Abstract
Cardiovascular disease is the leading cause of death among patients with chronic kidney disease (CKD). Vascular calcification (VC) is one of the independent risk factors associated with cardiovascular disease and cardiovascular mortality in both the general population and CKD patients. Earlier evidence revealed substantially higher prevalence of VC in young adults on chronic hemodialysis compared to the general population in the same age range, indicating the influence of CKD-related risk factors on the development of VC. Pathogenesis of VC involves an active, highly organized cellular transformation of vascular smooth muscle cells to bone forming cells evidenced by the presence of bone matrix proteins in the calcified arterial wall. VC occurs in both the intima and the media of arterial wall with medial calcification being more prevalent in CKD. In addition to traditional cardiovascular risks, risk factors specific to CKD such as phosphate retention, excess of calcium, history of dialysis, active vitamin D therapy in high doses and deficiency of calcification inhibitors play important roles in promoting the development of VC. Non-contrast multi-slice computed tomography has often been used to detect coronary artery calcification. Simple plain radiographs of the lateral lumbar spine and pelvis can also detect VC in the abdominal aorta and femoral and iliac arteries. Currently, there is no specific therapy to reverse VC. Reduction of calcium load, lowering phosphate retention using non-calcium containing phosphate binders, and moderate doses of active vitamin D may attenuate progression. Parenteral sodium thiosulfate has also been shown to delay VC progression.
Keywords: Coronary calcification, Cardiovascular, Vascular smooth muscle cells, Osteoblast, Bone, Phosphate, Vitamin D
INTRODUCTION
Cardiovascular disease is the leading cause of death among patients with chronic kidney disease (CKD)[1]. Vascular calcification (VC) in one of the independent risk factors associated with cardiovascular disease and mortality[2-4]. Earlier evidence revealed substantially higher prevalence of VC in young adults on chronic hemodialysis compared to the general population in the same age range, indicating the influence of CKD-related risk factors on the development of VC[5]. The following review will cover the pathology, pathogenesis, clinical significance, diagnosis modalities, role of screening and available therapies of VC.
PATHOLOGY OF VASCULAR CALCIFICATION
Rudolf Ludwig Karl Virchow, the father of cellular pathology, first noted the presence of active ossification and de novo bone formation in atheroma in 1863[6]. VC occurs in 2 layers of arterial wall: tunica intima and tunica media. Tunica intima is a layer of endothelial cells supported by internal elastic lamina. Tunica media comprises a smooth muscle layer and elastic tissue. In atherosclerosis, endothelial injury results in an adhesion of blood leukocytes and in maturation of monocytes into macrophages with lipid uptake. Smooth muscle cells migrate from the media to intima and proliferate. Fatty streaks and fibrous plaques enlarge and bulge into the arterial wall in which calcification causes narrowing of the lumen[7]. Intimal or atherosclerotic calcification is more prevalent in large arteries such as aorta, and occurs more frequently in elderly, hypertensive, dyslipidemic and diabetic patients.
Medial calcification or Mönckeberg’s arteriosclerosis was first described in 1903, by the German pathologist, as a sheet-like calcification in the smooth muscle layer of arterial wall without lipid or cholesterol deposit and, therefore, without lumen narrowing[8]. However, the increase in arterial stiffness can result in poor arterial compliance. Medial calcification is particularly common in patients with CKD and frequently found in peripheral arteries, such as epigastric, femoral, and radial arteries[9-11]. Another type of calcification described almost exclusively in CKD patients is calcific uremic arteriolopathy (CUA), previously referred to as calciphylaxis. The occurrence of CUA in non-CKD patients has also been reported[12]. Calciphylaxis was originally described by Hans Seyle et al[13] in 1961. Histologically, the calcification occurs in small arteries and arterioles within the dermal layers of the skin. Abnormalities include intimal hyperplasia, inflammation, obliterative endovascular fibrosis, arteriolar medial calcification, and thrombotic cutaneous ischemia. The result is dermal, subdermal and adipose tissue necrosis with subsequent skin ulceration[14]. Female gender, hyperphosphatemia, high alkaline phosphatase, and low serum albumin are risk factors of CUA[15].
CELLULAR PATHOGENESIS OF VASCULAR CALCIFICATION
VC is a highly controlled, active cell-mediated process which involves a phenotypic change of vascular smooth muscle cells (VSMCs) into bone forming cells (Figure 1). Production of extracellular matrix proteins and release of matrix vesicles and apoptotic bodies result in matrix mineralization with hydroxyapatite crystals. Since both smooth muscle cells and osteoblasts are derived from mesenchymal stem cells, phenotypic transformation between the two types of cells under appropriate stimuli is conceivable[16]. Explanted VSMCs from calcified arteries exhibit osteoblastic properties with an ability to mineralize in vitro[17,18]. Several factors relevant to CKD have been shown promote VSMC transformation. For example, a high phosphate environment enhances the expression of osteoblastic markers: runx-2, alkaline phosphatase and osteocalcin, and stimulates mineralization of VSMCs[19]. Increasing extracellular calcium while keeping constant phosphate concentration heightens mineral deposition on VSMCs[20]. Moreover, calcitriol, advance glycation product and homocysteine have been shown to induce calcification of VSMCs in vitro[21-24]. BMP-2, a potent osteogenic differentiation factor, also plays role in the development of VC in CKD. VSMCs cultured in the presence of uremic serum show an increase in runx-2 expression, which is independent of serum phosphate[25]. Addition of Noggin, an inhibitor of BMP binding to its receptor, ameliorates the upregulation of runx-2. Measurement of BMP-2 level in the uremic serum reveals a significant elevation compared to normal serum, suggesting a role of accumulated BMP-2 in the development of VC[26]. In fact, BMP-2 mRNA is present in the section of calcified artery[17]. In addition to BMP-2, other osteoblastic genes such as runx-2, alkaline phosphatase, osteopontin, bone sialoprotein and osteocalcin have been detected in calcified arterial wall, confirming the process of active ossification[10,18].
Figure 1.
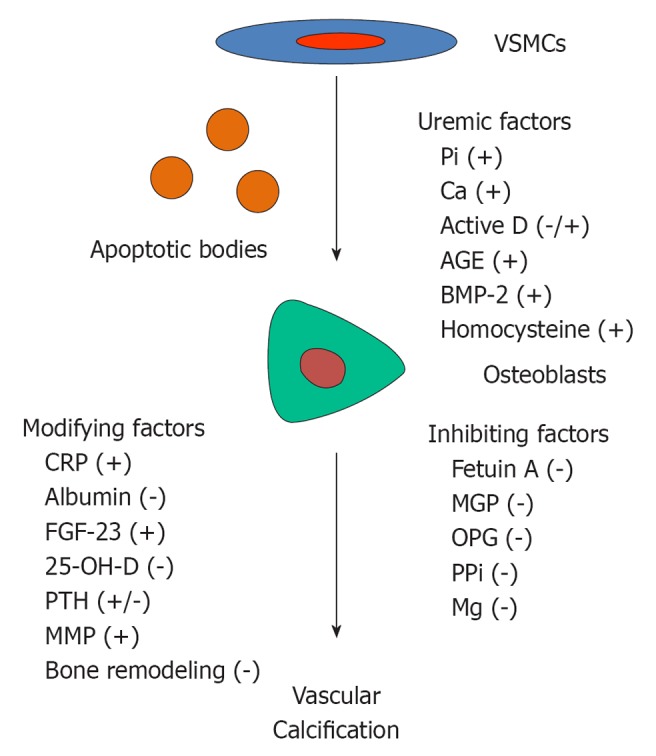
Factors associated with the development of vascular calcification in chronic kidney disease. VSMCs: Vascular smooth muscle cells; PPi: Pyrophosphate; MGP: Matrix-gla protein; OPG: Osteoprotegerin; MMP: Matrix matalloproteases.
After the phenotypic change of VSMCs toward osteoblasts and the production of bone matrix protein, mineral crystals are deposited by another organized process, biomineralization. Matrix vesicles are required to concentrate calcium and phosphate in preparation for mineralization. The release of these matrix vesicles enables nucleation of mineral crystals by the matrix proteins[27]. In VC, in addition to matrix vesicles, apoptotic bodies derived from dying VSMCs have also been detected[28,29]. Evidence of apoptosis was observed in post-confluent VSMCs cultures prior to the onset of calcification. These apoptotic bodies were able to concentrate calcium in the same fashion as matrix vesicles[28]. Vesicles released by VSMCs are calcified extensively after a prolonged exposure to calcium and phosphate[29]. From electron microscopy analysis, calcification appears to occur within and on the surface of the vesicles confirming the process of vesicle-mediated mineralization. Using synchrotron radiation analysis, many of the calcifications show a hydroxyapatite and whitlockite (magnesium-substituted crystal) crystalline structure and a mineral phase resembling that in mineralized bone[30].
Role of elastin
Elastin, a key constituent of the extracellular matrix of elastic arteries which is secreted by VSMCs, contributes to the tensile strength of blood vessels. Elastin also has calcium binding properties that may facilitate the development of arterial medial calcification[31]. Evidence suggests an association between elastin degradation and the development of VC. In a uremic mouse model of CKD, elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification[32]. Elastin degradation is mediated by matrix matalloproteases (MMP). Tissue inhibitors of MMP, in turn, regulate MMP activity in order to prevent excessive degradation of elastin[33]. In the arteries of patients on hemodialysis and peritoneal dialysis, MMP-2 is upregulated in association with medial elastic fiber fragmentation. The increase in MMP-2 activity correlates with an increase in arterial stiffness and the severity of medial calcium deposition[34]. Circulating MMP-2 is also elevated in advance stages of CKD[35]. Administration of MMP inhibitors to calcified rat aortic ring in culture results in a reduction in calcification confirming that blockade of MMP activity can inhibit arterial calcification[36].
Role of magnesium
Decreased intracellular and extracellular magnesium augments oxidative stress, promotes inflammation, impairs endothelial function, increases vasospasm and accelerates atherogenesis[37]. In hemodialysis and peritoneal dialysis patients, low serum magnesium is associated with an increased VC burden[38,39]. Magnesium supplementation may improve carotid intima-media thickness in hemodialysis patients[40]. In a small observational study, administration of magnesium carbonate as a phosphate binder to hemodialysis patients ameliorated the progression of coronary calcification (CAC) after 18 mo[41]. Higher magnesium levels also prevented VSMCs calcification in vitro through a negative regulation of osteogenic differentiation and an increased expression of anti-calcification proteins, including osteopontin, BMP-7 and matrix Gla protein[42,43].
CALCIFICATION INHIBITORS
Calcium and phosphate ions are kept soluble in the extracellular fluid by ionic strength, pH and body temperature. The concentration of calcium and phosphate in circulation exceeds the solubility product for spontaneous precipitation, suggesting the presence of endogenous mineralization inhibitors that prevent the development of calcification[44,45]. In CKD, reduced concentrations or abnormal metabolism of such inhibitors have been reported.
Fetuin A (α2-Heremans Schmid glycoprotein) is a negative acute phase reactant protein produced by the liver. Its level decreases in systemic inflammation and correlates negatively with c-reactive protein. Fetuin-A is a powerful inhibitor of hydroxyapatite formation. Fetuin- A limits matrix vesicle formation and enhances phagocytosis of matrix vesicles by VSMCs[46]. Fetuin-A-/- mice are phenotypically normal, but develop severe calcification of various organs. This phenotype was not associated with apparent changes in calcium and phosphate homeostasis, but with a decreased inhibitory activity of the fetuin-A deficient extracellular fluid on mineral formation[47]. In hemodialysis patients, a significant decrease in fetuin-A level was observed in association with an increase in IC50 for Ca × PO4 precipitation inhibition. Patients belonging to the lowest tertile of fetuin A also experienced the highest all-cause and cardiovascular mortality[48].
Matrix-gla protein (MGP) is a small molecular weight protein expressed in chondrocytes and VSMCs in the arterial media[18,49]. It is a member of the N-terminal γ-carboxylated (Gla) protein family that requires vitamin K-dependent γ-carboxylation prior to becoming biologically active. MGP-/- mice were found to develop to term, but died within 2 mo as a result of medial arterial calcification and arterial rupture. Other evidence supports the role of MGP as an inhibitor of calcification[50]. MGP mRNA is expressed abundantly in the area of calcified arterial wall[51]. While systemic administration of MGP fails to prevent VC, restoration of its expression in the arterial wall rescues arterial mineralization, suggesting that MGP acts locally to prevent calcification[52]. Warfarin, an antagonist to vitamin K, has been shown to promote calcification of elastic lamellae in the media of major arteries and in aortic heart valves in a similar fashion to what is seen in MGP-/- mice[53]. In patients on long-term warfarin therapy, aortic valve and CAC is significantly increased compared with patients without anticoagulation treatment[54]. In CKD, the circulating level of an inactive form of MGP is augmented progressively with advancing CKD stages in association with an increase in the severity of aortic calcification[55]. Daily supplementation with vitamin K2 for 6 wk lowers the level of inactive MGP. Likewise, low circulating level of carboxylated MGP (active form) is associated with a higher calcification score and increases the risk for all-cause and cardiovascular mortality[56].
Osteoprotegerin (OPG), a product of osteoblasts, acts as a decoy receptor preventing RANKL binding to its receptor on osteoclasts. OPG is essential in a bone remodeling process as it prohibits excessive osteoclast differentiation and bone resorption. OPG-/- mice develop osteoporosis and medial calcification of aorta and renal arteries suggesting the role of OPG as an inhibitor of calcification[57,58]. OPG transcript is detected in calcified arterial wall and increased OPG levels are associated with inflammation and atherosclerosis[51,59,60]. However, systemic administration of OPG protein fails to reverse arterial calcification in OPG-/- mice[59]. In CKD, OPG accumulates as a result of impaired excretion and augmented release in response to inflammation, atherosclerosis and VC[61,62]. Serum OPG correlates with the extent of aortic calcification, CAC and arterial stiffness. Increased OPG level also has the ability to predict cardiovascular and all-cause mortality in hemodialysis patients[63-67].
Inorganic pyrophosphate (PPi) is a naturally occurring inhibitor of hydroxyapatite formation by arterial smooth muscle cells. Addition of PPi can prevent calcification of rat aorta in culture[68]. PPi production is dependent on a rate limiting enzyme, nucleotide pyrophosphatasephosphodiesterase (Enpp1). In a child with Enpp1 mutation, arterial calcification was present early in life (idiopathic infantile arterial calcification) and the child died at a very young age[69]. Since PPi is hydrolyzed by alkaline phosphatase,heightened alkaline phosphatase expression found in the calcified arterial wall could, therefore, cause worsening of VC[68]. Systemic administration of sodium PPi to uremic rats with VC significantly reduces both the incidence and the amount of calcification without affecting bone formation and mineralization[70]. In CKD, the baseline calcification score and the change in calcification score at 1 year was found to decrease with increasing quartiles of plasma PPi[71]. ESRD patients with heterozygous ENPP1 mutation also have higher CAC scores and increased aortic stiffness[72].
PREVALENCE AND CLINICAL IMPACT OF VASCULAR CALCIFICATION
In 1979, Ibels et al[73] studied the pathology of arteries obtained from dialysis patients and discovered an increase in arterial calcification compared with a normal population of the same age. A study in young hemodialysis and peritoneal dialysis patients aged 20-30 years revealed that over 80% had CAC[5]. In CKD, studies reported prevalence of VC ranges from 47%-92%[74-78]. In a large cohort of CKD patients, the magnitude of CAC is independently and inversely associated with the estimated GFR[79]. VC has been shown to predict cardiovascular events and mortality in the entire spectrum of patients with CKD as well as in kidney allograft recipients[2-4]. In addition to traditional cardiovascular risk factors including aging, smoking, diabetes, dyslipidemia, inflammation, hypoalbuminemia and elevated c-reactive protein, CKD related risks such as phosphate retention, excessive calcium intake, past dialysis experience, decreased calcification inhibitors, vitamin D deficiency and increased FGF-23 are also associated with the severity and progression of VC[75,80-83]. The presence of arterial calcification increases arterial stiffness, which can be identified clinically by a functional increase in pulse wave velocity or cardiovascular ankle index[84-86]. Arterial stiffness is more closely related to calcified atheromatous plaque than to non-calcified atheroma[85,87]. The increase in arterial stiffness leads to widening of pulse pressure, left ventricular hypertrophy, impaired coronary perfusion and myocardial ischemia. Arterial stiffness is a predictor of mortality in hemodialysis and peritoneal dialysis patients[83,88].
As mentioned earlier, medial calcification is prevalent among patients with CKD. The impact of intimal and medial calcification on outcomes has been evaluated in dialysis patients. While intimal calcification is largely associated with traditional cardiovascular risks, CKD-related factors such as duration of dialysis, increasing serum calcium and hyperparathyroidism have been shown to correlate with medial calcification. Hemodialysis patients with intimal or medial calcification exhibit a decrease in all-cause and cardiovascular survival with patients with intimal calcification showing the lowest survival rate[83].
VC and osteoporosis
Relationship between the severity of VC and osteoporosis is well documented in the general population[89]. As mentioned earlier, OPG-/- animals develop osteoporosis and VC. Therefore, the OPG/RANK/RANKL system has been suggested as a common link between the bone and arteries[58,59,90]. The relationship between VC and low bone formation or adynamic bone disease has also been described in CKD[91,92]. Systemic administration of BMP-7, an inducer of bone formation, to uremic atherosclerotic animals improves bone formation and ameliorates VC[93,94]. It is believed that the uptake of calcium and phosphate by forming bone limits the availability of these minerals for the development and progression of VC. In the diabetic population, polymorphisms in BMP-7 gene are associated with the inverse relationship between bone mineralization and VC[95]. Similarly, intermittent PTH administration, an anabolic therapy for osteoporosis, has been shown to protect against VC and bone demineralization in experimental renal failure[96]. It appears that the link between bone and arteries exists but the underlying mechanism remains to be elucidated.
DIAGNOSIS AND SCREENING OF VASCULAR CALCIFICATION
There are several ways to detect VC ranging from simple plain radiographs, two-dimensional ultrasound to multi-slice computed tomography (MSCT). MSCT, a newer generation of electron beam CT, is commonly used for diagnosis and follow-up of VC progression. Its improved image quality allow efficient imaging of and precise determination of the amount of VC[97]. The machine is equipped with a computer program which can accurately quantify the area and density of calcification by means of Agatston and volume score (Figure 2A)[98,99]. Determination of the extent of CAC is particularly useful in clinical practice as the result can be used to stratify cardiovascular risk of an individual patient. However, MSCT cannot differentiate between intimal and medial calcification. Ultrasonography is widely available, inexpensive, and therefore offers a convenient alternative for evaluation of VC. Ultrasonography is useful in detection of VC in superficial arteries, such as the carotid and femoral arteries. Moreover, newer generation ultrasound machines have the ability to measure pulse wave velocity, which is an index of arterial compliance and stiffness. However, the data obtained from ultrasonography is qualitative or semiquantitative at best. Similar to MSCT, ultrasonography cannot differentiate between intimal and medial calcification[97].
Figure 2.
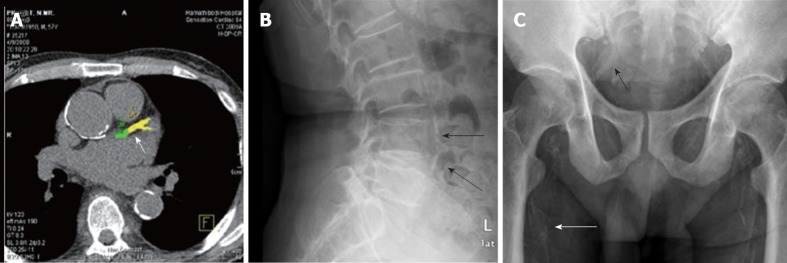
Imaging techniques for detection of vascular calcification. A: Multi-slice computed tomography demonstrating coronary artery calcification (arrow); B: Lateral abdominal radiograph displaying aortic calcification (arrows); C: Pelvic radiograph revealing iliac (black arrow) and femoral (white arrow) arterial calcification.
In the past few years plain radiographs have been increasingly used for detection and quantitation of VC. Calcification in large arteries such as the aorta can be evaluated using chest or lateral abdominal radiographs. Simple measurement methods have been devised to quantitate the amount of calcification. In hemodialysis patients, determination of aortic arch calcification in chest radiographs has been validated with that obtained by MSCT[100]. Aortic arch calcification score can also predict cardiovascular events and mortality[101,102]. Lateral abdominal radiography has been utilized in determination and quantitation of abdominal aortic calcification in the general population (Figure 2B)[103]. This same technique has been validated in hemodialysis patients, showing high correlation with CAC scores obtained by EBCT[104]. Attempts have been made to differentiate between intimal and medial calcification based on patterns of calcification appearing on plain radiograph: Discrete intimal-like plaques with irregular and patchy distribution were identified as intimal calcification and uniform linear railroad track-type plaques as medial calcification[83,105]. Since medial calcification is common among CKD patients and occurs more frequently in peripheral muscular arteries, plain radiographs of pelvis and hands have been used to evaluate calcification in iliac, femoral, radial and digital arteries (Figure 2C). In hemodialysis patients, simple VC scores obtained by these radiographs have been shown to predict fatal and non-fatal cardiovascular events[9]. Plain radiographs are subjective, semiquantitative and less sensitive than MSCT. As a result, the possibility of accurate assessment of changes of calcification burden, especially over a short period of time, may be limited.
At the time of this review, controversies exist regarding the benefit of screening for VC. Data on the epidemiological relationship between VC and poor outcomes in CKD patients is strong. Simple image screening with lateral abdominal radiograph every 1-2 years to detect patients at risk is inexpensive and may alert physicians to be more rigorous in modification of risk factors. Patients in lower risk categories will have lower morbidity and require less expenditure[106]. On the other hand, the lack of evidence that routine testing for VC helps identify CKD patients in whom subsequent modification of therapy can favorably impact clinical outcomes makes it difficult to justify the use of screening. Moreover, there is no strong evidence that modification of risk factors improves clinical outcomes[107]. The Kidney Disease Improving Global Outcome work group graded the evidence for recommendation of VC screening as 3c (weak and low quality of evidence) with lateral abdominal radiograph as a reasonable alternative to computed tomography[108,109].
TREATMENT
Currently, there is no therapy to reverse arterial calcification. Available treatment modalities can at best attenuate the progression of VC. Modification of risk factors has also been attempted.
Non-calcium containing phosphate binders
These drugs bind phosphate in the gastrointestinal tract as efficiently as calcium-containing phosphate binders but with the benefit of not increasing calcium load. In a model of atherosclerotic uremic mice, non-calcium containing phosphate binders were able to decrease calcification at both intimal and medial aortic sites[110]. Sevelamer hydrochloride, a non-absorbed cationic polymer that binds phosphate anions through ion exchange, was the first drug available in the market. Due to the side effect of metabolic acidosis, the manufacturer later substituted this formula with sevelamer carbonate. In hemodialysis patients, sevelamer hydrochloride is less likely to cause progressive CAC than calcium-based phosphate binders[111,112]. Sevelamer can also lower LDL cholesterol. In combination with its phosphate lowering effect and metabolic acidosis, sevelamer appears to have a favorable impact on VC progression[113]. Randomized controlled trials in hemodialysis patients have revealed a trend toward a better survival with sevelamer when compared to calcium-based binder, especially in patients older than 65 years of age[3,114]. However, according to the systematic review of randomized controlled trials, the beneficial effects of sevelamer on hard outcomes appears to be inconsistent and variable[115]. Lanthanum carbonate, the second drug available in the market, was introduced as an alternative to sevelamer for phosphate lowering in CKD. Lanthanum is a naturally occurring rare earth elements. In animal studies, long-term use of lanthanum can cause accumulation in various organs, such as bone, liver and brain but this appears to have no clinical significance in human[116,117]. Hemodialysis patients who received lanthanum carbonate for up to 2 years did not develop osteomalacia, adynamic bone disease, cognitive decline or abnormal liver enzymes[118,119]. In a head to head comparison, lanthanum carbonate was superior to sevelamer in lowering the amount of phosphate absorption[120]. A small randomized controlled trial in hemodialysis patients, indicated that lanthanum carbonate may reduce the progression of aortic calcification more than a calcium-based binder[121]. In a post hoc survival analysis, a survival benefit associated with lanthanum carbonate treatment for dialysis patients aged > 5 years was also suggested[122].
Active vitamin D
Active vitamin D formulations including calcitriol, alfacalcidol, doxercalciferol, and paricalcitol, are prescribed in the treatment of hyperparathyroidism in CKD. Hemodialysis and peritoneal dialysis patients who receive active vitamin D experience better survival than those who do not, regardless of PTH levels[123-125]. The earlier active vitamin D drugs, calcitriol, alfacalcidol and doxercalciferol, can effectively lower PTH but have the side effect of heightening gastrointestinal calcium and phosphate absorption resulting in an undesirable increase in calcium and phosphate load. The newer active vitamin D analog, paricalcitol, preferentially targets parathyroid gland while sparing the gastrointestinal effect[126]. Studies in uremic animals have revealed an association between supraphysiological doses of active vitamin D, especially calcitriol, and the presence of VC[127-129]. On the other hand, low, more clinically relevant doses of both calcitriol and paricalcitol may protect against VC[130]. In a 2-year follow up study in dialysis patients, progression of aortic calcification was associated with higher accumulative doses of alfacalcidol, a derivative of calcitriol[131]. In predialysis CKD patients, higher 25-OHD and 1, 25-OHD levels were associated with less VC[80,132]. The above data suggest that physiologic doses of calcitriol and its derivatives may not be detrimental, but may even protect the vascular bed and improve survival. However, randomized controlled trials are necessary to confirm this observation.
Bisphosphonates
Bisphosphonates are analogs of PPi that inhibit osteoclast function and bone resorption. Etidronate administration for 6 mo in hemodialysis patients has been found to attenuate CAC progression and aortic calcification[76,133]. In uremic animals, pamidronate and etidronate can directly inhibit VC independent of bone resorption[134]. However, in a randomized controlled trial in predialysis CKD stage 3-4, alendronate failed to decrease progression of aortic calcification more than placebo[135]. Analysis of the relationship between bisphosphonate use and the prevalence of vascular and valvular calcification in 3710 women in the MESA (Multi-Ethnic Study of Atherosclerosis) cohort revealed a decrease in prevalence of cardiovascular calcification in older subjects but an increased prevalence in the younger ones[136]. The effect of bisphosphonates on VC varies from population to population, and the data pertaining patient outcomes are still limited.
Sodium thiosulfate
Sodium thiosulfate (STS) is a chelating, reducing and antioxidant agent. STS is used as an antidote for cyanide poisoning and in prevention of cisplatin nephrotoxicity[137,138]. It has the ability of chelate calcium in precipitated minerals giving rise to calcium thiosulfate, which is several fold more soluble than calcium phosphate or calcium oxalate. As an antioxidant, STS has been shown to improve endothelial function[139]. STS has been used successfully in conditions with increased calcification burden, such as nephrolithiasis, soft tissue calcification and especially CUA[140-145]. In hemodialysis and peritoneal dialysis patients with CUA, parenteral STS is able to reduce skin necrosis and calcium deposit within 3 mo of administration. Long-term intravenous infusion of STS in hemodialysis and intraperitoneal administration in peritoneal dialysis patients are safe and well tolerated[145-147]. In an experimental model of uremic rats, STS was able to prevent VC but with the untoward effect of decreased bone strength[148]. In two preliminary studies in hemodialysis patients, STS was able to delay the progression of CAC after 4-5 mo of intravenous administration but with a decline in bone mineral density of the hip in one study[146,149]. The possible effect of STS in delaying the progression of CAC will require larger studies and determination of the safe therapeutic window is necessary in order to avoid bone demineralization.
In conclusion, VC is common among patients with CKD. Pathogenesis of VC involves an active, highly organized cellular transformation of VSMCs into bone forming cells. CKD-related factors such as phosphate retention, excess of calcium, history of dialysis, active vitamin D therapy and deficiency of calcification inhibitors play important roles in promoting the development of VC. Non-contrast CT scans as well as simple plain radiographs can also be used to detect VC. Controversies exist regarding benefits of VC screening in CKD populations. Currently, there is no specific therapy to reverse VC and available treatment modalities can at best attenuate its progression.
Footnotes
Supported by The Kidney Foundation of Thailand
Peer reviewer: Robert Gordon Fassett, PhD, Professor, Department of Renal Medicine, Level 9 Ned Hanlon Building, Royal Brisbane and Women’s Hospital, Brisbane, Queensland 4029, Australia
S- Editor Zhang DN L- Editor Hughes D E- Editor Zheng XM
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 2.Chiu YW, Adler SG, Budoff MJ, Takasu J, Ashai J, Mehrotra R. Coronary artery calcification and mortality in diabetic patients with proteinuria. Kidney Int. 2010;77:1107–1114. doi: 10.1038/ki.2010.70. [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 4.Roe P, Wolfe M, Joffe M, Rosas SE. Inflammation, coronary artery calcification and cardiovascular events in incident renal transplant recipients. Atherosclerosis. 2010;212:589–594. doi: 10.1016/j.atherosclerosis.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 6.Virchow RLK Cellular pathology as based upon physiological and pathological histology. Philadelphia: J. B. Lippincott, 1863. doi: 10.1111/j.1753-4887.1989.tb02747.x. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 8.Monckeberg JG. Uber die reine Mediaverkalkung der Exrtremittenarterien und ihr Verhalten zur Arteriosklerose. Virchows Arch Pathol Anat. 1903;171:141–167. [Google Scholar]
- 9.Adragao T, Pires A, Lucas C, Birne R, Magalhaes L, Gonçalves M, Negrao AP. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1480–1488. doi: 10.1093/ndt/gfh217. [DOI] [PubMed] [Google Scholar]
- 10.Moe SM, O'Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, Fineberg N, Kopecky K. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61:638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 11.Ballanti P, Silvestrini G, Pisanò S, De Paolis P, Di Giulio S, Mantella D, Iappelli M, Favarò A, Bonucci E, Coen G. Medial artery calcification of uremic patients: a histological, histochemical and ultrastructural study. Histol Histopathol. 2011;26:191–200. doi: 10.14670/HH-26.191. [DOI] [PubMed] [Google Scholar]
- 12.Nigwekar SU, Wolf M, Sterns RH, Hix JK. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139–1143. doi: 10.2215/CJN.00530108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selye H, Gentile G, Prioreschi P. Cutaneous molt induced by calciphylaxis in the rat. Science. 1961;134:1876–1877. doi: 10.1126/science.134.3493.1876. [DOI] [PubMed] [Google Scholar]
- 14.Sowers KM, Hayden MR. Calcific uremic arteriolopathy: pathophysiology, reactive oxygen species and therapeutic approaches. Oxid Med Cell Longev. 2010;3:109–121. doi: 10.4161/oxim.3.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazhar AR, Johnson RJ, Gillen D, Stivelman JC, Ryan MJ, Davis CL, Stehman-Breen CO. Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int. 2001;60:324–332. doi: 10.1046/j.1523-1755.2001.00803.x. [DOI] [PubMed] [Google Scholar]
- 16.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 17.Boström K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Mönckeberg's sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 19.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Curinga G, Giachelli CM. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int. 2004;66:2293–2299. doi: 10.1111/j.1523-1755.2004.66015.x. [DOI] [PubMed] [Google Scholar]
- 21.Jono S, Nishizawa Y, Shioi A, Morii H. 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation. 1998;98:1302–1306. doi: 10.1161/01.cir.98.13.1302. [DOI] [PubMed] [Google Scholar]
- 22.Yamagishi S, Fujimori H, Yonekura H, Tanaka N, Yamamoto H. Advanced glycation endproducts accelerate calcification in microvascular pericytes. Biochem Biophys Res Commun. 1999;258:353–357. doi: 10.1006/bbrc.1999.0625. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Chai S, Tang C, Du J. Homocysteine potentiates calcification of cultured rat aortic smooth muscle cells. Life Sci. 2003;74:451–461. doi: 10.1016/j.lfs.2003.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Suga T, Iso T, Shimizu T, Tanaka T, Yamagishi S, Takeuchi M, Imaizumi T, Kurabayashi M. Activation of receptor for advanced glycation end products induces osteogenic differentiation of vascular smooth muscle cells. J Atheroscler Thromb. 2011;18:670–683. doi: 10.5551/jat.7120. [DOI] [PubMed] [Google Scholar]
- 25.Moe SM, Duan D, Doehle BP, O'Neill KD, Chen NX. Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int. 2003;63:1003–1011. doi: 10.1046/j.1523-1755.2003.00820.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen NX, Duan D, O'Neill KD, Wolisi GO, Koczman JJ, Laclair R, Moe SM. The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney Int. 2006;70:1046–1053. doi: 10.1038/sj.ki.5001663. [DOI] [PubMed] [Google Scholar]
- 27.Boskey AL. Biomineralization: conflicts, challenges, and opportunities. J Cell Biochem Suppl. 1998;30-31:83–91. [PubMed] [Google Scholar]
- 28.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87:1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 30.Schlieper G, Aretz A, Verberckmoes SC, Krüger T, Behets GJ, Ghadimi R, Weirich TE, Rohrmann D, Langer S, Tordoir JH, et al. Ultrastructural analysis of vascular calcifications in uremia. J Am Soc Nephrol. 2010;21:689–696. doi: 10.1681/ASN.2009080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pai AS, Giachelli CM. Matrix remodeling in vascular calcification associated with chronic kidney disease. J Am Soc Nephrol. 2010;21:1637–1640. doi: 10.1681/ASN.2010040349. [DOI] [PubMed] [Google Scholar]
- 32.Pai A, Leaf EM, El-Abbadi M, Giachelli CM. Elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification in a uremic mouse model of chronic kidney disease. Am J Pathol. 2011;178:764–773. doi: 10.1016/j.ajpath.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung AW, Yang HH, Kim JM, Sigrist MK, Chum E, Gourlay WA, Levin A. Upregulation of matrix metalloproteinase-2 in the arterial vasculature contributes to stiffening and vasomotor dysfunction in patients with chronic kidney disease. Circulation. 2009;120:792–801. doi: 10.1161/CIRCULATIONAHA.109.862565. [DOI] [PubMed] [Google Scholar]
- 35.Peiskerová M, Kalousová M, Kratochvílová M, Dusilová-Sulková S, Uhrová J, Bandúr S, Malbohan IM, Zima T, Tesar V. Fibroblast growth factor 23 and matrix-metalloproteinases in patients with chronic kidney disease: are they associated with cardiovascular disease. Kidney Blood Press Res. 2009;32:276–283. doi: 10.1159/000243050. [DOI] [PubMed] [Google Scholar]
- 36.Chen NX, O'Neill KD, Chen X, Kiattisunthorn K, Gattone VH, Moe SM. Activation of arterial matrix metalloproteinases leads to vascular calcification in chronic kidney disease. Am J Nephrol. 2011;34:211–219. doi: 10.1159/000330175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maier JA. Low magnesium and atherosclerosis: an evidence-based link. Mol Aspects Med. 2003;24:137–146. doi: 10.1016/s0098-2997(02)00095-x. [DOI] [PubMed] [Google Scholar]
- 38.Meema HE, Oreopoulos DG, Rapoport A. Serum magnesium level and arterial calcification in end-stage renal disease. Kidney Int. 1987;32:388–394. doi: 10.1038/ki.1987.222. [DOI] [PubMed] [Google Scholar]
- 39.Ishimura E, Okuno S, Kitatani K, Tsuchida T, Yamakawa T, Shioi A, Inaba M, Nishizawa Y. Significant association between the presence of peripheral vascular calcification and lower serum magnesium in hemodialysis patients. Clin Nephrol. 2007;68:222–227. doi: 10.5414/cnp68222. [DOI] [PubMed] [Google Scholar]
- 40.Turgut F, Kanbay M, Metin MR, Uz E, Akcay A, Covic A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol. 2008;40:1075–1082. doi: 10.1007/s11255-008-9410-3. [DOI] [PubMed] [Google Scholar]
- 41.Spiegel DM, Farmer B. Long-term effects of magnesium carbonate on coronary artery calcification and bone mineral density in hemodialysis patients: a pilot study. Hemodial Int. 2009;13:453–459. doi: 10.1111/j.1542-4758.2009.00364.x. [DOI] [PubMed] [Google Scholar]
- 42.Kircelli F, Peter ME, Sevinc Ok E, Celenk FG, Yilmaz M, Steppan S, Asci G, Ok E, Passlick-Deetjen J. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol Dial Transplant. 2012;27:514–521. doi: 10.1093/ndt/gfr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montezano AC, Zimmerman D, Yusuf H, Burger D, Chignalia AZ, Wadhera V, van Leeuwen FN, Touyz RM. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. 2010;56:453–462. doi: 10.1161/HYPERTENSIONAHA.110.152058. [DOI] [PubMed] [Google Scholar]
- 44.Schlieper G, Westenfeld R, Brandenburg V, Ketteler M. Inhibitors of calcification in blood and urine. Semin Dial. 2007;20:113–121. doi: 10.1111/j.1525-139X.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- 45.Schoppet M, Shroff RC, Hofbauer LC, Shanahan CM. Exploring the biology of vascular calcification in chronic kidney disease: what's circulating. Kidney Int. 2008;73:384–390. doi: 10.1038/sj.ki.5002696. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds JL, Skepper JN, McNair R, Kasama T, Gupta K, Weissberg PL, Jahnen-Dechent W, Shanahan CM. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol. 2005;16:2920–2930. doi: 10.1681/ASN.2004100895. [DOI] [PubMed] [Google Scholar]
- 47.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Böhm R, Metzger T, Wanner C, Jahnen-Dechent W, Floege J. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361:827–833. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 49.Prince RL, Langton SR. Clinical validation of dialysable calcium in relation to other methods of serum calcium measurement. Br Med J (Clin Res Ed) 1985;290:735–739. doi: 10.1136/bmj.290.6470.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 51.Moe SM, Reslerova M, Ketteler M, O'neill K, Duan D, Koczman J, Westenfeld R, Jahnen-Dechent W, Chen NX. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD) Kidney Int. 2005;67:2295–2304. doi: 10.1111/j.1523-1755.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 52.Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–630. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–1407. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- 54.Koos R, Mahnken AH, Mühlenbruch G, Brandenburg V, Pflueger B, Wildberger JE, Kühl HP. Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am J Cardiol. 2005;96:747–749. doi: 10.1016/j.amjcard.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G, Massy ZA. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010;5:568–575. doi: 10.2215/CJN.07081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schlieper G, Westenfeld R, Krüger T, Cranenburg EC, Magdeleyns EJ, Brandenburg VM, Djuric Z, Damjanovic T, Ketteler M, Vermeer C, et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol. 2011;22:387–395. doi: 10.1681/ASN.2010040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 58.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192:463–474. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venuraju SM, Yerramasu A, Corder R, Lahiri A. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J Am Coll Cardiol. 2010;55:2049–2061. doi: 10.1016/j.jacc.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Kazama JJ, Shigematsu T, Yano K, Tsuda E, Miura M, Iwasaki Y, Kawaguchi Y, Gejyo F, Kurokawa K, Fukagawa M. Increased circulating levels of osteoclastogenesis inhibitory factor (osteoprotegerin) in patients with chronic renal failure. Am J Kidney Dis. 2002;39:525–532. doi: 10.1053/ajkd.2002.31402. [DOI] [PubMed] [Google Scholar]
- 62.Gonnelli S, Montagnani A, Caffarelli C, Cadirni A, Campagna MS, Franci MB, Lucani B, Gaggiotti E, Nuti R. Osteoprotegerin (OPG) and receptor activator of NF-kB ligand (RANK-L) serum levels in patients on chronic hemodialysis. J Endocrinol Invest. 2005;28:534–539. doi: 10.1007/BF03347242. [DOI] [PubMed] [Google Scholar]
- 63.Nitta K, Akiba T, Uchida K, Otsubo S, Takei T, Yumura W, Kabaya T, Nihei H. Serum osteoprotegerin levels and the extent of vascular calcification in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1886–1889. doi: 10.1093/ndt/gfh263. [DOI] [PubMed] [Google Scholar]
- 64.Mazzaferro S, Pasquali M, Pugliese F, Barresi G, Carbone I, Francone M, Sardella D, Taggi F. Serum levels of calcification inhibition proteins and coronary artery calcium score: comparison between transplantation and dialysis. Am J Nephrol. 2007;27:75–83. doi: 10.1159/000099095. [DOI] [PubMed] [Google Scholar]
- 65.Morena M, Terrier N, Jaussent I, Leray-Moragues H, Chalabi L, Rivory JP, Maurice F, Delcourt C, Cristol JP, Canaud B, et al. Plasma osteoprotegerin is associated with mortality in hemodialysis patients. J Am Soc Nephrol. 2006;17:262–270. doi: 10.1681/ASN.2005030260. [DOI] [PubMed] [Google Scholar]
- 66.Nishiura R, Fujimoto S, Sato Y, Yamada K, Hisanaga S, Hara S, Nakao H, Kitamura K. Elevated osteoprotegerin levels predict cardiovascular events in new hemodialysis patients. Am J Nephrol. 2009;29:257–263. doi: 10.1159/000157629. [DOI] [PubMed] [Google Scholar]
- 67.Nakashima A, Carrero JJ, Qureshi AR, Hirai T, Takasugi N, Ueno T, Taniguchi Y, Lindholm B, Yorioka N. Plasma osteoprotegerin, arterial stiffness, and mortality in normoalbuminemic Japanese hemodialysis patients. Osteoporos Int. 2011;22:1695–1701. doi: 10.1007/s00198-010-1377-0. [DOI] [PubMed] [Google Scholar]
- 68.Lomashvili KA, Cobbs S, Hennigar RA, Hardcastle KI, O'Neill WC. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 69.Rutsch F, Vaingankar S, Johnson K, Goldfine I, Maddux B, Schauerte P, Kalhoff H, Sano K, Boisvert WA, Superti-Furga A, et al. PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am J Pathol. 2001;158:543–554. doi: 10.1016/S0002-9440(10)63996-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Neill WC, Lomashvili KA, Malluche HH, Faugere MC, Riser BL. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int. 2011;79:512–517. doi: 10.1038/ki.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Neill WC, Sigrist MK, McIntyre CW. Plasma pyrophosphate and vascular calcification in chronic kidney disease. Nephrol Dial Transplant. 2010;25:187–191. doi: 10.1093/ndt/gfp362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eller P, Hochegger K, Feuchtner GM, Zitt E, Tancevski I, Ritsch A, Kronenberg F, Rosenkranz AR, Patsch JR, Mayer G. Impact of ENPP1 genotype on arterial calcification in patients with end-stage renal failure. Nephrol Dial Transplant. 2008;23:321–327. doi: 10.1093/ndt/gfm566. [DOI] [PubMed] [Google Scholar]
- 73.Ibels LS, Alfrey AC, Huffer WE, Craswell PW, Anderson JT, Weil R. Arterial calcification and pathology in uremic patients undergoing dialysis. Am J Med. 1979;66:790–796. doi: 10.1016/0002-9343(79)91118-5. [DOI] [PubMed] [Google Scholar]
- 74.Stompór T. An overview of the pathophysiology of vascular calcification in chronic kidney disease. Perit Dial Int. 2007;27 Suppl 2:S215–S222. [PubMed] [Google Scholar]
- 75.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease. J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 76.Nitta K, Akiba T, Suzuki K, Uchida K, Watanabe R, Majima K, Aoki T, Nihei H. Effects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysis. Am J Kidney Dis. 2004;44:680–688. [PubMed] [Google Scholar]
- 77.Matsuoka M, Iseki K, Tamashiro M, Fujimoto N, Higa N, Touma T, Takishita S. Impact of high coronary artery calcification score (CACS) on survival in patients on chronic hemodialysis. Clin Exp Nephrol. 2004;8:54–58. doi: 10.1007/s10157-003-0260-0. [DOI] [PubMed] [Google Scholar]
- 78.Porter CJ, Stavroulopoulos A, Roe SD, Pointon K, Cassidy MJ. Detection of coronary and peripheral artery calcification in patients with chronic kidney disease stages 3 and 4, with and without diabetes. Nephrol Dial Transplant. 2007;22:3208–3213. doi: 10.1093/ndt/gfm377. [DOI] [PubMed] [Google Scholar]
- 79.Budoff MJ, Rader DJ, Reilly MP, Mohler ER, Lash J, Yang W, Rosen L, Glenn M, Teal V, Feldman HI. Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2011;58:519–526. doi: 10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matias PJ, Ferreira C, Jorge C, Borges M, Aires I, Amaral T, Gil C, Cortez J, Ferreira A. 25-Hydroxyvitamin D3, arterial calcifications and cardiovascular risk markers in haemodialysis patients. Nephrol Dial Transplant. 2009;24:611–618. doi: 10.1093/ndt/gfn502. [DOI] [PubMed] [Google Scholar]
- 81.Nasrallah MM, El-Shehaby AR, Salem MM, Osman NA, El Sheikh E, Sharaf El Din UA. Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant. 2010;25:2679–2685. doi: 10.1093/ndt/gfq089. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4:1892–1900. doi: 10.2215/CJN.04320709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 84.Ng K, Hildreth CM, Phillips JK, Avolio AP. Aortic stiffness is associated with vascular calcification and remodeling in a chronic kidney disease rat model. Am J Physiol Renal Physiol. 2011;300:F1431–F1436. doi: 10.1152/ajprenal.00079.2011. [DOI] [PubMed] [Google Scholar]
- 85.Cecelja M, Jiang B, Bevan L, Frost ML, Spector TD, Chowienczyk PJ. Arterial stiffening relates to arterial calcification but not to noncalcified atheroma in women. A twin study. J Am Coll Cardiol. 2011;57:1480–1486. doi: 10.1016/j.jacc.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mineoka Y, Fukui M, Tanaka M, Tomiyasu K, Akabame S, Nakano K, Yamazaki M, Hasegawa G, Oda Y, Nakamura N. Relationship between cardio-ankle vascular index (CAVI) and coronary artery calcification (CAC) in patients with type 2 diabetes mellitus. Heart Vessels. 2012;27:160–165. doi: 10.1007/s00380-011-0138-0. [DOI] [PubMed] [Google Scholar]
- 87.Rattanasompattikul M, Chanchairujira K, On-Ajyooth L, Chanchairujira T. Evaluation of atherosclerosis, arterial stiffness and related risk factors in chronic hemodialysis patients in Siriraj Hospital. J Med Assoc Thai. 2011;94 Suppl 1:S117–S124. [PubMed] [Google Scholar]
- 88.Verbeke F, Van Biesen W, Honkanen E, Wikström B, Jensen PB, Krzesinski JM, Rasmussen M, Vanholder R, Rensma PL. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: outcome of the calcification outcome in renal disease (CORD) study. Clin J Am Soc Nephrol. 2011;6:153–159. doi: 10.2215/CJN.05120610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 90.D'Amelio P, Isaia G, Isaia GC. The osteoprotegerin/RANK/RANKL system: a bone key to vascular disease. J Endocrinol Invest. 2009;32:6–9. [PubMed] [Google Scholar]
- 91.Barreto DV, Barreto Fde C, Carvalho AB, Cuppari L, Draibe SA, Dalboni MA, Moyses RM, Neves KR, Jorgetti V, Miname M, et al. Association of changes in bone remodeling and coronary calcification in hemodialysis patients: a prospective study. Am J Kidney Dis. 2008;52:1139–1150. doi: 10.1053/j.ajkd.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 92.Tomiyama C, Carvalho AB, Higa A, Jorgetti V, Draibe SA, Canziani ME. Coronary calcification is associated with lower bone formation rate in CKD patients not yet in dialysis treatment. J Bone Miner Res. 2010;25:499–504. doi: 10.1359/jbmr.090735. [DOI] [PubMed] [Google Scholar]
- 93.Davies MR, Lund RJ, Hruska KA. BMP-7 is an efficacious treatment of vascular calcification in a murine model of atherosclerosis and chronic renal failure. J Am Soc Nephrol. 2003;14:1559–1567. doi: 10.1097/01.asn.0000068404.57780.dd. [DOI] [PubMed] [Google Scholar]
- 94.Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092–1105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Freedman BI, Bowden DW, Ziegler JT, Langefeld CD, Lehtinen AB, Rudock ME, Lenchik L, Hruska KA, Register TC, Carr JJ. Bone morphogenetic protein 7 (BMP7) gene polymorphisms are associated with inverse relationships between vascular calcification and BMD: the Diabetes Heart Study. J Bone Miner Res. 2009;24:1719–1727. doi: 10.1359/JBMR.090501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sebastian EM, Suva LJ, Friedman PA. Differential effects of intermittent PTH(1-34) and PTH(7-34) on bone microarchitecture and aortic calcification in experimental renal failure. Bone. 2008;43:1022–1030. doi: 10.1016/j.bone.2008.07.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bellasi A, Raggi P. Techniques and technologies to assess vascular calcification. Semin Dial. 2007;20:129–133. doi: 10.1111/j.1525-139X.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 98.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 99.Hokanson JE, MacKenzie T, Kinney G, Snell-Bergeon JK, Dabelea D, Ehrlich J, Eckel RH, Rewers M. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol. 2004;182:1327–1332. doi: 10.2214/ajr.182.5.1821327. [DOI] [PubMed] [Google Scholar]
- 100.Ogawa T, Ishida H, Matsuda N, Fujiu A, Matsuda A, Ito K, Ando Y, Nitta K. Simple evaluation of aortic arch calcification by chest radiography in hemodialysis patients. Hemodial Int. 2009;13:301–306. doi: 10.1111/j.1542-4758.2009.00366.x. [DOI] [PubMed] [Google Scholar]
- 101.Ogawa T, Ishida H, Akamatsu M, Matsuda N, Fujiu A, Ito K, Ando Y, Nitta K. Progression of aortic arch calcification and all-cause and cardiovascular mortality in chronic hemodialysis patients. Int Urol Nephrol. 2010;42:187–194. doi: 10.1007/s11255-009-9574-5. [DOI] [PubMed] [Google Scholar]
- 102.Inoue T, Ogawa T, Ishida H, Ando Y, Nitta K. Aortic arch calcification evaluated on chest X-ray is a strong independent predictor of cardiovascular events in chronic hemodialysis patients. Heart Vessels. 2012;27:135–142. doi: 10.1007/s00380-011-0129-1. [DOI] [PubMed] [Google Scholar]
- 103.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132:245–250. doi: 10.1016/s0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 104.Bellasi A, Ferramosca E, Muntner P, Ratti C, Wildman RP, Block GA, Raggi P. Correlation of simple imaging tests and coronary artery calcium measured by computed tomography in hemodialysis patients. Kidney Int. 2006;70:1623–1628. doi: 10.1038/sj.ki.5001820. [DOI] [PubMed] [Google Scholar]
- 105.Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 106.Block GA. Screening dialysis patients for vascular calcification. Semin Dial. 2010;23:271–276. doi: 10.1111/j.1525-139X.2010.00727.x. [DOI] [PubMed] [Google Scholar]
- 107.Uhlig K. There is no practical utility in routinely screening dialysis patients for vascular calcification. Semin Dial. 2010;23:277–279. doi: 10.1111/j.1525-139X.2010.00732.x. [DOI] [PubMed] [Google Scholar]
- 108.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 109.Goldsmith D. The case against routine screening for vascular calcification in chronic kidney disease. Semin Dial. 2010;23:280–282. doi: 10.1111/j.1525-139X.2010.00729.x. [DOI] [PubMed] [Google Scholar]
- 110.Nikolov IG, Joki N, Nguyen-Khoa T, Guerrera IC, Maizel J, Benchitrit J, Machado dos Reis L, Edelman A, Lacour B, Jorgetti V, et al. Lanthanum carbonate, like sevelamer-HCl, retards the progression of vascular calcification and atherosclerosis in uremic apolipoprotein E-deficient mice. Nephrol Dial Transplant. 2012;27:505–513. doi: 10.1093/ndt/gfr254. [DOI] [PubMed] [Google Scholar]
- 111.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 112.Kakuta T, Tanaka R, Hyodo T, Suzuki H, Kanai G, Nagaoka M, Takahashi H, Hirawa N, Oogushi Y, Miyata T, et al. Effect of sevelamer and calcium-based phosphate binders on coronary artery calcification and accumulation of circulating advanced glycation end products in hemodialysis patients. Am J Kidney Dis. 2011;57:422–431. doi: 10.1053/j.ajkd.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 113.Qunibi W, Moustafa M, Muenz LR, He DY, Kessler PD, Diaz-Buxo JA, Budoff M. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51:952–965. doi: 10.1053/j.ajkd.2008.02.298. [DOI] [PubMed] [Google Scholar]
- 114.Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, Ling BN, Chasan-Taber S, Dillon MA, Blair AT, et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 2007;72:1130–1137. doi: 10.1038/sj.ki.5002466. [DOI] [PubMed] [Google Scholar]
- 115.McCullough PA, Chinnaiyan KM. Annual progression of coronary calcification in trials of preventive therapies: a systematic review. Arch Intern Med. 2009;169:2064–2070. doi: 10.1001/archinternmed.2009.382. [DOI] [PubMed] [Google Scholar]
- 116.Behets GJ, Verberckmoes SC, Oste L, Bervoets AR, Salomé M, Cox AG, Denton J, De Broe ME, D'Haese PC. Localization of lanthanum in bone of chronic renal failure rats after oral dosing with lanthanum carbonate. Kidney Int. 2005;67:1830–1836. doi: 10.1111/j.1523-1755.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 117.Lacour B, Lucas A, Auchère D, Ruellan N, de Serre Patey NM, Drüeke TB. Chronic renal failure is associated with increased tissue deposition of lanthanum after 28-day oral administration. Kidney Int. 2005;67:1062–1069. doi: 10.1111/j.1523-1755.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 118.Altmann P, Barnett ME, Finn WF. Cognitive function in Stage 5 chronic kidney disease patients on hemodialysis: no adverse effects of lanthanum carbonate compared with standard phosphate-binder therapy. Kidney Int. 2007;71:252–259. doi: 10.1038/sj.ki.5001932. [DOI] [PubMed] [Google Scholar]
- 119.D'Haese PC, Spasovski GB, Sikole A, Hutchison A, Freemont TJ, Sulkova S, Swanepoel C, Pejanovic S, Djukanovic L, Balducci A, et al. A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int Suppl. 2003:S73–S78. doi: 10.1046/j.1523-1755.63.s85.18.x. [DOI] [PubMed] [Google Scholar]
- 120.Martin P, Wang P, Robinson A, Poole L, Dragone J, Smyth M, Pratt R. Comparison of dietary phosphate absorption after single doses of lanthanum carbonate and sevelamer carbonate in healthy volunteers: a balance study. Am J Kidney Dis. 2011;57:700–706. doi: 10.1053/j.ajkd.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 121.Toussaint ND, Lau KK, Polkinghorne KR, Kerr PG. Attenuation of aortic calcification with lanthanum carbonate versus calcium-based phosphate binders in haemodialysis: A pilot randomized controlled trial. Nephrology (Carlton) 2011;16:290–298. doi: 10.1111/j.1440-1797.2010.01412.x. [DOI] [PubMed] [Google Scholar]
- 122.Wilson R, Zhang P, Smyth M, Pratt R. Assessment of survival in a 2-year comparative study of lanthanum carbonate versus standard therapy. Curr Med Res Opin. 2009;25:3021–3028. doi: 10.1185/03007990903399398. [DOI] [PubMed] [Google Scholar]
- 123.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 124.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernán MA, Camargo CA, Thadhani R. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 125.Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, Johnson HK, Zager PG. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 126.Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003;63:1483–1490. doi: 10.1046/j.1523-1755.2003.00878.x. [DOI] [PubMed] [Google Scholar]
- 127.Cardús A, Panizo S, Parisi E, Fernandez E, Valdivielso JM. Differential effects of vitamin D analogs on vascular calcification. J Bone Miner Res. 2007;22:860–866. doi: 10.1359/jbmr.070305. [DOI] [PubMed] [Google Scholar]
- 128.Mizobuchi M, Finch JL, Martin DR, Slatopolsky E. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int. 2007;72:709–715. doi: 10.1038/sj.ki.5002406. [DOI] [PubMed] [Google Scholar]
- 129.Becker LE, Koleganova N, Piecha G, Noronha IL, Zeier M, Geldyyev A, Kökeny G, Ritz E, Gross ML. Effect of paricalcitol and calcitriol on aortic wall remodeling in uninephrectomized ApoE knockout mice. Am J Physiol Renal Physiol. 2011;300:F772–F782. doi: 10.1152/ajprenal.00042.2010. [DOI] [PubMed] [Google Scholar]
- 130.Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol. 2008;19:1509–1519. doi: 10.1681/ASN.2007080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ogawa T, Ishida H, Akamatsu M, Matsuda N, Fujiu A, Ito K, Ando Y, Nitta K. Relation of oral 1alpha-hydroxy vitamin D3 to the progression of aortic arch calcification in hemodialysis patients. Heart Vessels. 2010;25:1–6. doi: 10.1007/s00380-009-1151-4. [DOI] [PubMed] [Google Scholar]
- 132.Petchey WG, Hawley CM, Johnson DW, Haluska BA, Watkins TW, Isbel NM. Multimodality vascular imaging in CKD: divergence of risk between measured parameters. Nephrol Dial Transplant. 2012;27:1004–1012. doi: 10.1093/ndt/gfr397. [DOI] [PubMed] [Google Scholar]
- 133.Hashiba H, Aizawa S, Tamura K, Shigematsu T, Kogo H. Inhibitory effects of etidronate on the progression of vascular calcification in hemodialysis patients. Ther Apher Dial. 2004;8:241–247. doi: 10.1111/j.1526-0968.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 134.Lomashvili KA, Monier-Faugere MC, Wang X, Malluche HH, O'Neill WC. Effect of bisphosphonates on vascular calcification and bone metabolism in experimental renal failure. Kidney Int. 2009;75:617–625. doi: 10.1038/ki.2008.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Effect of alendronate on vascular calcification in CKD stages 3 and 4: a pilot randomized controlled trial. Am J Kidney Dis. 2010;56:57–68. doi: 10.1053/j.ajkd.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 136.Elmariah S, Delaney JA, O'Brien KD, Budoff MJ, Vogel-Claussen J, Fuster V, Kronmal RA, Halperin JL. Bisphosphonate Use and Prevalence of Valvular and Vascular Calcification in Women MESA (The Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56:1752–1759. doi: 10.1016/j.jacc.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baskin SI, Horowitz AM, Nealley EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol. 1992;32:368–375. doi: 10.1002/j.1552-4604.1992.tb03849.x. [DOI] [PubMed] [Google Scholar]
- 138.Pfeifle CE, Howell SB, Felthouse RD, Woliver TB, Andrews PA, Markman M, Murphy MP. High-dose cisplatin with sodium thiosulfate protection. J Clin Oncol. 1985;3:237–244. doi: 10.1200/JCO.1985.3.2.237. [DOI] [PubMed] [Google Scholar]
- 139.Hayden MR, Tyagi SC, Kolb L, Sowers JR, Khanna R. Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: the emerging role of sodium thiosulfate. Cardiovasc Diabetol. 2005;4:4. doi: 10.1186/1475-2840-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Araya CE, Fennell RS, Neiberger RE, Dharnidharka VR. Sodium thiosulfate treatment for calcific uremic arteriolopathy in children and young adults. Clin J Am Soc Nephrol. 2006;1:1161–1166. doi: 10.2215/CJN.01520506. [DOI] [PubMed] [Google Scholar]
- 141.Asplin JR, Donahue SE, Lindeman C, Michalenka A, Strutz KL, Bushinsky DA. Thiosulfate reduces calcium phosphate nephrolithiasis. J Am Soc Nephrol. 2009;20:1246–1253. doi: 10.1681/ASN.2008070754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cicone JS, Petronis JB, Embert CD, Spector DA. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104–1108. doi: 10.1053/j.ajkd.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 143.Kyriakopoulos G, Kontogianni K. Sodium thiosulfate treatment of tumoral calcinosis in patients with end-stage renal disease. Ren Fail. 1990;12:213–219. doi: 10.3109/08860229009060727. [DOI] [PubMed] [Google Scholar]
- 144.Tokashiki K, Ishida A, Kouchi M, Ishihara S, Tomiyama N, Kohagura K, Iseki K, Takishita S. Successful management of critical limb ischemia with intravenous sodium thiosulfate in a chronic hemodialysis patient. Clin Nephrol. 2006;66:140–143. doi: 10.5414/cnp66140. [DOI] [PubMed] [Google Scholar]
- 145.Mataic D, Bastani B. Intraperitoneal sodium thiosulfate for the treatment of calciphylaxis. Ren Fail. 2006;28:361–363. doi: 10.1080/08860220600583781. [DOI] [PubMed] [Google Scholar]
- 146.Adirekkiat S, Sumethkul V, Ingsathit A, Domrongkitchaiporn S, Phakdeekitcharoen B, Kantachuvesiri S, Kitiyakara C, Klyprayong P, Disthabanchong S. Sodium thiosulfate delays the progression of coronary artery calcification in haemodialysis patients. Nephrol Dial Transplant. 2010;25:1923–1929. doi: 10.1093/ndt/gfp755. [DOI] [PubMed] [Google Scholar]
- 147.Brucculeri M, Cheigh J, Bauer G, Serur D. Long-term intravenous sodium thiosulfate in the treatment of a patient with calciphylaxis. Semin Dial. 2005;18:431–434. doi: 10.1111/j.1525-139X.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 148.Pasch A, Schaffner T, Huynh-Do U, Frey BM, Frey FJ, Farese S. Sodium thiosulfate prevents vascular calcifications in uremic rats. Kidney Int. 2008;74:1444–1453. doi: 10.1038/ki.2008.455. [DOI] [PubMed] [Google Scholar]
- 149.Mathews SJ, de Las Fuentes L, Podaralla P, Cabellon A, Zheng S, Bierhals A, Spence K, Slatopolsky E, Davila-Roman VG, Delmez JA. Effects of sodium thiosulfate on vascular calcification in end-stage renal disease: a pilot study of feasibility, safety and efficacy. Am J Nephrol. 2011;33:131–138. doi: 10.1159/000323550. [DOI] [PMC free article] [PubMed] [Google Scholar]


