Abstract
Tissues are equipped with reasonable strategies for repair and regeneration and the renal proximal tubule (PT) is no exception. New information has become available on the mode of PT regeneration in mammals. Unlike the intestinal epithelium with a high rate of turnover maintained by the stem cell system, the kidney has low turnover under normal physiological conditions. The PT seems to be maintained physiologically by hyperplasia, a regenerating system with self-renewal of mature tubular cells. This mode of regeneration is advantageous for effective replenishment of randomly isolated and eliminated tubular cells by self-renewal of adjacent cells. On the other hand, it has been suggested that dedifferentiation of mature tubular cells plays a role in regeneration after acute kidney injury. Recent studies employing genetic labeling and DNA-labeling techniques have confirmed that the proliferation of preexisting injured mature tubular cells contributes mainly to PT regeneration in ischemic reperfusion injury. This mode of regeneration is beneficial with regard to the rapid reparation of focally injured tubules often induced by ischemic reperfusion injury. What happens, however, when the PT is homogeneously injured with almost no remaining surviving cells Is the PT equipped with another backup regeneration system, e.g., the stem cell system Is it possible that certain types of renal injuries evoke a stem cell response whereas others do not This review focuses on all three possible modes of tissue regeneration (compensatory hyperplasia, dedifferentiation and stem cell system) in mammals and their involvement in PT regeneration in health and disease.
Keywords: Proximal tubule, Regeneration, Compensatory hyperplasia, Dedifferentiation, Stem cell, Progenitor cell
MECHANISMS OF RENAL TUBULE REGENERATION
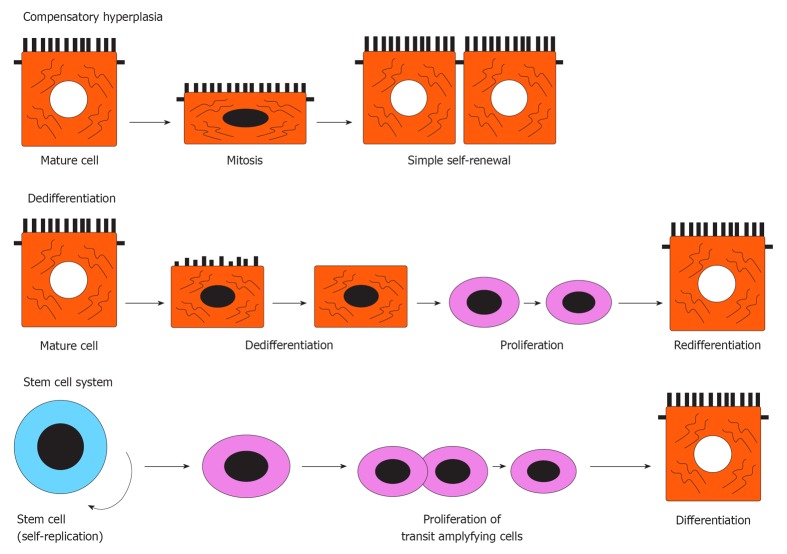
There are three mechanisms of tissue regeneration in vertebrates[1], as illustrated in Figure 1: (1) compensatory hyperplasia, where mitosis of cells occurs during the differentiation state (e.g., liver, pancreas[2,3]); (2) dedifferentiation of mature cells where stem-like cells are raised by the dedifferentiation of differentiated cells (e.g., myofibers, lens[4-6]); and (3) activation of undifferentiated adult stem cells sequestered during tissue development, where the stem cell divides to produce one daughter cell committed to specific lineage differentiation, while another daughter cell is renewed as a stem cell (e.g., skin epidermis, hair follicles, epithelium of the digestive tract[7-9]). In general, epithelial tissues equipped with a stem cell system may use such a system to maintain cell turnover under both physiological and pathological conditions.
Figure 1.
Three mechanisms of tissue regeneration in vertebrates.
Like other organs, the kidney is also known to regenerate completely in lower vertebrates, such as teleost fish, the skate, elasmobranch fish and zebrafish, during which the entire nephron regenerates following injury or partial removal of the kidney[10-13]. The source of the new nephrons is a population of stem cells that exist in the special nephrogenic zone[14]. On the other hand, the regenerative capacity of the mammalian kidney is limited compared to that of lower vertebrates. However, it is well known that even in mammalian kidney, renal tubules have regenerative capacity, especially after acute kidney injury such as acute tubular necrosis[15], through yet unknown regeneration mechanisms.
Recent interest in stem cell-based therapy led many investigators to study the source of regenerating tubular cells and the role of stem cells after acute kidney injury in mammalian kidneys[16-33]. However, accumulating evidence indicated that the main source of regenerating cells is resident kidney cells, not bone marrow-derived cells (e.g., hematopoietic stem cells, mesenchymal stromal cells and endothelial progenitor cells)[34,35]. Moreover, resident kidney cells, rather than bone marrow-derived cells, were the main contributors to the tubular repair in the ischemic reperfusion injury model[34,35]. However, recent reports have demonstrated that the bone marrow-derived mesenchymal stromal cells play renoprotective roles in tubular repair and/or recovery by producing various humoral factors not at a cell basis[36-41]. More recent studies of rats with ischemic reperfusion injury indicated that the source of tubular regenerating cells that contribute to the repair of renal tubules is limited to resident (pre-existing) tubular cells[42] and that the intratubular stem cell system is not involved in tubular regeneration[43]. However, it is too early to conclude that there is no intratubular stem cell system in mammalian kidneys.
In this review, we discuss the modes of regeneration of proximal tubules (PTs) and their implication in health and disease.
REGENERATION OF PT CELL UNDER PHYSIOLOGICAL CONDITIONS
The physiological processes of renal tubular cell turnover play an important role in the maintenance of normal tissue function and architecture, which is achieved by a dynamic balance between the rate of cell elimination and the rate of cell proliferation. In 1959, McCreight and Sulkin counted the number of mitotic figures and calculated the proliferation index of PT cells to be 0.1% in the normal rat kidney, and hence concluded that the kidney has a low cell turnover under physiological conditions[44]. In another study, the estimated proliferation indexes of PT cells stained for S-phase markers in paraffin sections (percentages of cells positive for the proliferation cell nuclear antigen and Ki67 antibodies) were 0.22 and 0.24, respectively[45]. In a study from our laboratory[46], about 40% of S3 segment of PT cells were labeled by the S-phase marker, bromodeoxyuridine (BrdU), when adult normal rats were treated with BrdU by osmotic mini-pump for 2 wk. Since the number of eliminated cells should be substituted by the same number of newly regenerating tubular cells to maintain normal tissue function and architecture, at most about 20% of tubular cells in the S3 segment should divide into two cells during a 2-wk period if they divided only once during the period. This suggests that the proliferation index 1 h after BrdU administration may be about 0.06% in the S3 segment in adult rats. Considered together, the above studies indicate that PT cell proliferation or turnover is slow in adults.
Recently, Vogetseder et al[47,48] concluded that the S3 segment of PT is maintained by a physiological regenerating system with self-renewal of mature tubular cells. Their conclusion was based on the finding of numerous cells with proliferative potency (retaining positivity for the proliferation marker, BrdU). Both cycling and non-cycling cells remained morphologically and phenotypically fully differentiated PT cells and cycling cells did not show the characteristics of transit amplifying cells[47,48], which can expand the number of cells by rapid cycling after division from stem cell[49]. This does not support the notion that stem cell system ensures turnover of tubular cells under physiological conditions.
In our study, we also found that BrdU+ proliferating PT cells in the S3 segment of normal rat nephron exhibited a mature PT phenotype, such as staining for megalin, aquaporin 1 and Na+K+-ATPase, and also maintained a mature PT ultrastructure[50]. However, these cells did not express vimentin, a marker of mesenchyme or dedifferentiated PT cells[51]. These findings suggest that normal PT cells can undergo cell division without dedifferentiation.
In the liver, cell marking studies indicated that during normal liver turnover and after partial hepatectomy, hepatocytes are replaced by compensatory hyperplasia of existing hepatocytes[52]. Interestingly, mature hepatocytes can replicate during normal liver growth, but the newly formed cells do not migrate[53]. Since cells generated by simple self-renewal through compensatory hyperplasia cannot migrate, it is unlikely that these newly regenerating cells can repair largely damaged areas in a mode of simple self-renewal. This may also be the case in renal PT cells. Thus, compensatory hyperplasia provides effective replenishment of randomly eliminated tubular cells by self-renewal of adjacent cells under physiological conditions. However, other modes of regeneration are required under pathological conditions.
REPAIR AFTER ACUTE TUBULAR INJURY
The mammalian kidney is classically regarded as an organ that cannot truly regenerate. In the past, it was thought that acutely injured tubular cells slough off the tubular basement membrane and that the surviving tubular cells undergo migration, dedifferentiation, proliferation and redifferentiation to reline the injured tubules[54,55]. Vogetseder et al[48] reported that in rats treated with potent proliferative agents (lead acetate injection[56]), PT proliferation did not require stem cells but involved proliferation of preexisting differentiated tubular cells. Interestingly, they concluded that PT cells were probably not quiescent but resting in G1-phase of the cell cycle, i.e., they could divide rapidly in response to injury. Recently, Humphreys et al[42] used sophisticated technology to demonstrate that tubular cells per se are the source of regenerating tubular cells. They prepared transgenic mouse strains in which all cells involved in nephrogenesis were lineage labeled. Using these mice, they tested whether any endogenous cell type entered the tubules and contributed in the repair process of tubules after ischemic reperfusion injury. Their data showed a lack of non-tubular cells in renal tubules before as well as after ischemic reperfusion injury. However, this finding neither excludes the possibility of the existence of intratubular stem cells/progenitor cells nor the proliferation of preexisting differentiated cells within the tubules.
More recently, Humphreys et al[43] used a DNA analog-labeled approach to chase multiple rounds of cell divisions in mice after ischemic reperfusion injury and demonstrated that PT cell division in the cortex and outer medulla occurred predominantly in injured and dedifferentiated PT cells. PT cell injury was confirmed by Kim-1 expression[57] and dedifferentiated cells by both PAX-2 expression[58] and reduction in Na+K+-ATPase expression in proliferating cells labeled with DNA analog. A stochastic kinetics of proliferation was identified, probably reflecting simple self-duplication rather than selective activation of an intratubular progenitor population. The findings of Humphreys et al[43] strongly suggest that proliferation of preexisting differentiated cells within the tubules is the main event in PT regeneration in ischemic reperfusion injury.
We also examined the importance of dedifferentiation in the initiation of cell division of PT cells after acute PT injury induced by uranyl acetate (UA), a nephrotoxic agent[50]. High-dose UA induced severe PT injury of the S3 segment and the first proliferating PT cells showed loss of PT cell protein phenotype (megalin, aquaporin 1 and Na+K+-ATPase) but became positively stained for vimentin. In comparison, low-dose UA induced focal PT injury of the S3 segment, with the first proliferating PT cells still exhibiting the PT phenotype and not staining for vimentin. Subsequently, the proliferating PT cells showed loss of PT cell phenotype and expressed vimentin. Thus, similar to the changes seen under physiological conditions, the PT cells can enter the cell cycle without apparent dedifferentiation after low-dose UA-induced focal PT injury. However, dedifferentiation with vimentin expression may follow after initial cell division. Interestingly, continuously proliferating tubular cells tend to express vimentin unlike regenerating cells under physiological conditions[54,55]. Since vimentin is a major intermediate filament protein and is associated with the development of migratory capacity[59,60], it is conceivable that proliferating PT cells can acquire vimentin expression to undergo cell division more than once and to migrate to cover the denuded tubular basement membrane. This may not be the case in regenerating PT cells under physiological conditions.
Thus, dedifferentiation must be a beneficial mode of regeneration for rapid reparation of focal areas following focal injury of the tubule, such as after ischemic reperfusion injury[61]. However, questions remain on whether all PT cells possess the ability to enter the cell cycle and acquire dedifferentiation property (i.e., is a stem-like cell) and whether the insult of ischemic reperfusion injury is adequate to activate intratubular progenitor cells, if they do exist. It is also possible that certain forms of renal damage can evoke a stem cell response whereas others do not.
DIFFERENT REPAIR PROCESSES OF PT AFTER ACUTE TUBULAR INJURY
The study of Oliver and colleagues[61] indicated that the main site of tubular injury following traumatic and toxic insults is PTs, based on histopathological examinations of cadaver kidneys in patients with severe fatal acute renal failure. They also found two types of tubular injuries. The first was nephrotoxic necrosis limited to that part of the nephron in the PT that is functionally concerned with the handling of poisons; the necrosis was homogeneous in that part of the nephron. The second type of lesion was disruption of the renal tubule due to focal cortical ischemia. It occurs at random among nephrons. The authors suggested that, in the kidney of any case of fatal acute renal failure arising under various clinical circumstances, these two types of lesions appear in varying proportions depending on the nature of the renal insult, whether toxic or circulatory or both. Thus, the number and distribution of surviving tubular cells after acute tubular injury must be highly variable among the different causes of acute tubular injury. It is also conceivable that different repair processes of tubules also occur under different pathological conditions.
In fact, we found two different modes of repair processes of PT after acute tubular injury induced even by the same nephrotoxic agent, low- or high-dose of UA in rats using the 3H-thymidine pulse/chase approach[62] for the detection of early regenerating PT cells[63]. In these studies, low-dose UA (0.25 or 0.5 mg/kg) induced mild and focal PT depletion in S3 segment without significant increase in serum creatinine. Some of the surviving PT cells scattered in the proximal three quarters of the S3 segment became thymidine-incorporating (detected by grain on sections) early regenerating PT cells. They were increasingly found in the proximal three quarters of S3 and to a lesser extent in the distal S3 at day 7, and decreased in number by day 42. The number of label-retaining PT cells increased in the entire S3 and the number of label-diluted PT cells was significantly increased, mainly in the proximal three quarters of S3, and both were decreased in parallel at day 42. Early regenerating cells maintained the differentiated phenotype initially then loss of the phenotype was noted shortly after the initial regeneration[50,63]. Taken together, the surviving PT cells contributed to the repair of focal PT injury, suggesting that dedifferentiated PT cells, derived from preexisting mature PT cells are responsible for focal repair of the S3 segment.
On the other hand, high-dose UA (1 or 5 mg/kg) induced a significant increase in serum creatinine and necrotic PT started to appear at the corticomedullary junction as early as day 2 after injection of UA, and then maximally spread in the entire S3 segment with almost complete PT depletion in three-quarters of the S3 segment with less PT depletion in the distal quarter of S3 by day 5[63,64]. The BrdU or thymidine-incorporating early regenerating cells were limited to the distal area of the S3 segment from days 2 to 3, remote from the initial site of damage, then upstream proliferation of PT cells occurred along the denuded tubular basement membrane, which was almost completed by day 7[63,64]. Thymidine-labeled PT cells were increasingly found in the entire S3 at day 7 during the repair phase. Label retaining PT cells were increased in the entire S3 and to a significantly greater extent in the distal S3. They were rapidly decreased in number in the proximal three quarters of S3 by day 21, but their number remained constant in the distal S3 until day 42. In contrast, the label-diluted PT cell population increased in the entire S3, although to a significantly lesser extent in the distal S3 at day 7, and their numbers decreased markedly in the entire S3 by day 42[63]. Early regenerating cells after high-dose UA insult seem to be the cellular source of regenerating tubules with high proliferative properties to repair the entire S3 segment with infrequent cycling after completion of the repair process of PT. Thus, we hypothesized that these cells might be slow cycling cells responsible for the repair of the entire S3. Next, we examined whether they could be designated the “target cells” and have intratubular progenitor-like properties.
POSSIBLE EXISTENCE OF RENAL TUBULAR PROGENITOR-LIKE CELLS
No specific renal tubular stem/progenitor cell markers are currently available. Therefore, indirect markers of slow cell cycling properties (label retention) have so far been used to search for potential population of intratubular progenitor cells. These include transcription factors and cell surface expression markers. However, once the PT cells are injured in vivo or isolated into a culture system, they also express genes and proteins of earlier stages of development[65], which makes it difficult to distinguish dedifferentiated tubular cells from mature differentiated tubular cells and intratubular progenitor cells. Therefore, at present there seems to be no reliable method to prove the existence of intratubular stem cells, both in vivo and in vitro.
To examine the specific properties of the early regenerating cells (designated as “target cells”) in the S3 segment of rats with high-dose UA-induced acute tubular injury, we searched for possible cell features that could define progenitor-like cells in vivo among the different tubular cells in the S3 segment. Our studies yielded the following conclusions regarding target cell characteristics: (1) The target cells (i.e., thymidine-labeled cells) were persistently present in the distal area of the S3 up to and including week 40[66], further suggesting slow-cycling cells; (2) About 60% of PT cells in the S3 segment were “thymidine-labeled cells” at day 7 after high-dose UA-induced acute renal failure[66], suggesting that the majority of the regenerating cells in the S3 were newly synthesized following injury and originated from the target cells in the distal area of S3; (3) Some target cells reproliferated after a second high-dose UA insult[66]; (4) The target cells were resistant to 5-fluorouracil (5-FU) in vivo and showed restoration of regenerative property after withdrawal of 5-FU and were also reactivated by the second UA insult[66]. Whereas there is substantial information on the response of hematopoietic stem cells to 5-FU[67], there is little information on the response of epithelial stem/progenitor cells to 5-FU. Previous studies reported that 5-FU is cytotoxic to proliferating epithelial cells such as retinal pigment epithelial cells[68] and lens epithelial cells[69]. Thus, the findings suggest that the target cells may be in some way unique with possible progenitor-like cell properties; (5) The target cells showed weak or no staining for all three markers of mature PT phenotype (megalin, aquaporin1 and Na+K+-ATPase) but became positive for a mesenchymal marker (vimentin)[50]. On the other hand, following acute tubular injury induced by low-dose UA, the initial proliferating PT cells divided while keeping the mature PT phenotype, but subsequently showed regression of this phenotype[50]. Unlike other PT cells, the target cells could undergo cell cycle progression without accumulation of heat shock protein 27[70], which is thought to provide partial protection for PT cells against injury or death by acting as a molecular chaperone and thus promotes the stabilization, repair and/or disposal of denatured proteins[71]. The data showed that the PT cell phenotype at the time of initial cell division was different between the target cell and other tubular cells, suggesting that the target cells are probably unique; (6) The target cells exhibited morphological features of dedifferentiated/undifferentiated cells, such as smaller brush-border, large nuclei, fewer cytoplasmic organelles and spindle-like morphology[66], compatible with the features of progenitor cells[72]. At present, there are no reports on the existence of morphologically and phenotypically unique cells (e.g., cells lacking brush-border or cells negative for markers of mature PT) among PTs based on histological examination under physiological conditions. However, progenitor cells usually exhibit spindle-like morphology with small length; thus, it is difficult to detect them when they are sequestered and/or buried among other PT cells without proper labeling such as BrdU; (7) A proportion of the target cells were localized at the transition zone between PT and the thin descending limb of Henle[66]. The target cells might have a bipotential differentiation because they exist at a unique location where cells can differentiate into both PT cells and thin descending limb of Henle; and (8) Under physiological conditions, most target cells did not enter the cell cycle based on BrdU-labeling[66], probably being different from the previously reported progenitor-like cells, which can be labeled with BrdU, during a 2 wk observation under physiological conditions[22,23]. This also suggests that the target cells do not contribute to the maintenance of cell turnover under physiological conditions but may be activated after severe PT injury in the S3 segment.
As mentioned earlier, we cannot confirm the existence of intratubular progenitor cells due to the lack of definitive markers for these cells, although some recent reports have provided some evidence for the existence of intratubular progenitor-like cells[22-30,33]. Therefore, it is not clear at this stage whether our “target cells” are truly progenitor-like cells or merely dedifferentiated PT cells that can acquire progenitor-like properties. However, our findings suggest the presence of a distinct population of tubular cells in the distal area of the S3 segment or at the transition zone between PT and thin descending limb of Henle. This cell population can be activated and stimulated to proliferate for adequate repair of PTs after severe impairment of the replicative capacity of PT cells in S3 segment or upon depletion of surviving PT following acute tubular injury.
PERSPECTIVES
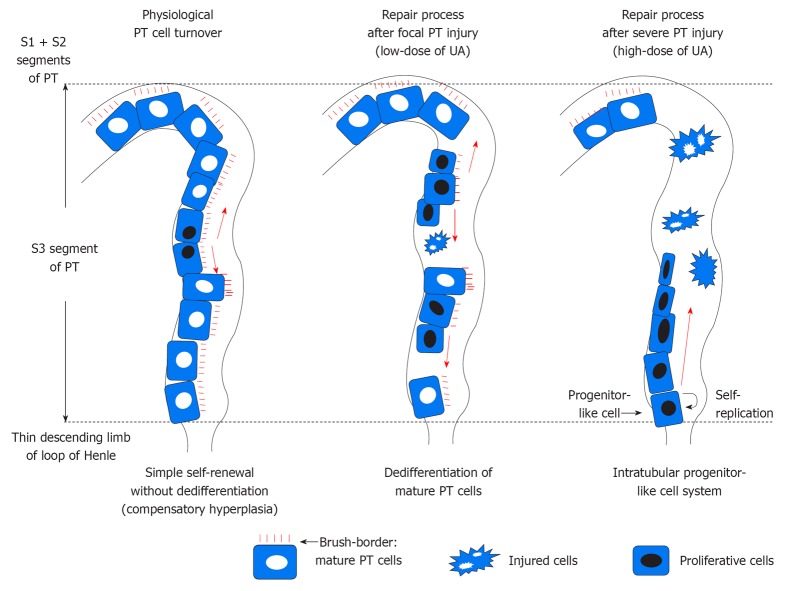
Based on our data, we conclude that the three modes of regeneration, compensatory hyperplasia, dedifferentiation of mature tubular cells and intratubular progenitor-like cell system, as illustrated in Figure 2, may be involved in PT repair. Compensatory hyperplasia provides effective PT cell turnover by self-renewal of adjacent cells without dedifferentiation under physiological conditions. However, PT cells are vulnerable because they are exposed to a variety of toxins and are susceptible to ischemic injury. This might explain why PT cells can regenerate through dedifferentiation of mature tubular cells, which can result in effective and rapid repair of focal PT lesions. Intratubular progenitor-like cells can play a role as a backup system to repair severely injured PTs. This does not exclude the possibility that both dedifferentiation and intratubular progenitor-like cells also contribute together to repair PTs in certain types of tubular injury. Interestingly, evidence points to the presence of stem cells in the liver of several rat models of liver injury, which promote tissue regeneration as a second backup system for liver regeneration when the proliferative capacity of hepatocytes via compensatory hyperplasia is compromised[52]. The PTs also seem to be equipped with the same backup system for PT regeneration, including intratubular progenitor-like cells at different locations. For instance, severe injury in S1 and S2, but not S3 segment, of PT induced by gentamicin[73] might evoke different progenitor-like cells than in other intratubular locations.
Figure 2.
Three modes of regeneration of tubular cells in S3 segment of proximal tubule. PT: Proximal tubule.
Unfortunately, there is only a limited knowledge about the modes of regeneration of tubular cells and the factors that induce regeneration. Understanding tubular regeneration in health and disease can potentially allow the design of new therapeutic strategies against various tubular diseases.
Footnotes
Supported by A Grant-In-Aid for Scientific Research (C; No. 22590884) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan
Peer reviewer: Keiju Hiromura, MD, PhD, Associate Professor, Department of Medicine and Clinical Science, Gunma University Graduate School of Medicine, 3-39-22 Showa, Maebashi, Gunma 371-8511, Japan
S- Editor Zhang DN L- Editor Roemmele A E- Editor Zheng XM
References
- 1.Stocum DL. Regenerative biology and medicine. An overview of regenerative biology and medicine. Canada: Academic Press; 2006. pp. 1–20. [Google Scholar]
- 2.Kren BT, Trembley JH, Fan G, Steer CJ. Molecular regulation of liver regeneration. Ann N Y Acad Sci. 1997;831:361–381. doi: 10.1111/j.1749-6632.1997.tb52211.x. [DOI] [PubMed] [Google Scholar]
- 3.Zenilman ME, Perfetti R, Swinson K, Magnuson T, Shuldiner AR. Pancreatic regeneration (reg) gene expression in a rat model of islet hyperplasia. Surgery. 1996;119:576–584. doi: 10.1016/s0039-6060(96)80270-4. [DOI] [PubMed] [Google Scholar]
- 4.Casimir CM, Gates PB, Patient RK, Brockes JP. Evidence for dedifferentiation and metaplasia in amphibian limb regeneration from inheritance of DNA methylation. Development. 1988;104:657–668. doi: 10.1242/dev.104.4.657. [DOI] [PubMed] [Google Scholar]
- 5.Carlson BM. Some principles of regeneration in mammalian systems. Anat Rec B New Anat. 2005;287:4–13. doi: 10.1002/ar.b.20079. [DOI] [PubMed] [Google Scholar]
- 6.Yamada T, McDevitt DS. Conversion of iris epithelial cells as a model of differentiation control. Differentiation. 1984;27:1–12. doi: 10.1111/j.1432-0436.1984.tb01402.x. [DOI] [PubMed] [Google Scholar]
- 7.Potten CS, Morris RJ. Epithelial stem cells in vivo. J Cell Sci Suppl. 1988;10:45–62. doi: 10.1242/jcs.1988.supplement_10.4. [DOI] [PubMed] [Google Scholar]
- 8.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 9.Brittan M, Wright NA. The gastrointestinal stem cell. Cell Prolif. 2004;37:35–53. doi: 10.1111/j.1365-2184.2004.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salice CJ, Rokous JS, Kane AS, Reimschuessel R. New nephron development in goldfish (Carassius auratus) kidneys following repeated gentamicin-induced nephrotoxicosis. Comp Med. 2001;51:56–59. [PubMed] [Google Scholar]
- 11.Elger M, Hentschel H, Litteral J, Wellner M, Kirsch T, Luft FC, Haller H. Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J Am Soc Nephrol. 2003;14:1506–1518. doi: 10.1097/01.asn.0000067645.49562.09. [DOI] [PubMed] [Google Scholar]
- 12.Drummond I. The skate weighs in on kidney regeneration. J Am Soc Nephrol. 2003;14:1704–1705. doi: 10.1097/01.asn.0000072725.30005.03. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol. 2010;299:F1040–F1047. doi: 10.1152/ajprenal.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown AC, Blank U, Adams DC, Karolak MJ, Fetting JL, Hill BL, Oxburgh L. Isolation and culture of cells from the nephrogenic zone of the embryonic mouse kidney. J Vis Exp. 2011;50:e2555. doi: 10.3791/2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 16.Grimm PC, Nickerson P, Jeffery J, Savani RC, Gough J, McKenna RM, Stern E, Rush DN. Neointimal and tubulointerstitial infiltration by recipient mesenchymal cells in chronic renal-allograft rejection. N Engl J Med. 2001;345:93–97. doi: 10.1056/NEJM200107123450203. [DOI] [PubMed] [Google Scholar]
- 17.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002;62:1285–1290. doi: 10.1111/j.1523-1755.2002.kid569.x. [DOI] [PubMed] [Google Scholar]
- 19.Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14:1188–1199. doi: 10.1097/01.asn.0000061595.28546.a0. [DOI] [PubMed] [Google Scholar]
- 20.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112:42–49. doi: 10.1172/JCI17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeshima A, Yamashita S, Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol. 2003;14:3138–3146. doi: 10.1097/01.asn.0000098685.43700.28. [DOI] [PubMed] [Google Scholar]
- 23.Maeshima A, Sakurai H, Nigam SK. Adult kidney tubular cell population showing phenotypic plasticity, tubulogenic capacity, and integration capability into developing kidney. J Am Soc Nephrol. 2006;17:188–198. doi: 10.1681/ASN.2005040370. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura S, Yamasaki Y, Kinomura M, Sugaya T, Sugiyama H, Maeshima Y, Makino H. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 2005;19:1789–1797. doi: 10.1096/fj.05-3942com. [DOI] [PubMed] [Google Scholar]
- 25.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y, et al. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17:3028–3040. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 27.Langworthy M, Zhou B, de Caestecker M, Moeckel G, Baldwin HS. NFATc1 identifies a population of proximal tubule cell progenitors. J Am Soc Nephrol. 2009;20:311–321. doi: 10.1681/ASN.2008010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallustio F, De Benedictis L, Castellano G, Zaza G, Loverre A, Costantino V, Grandaliano G, Schena FP. TLR2 plays a role in the activation of human resident renal stem/progenitor cells. FASEB J. 2010;24:514–525. doi: 10.1096/fj.09-136481. [DOI] [PubMed] [Google Scholar]
- 29.Lindgren D, Boström AK, Nilsson K, Hansson J, Sjölund J, Möller C, Jirström K, Nilsson E, Landberg G, Axelson H, et al. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am J Pathol. 2011;178:828–837. doi: 10.1016/j.ajpath.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Kim JI, Na YK, Park KM. Intra-renal slow cell-cycle cells contribute to the restoration of kidney tubules injured by ischemia/reperfusion. Anat Cell Biol. 2011;44:186–193. doi: 10.5115/acb.2011.44.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells. 2012;30:1714–1725. doi: 10.1002/stem.1130. [DOI] [PubMed] [Google Scholar]
- 34.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest. 2005;115:1756–1764. doi: 10.1172/JCI23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 37.Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613–1617. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 38.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 39.Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 40.Mias C, Trouche E, Seguelas MH, Calcagno F, Dignat-George F, Sabatier F, Piercecchi-Marti MD, Daniel L, Bianchi P, Calise D, et al. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26:1749–1757. doi: 10.1634/stemcells.2007-1000. [DOI] [PubMed] [Google Scholar]
- 41.Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation. 2010;121:2211–2220. doi: 10.1161/CIRCULATIONAHA.109.928796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA. 2011;108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCreight CE, Sulkin NM. Cellular proliferation in the kidneys of young and senile rats following unilateral nephrectomy. J Gerontol. 1959;14:440–443. [Google Scholar]
- 45.Nadasdy T, Laszik Z, Blick KE, Johnson LD, Silva FG. Proliferative activity of intrinsic cell populations in the normal human kidney. J Am Soc Nephrol. 1994;4:2032–2039. doi: 10.1681/ASN.V4122032. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, Fujigaki Y, Sakakima M, Hishida A. Acquired resistance to rechallenge injury in rats recovered from subclinical renal damage with uranyl acetate--Importance of proliferative activity of tubular cells. Toxicol Appl Pharmacol. 2010;243:104–110. doi: 10.1016/j.taap.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Vogetseder A, Palan T, Bacic D, Kaissling B, Le Hir M. Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am J Physiol Cell Physiol. 2007;292:C807–C813. doi: 10.1152/ajpcell.00301.2006. [DOI] [PubMed] [Google Scholar]
- 48.Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol. 2008;294:C22–C28. doi: 10.1152/ajpcell.00227.2007. [DOI] [PubMed] [Google Scholar]
- 49.Alison MR, Poulsom R, Forbes S, Wright NA. An introduction to stem cells. J Pathol. 2002;197:419–423. doi: 10.1002/path.1187. [DOI] [PubMed] [Google Scholar]
- 50.Fujigaki Y, Sakakima M, Sun Y, Fujikura T, Tsuji T, Yasuda H, Hishida A. Cell division and phenotypic regression of proximal tubular cells in response to uranyl acetate insult in rats. Nephrol Dial Transplant. 2009;24:2686–2692. doi: 10.1093/ndt/gfp199. [DOI] [PubMed] [Google Scholar]
- 51.Gröne HJ, Weber K, Gröne E, Helmchen U, Osborn M. Coexpression of keratin and vimentin in damaged and regenerating tubular epithelia of the kidney. Am J Pathol. 1987;129:1–8. [PMC free article] [PubMed] [Google Scholar]
- 52.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 53.Grisham JW. Migration of hepatocytes along hepatic plates and stem cell-fed hepatocyte lineages. Am J Pathol. 1994;144:849–854. [PMC free article] [PubMed] [Google Scholar]
- 54.Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93:2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14 Suppl 1:S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 56.Choie DD, Richter GW. Cell proliferation in rat kidney induced by lead acetate and effects of uninephrectomy on the proliferation. Am J Pathol. 1972;66:265–275. [PMC free article] [PubMed] [Google Scholar]
- 57.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–F563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 58.Torban E, Goodyer P. What PAX genes do in the kidney. Exp Nephrol. 1998;6:7–11. doi: 10.1159/000020498. [DOI] [PubMed] [Google Scholar]
- 59.Gilles C, Polette M, Zahm JM, Tournier JM, Volders L, Foidart JM, Birembaut P. Vimentin contributes to human mammary epithelial cell migration. J Cell Sci. 1999;112(Pt 24):4615–4625. doi: 10.1242/jcs.112.24.4615. [DOI] [PubMed] [Google Scholar]
- 60.Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. 2007;313:2050–2062. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 61.Oliver J, MacDowell M, Tracy A. The pathogenesis of acute renal failure associated with traumatic and toxic injury; renal ischemia, nephrotoxic damage and the ischemic episode. J Clin Invest. 1951;30:1307–1439. doi: 10.1172/JCI102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hattori T, Niki H, Fujita S. Tritiated thymidine autoradiographic study on the origin and renewal of argentaffin cells in the pyloric gland of hamsters. Cell Tissue Res. 1977;181:15–25. doi: 10.1007/BF00222771. [DOI] [PubMed] [Google Scholar]
- 63.Fujigaki Y, Goto T, Sakakima M, Fukasawa H, Miyaji T, Yamamoto T, Hishida A. Kinetics and characterization of initially regenerating proximal tubules in S3 segment in response to various degrees of acute tubular injury. Nephrol Dial Transplant. 2006;21:41–50. doi: 10.1093/ndt/gfi035. [DOI] [PubMed] [Google Scholar]
- 64.Sun DF, Fujigaki Y, Fujimoto T, Yonemura K, Hishida A. Possible involvement of myofibroblasts in cellular recovery of uranyl acetate-induced acute renal failure in rats. Am J Pathol. 2000;157:1321–1335. doi: 10.1016/S0002-9440(10)64647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallin A, Zhang G, Jones TW, Jaken S, Stevens JL. Mechanism of the nephrogenic repair response. Studies on proliferation and vimentin expression after 35S-1,2-dichlorovinyl-L-cysteine nephrotoxicity in vivo and in cultured proximal tubule epithelial cells. Lab Invest. 1992;66:474–484. [PubMed] [Google Scholar]
- 66.Sakakima M, Fujigaki Y, Yamamoto T, Hishida A. A distinct population of tubular cells in the distal S3 segment contributes to S3 segment regeneration in rats following acute renal failure induced by uranyl acetate. Nephron Exp Nephrol. 2008;109:e57–e70. doi: 10.1159/000142100. [DOI] [PubMed] [Google Scholar]
- 67.Hodgson GS, Bradley TR. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell. Nature. 1979;281:381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- 68.Mannerström M, Zorn-Kruppa M, Diehl H, Engelke M, Toimela T, Mäenpää H, Huhtala A, Uusitalo H, Salminen L, Pappas P, et al. Evaluation of the cytotoxicity of selected systemic and intravitreally dosed drugs in the cultures of human retinal pigment epithelial cell line and of pig primary retinal pigment epithelial cells. Toxicol In Vitro. 2002;16:193–200. doi: 10.1016/s0887-2333(01)00113-8. [DOI] [PubMed] [Google Scholar]
- 69.McDonnell PJ, Krause W, Glaser BM. In vitro inhibition of lens epithelial cell proliferation and migration. Ophthalmic Surg. 1988;19:25–30. [PubMed] [Google Scholar]
- 70.Fujigaki Y, Sun Y, Fujikura T, Sakao Y, Togawa A, Suzuki H, Yasuda H, Hishida A. Immunohistochemical study of heat shock protein 27 with respect to survival and regeneration of proximal tubular cells after uranyl acetate-induced acute tubular injury in rats. Ren Fail. 2010;32:119–125. doi: 10.3109/08860220903367569. [DOI] [PubMed] [Google Scholar]
- 71.Riordan M, Sreedharan R, Kashgarian M, Siegel NJ. Modulation of renal cell injury by heat shock proteins: lessons learned from the immature kidney. Nat Clin Pract Nephrol. 2006;2:149–156. doi: 10.1038/ncpneph0117. [DOI] [PubMed] [Google Scholar]
- 72.Karam SM, Straiton T, Hassan WM, Leblond CP. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells. 2003;21:322–336. doi: 10.1634/stemcells.21-3-322. [DOI] [PubMed] [Google Scholar]
- 73.Houghton DC, Hartnett M, Campbell-Boswell M, Porter G, Bennett W. A light and electron microscopic analysis of gentamicin nephrotoxicity in rats. Am J Pathol. 1976;82:589–612. [PMC free article] [PubMed] [Google Scholar]