping styles and alexithymia. On a neural level, RD participants showed reduced activations in the pregenual anterior cingulate cortex (pgACC) in response to pleasant [parameter estimates: -0.78 vs 0.32; t(26) = -3.41, P < 0.05] and unpleasant [parameter estimates: -0.88 vs 0.56; t(26)= -4.02, P < 0.05] emotional stimuli. Linear regression analysis revealed that pgACC activity was modulated by early life stress [β = -0.48; R2 = 0.23, F(1,27) = 7.83, P < 0.01] and task-oriented coping style [β = 0.63; R2 = 0.37, F(1,27) = 16.91, P < 0.001]. Trait anxiety modulated hippocampal responses to unpleasant stimuli [β = 0.62; R2 = 0.38, F(1,27) = 15.95, P < 0.001]. Interestingly, in their reported experiences of pleasantness, arousal, happiness and fear in response to pleasant, unpleasant and neutral stimuli, RD participants did not differ significantly from healthy control participants. Adding trait anxiety or alexithymia as a covariate did not change the results.
CONCLUSION: The present study indicates that, in euthymic individuals, depression history alters neural correlates, but not the subjective dimension of pleasant and unpleasant emotional experiences.
Keywords: Mood disorders, Remission, Emotion, Anterior cingulate cortex, Early life stress, Music, Functional magnetic resonance imaging
Core tip: A profound disturbance of emotional experiences is one of the key symptoms of major depressive disorder. Therefore, we investigated alterations in neural correlates of emotional experiences in individuals at risk for major depression to identify potential vulnerability markers for depression. Pleasant and unpleasant emotional states were induced via music and emotional faces while recording brain responses via functional magnetic resonance imaging. Our study shows that pregenual anterior cingulate cortex reactivity in response to emotional stimuli can serve as a “neural marker” for depression vulnerability - a finding that, if replicated, could be important for innovations in early diagnosis or therapy.
INTRODUCTION
Major depressive disorder (MDD) is associated with a profound disturbance of emotional experiences. Recent studies revealed increased limbic activity in response to emotional information processing and decreased prefrontal activity in response to cognitive tasks[1,2] in patients during an acute major depressive episode. In particular, the anterior cingulate cortex (ACC) has been discussed as a key brain region[3] showing alterations of connectivity[4] and activity[5] in patients with MDD.
However, less is known about abnormalities of emotion processing present in the euthymic state, which might serve as a risk and vulnerability marker for the recurrence of affective disorders. A few studies have drawn attention to patients with remitted depression, reporting alterations in distinct domains of the human emotion system similar to those reported for acutely depressed individuals. As recently suggested, the degree to which behavioral alterations and their neuronal correlates persist after remission might depend on a number of trait markers that are related to the vulnerability to relapse[6]. Among these variables are early life stress[7], trait anxiety[8] and coping style[9]. Given the current state of knowledge, remitted depression is associated with a selective deficit in cognitive control over sad stimuli[10], higher self-reported negative affect in response to stress[11], biased processing of emotional faces[12], and reduced orbitofrontal responses to social interaction images[13]. These behavioral and neurobiological markers are partly modulated by trait variables (e.g., extraversion[13]) and are supposed to influence an individual’s vulnerability to MDD.
However, it is surprising that alterations in neural and behavioral correlates of emotional experiences have hardly been investigated as a marker for depression vulnerability so far, given the considerable impact of depression on experiencing emotions. A recent study found the inability to experience positive emotions, anhedonia, to be associated with altered ACC white matter microstructure and positive family history of depression in healthy individuals[14]. Beside the reduced capacity for pleasure as a key symptom of MDD, other facets of disturbed emotional experience such as feelings of emotional emptiness (often described as “feeling numb” or “not being able to feel anything at all”) or strong experiences of fear are frequently reported. We therefore consider neural and behavioral alterations in experiencing both pleasant and unpleasant emotions, besides their regulation[15] and processing[12], which previous studies on remitted depression already addressed, as a promising vulnerability marker.
Thus, the two main goals of the present study were to better understand emotional experiences on the behavioral and neural level in remitted depressed (RD) patients and to investigate the impact of MDD relevant psychosocial and cognitive-interpersonal variables on emotional experiences. We used functional magnetic resonance imaging (fMRI) and induced pleasant and unpleasant emotional experiences by combining music with emotionally congruent pictures of human faces. Music is related to strong emotional experiences on the behavioral and neural level[16,17], and the processing of its emotional content seems to be affected by acute depression[18]. To explain potential neural differences between RD and healthy participants, we assessed early life stress, trait anxiety and coping style and also controlled for alexithymia, a personality trait potentially influencing the nature of an emotional experience[19]. We hypothesized that participants with a history of MDD showed alterations in behavioral and brain (especially ACC) responses to emotion-inducing stimuli of both positive and negative valence. We expected to explain these differences with early life stress, coping style and trait anxiety, providing a deeper insight into vulnerability factors for depression and their neural basis.
MATERIALS AND METHODS
Participants
Twenty-eight German native volunteers with an age range between 27 and 69 years were investigated regarding depression and neural processing of human emotional faces and emotional music via fMRI. There were 14 RD participants and 14 healthy control participants matched for sex and age.
Procedure
A total of fifty volunteers potentially suitable for the RD group received an invitation letter that briefly described the aims of the study and were offered an e-mail address to register for participation without commitment. All addressees were former in-patients at the psychiatric hospital of Charité Berlin and had been treated for depression at least once within the past 5 years. After registration, written informed consent was obtained from prospective participants and they were invited for an interview by an experienced psychiatrist. A total of 14 patients with a history of depression fulfilled the criteria of remission and were qualified for MRI investigation. Control participants were recruited using a newsletter announcing studies and were interviewed by an experienced psychiatrist using the M.I.N.I.[20]. A total of 14 closely matched healthy individuals were included in the study. The study protocol was approved by the local ethics committee and carried out in accordance with the declaration of Helsinki (as revised in 2004).
On the day of fMRI assessment, participants were informed about the course of the experiment. The investigation started with five questionnaires (see Measures section) including a reassessment of depression to avoid testing participants that were no longer remitted. Then participants rated the music stimuli with respect to familiarity on a four-point scale [from 1 = “Never heard it before” to 4 = “I know this song, it is by (name of composer or interpreter)”]. Because familiarity decreases the arousal potential of an emotional stimulus[21] and memory is known to affect the emotional value of music[22], we controlled for familiarity and associated memory processes. None of our subjects was a professional musician or knew any of the experimental music stimuli. Participants then completed several test trials to get used to the experimental design outside the MR scanner. The stimuli used in the test trials were not included in the final experiment to prevent familiarity effects. All subjects were reimbursed for participation.
Measures
Assessment of depression: We used the 21-item Hamilton Depression Rating Scale (HDRS[23]), the Montgomery-Asberg Depression Rating Scale (MADRS[24]), and the Beck Depression Inventory (BDI[25]) to assess depression severity. Cutoff points were a total score of 7 for HDRS, 10 for MADRS and 12 for BDI to exclude subjects with a potentially clinically relevant depressive episode[26].
Assessment of early life stress: Early childhood adversities were assessed in retrospect using the Childhood Trauma Questionnaire (CTQ[27]) with 28 items that are assigned to the following five subscales: emotional neglect, emotional abuse, physical neglect, physical abuse and sexual abuse.
Assessment of trait anxiety: Trait anxiety was assessed using the 40-item State and Trait Anxiety Inventory (STAI[28]). The inventory consists of two separate questionnaires, one assessing the participants’ current degree of anxiety (“state”) and the other measuring habitual anxiety (“trait”).
Assessment of coping style: We used the Coping Inventory for Stressful Situations (CISS[29]), a 48-item self-report measure assessing the following three ways of coping: task-oriented, emotion-oriented and avoidance-oriented coping. The items evaluate the use of different coping behaviors in response to stressful experiences, which participants rate with respect to their own habits.
Assessment of alexithymia: Alexithymia was assessed using the elaborated 40-item Bermond-Vorst Alexithymia Questionnaire (BVAQ[30]). The scale is known to provide a comprehensive operationalization of alexithymia[31]. The BVAQ was favored over the more popular Toronto Alexithymia Scale (TAS-20[32]), which might primarily measure general psychological distress[33].
fMRI
Stimulus material: We used music and human emotional faces to induce pleasant and unpleasant emotional experiences in our participants; neutral stimuli were used as a control condition. The combination of auditory and visual emotional stimuli has been previously described as particularly effective in evoking emotional experiences in humans[34]. For visual stimulation we used pictures of facial affect from the recently developed database “FACES”[35], including naturalistic emotional faces of young, middle-aged and older women and men that correspond well to the age distribution of our sample. Previous studies report an own-age bias in face recognition[36], and face recognition, in turn, was shown to be essential for emotional contagion through others’ facial expressions[37]. The chosen faces expressed joy (pleasant condition), fear (unpleasant condition) or showed a neutral expression (control condition). For auditory stimulation we used music from different epochs and musical genres to induce pleasant (e.g., classical music, jazz, Irish dances) and unpleasant (music taken from horror movies) emotional experiences. Sequences of random tones were used as neutral control stimuli, carefully matched with each pleasant/unpleasant piece of music with regard to mean pitch, pitch variation, spectral complexity, instrumentation, and tempo (beats per minute), because previous studies report an influence of these parameters on emotional responses[38]. The analysis of these parameters was completed using “Essentia”, an in-house library for extracting audio and music features from audio files (http://mtg.upf.edu/technologies/essentia). We tested a large set of stimuli in a behavioral pre-study with 30 healthy volunteers who did not participate in the final fMRI study. The purpose was to exclude stimuli eliciting ambiguous emotional reactions and to select a homogeneous set of equally arousing stimuli with the best possible distance on the valence dimension. Behavioral data reported in the RESULTS section show that the stimuli induced the intended emotional states in our study population.
MRI design: We used a block design with the three conditions “pleasant”, “unpleasant” and “neutral”. One trial consisted of 30 s of stimulus presentation and 4 × 3 s of rating, with a 2- and a 4-s pause before and after the rating. During these pauses, participants looked at a white fixation cross on a grey screen. Following stimulus presentation in each trial, participants rated their current emotional experience (i.e., emotional state evoked by the stimulus) with respect to pleasantness, arousal, happiness and fear on six-point scales ranging from “very strong” (= 6) to “not at all” (= 1). Therefore, a response box that participants operated with their right hand was implemented inside the scanner. The total duration per trial was 48 s and the experiment comprised three functional runs with 18 trials each. Total scanning time was 43.2 min.
Scanning procedure: Subjects were scanned in a 3 T Siemens Magnetom TimTrio system. Structural image acquisition consisted of 176 T1-weighted images with a slice thickness of 1 mm. Blood oxygenation level dependent contrast was obtained with an echo-planar imaging sequence (TR = 2000 ms, TE = 30 ms, flip angle = 90°, field of view = 192 mm). Thirty-seven axial slices were acquired covering the whole brain (3 mm slice thickness). The orientation of slices was parallel to the AC-PC line. The experiment was programmed using Presentation (NeuroBehavioral Systems, Albany, CA, United States). The audio-visual stimuli were presented using goggles and noise shielded earphones by Visual Stim Digital for fMRI (Resonance Technology Inc., Northridge, CA, United States).
Statistical analysis
MRI data analysis: MRI data were analyzed using the statistical parametric mapping software package (SPM 8, Wellcome Department of Cognitive Neurology, London, United Kingdom) implemented in Matlab (version 2011a; The MathWorks Inc., Natick, Massachusetts, United States). Preprocessing of functional scans included realignment, motion correction, normalization (3 mm3) into MNI space and smoothing using an 8 mm full-width-at-half-maximum Gaussian kernel. The experimental conditions were modeled with a box-car function convolved with a hemodynamic response function in the General Linear Model of SPM. T-contrast images were calculated at the individual level regarding valence (e.g., pleasant > neutral). Two-sample t-tests were calculated to test for group differences between RD and healthy participants. Linear regression analysis was used to further explain brain activity with behavioral variables. A P value < 0.05 was considered as statistically significant, false discovery rate (FDR) control was applied to correct for multiple comparisons.
Behavioral data analysis: Behavioral data were analyzed using SPSS 19.0 (SPSS Inc., Chicago, Illinois, United States). In all analyses, a P value < 0.05 was considered as statistically significant. When indicated, Bonferroni corrections were used to counteract the problem of multiple comparisons. All behavioral variables were normally distributed in the sample (according to Kolomogorov-Smirnoff-Tests, all P > 0.05). Two-sample t-tests were calculated to test for group differences.
RESULTS
Descriptive statistics
The sample consisted of 28 healthy volunteers with a mean age of 54.9 years (± 11.3; range: 27-69). There were no differences between RD (n = 14; 9 females and 5 males) and healthy participants (n = 14; 8 females and 6 males) regarding age, current degree of depression (BDI), early life stress (CTQ), coping styles (CISS) and alexithymia (BVAQ). RD participants showed higher trait anxiety than controls [t(26) = 3.93, P < 0.001; Table 1 for means and standard deviations]. There were no sex differences regarding all tested variables in either group.
Table 1.
Group differences regarding age, depression, early life stress, and personality variables (n = 28)
| Measure |
Remitted depressed (n = 14) |
Healthy controls (n = 14) |
t | v | P value | ||
| mean | SD | mean | SD | ||||
| Age (yr) | 55.1 | 11.3 | 54.9 | 11.8 | 0.07 | 26 | 0.948 |
| BDI | 8.2 | 4.5 | 5.0 | 5.2 | 2.1 | 26 | 0.046 |
| HDRS | 3.8 | 1.5 | - | - | - | - | - |
| MADRS | 5.2 | 2.9 | - | - | - | - | - |
| No. of episodes1 | 3.9 | 1.9 | - | - | - | - | - |
| CTQ | 53.4 | 25.4 | 36.1 | 10.2 | 2.3 | 26 | 0.026 |
| total score | |||||||
| STAI state | 41.6 | 11.9 | 32.5 | 8.9 | 2.3 | 26 | 0.031 |
| STAI trait | 29.9 | 9.1 | 18.9 | 5.4 | 3.9 | 26 | < 0.001 |
| CISS task | 27.5 | 6.5 | 31.3 | 4.9 | -1.7 | 26 | 0.093 |
| CISS emotion | 22.3 | 4.2 | 22.0 | 5.3 | 0.51 | 26 | 0.612 |
| CISS avoidance | 22.2 | 5.8 | 24.9 | 8.7 | -0.95 | 26 | 0.353 |
| BVAQ total score | 112.6 | 17.3 | 100.4 | 18.8 | 1.7 | 26 | 0.093 |
Only available from 10 patients. BDI: Beck Depression Inventory; HDRS: Hamilton Depression Rating Scale (remitted depressed group only); MADRS: Montgomery-Asberg Depression Rating Scale (remitted depressed group only); CTQ: Childhood Trauma Questionnaire; STAI: State Trait Anxiety Inventory; CISS: Coping Inventory for Stressful Situation (task, emotion and avoidance oriented coping style); BVAQ: Bermond-Vorst Alexithymia Questionnaire; Bonferroni adjusted α levels of 0.006 per test (0.05/9).
Within the patient group, the correlation between the two rating measures of depression was satisfactory (MADRS - HDRS: r = 0.85, P < 0.001). Current degree of depression as measured by self-report on the day of MRI assessment was associated with depression ratings obtained by clinical interview (BDI - HDRS: r = 0.71, P < 0.01, and BDI - MADRS: r = 0.77, P < 0.01; significant after Bonferroni adjusted α levels of 0.017 per test). Ten patients were medicated at the time of study participation. All of them were treated with selective serotonin reuptake inhibitors (SSRIs), and 50% received atypical neuroleptics as add-on therapy. Three patients were additionally augmented with lithium as mood stabilizer.
Subjective ratings of emotional experience
Participants rated their emotional experience with regard to pleasantness, arousal, joy and fear on a six-point Likert scale. In the total sample (n = 28), pleasant stimuli were experienced to be more pleasant (M = 4.7 ± 0.64) and happier (M = 4.6 ± 0.73) than unpleasant (M = 2.3 ± 1.0; M = 2.1 ± 0.83) or neutral stimuli (M = 2.8 ± 0.87; M = 2.4 ± 0.79) (P < 0.001 in each test). In response to unpleasant stimuli, participants reported to feel more anxious (M = 3.1 ± 1.3) than in response to pleasant (M = 1.7 ± 0.86) or neutral stimuli (M = 2.6 ± 1.0) (P < 0.001 in each test). There were no differences between pleasant and unpleasant stimuli regarding reported experience of arousal, either in RD or in healthy participants. Furthermore, RD participants did not report different experiences of pleasantness, arousal, happiness or fear compared to healthy control participants regarding pleasant, unpleasant and neutral stimuli. Adding trait anxiety or alexithymia as a covariate did not change the results.
fMRI results
To validate the emotion-inducing character of our stimuli, we calculated a one-sample t-test (of all 28 statistical parametric maps of “pleasant > neutral”) showing stronger responses in the superior temporal gyrus, Rolandic Operculum, occipital lobe, right Insula, Heschl’s gyrus, right hippocampus and fusiform gyrus (see Table 2 for MNI coordinates and Z scores). Stronger responses to unpleasant than neutral stimuli were found in the superior temporal gyrus, Heschl’s gyrus, hippocampal formation and angular gyrus (Table 3).
Table 2.
Brain regions activated in response to pleasant > neutral stimuli (n = 28)
| Brain regions |
MNI coordinates |
Z-score | Voxels | ||
| x | y | z | |||
| Superior temporal gyrus/rolandic operculum (BA41) | -44 | -24 | 10 | 7.29 | 4250 |
| 54 | -12 | 2 | 7.58 | 3935 | |
| Occipital lobe/cuneus (BA18) | 8 | -88 | 18 | 5.86 | 1808 |
| Insula/heschl’s gyrus (BA22) | 48 | -18 | 8 | 3.78 | 446 |
| Angular gyrus (BA39) | -50 | -68 | 34 | 3.32 | 49 |
| Hippocampal formation | 29 | -14 | -14 | 2.83 | 32 |
| Fusiform gyrus (BA37) | 40 | -44 | 20 | 2.92 | 15 |
| Remitted depressed < Healthy controls | |||||
| Anterior cingulate cortex | -6 | 44 | 10 | 2.78 | 62 |
| Middle temporal gyrus (BA21) | 62 | -18 | -6 | 3.67 | 21 |
| Remitted depressed > Healthy controls | |||||
| Inferior frontal gyrus | -44 | 20 | 16 | 4.74 | 88 |
Height and extend threshold: P < 0.05, false discovery rate corrected on peak-level, and k = 10 voxels.
Table 3.
Brain regions activated in response to unpleasant > neutral stimuli (n = 28)
| Brain regions |
MNI coordinates |
Z-score | Voxels | ||
| x | y | z | |||
| Superior temporal gyrus/Heschl’s gyrus (BA22) | 50 | -14 | 4 | 7.66 | 3232 |
| -50 | -18 | 6 | 7.65 | 3060 | |
| Hippocampal formation | -24 | -16 | -16 | 4.35 | 110 |
| Angular gyrus (BA39) | -52 | -68 | 30 | 3.72 | 93 |
| Remitted depressed < healthy controls | |||||
| Anterior cingulate cortex | -3 | 43 | 11 | 3.43 | 264 |
| Superior temporal gyrus (BA21) | 62 | -18 | -4 | 3.34 | 29 |
| Remitted depressed > healthy controls | |||||
| Medial globus pallidus | 18 | -6 | 2 | 2.95 | 30 |
Height and extend threshold: P < 0.05, false discovery rate corrected on peak-level, and k = 10 voxels.
RD participants showed reduced activations in the pregenual ACC (pgACC) (Figure 1) and in middle temporal gyrus as compared to control participants [pleasant > neutral, parameter estimates: -0.78 vs 0.32; t(26) = -3.41, P < 0.05, FDR corrected]. Brain activations were higher for RD participants in the inferior frontal gyrus (pleasant > neutral, P < 0.05, FDR corrected; Table 2). Linear regression analysis revealed that 37% of variance in pgACC activity in response to pleasant > neutral stimuli was explained by task oriented coping style [β = 0.63; R2 = 0.37, F(1,27)= 16.91, P < 0.001].
Figure 1.
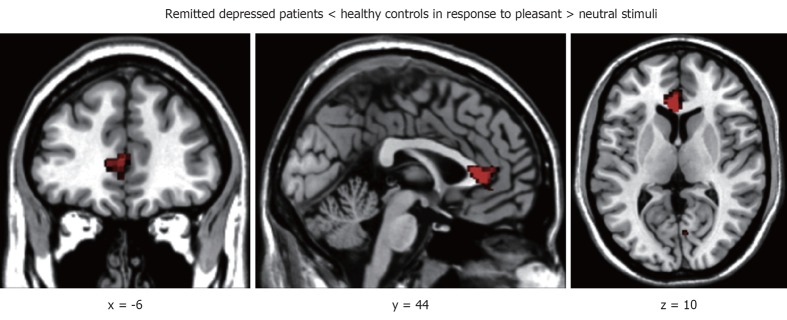
Reduced pg anterior cingulate cortex activation in response to pleasant > neutral stimuli in remitted depressed patients as compared to healthy controls. Left side corresponds to left hemisphere, MNI coordinates [-6 44 10], height and extend threshold: P < 0.05, false discovery rate corrected on peak-level, k = 62.
In response to unpleasant stimuli, RD participants showed reduced activations in the pgACC and superior temporal gyrus, but increased activations in the globus pallidus as compared to control participants [unpleasant > neutral, parameter estimates: -0.88 vs 0.56; t(26)= -4.02, P < 0.05, FDR corrected; Table 3]. Linear regression analysis revealed that 23% of variance in pgACC activity in response to unpleasant > neutral stimuli was explained by early life stress [β = -0.48; R2 = 0.23, F(1,27) = 7.83, P < 0.01]. Furthermore, 38% of variance in hippocampal activation in response to unpleasant > neutral stimuli was explained by trait anxiety [β = 0.62; R2= 0.38, F(1,27) = 15.95, P < 0.001; Figure 2].
Figure 2.
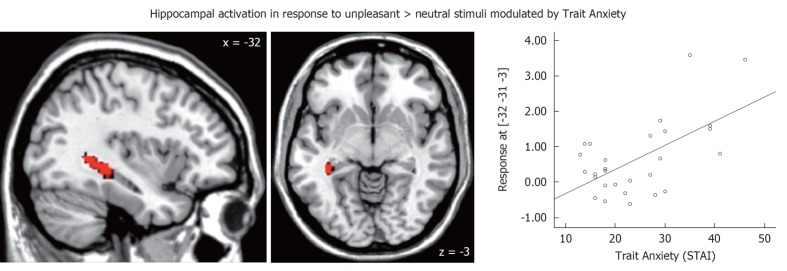
Hippocampal activation in response to unpleasant > neutral stimuli explained by trait anxiety in remitted depression [linear regression analysis; β = 0.62; R2 = 0.38, F(1,27) = 15.95, P < 0.001]. Left side corresponds to left hemisphere, MNI coordinates [-32 -31 -3], k = 46. The result remains significant (P < 0.05) after excluding the two participants with the strongest hippocampal responses from the analysis. STAI: State and Trait Anxiety Inventory.
DISCUSSION
The present study is the first to investigate neural correlates of emotional experiences induced by audio-visual stimuli in RD patients as compared to healthy participants. We hypothesized finding differences between euthymic participants with and without a history of MDD regarding behavioral and brain responses to emotion inducing stimuli. We also expected to explain these differences with variables relevant to the development of MDD. Our main finding is that RD participants showed reduced activations in the pgACC in response to both pleasant and unpleasant emotional stimuli, and that early life stress, coping style and trait anxiety moderated these effects.
The ACC comprises a variety of cognitive and emotional functions, such as attention and conflict monitoring[39] and emotion regulation[40]. It is also an important structure to integrate cognitive and affective information and to regulate and synchronize autonomic responses[17]. In particular, the pre- and subgenual ACC have been elaborately discussed in the depression literature and are target areas for chronic deep brain stimulation in treatment-resistant depression[41,42]. In acute MDD, the pgACC usually shows a decrease of activation during emotion processing[1]. Interestingly, in the present study, the pgACC was underactive in RD patients independent from stimulus valence. As Mayberg[3] suggested, pgACC hypoactivation might be a compensatory or adaptive process to maintain remission from MDD. In addition, previous studies showed that ACC activity was positively related to high emotional awareness[43]. Reduced ACC activation in response to pleasant and unpleasant stimuli might indicate lower emotional awareness and less integration of emotionally relevant information in individuals with a history of depression. Furthermore, reduced ACC activation is one of the most consistently reported results in study populations with high alexithymia, a personality trait associated with difficulties identifying and describing one’s own emotions as well as low emotional awareness[19,44]. Previous studies found a close link between alexithymia and MDD on the phenomenological level[45], however, the shared variance of depression and alexithymia is mainly due to one alexithymic facet (“difficulty in identifying feelings”[46]) and its role in the etiology of MDD is controversial[47]. Consistent with this, we did not find any differences between RD and control participants regarding alexithymia. Moreover, alexithymia did not explain any variance in our analyses performed with behavioral and fMRI data. Thus, it might be a variable relevant within depressive episodes rather than a vulnerability marker.
Examining potential variables to explain pgACC activation differences between RD and healthy participants, we found that 23% of variance in response to unpleasant stimuli can be explained by early life stress. Early life stress is a well-established risk factor for MDD, particularly in combination with moderating genetic predispositions[7]. Furthermore, it is associated with reduced ACC volume in healthy individuals, indicating that the experience of early life stress can affect brain development in the absence of psychological disorders[48]. The majority of studies examining the relationship between early life stress and depression on a neural level, however, have focused on the hippocampus, a brain region particularly vulnerable to early stress conditions[49] which also shows abnormal activity in acute MDD[1]. As a part of the limbic system, the hippocampus is involved in affective processing[5] and memory[50]. Looking at this brain structure in our sample, we found higher activity in the RD group as compared to controls in response to unpleasant stimuli. However, this result did not survive a correction for multiple comparisons. Interestingly, linear regression analysis revealed that 38% of variance in hippocampal activity in response to unpleasant stimuli can be explained by trait anxiety - a variable that has previously been implicated as a vulnerability factor associated with the development of MDD in response to stress[51]. One could hypothesize that the influence of trait anxiety on hippocampal activity shown in our data might be modulated by rumination. Rumination is a cognitive style reported to be associated with high hippocampal activity[52] and anxiety[53]. Moreover, it is a key symptom of MDD and an important mediator of the risk of relapse[54]. Although in our data there was no association between early life stress and trait anxiety, our analyses show a potential involvement of both variables in the effect of depression vulnerability on emotional experiences on the neural level. However, there might still be additional modulating factors such as rumination that need to be considered in future investigations.
In response to pleasant stimuli, 37% of pgACC variance was explained by an individual’s tendency towards task-oriented coping. This coping style is considered a proactive approach to external signals and is associated with higher coping effectiveness, less depression and high resilience[55]. Previous studies have shown that MDD is associated with low task-oriented and high emotion-oriented coping tendencies[56] and that emotion- and avoidance-oriented strategies are associated with relapse of depressive episodes[57]. Interestingly, we did not find any group differences regarding any of the three coping styles assessed. However, all RD participants reported a history of psychotherapeutic treatment (3.0 years on average) and 46% received psychotherapy at the time of MRI assessment, indicating that the variable “coping style” might have been subject to change. In addition to coping styles, reduced pgACC activity might also be related to anhedonia, a trait associated with ACC alterations regarding white matter microstructure (in individuals at risk for MDD[14]) and resting activity[58]. However, anhedonia scores (calculated using a 3-factor solution of the BDI[59]) did not explain any variance in our analyses performed with behavioral and fMRI data.
Although neural correlates of emotional experiences differed considerably between RD and healthy participants, we did not find any group differences regarding reported experiences of pleasantness, arousal, happiness or fear. Even a separate analysis of neutral control stimuli did not reveal differences in emotion ratings between the two groups. This again shows that our patient group was actually in remission, otherwise the ratings would probably have shown a negative processing bias as previously described for patients with MDD[60].
Conclusions regarding the role of the pgACC in MDD and remission must be tempered by several limitations of the present study. Although investigating remitted patients with a history of MDD is an established way to examine vulnerability factors for depression, some factors revealed by such an experiment might still have developed as a consequence of MDD instead of anteceding the first episode. Thus, they might be a residual rather than a risk factor. The strength of the present study, however, is the careful selection of patients, closely following the recommendations by the ACNP task force[26]. The modest sample size is also a result of these strict inclusion criteria, which, in our opinion, were necessary to study the unbiased influence of depression history on neural correlates of emotional experiences. Ten of the 14 RD patients were stably medicated with SSRIs for at least 1 year. Previous studies report that SSRIs increase limbic sensitivity to positive stimuli and decrease limbic responses to negative stimuli[12]. To explore potential effects of antidepressant treatment on brain activity, we compared medicated to unmedicated patients regarding the contrasts reported above. We did not find any significant voxels in these group comparisons (even at an uncorrected significance level of P < 0.005). However, due to the small sample size and the resulting restrictions for data interpretation, we cannot preclude that antidepressant medication might have modulated brain responses to emotional stimuli in our patients.
The present study indicates that, in euthymic individuals, depression history modulates neural correlates, but not the subjective dimension of an emotional experience. Our results provide evidence that the ACC plays a key role not only during depressive episodes but also in remission. In addition, we demonstrated that its activity is modulated by depression relevant trait variables, such as early life stress, anxiety and task-oriented coping.
ACKNOWLEDGMENTS
We thank the former patients of Charité Berlin for their willingness to take part in our study, Dr. Lara Rzesnitzek and Dr. Alexander Luborzewski for help with data acquisition, James Kerr for language editing, and the team of the Dahlem Institute for Neuroimaging of Emotion for their technical support.
COMMENTS
Background
Major depression is a psychiatric disorder characterized by discrete, recurrent episodes of low mood with a number of affective symptoms such as anhedonia, feelings of helplessness and worthlessness, suicidal ideation, as well as low motivation and anergia. A large number of studies have investigated its neural and behavioral correlates. However, less is known about brain abnormalities putting individuals at risk for depression. Therefore, the authors wanted to know whether an individual’s way of experiencing emotions as well as emotion-related activity in the brain could serve as a biomarker for an increased risk for depression.
Research frontiers
As estimated by the World Health Organization, major depression will become one of the main causes of premature death and disability by the year 2020. Therefore, identifying its risk factors has become a highly relevant topic in psychiatry research. As summarized in so-called “vulnerability-stress models”, a variety of biological, interpersonal and environmental factors contribute to the development of major depression. Consequently, investigating neural correlates of these risk factors is necessary to provide critical insight into the development, the course and the nature of the disorder.
Innovations and breakthroughs
Previous studies have identified a number of factors increasing an individuals’ risk for depression. Genetic predispositions play an important role, especially when adverse environmental factors such as early life stress occur. Moreover, interpersonal factors, such as neuroticism, high trait anxiety and specific thought patterns have also been identified as risk factors. There is evidence that all these variables interdependently influence an individual’s vulnerability to depression. On a neural level, recent studies have shown that a high risk for depression is associated with a selective deficit in cognitive control over stimuli inducing sadness. In addition, individuals at risk show brain abnormalities in the processing of emotional faces and images with scenes of social interaction. In this study, the authors carefully investigate the emotional-experiential domain to extend these findings. Examining neural and behavioral correlates of emotional experiences to further clarify the role of limbic and frontal brain regions in the development of major depression seemed promising, given the considerable impact of depression on experiencing emotions.
Applications
This study shows that pregenual anterior cingulate cortex (pgACC) reactivity in response to emotional stimuli can serve as a vulnerability factor or “neural marker” for depression, although individuals at risk did not report any alterations with regard to their emotional experiences. Future studies are necessary to clarify whether the authors found a real biomarker here or if these alterations in brain function occurred as a consequence of recurrent episodes of major depression. If future studies confirm that reduced pgACC reactivity is a neural marker for an increased risk or vulnerability for depression, this knowledge could be integrated in programs for early diagnosis or in the assessment of psychotherapeutic or psychiatric treatment responses.
Terminology
When an individual is considered “remitted”, he or she had one or several major depressive episodes in the past, but has now recovered completely. However, the possibility of return of the disorder remains, implying that remitted depressed individuals show an increased risk for future depressive episodes when compared to healthy, never depressed individuals.
Peer review
This is a well planned study with sound methodology.
Footnotes
Supported by German Research Foundation (Cluster of Excellence “Languages of Emotion”, EXC302 and KFO247)
P- Reviewer Trivedi JK S- Editor Song XX L- Editor A E- Editor Zheng XM
References
- 1.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 4.Carballedo A, Scheuerecker J, Meisenzahl E, Schoepf V, Bokde A, Möller HJ, Doyle M, Wiesmann M, Frodl T. Functional connectivity of emotional processing in depression. J Affect Disord. 2011;134:272–279. doi: 10.1016/j.jad.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Lee BT, Seok JH, Lee BC, Cho SW, Yoon BJ, Lee KU, Chae JH, Choi IG, Ham BJ. Neural correlates of affective processing in response to sad and angry facial stimuli in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:778–785. doi: 10.1016/j.pnpbp.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Thomas EJ, Elliott R, McKie S, Arnone D, Downey D, Juhasz G, Deakin JF, Anderson IM. Interaction between a history of depression and rumination on neural response to emotional faces. Psychol Med. 2011;41:1845–1855. doi: 10.1017/S0033291711000043. [DOI] [PubMed] [Google Scholar]
- 7.Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Min JA, Lee NB, Lee CU, Lee C, Chae JH. Low trait anxiety, high resilience, and their interaction as possible predictors for treatment response in patients with depression. J Affect Disord. 2012;137:61–69. doi: 10.1016/j.jad.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Holahan CJ, Moos RH, Holahan CK, Brennan PL, Schutte KK. Stress generation, avoidance coping, and depressive symptoms: a 10-year model. J Consult Clin Psychol. 2005;73:658–666. doi: 10.1037/0022-006X.73.4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanderhasselt MA, De Raedt R, Dillon DG, Dutra SJ, Brooks N, Pizzagalli DA. Decreased cognitive control in response to negative information in patients with remitted depression: an event-related potential study. J Psychiatry Neurosci. 2012;37:250–258. doi: 10.1503/jpn.110089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagley SL, Weaver TL, Buchanan TW. Sex differences in physiological and affective responses to stress in remitted depression. Physiol Behav. 2011;104:180–186. doi: 10.1016/j.physbeh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Anderson IM, Juhasz G, Thomas E, Downey D, McKie S, Deakin JF, Elliott R. The effect of acute citalopram on face emotion processing in remitted depression: a pharmacoMRI study. Eur Neuropsychopharmacol. 2011;21:140–148. doi: 10.1016/j.euroneuro.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Elliott R, Lythe K, Lee R, McKie S, Juhasz G, Thomas EJ, Downey D, Deakin JF, Anderson IM. Reduced medial prefrontal responses to social interaction images in remitted depression. Arch Gen Psychiatry. 2012;69:37–45. doi: 10.1001/archgenpsychiatry.2011.139. [DOI] [PubMed] [Google Scholar]
- 14.Keedwell PA, Chapman R, Christiansen K, Richardson H, Evans J, Jones DK. Cingulum white matter in young women at risk of depression: the effect of family history and anhedonia. Biol Psychiatry. 2012;72:296–302. doi: 10.1016/j.biopsych.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Kanske P, Heissler J, Schönfelder S, Wessa M. Neural correlates of emotion regulation deficits in remitted depression: the influence of regulation strategy, habitual regulation use, and emotional valence. Neuroimage. 2012;61:686–693. doi: 10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- 16.Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 1999;2:382–387. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- 17.Koelsch S. Towards a neural basis of music-evoked emotions. Trends Cogn Sci. 2010;14:131–137. doi: 10.1016/j.tics.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Naranjo C, Kornreich C, Campanella S, Noël X, Vandriette Y, Gillain B, de Longueville X, Delatte B, Verbanck P, Constant E. Major depression is associated with impaired processing of emotion in music as well as in facial and vocal stimuli. J Affect Disord. 2011;128:243–251. doi: 10.1016/j.jad.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson H, Näätänen P, Stenman H. Cortical activation in alexithymia as a response to emotional stimuli. Br J Psychiatry. 2008;192:32–38. doi: 10.1192/bjp.bp.106.034728. [DOI] [PubMed] [Google Scholar]
- 20.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–33; quiz 34-57. [PubMed] [Google Scholar]
- 21.Schellenberg EG, Peretz I, Vieillard S. Liking for happy- and sad-sounding music: Effects of exposure. Cogn Emot. 2008;22:218–237. [Google Scholar]
- 22.Jäncke L. Music, memory and emotion. J Biol. 2008;7:21. doi: 10.1186/jbiol82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 26.Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Ninan PT, Thase ME, Gelenberg AJ, Kupfer DJ, et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31:1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein DP, Fink L. Childhood Trauma Questionnaire: A retrospective self-report questionnaire and manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 28.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 29.Endler NS, Parker JD. Multidimensional assessment of coping: a critical evaluation. J Pers Soc Psychol. 1990;58:844–854. doi: 10.1037//0022-3514.58.5.844. [DOI] [PubMed] [Google Scholar]
- 30.Vorst HCM, Bermond B. Validity and reliability of the Bermond-Vorst Alexithymia Questionnaire. Pers Individ Dif. 2001;30:413–434. [Google Scholar]
- 31.Müller J, Bühner M, Ellgring H. The assessment of alexithymia: psychometric properties and validity of the Bermond-Vorst alexithymia questionnaire. Pers Individ Dif. 2004;37:373–391. [Google Scholar]
- 32.Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. J Psychosom Res. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 33.Leising D, Grande T, Faber R. The Toronto Alexithymia Scale (TAS-20): A measure of general psychological distress. J Res Pers. 2009;43:707–710. [Google Scholar]
- 34.Baumgartner T, Esslen M, Jäncke L. From emotion perception to emotion experience: emotions evoked by pictures and classical music. Int J Psychophysiol. 2006;60:34–43. doi: 10.1016/j.ijpsycho.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Ebner NC, Riediger M, Lindenberger U. FACES--a database of facial expressions in young, middle-aged, and older women and men: development and validation. Behav Res Methods. 2010;42:351–362. doi: 10.3758/BRM.42.1.351. [DOI] [PubMed] [Google Scholar]
- 36.Anastasi JS, Rhodes MG. Evidence for an own-age bias in face recognition. N Am J Psychol. 2006;8:237–253. [Google Scholar]
- 37.Hatfield E, Rapson RL, Le YL. Primitive emotional contagion: Recent research. In: Decety J, Ickes W, editors. The social neuroscience of empathy. Boston, MA: MIT Press; 2009. pp. 19–30. [Google Scholar]
- 38.Khalfa S, Roy M, Rainville P, Dalla Bella S, Peretz I. Role of tempo entrainment in psychophysiological differentiation of happy and sad music. Int J Psychophysiol. 2008;68:17–26. doi: 10.1016/j.ijpsycho.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Mohanty A, Engels AS, Herrington JD, Heller W, Ho MH, Banich MT, Webb AG, Warren SL, Miller GA. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 40.Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- 41.Bajbouj M, Heuser I. Stimulating the brain to treat depression. Exp Neurol. 2009;219:1. doi: 10.1016/j.expneurol.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 42.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci. 1998;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- 44.Heinzel A, Schäfer R, Müller HW, Schieffer A, Ingenhag A, Eickhoff SB, Northoff G, Franz M, Hautzel H. Increased activation of the supragenual anterior cingulate cortex during visual emotional processing in male subjects with high degrees of alexithymia: an event-related fMRI study. Psychother Psychosom. 2010;79:363–370. doi: 10.1159/000320121. [DOI] [PubMed] [Google Scholar]
- 45.Hintikka J, Honkalampi K, Lehtonen J, Viinamäki H. Are alexithymia and depression distinct or overlapping constructs: a study in a general population. Compr Psychiatry. 2001;42:234–239. doi: 10.1053/comp.2001.23147. [DOI] [PubMed] [Google Scholar]
- 46.Luminet O. Commentary on the paper “Is alexithymia a risk factor for major depression, personality disorder, or alcohol use disorders A prospective population-based study”. J Psychosom Res. 2010;68:275–277. doi: 10.1016/j.jpsychores.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Honkalampi K, Koivumaa-Honkanen H, Antikainen R, Haatainen K, Hintikka J, Viinamäki H. Relationships among alexithymia, adverse childhood experiences, sociodemographic variables, and actual mood disorder: a 2-year clinical follow-up study of patients with major depressive disorder. Psychosomatics. 2004;45:197–204. doi: 10.1176/appi.psy.45.3.197. [DOI] [PubMed] [Google Scholar]
- 48.Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59:975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 49.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adolphs R. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behav Cogn Neurosci Rev. 2002;1:21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- 51.Sandi C, Richter-Levin G. From high anxiety trait to depression: a neurocognitive hypothesis. Trends Neurosci. 2009;32:312–320. doi: 10.1016/j.tins.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Denson TF, Pedersen WC, Ronquillo J, Nandy AS. The angry brain: neural correlates of anger, angry rumination, and aggressive personality. J Cogn Neurosci. 2009;21:734–744. doi: 10.1162/jocn.2009.21051. [DOI] [PubMed] [Google Scholar]
- 53.Liao KY, Wei M. Intolerance of uncertainty, depression, and anxiety: the moderating and mediating roles of rumination. J Clin Psychol. 2011;67:1220–1239. doi: 10.1002/jclp.20846. [DOI] [PubMed] [Google Scholar]
- 54.Spasojević J, Alloy LB. Rumination as a common mechanism relating depressive risk factors to depression. Emotion. 2001;1:25–37. doi: 10.1037/1528-3542.1.1.25. [DOI] [PubMed] [Google Scholar]
- 55.Campbell-Sills L, Cohan SL, Stein MB. Relationship of resilience to personality, coping, and psychiatric symptoms in young adults. Behav Res Ther. 2006;44:585–599. doi: 10.1016/j.brat.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Wingenfeld K, Mensebach C, Rullkoetter N, Schlosser N, Schaffrath C, Beblo T, Driessen M. Relationship between coping with negative life-events and psychopathology: major depression and borderline personality disorder. Psychol Psychother. 2009;82:421–425. doi: 10.1348/147608309X452416. [DOI] [PubMed] [Google Scholar]
- 57.Christensen MV, Kessing LV. Clinical use of coping in affective disorder, a critical review of the literature. Clin Pract Epidemiol Ment Health. 2005;1:20. doi: 10.1186/1745-0179-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46:327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schotte CK, Maes M, Cluydts R, De Doncker D, Cosyns P. Construct validity of the Beck Depression Inventory in a depressive population. J Affect Disord. 1997;46:115–125. doi: 10.1016/s0165-0327(97)00094-3. [DOI] [PubMed] [Google Scholar]
- 60.Leppänen JM, Milders M, Bell JS, Terriere E, Hietanen JK. Depression biases the recognition of emotionally neutral faces. Psychiatry Res. 2004;128:123–133. doi: 10.1016/j.psychres.2004.05.020. [DOI] [PubMed] [Google Scholar]


