Abstract
Chronic kidney disease (CKD) is associated with a high burden of coronary artery disease. In patients with acute coronary syndromes (ACS), CKD is highly prevalent and associated with poor short- and long-term outcomes. Management of patients with CKD presenting with ACS is more complex than in the general population because of the lack of well-designed randomized trials assessing therapeutic strategies in such patients. The almost uniform exclusion of patients with CKD from randomized studies evaluating new targeted therapies for ACS, coupled with concerns about further deterioration of renal function and therapy-related toxic effects, may explain the less frequent use of proven medical therapies in this subgroup of high-risk patients. However, these patients potentially have much to gain from conventional revascularization strategies used in the general population. The objective of this review is to summarize the current evidence regarding the epidemiology and the clinical and prognostic relevance of CKD in ACS patients, in particular with respect to unresolved issues and uncertainties regarding recommended medical therapies and coronary revascularization strategies.
Keywords: Chronic kidney disease, Acute coronary syndromes, Non-ST-elevation myocardial infarction, ST-elevation myocardial infarction, Percutaneous coronary intervention, Renal insufficiency
INTRODUCTION
Chronic kidney disease (CKD) is associated with accelerated atherogenesis, due to the presence of both traditional and non-traditional (related to the underlying uremic state) risk factors, and any degree of renal insufficiency portends a worsened prognosis in patients with coronary artery disease[1,2]. The adverse influence of CKD has been also demonstrated in the setting of acute coronary syndromes (ACS)[3-11]. Indeed, among ACS patients, CKD doubles mortality rates and is third only to cardiogenic shock and congestive heart failure as a predictor of mortality[12]. Antithrombotic agents and percutaneous coronary interventions (PCI) are clearly emerging as the cornerstones of treatment patterns in patients presenting with ACS[13]. However, despite the increasing number of CKD patients with a broad range of ACS at presentation, evidence-based data with established or newer drugs and interventional strategies are still lacking in this population because CKD patients have typically been excluded from randomized trials. Ideally, these are the patients to whom recent therapeutic advances should be aggressively applied, in order to minimize their increased risk. However, application of strategies for reducing cardiovascular morbidity and mortality seem to be limited in CKD patients, when compared to patients with normal renal function.
The currently high prevalence of CKD patients in the setting of ACS represents a strong incentive to development of targeted strategies from well-designed research, which will ultimately reduce the burden of risk in this population and achieve improved outcomes. CKD is present in a substantial proportion of patients with ACS; indeed, large registries report that almost 40% of patients with non-ST-elevation myocardial infarction (NSTEMI), and 30% of those with ST-elevation myocardial infarction (STEMI), have CKD, as defined by an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2[14,15].
PROGNOSTIC RELEVANCE OF CKD IN ACUTE CORONARY SYNDROMES
In ACS, CKD represents a potent and independent risk factor for adverse outcome. Although the mechanisms underlying the poor prognosis of this vulnerable population are not fully understood, it is conceivable that the interplay between extensive comorbidities, more severe disease on presentation with ACS, underutilization of known cardio-protective therapies, less aggressive treatment, more frequent errors in dosing with excess toxicity from conventional therapies, and unique pathobiology of CKD, has a considerable role (Table 1). Important differences in the extent of coronary atherosclerosis and coronary plaque morphology between patients with and without CKD have been recently reported[16]. Coronary lesions in CKD patients were longer with greater luminal encroachment and higher plaque burden compared with their non-CKD counterparts. Moreover, coronary atherosclerotic plaque composition assessed with radiofrequency IVUS demonstrated greater necrotic core and dense calcium with less fibrous tissue in CKD, suggesting that renal dysfunction not only results in greater coronary atherosclerotic plaque burden and luminal encroachment, but it may also modulate coronary atherosclerotic plaque composition to a less stable phenotype. Thus, it is possible that unique and independent mechanisms may alter atherosclerotic plaque composition in the setting of CKD. Serum levels of matrix metalloproteinases, for example, are elevated in patients with moderate to severe renal impairment and are associated with plaque progression and fibrous cap degradation[17]. In addition, experimental data suggest that CKD potentiates foam cell generation by enhancing macrophage entry into the vascular wall and inhibiting cholesterol efflux[18].
Table 1.
Most important studies on the in-hospital and long-term prognostic relevance of chronic kidney disease in acute coronary syndromes
| Source | Year | Kind of ACS | No. of patients | Evaluated mortality | CKD definition and severity | Mortality rate in CKD patients (%) | Mortality rate in no-CKD patients (%) | Comment |
| Herzog et al[3] | 1998 | STEMI | 34 189 | In-hospital | Patients in dialysis | 26 | - | Included pts with a first STEMI after dialysis initiation |
| 1-yr | 59 | |||||||
| 2-yr | 73 | |||||||
| 3-yr | 81 | |||||||
| 5-yr | 90 | |||||||
| 10-yr | 97 | |||||||
| Chertow et al[4] | 2000 | STEMI | 640 | 30-d (1-yr) | Patients in dialysis | 20 (53) | - | Only 7% of pts were treated with PCI and 5% with CABG |
| McCullough et al[20] | 2000 | STEMI/NSTEMI | 9544 | Mean follow-up: 27.1 mo | CrCl > 81.5 mL/min | 30.5 | 14.6 | - |
| 81.5-63.1 mL/min | 54.5 | 22.7 | ||||||
| 63.1-46.2 mL/min | 65 | |||||||
| < 46.2 mL/min | ||||||||
| Dialysis | ||||||||
| Beattie et al[5] | 2001 | STEMI | 1724 | In-hospital | CrCl > 81 mL/min | OR 3.88 | OR 1 | Age- and sex-adjusted risks for in-hospital death |
| 80-63 mL/min | OR 8.76 | OR 2.45 | ||||||
| 62-46 mL/min | OR 5.43 | |||||||
| < 46 mL/min | ||||||||
| HD | ||||||||
| Wright et al[6] | 2002 | STEMI | 3106 | In-hospital | ClCl > 75 mL/min | 6 | 2 | - |
| 75-51 mL/min | 14 | |||||||
| 50-35 mL/min | 21 | |||||||
| < 35 mL/min | 30 | |||||||
| HD | ||||||||
| Shlipak et al[7] | 2002 | STEMI | 130 099 | 1-yr | sCr < 1.5 mg/dL | 46 | 24 | Included pts with > 65 yr |
| 1.5-2.4 mg/dL | 66 | |||||||
| 2.5-3.9 mg/dL | ||||||||
| Sorensen et al[22] | 2002 | STEMI | 6252 | 6-yr | CrCl > 85 mL/min | 62 | 22.5 | - |
| 85-71 mL/min | 83 | 32 | ||||||
| 70-56 mL/min | 46 | |||||||
| 55-41 mL/min | ||||||||
| < 40 mL/min | ||||||||
| Santopinto et al[8] | 2003 | STEMI | 4716 | In-hospital | CrCl > 60 mL/min | 8.1 | 2.1 | - |
| 30-60 mL/min | 18.1 | |||||||
| < 30 mL/min | ||||||||
| Santopinto et al[8] | 2003 | NSTEMI/UA | 7058 | In-hospital | CrCl > 60 mL/min | 3.8 | 0.9 | |
| 30-60 mL/min | 8.5 | |||||||
| < 30 mL/min | ||||||||
| Sadeghi et al[34] | 2003 | STEMI | 2082 | 30-d (1 yr) | CrCl < 60 mL/min | 7.5 (12.7) | 0.8 (7.5) | Primary PCI Excluded pts with sCr > 2 mg/dL or CS |
| Hobbach et al[38] | 2003 | STEMI | 352 | 30-d (6-mo) | sCr > 1.2-2.8 mg/dL | 16 (19.5) | 3.4 (4.5) | Included pts treated with systemic thrombolysis |
| Berger et al[43] | 2003 | STEMI | 146 765 | 30-d | Dialysis vs no dialysis patients | 29 | 18.3 (no dialysis) | Included pts with > 65 yr |
| Freeman et al[61] | 2003 | STEMI/NSTEMI | 889 | In-hospital | sCr > 1.5 mg/dL | 8.1 | 2.6 | - |
| Anavekar et al[23] | 2004 | STEMI | 14 527 | 3-yr | eGFR > 75 mL/min per 1.73 m2 | 28.9 | 14.1 | Included pts with HF and/or LV systolic dysfunction |
| 74.9-60 mL/min per 1.73 m2 | 45.5 | 20.5 | ||||||
| 59.9-45 mL/min per 1.73 m2 | ||||||||
| < 45 mL/min per 1.73 m2 | ||||||||
| Gibson et al[11] | 2004 | NSTEMI/UA | 13 307 | 30-d (6-mo) | eGFR > 90 mL/min per 1.73 m2 | 2.1 (3.8) | 1.3 (2.5) | - |
| 89-60 mL/min per 1.73 m2 | 5 (9.5) | |||||||
| < 60 mL/min per 1.73 m2 | ||||||||
| Masoudi et al[12] | 2004 | STEMI/NSTEMI/UA | 2706 | 7-mo | eGFR > 90 mL/min per 1.73 m2 | 15 | 3 | - |
| 89-60 mL/min per 1.73 m2 | 39 | 6 | ||||||
| 59-30 mL/min per 1.73 m2 | ||||||||
| < 30 mL/min per 1.73 m2 | ||||||||
| Mueller et al[29] | 2004 | NSTEMI/UA | 1400 | In-hospital (3-yr) | eGFR > 130 mL/min per 1.73 m2 | 5.1 (23.2) | 0 (7.4) | Included pts with PCI performed within 24 h |
| 129-90 mL/min per 1.73 m2 | 0.4 (4.5) | |||||||
| 89-60 mL/min per 1.73 m2 | 2.6 (8.1) | |||||||
| < 60 mL/min per 1.73 m2 | ||||||||
| Dumaine et al[66] | 2004 | ACS | 496 | In-hospital | CrCl < 60 mL/min | 4.8 | 0.9 | - |
| Goldberg et al[73] | 2005 | STEMI | 1038 | In-hospital (1-yr) | eGFR > 90 mL/min per 1.73 m2 | 19.9 (29.9) | 4.7 (6.7) | - |
| 89-60 mL/min per 1.73 m2 | 6.3 (10) | |||||||
| < 60 mL/min per 1.73 m2 | ||||||||
| Han et al[74] | 2006 | NSTEMI/ UA | 45 343 | In-hospital | sCr > 2.0 mg/dL or dialysis | 9 | 3.6 | - |
| Marenzi et al[40] | 2007 | STEMI | 467 | In-hospital | CrCl < 60 mL/min | 8.9 | 3.7 | Primary PCI |
| Fox et al[57] | 2007 | STEMI | 20 479 | 30-d | CrCl > 90 mL/min | 15.30 | 2.6 | Thrombolysis Excluded pts with sCr > 2.5 (men) and > 2.0 (women) |
| 90-61 mL/min | 31.10 | 7.1 | ||||||
| 60-30 mL/min | ||||||||
| < 30 mL/min | ||||||||
| Pitsavos et al[75] | 2007 | STEMI/NSTEMI | 2172 | In-hospital | CrCl > 60 mL/min | 7 | 2 | - |
| 60-30 mL/min | 16 | |||||||
| < 30 mL/min | ||||||||
| Inrig et al[76] | 2008 | ACS | 9190 | 1-yr | eGFR > 90 mL/min per 1.73 m2 | 6 | 1.7 | - |
| 90-75 mL/min per 1.73 m2 | 5.7 | 2 | ||||||
| 75-60 mL/min per 1.73 m2 | 3.3 | |||||||
| 60-45 mL/min per 1.73 m2 | ||||||||
| < 45 mL/min per 1.73 m2 | ||||||||
| Szummer et al[30] | 2009 | NSTEMI | 23 262 | 1-yr | eGFR > 90 mL/min per 1.73 m2 | 16.7 | 5 | - |
| 89-60 mL/min per 1.73 m2 | 38.2 | 5.8 | ||||||
| 59-30 mL/min per 1.73 m2 | 51.2 | |||||||
| 29-15 mL/min per 1.73 m2 | ||||||||
| < 15 mL/min per 1.73 m2/HD | ||||||||
| Mehran et al[65] | 2009 | NSTEMI/UA | 12 939 | 30-d (1-yr) | CrCl < 60 mL/min | 3 (7.9) | 1.1 (2.8) | Excluded pts with CrCl < 30 mL/min |
| James et al[50] | 2010 | STEMI/NSTEMI | 15 202 | 1-yr | CrCl < 60 mL/min | 12.1 | 3.3 | Included pts admitted < 24 h from symptoms onset |
| Fox et al[15] | 2010 | STEMI | 19 089 | In-hospital | Stage 3a | 8.8 | 2.3 | - |
| Stage 3b | 17.9 | |||||||
| Stage 4 | 27.3 | |||||||
| Stage 5 | 31.8 | |||||||
| Fox et al[15] | 2010 | NSTEMI | 30 462 | In-hospital | Stage 3a | 4.8 | 1.8 | - |
| Stage 3b | 8.6 | |||||||
| Stage 4 | 13.4 | |||||||
| Stage 5 | 12.4 | |||||||
| Hachinohe et al[42] | 2011 | NSTEMI | 3615 | 1-yr | eGFR > 90 mL/min per 1.73 m2 | 9.4 | 4 | Excluded pts with CS |
| 89-60 mL/min per 1.73 m2 | 30 | 3 | ||||||
| 59-30 mL/min per 1.73 m2 | ||||||||
| < 30 mL/min per 1.73 m2 | ||||||||
| Hanna et al[77] | 2011 | NSTEMI | 40 074 | In-hospital | eGFR > 60 mL/min per 1.73 m2 | 2.8 | 0.6 | All pts were treated with PCI |
| 59-30 mL/min per 1.73 m2 | 6.1 | |||||||
| 29-15 mL/min per 1.73 m2 | 3.9 | |||||||
| < 15 mL/min per 1.73 m2 |
ACS: Acute coronary syndromes; CABG: Coronary artery bypass graft; CKD: Chronic kidney disease; CrCl: Creatinine clearance; CS: Cardiogenic shock; eGFR: Estimated glomerular filtration rate; HD: Hemodialysis; HF: Heart failure; LV: Left ventricular; NSTEMI: Non-ST elevation myocardial infarction; OR: Odds ratio; PCI: Percutaneous coronary intervention; sCr: Serum creatinine concentration; STEMI: ST-elevation myocardial infarction; UA: Unstable angina.
Finally, patients with CKD have been shown to have an accelerated infarct expansion in association with enhanced inflammation and oxidative stress, as compared with non-CKD patients, suggesting an important role of CKD in the development of left ventricular remodeling after myocardial infarction, via enhanced inflammatory responses and oxidative stress[19].
ST-elevation myocardial infarction
The fundamental work of Herzog et al[3] was the initial observation that revealed the poor prognosis faced by patients with Stage 5 CKD who suffer from acute myocardial infarction. Using the US Renal Data System database, the investigators examined the outcome of 34 189 patients on long-term dialysis after a first episode of acute myocardial infarction, and documented an in-hospital mortality of 26% and 1-year and 2-year mortality rates of 59% and 73%, respectively. These observations were confirmed by Chertow et al[4] who reported a 30-d mortality rate of 20% and a 1-year mortality rate of 53% after acute myocardial infarction, in 640 patients with Stage 5 CKD. Beattie et al[5] extended the investigation to patients with CKD who were not on dialysis therapy. They analyzed a prospective coronary care unit registry of 1724 patients with STEMI admitted over an 8-year period at a single tertiary-care center. Patients were stratified into groups based on different corrected creatinine clearance (CrCl) values. A graded rise in in-hospital complications and death rate, as well as a reduction in long-term survival, were observed across increasing CKD strata. This study, as well as another one by McCullough et al[20] showed a similar graded increase in the relative risk of atrial and ventricular arrhythmias, heart block, asystole, pulmonary congestion, and cardiogenic shock in parallel with decrease in renal function. Two following large studies revealed the significant morbidity and mortality risk faced by STEMI patients with even minor CKD. Wright et al[6] examined treatment patterns, in-hospital complications, and short- and long-term survival in 3106 patients with STEMI in relation to their renal function. CrCl values, derived by the Cockcroft-Gault formula from serum creatinine (sCr) concentration[21], were used to stratify patients into five groups. They observed a gradient of increased risk of death in all groups of patients with CKD, even in those with mild renal impairment: in-hospital mortality was 2% in patients with normal renal function and progressively increased to 6%, 14%, 21% and 30% in parallel with renal function worsening. Patients with CKD also developed more STEMI-related complications, including atrial fibrillation, congestive heart failure, and mechanical complications.
Similar findings were also reported by Shlipak et al[7] in 130 099 elderly patients (age ≥ 65 years) with acute myocardial infarction (about 30% of whom had STEMI). In this large cohort study, CKD turned out to be strongly associated with survival after acute myocardial infarction. In particular, 1 mo after hospital admission, mortality for patients with moderate renal impairment (as defined by sCr level between 2.5 and 3.9 mg/dL) was 44%, compared with 13% for patients who had relatively normal renal function.
All these, as well as further studies, like a Danish study evaluating 6252 patients included in the TRAndopril Cardiac Evaluation register[22], and a recent large study, the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) registry, including 19 029 STEMI patients[15], support the evidence of a strikingly high mortality in STEMI patients with CKD.
Finally, in the Valsartan in Acute Myocardial Infarction Trial study the outcome of CKD patients remained worse also after hospital discharge, at a long-term (3-year) follow-up, despite an apparent optimal post-discharge treatment (all patients received valsartan, captopril, or a combination of both)[23]. These results suggest that even appropriate post-discharge therapy, given in a timely manner, may not be sufficient to improve post-acute myocardial infarction outcome in CKD population.
Non-ST elevation ACS
Several observational studies have found that, in the setting of non-ST elevation acute coronary syndromes (NSTE-ACS), in-hospital outcomes and mid- to long-term mortality are worse among patients with CKD[8,9,24]. The Global Registry of Acute Coronary Events (GRACE) study, a large prospective multinational registry, including the full spectrum of patients with ACS, evaluated the prognostic impact of sCr levels on hospital mortality and adverse outcomes in 11 774 NSTE-ACS patients[8]. Patients were divided into three groups according to their CrCl values: > 60 mL/min or normal renal function (including patients with minimally impaired renal function), 30-60 mL/min or moderate CKD, and < 30 mL/min or severe CKD. In comparison with patients with normal renal function, patients with moderate and severe CKD were at a significantly increased risk of hospital mortality and major bleeding episodes (Figure 1). Other studies have definitely confirmed the close association between CKD and increased risk of death in patients with NSTE-ACS at presentation[10,12,25]. The significant contribution of renal function evaluation to risk stratification was demonstrated by Gibson et al[11] who pooled data from five international multicenter trials (TIMI 11A and B, TIMI 12, OPUS-TIMI 16 and TACTICS-TIMI 18) and analyzed 13 307 patients with NSTE-ACS. Notably, these trials excluded patients with a sCr level above 2.0 mg/dL. As a consequence, moderate to severe CKD (eGFR < 60 mL/min per 1.73 m2) was present in 16% of patients, while only less than 1% of the patients had an eGFR < 30 mL/min per 1.73 m2. In this study, a significant and graded association was observed between reduced eGFR and short-term (30 d) and mid-term (6 mo) mortality: 1.3% and 2.5% for patients with normal renal function, 2.1% and 3.8% for patients with mild CKD, and 5% and 9.5% for patients with moderate to severe CKD, respectively (P for trend < 0.001). In parallel, there was a stepwise increase in the incidence of stroke, major bleeding, recurrent myocardial infarction, and recurrent ischemia with worsening eGFR (Figure 2). Interestingly, within each eGFR category, high thrombolysis in myocardial infarction (TIMI) risk scores and augmented levels of biomarkers (C-reactive protein, B-type natriuretic peptide, and troponin I) were associated with a significant increase in mortality as compared with low TIMI scores and normal levels of biomarkers (Figure 3), demonstrating the prognostic value of eGFR in addition to the traditional clinical risk stratification of patients with NSTE-ACS. The authors concluded that eGFR should be part of the evaluation of any patient presenting with an ACS.
Figure 1.
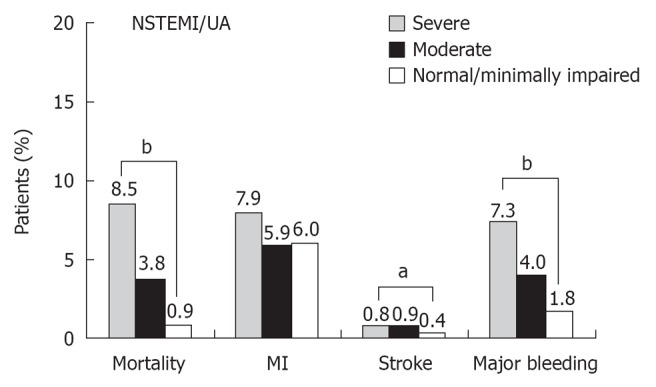
Hospital outcomes for patients with non-ST segment elevation myocardial infarction/unstable angina. aP < 0.05 and bP < 0.0001 across all categories of renal function. Modified from Santopinto et al[8]. NSTEMI: Non-ST-elevation myocardial infarction.
Figure 2.
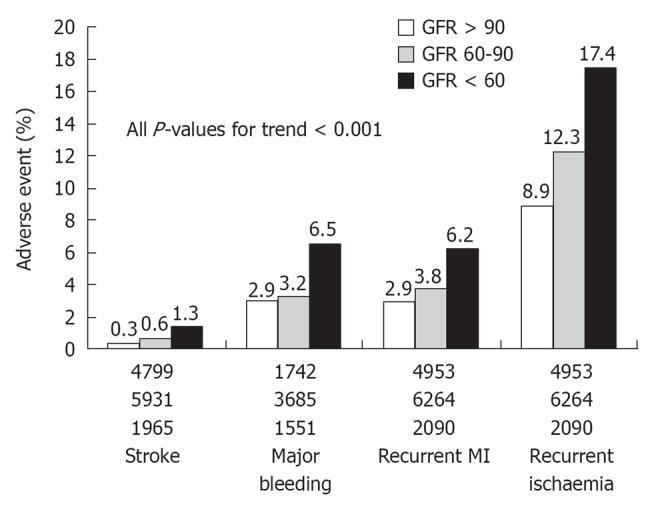
Incidence of adverse event by glomerular filtration rate groups: stroke, in-hospital thrombolysis in myocardial infarction major bleeding, recurrent myocardial infarction, and recurrent ischemia at 30 d. The number of patients within each subgroup is displayed at the bottom of each bar. Glomerular filtration rate is expressed in mL/min per 1.73 m2. From Gibson et al[11]. GFR: Glomerular filtration rate.
Figure 3.
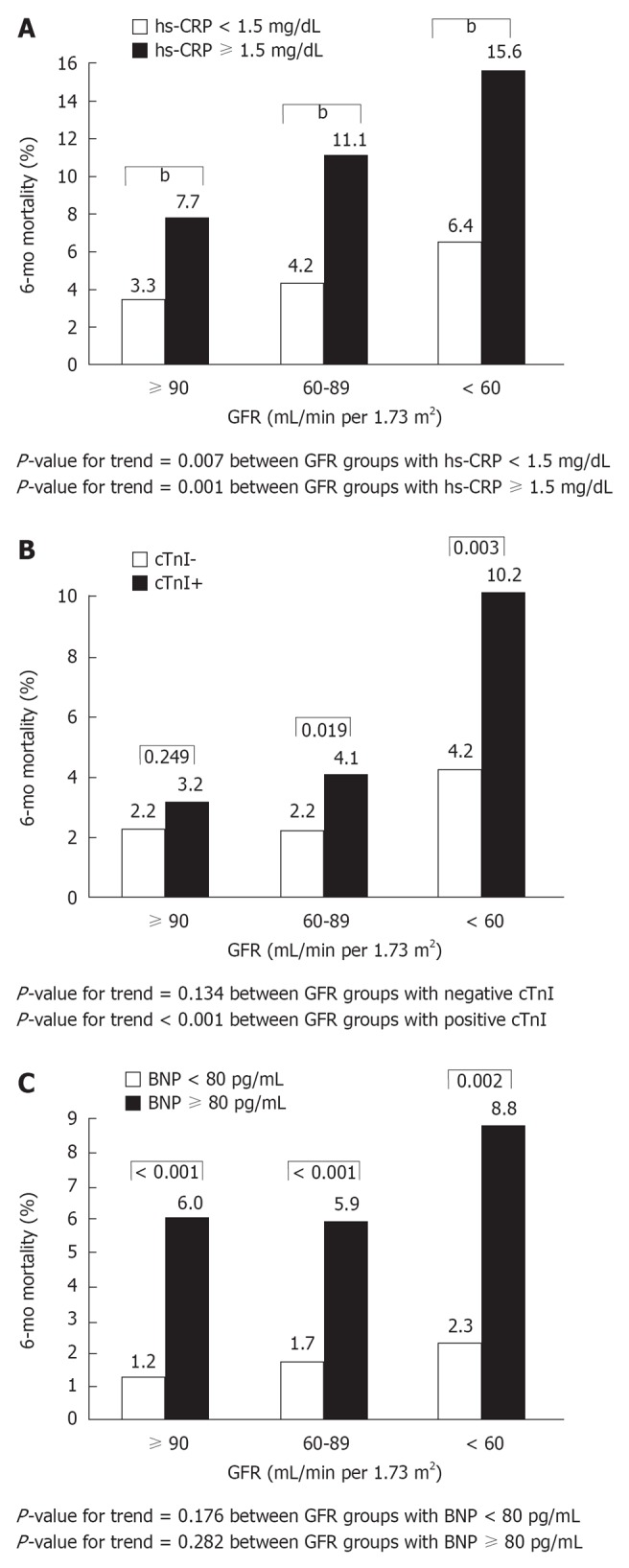
Six-month mortality, stratified by glomerular filtration rate and biomarker levels. A: Stratification by high-sensitivity C-reactive protein (hs-CRP) levels. bP < 0.001; B: Stratification by Troponin I (cTnI) levels; C: Stratification by B-type natriuretic peptide levels. From Gibson et al[11]. BNP: B-type natriuretic peptide; GFR: Glomerular filtration rate.
As early coronary angiography and revascularization has been shown to be a more effective strategy for high-risk patients with NSTE-ACS[13], a major issue in CKD patients is the question as to whether the decrease in renal function or the coronary revascularization procedure (PCI or coronary bypass surgery) may be the cause of their worsened hospital outcome. In most of the studies focusing on the prognostic role of CKD in NSTE-ACS, patients were more likely to be treated conservatively with anti-ischemic and antithrombotic agents, while PCI was performed only in patients with recurrent myocardial ischemia. Furthermore, in the studies in which the advantage of an early invasive strategy was demonstrated[26-28], patients with advanced CKD, as well as those with an increased risk of bleeding, were excluded. With these limitations, a retrospective study examined the interaction between CrCl, outcomes, and the use of an early invasive strategy in 2190 patients with NSTE-ACS enrolled in the Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative strategy trial[24]. Irrespective of treatment strategy, mild to moderate decrease in renal function was a potent risk factor for adverse outcome, with a concomitant increase in endpoints such as death, acute myocardial infarction, and rehospitalization at 30 and 180 d. Routine invasive management, however, was associated with a statistically significant reduction in the same end points, across most categories of CKD, at the predictable price of a significant increase in major and minor bleeding. Mueller et al[29] investigated the association between baseline renal function and mortality, after NSTE-ACS, in a cohort of 1400 consecutive, unselected patients treated uniformly very early (within 24 h of admission, with a median time interval of 5 h) and predominantly with PCI. Patients on dialysis, and with high bleeding risk, were also included in the study. A significantly higher in-hospital and long-term mortality rate was found among patients with a GFR < 60mL/min per 1.73 m2 than among patients with higher eGFR. Interestingly, renal function was predictive of long-term mortality, irrespective of the revascularization method applied. Thus, this study confirmed that baseline renal function is a strong independent predictor of in-hospital and long-term mortality, and extended this important finding to NSTE-ACS patients treated very early with PCI.
Two recent large studies, the ACTION registry[15] and the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) study[30], including 30 462 and 23 262 NSTEMI patients, respectively, confirmed the close association between CKD severity and increased in-hospital and 1-year mortality rates. In particular, the SWEDEHEART study demonstrated a gradient of progressively lower advantage from PCI vs medical therapy with no advantage, in terms of 1-year mortality, going from patients with normal renal function to patients with increasing CKD severity, with no benefit at all for those with severe (Stage IV) CKD[30]. The lack of overall benefit in this subset of patients is possibly explained by a higher incidence of PCI-associated complications that may overweight the advantage deriving from coronary revascularization. Indeed, most post-PCI complications associated with poor short-term and long-term outcomes, such as contrast-induced nephropathy (CIN)[31,32], coronary stent thrombosis and restenosis[33,34], underlying coronary artery disease progression[1], and bleedings[8], have been found to occur more frequently in CKD than in no CKD patients.
TREATMENT OF PATIENTS WITH ACS AND CKD
Treatment of ACS in patients with CKD is particularly problematic. Traditionally, patients with advanced CKD and those receiving dialysis have not been included in randomized ACS trials evaluating either medical or interventional therapies. Thus, only scarce data deriving from limited observational studies are available and, to date, no optimal treatment strategy has been defined for this subgroup of patients.
Coronary reperfusion strategies
Doubts still exist on how CKD patients should be treated in the early phase of STEMI. In particular, there are concerns about the use of aggressive reperfusion strategy (fibrinolytic therapy and primary PCI). Undoubtedly, landmark megatrials, such as the Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico, the International Study of Infarct Survival, and the Global Utilization of Streptokinase and Tissue Plasminogen for Occluded Coronary Arteries (GUSTO) trials, have shown the benefit of thrombolytic agents in reducing mortality in patients with STEMI[35-37]. However, in all these trials, no subgroup analysis was performed in patients with CKD, and scarce data have been published on the use of thrombolytics in these patients. In the study by Wright et al[6], 13% of the total population received intravenous fibrinolytic therapy and 10% received PCI as a primary treatment. Reperfusion therapy was used less frequently in patients with any degree of CKD than in patients without CKD. In the Beattie et al[5] study, the use of mortality-reducing treatments, including primary angioplasty and thrombolysis, decreased with the progressive decline of renal function, suggesting a treatment bias in favor of patients with less advanced renal dysfunction.
While the lower rates of PCI may be rationally explained by the fear of an increased risk of CIN and of the associated high mortality rate[31,32], the potential risk of bleeding constitutes only a partial justification for the less frequent use of thrombolysis.
CKD should not preclude the success rate of percutaneous or pharmacological reperfusion therapies, but it may be associated with increased incidence of major adverse events. To evaluate the effects of an invasive management with additional early revascularization, the outcome of 352 patients with STEMI (sCr ranging from 1.2 to 2.8 mg/dL), was analyzed in a single-center retrospective study[38]. All patients received thrombolytic therapy, while early PCI or coronary bypass surgery were performed in 47% and 28% of patients with normal renal function and 32% and 30% of those with CKD. Despite the optimal guideline-based therapy, patients with CKD showed a significantly higher 30-d and 6-mo mortality rate than those with normal renal function (16% and 19% vs 3% and 4%, respectively, P < 0.001). It is noteworthy, however, that the 30-d and 6-mo mortality was reduced from 22% to 4% (P < 0.03) and from 25% to 7% (P < 0.05) among CKD patients who underwent PCI during hospitalization. Thus, this study confirms that mild to moderate CKD in the setting of STEMI is associated with increased mortality, despite extensive use of optimal therapy, but it also suggests that early PCI may be beneficial among such patients. The influence of CKD in patients with STEMI receiving fibrinolytic therapy was investigated by Gibson et al[39]. Again, despite appropriate treatment with thrombolytics and adjunctive therapies for acute myocardial infarction (including early PCI in many patients), and even though the epicardial and myocardial reperfusion rates were equivalent, there was a stepwise decrease in survival going from normal to mildly and severely reduced renal function that continued through up to two years of follow-up. The incidence of intracranial hemorrhage was also increased in patients with CKD, suggesting that primary PCI may represent a favorable alternative therapy. Nevertheless, the outcomes of primary PCI in patients with STEMI and CKD have not been well characterized, because such patients are typically excluded from clinical trials. Data from the Controlled Abcximab and Device Investigation to Lower Late Angioplasty Complications trial and from our institute clearly demonstrated a worse outcome of patients with CKD among those treated with primary PCI[34,40]. So, despite the evidence of a clear benefit deriving from pharmacologic and mechanical coronary reperfusion in STEMI, the best strategy for STEMI patients with CKD remains elusive. Indeed, data from the GRACE registry have recently showed similar in-hospital mortality rates in STEMI patients with severe CKD, regardless their treatment with primary PCI (29%), thrombolysis (32%), or medical therapy (31.5%)[41]. The SWEDEHEART study[30], and a recent Korean prospective multicenter study, the Korea Acute Myocardial Infarction Registry (KAMIR) registry[42], including 13 901 NSTEMI patients seem to confirm that the benefit of revascularization is uncertain in patients with severe CKD or on dialysis. In the management of NSTEMI, according to previous studies and guidelines, an invasive strategy is superior to an initial conservative strategy (invasive management only after failed medical therapy or for objective evidence of ischemia) and an early invasive strategy is superior to a delayed invasive strategy. In the KAMIR study, an invasive (within 24 h after admission) strategy decreased mortality compared to a conservative strategy except for severe CKD. In the timing of an invasive strategy, there were trends showing that an early invasive strategy was superior to a delayed strategy in patients with mild CKD, but this tendency decreased as renal function decreased[42].
Standard medical therapy
Berger et al[43] compared the patterns of care and the effect of standard STEMI therapy on 30-d mortality between 1025 patients on chronic dialysis (either peritoneal dialysis or hemodialysis) and 145 740 non-dialysis patients. They confirmed that aspirin, β-blockers, and ACE-inhibitors were less likely to be used in patients on dialysis, even among those considered “ideal candidates” for these medications, than in patients not receiving dialysis. Nevertheless, the authors observed a similar absolute reduction in short-term mortality with aspirin, β-blocker, and ACE-inhibitor therapy when comparing the dialysis and non-dialysis groups. Aspirin was associated with a 21% absolute reduction in mortality in dialysis patients, and a 23% reduction in non-dialysis patients. β-blocker therapy was associated with a 14% absolute reduction in mortality in both the dialysis and non-dialysis patients. The ACE-inhibitor use was associated with a 16% absolute reduction in 30-d mortality in dialysis patients and a 5% reduction in non-dialysis patients. In the Wright et al[6] and in the Shlipak et al[7] studies, the use of aspirin, β-blockers and intravenous heparin during the first 24 h of hospitalization was less frequent in patients in dialysis and un those with moderate to severe CKD; these trends persisted with therapies prescribed at hospital discharge, including aspirin, ACE-inhibitors and β-blockers. Recently, data from the ACTION registry documented, in both STEMI and NSTEMI patients, lower use of short-term therapies, in-hospital procedures, cardioprotective medications, and higher rates of medication overdosing among patients with CKD[15]. Finally, despite these high rates of adverse outcomes, patients with CKD were less likely to receive discharge counseling related to cardiovascular disease risk reduction. Thus, all these studies confirm less aggressive care in CKD patients with ACS that parallel the degree of renal dysfunction, and demonstrate that beneficial therapies are underutilized in patients with CKD despite increased prevalence of hypertension, congestive heart failure, and coronary artery disease, and despite the fact that these medications are associated with a substantial survival benefit in patients with normal renal function.
Antithrombotic therapy
Refinement of the antithrombotic strategies among CKD patients in ACS setting is still a major and unmet need. The challenge is daunting because, on the one hand, CKD is associated with prolongation of bleeding time and abnormal platelet aggregation and adhesion[44], and, on the other hand, a state of hypercoagulation has been demonstrated with high levels of von Willebrand factor[45], fibrinogen, factors VII, VIII, and XIII and enhanced thrombin generation[44]. The combination of these alterations puts the patient with CKD at risk, simultaneously, for thrombosis and hemorrhage. Thus, use of well-established antiplatelet drugs, such as aspirin and clopidogrel, should be weighed against bleeding risk in renal patients. Based on the benefit demonstrated in NSTE-ACS, it seems advisable to keep CKD patients on aspirin therapy, with a suggestion for low doses, despite no dose modification is required when given to patients with CKD. The UK Heart and Renal Protection-1 trial and the Dialysis Outcomes and Prescription Patterns Study showed that low-dose aspirin (100 mg/d) in CKD patients was not associated with increased major bleeding or progression of CKD[46,47]. In a recent meta-analysis by the Antithrombotic Trialists Collaboration low-dose aspirin (65 to 260 mg) was found to be as efficacious as high-dose aspirin (325 mg) beyond the acute phase for secondary prevention of coronary artery disease in patients with CKD and end-stage renal disease[48].
Current practice guidelines recommend the addition to aspirin of another antiplatelet agent (ticlopidine, clopidogrel, prasugrel, ticagrelor) for treatment of high-risk ACS patients. Despite never specifically investigated in CKD patients, some differences, in terms of bleeding risk, possibly exist among these drugs. In the Clopidogrel for the Reduction of Events During Observation trial the effectiveness of clopidogrel in reducing adverse cardiovascular events seemed to be greatest in patients with normal renal function, whereas patients with moderate CKD appeared to have experienced less benefit[49]. In The Platelet Inhibition and Patient Outcomes study clinical evidence was provided that ticagrelor is a more effective antiplatelet agent than clopidogrel in patients with ACS, regardless of renal function and without any need for dose reduction to prevent major bleeding[50]. A recent systematic review and meta-analysis, however, concluded that benefits for antiplatelet therapy among patients with CKD are uncertain and are potentially outweighed by bleeding hazards[51].
In addition to antiplatelet agents, heparin has become the standard of care in patients with ACS. The two preparations generally available are unfractionated heparin (UFH) and low-molecular weight heparin (LMWH). A major difference between these two therapeutic agents is their mechanism of clearance: at low doses, UHF is cleared primarily by macrophages and endothelial cell binding, whereas LMWH is cleared primarily by renal mechanisms[52]. The half-life of enoxaparin is 1.7 times longer in patients with renal insufficiency[53]. Clinical studies on enoxaparin, the most widely used LMWH in NSTE-ACS, excluded patients with CKD, so that the optimal dosing for renal patients has not been established[54,55]. A retrospective review showed a significant increase in bleeding events and death in patients with CKD treated with enoxaparin[56], suggesting that dosage adjustment is needed in these patients to minimize the bleeding risk. A substudy of the Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial infarction-TIMI 25 trial showed that with every 30 mL/min decrease in CrCl, the risk of major and minor bleeding increased by 50%[57]. Reduction in dose to half or reduction in frequency of administration to only once daily may be necessary. Until conclusive results are available regarding optimal dosing, it may be safer to use UFH in CKD patients presenting with ACS. It should be highlighted, however, that bleeding risk increases in parallel with the increasing severity of baseline renal insufficiency also in patients receiving UHF alone[58]. Finally, data from the Organization to Assess Strategies in Acute Ischemic Syndromes trial showed a lower bleeding risk profile for fondaparinux vs enoxaparin, two drugs that are mainly cleared by the kidneys, across all quartiles of renal dysfunction[59].
Impaired renal clearance of many pharmacological agents may increase the probability of overdosing in patients with reduced renal function, further increasing their bleeding risk[60]. This is particularly true for renally excreted antithrombotic agents, such as enoxaparin, tirofiban and eptifibatide that are recommended at a lower dose in CKD patients.
Use of antithrombotic agents, such as platelet glycoprotein (GP) IIb/IIIa receptor inhibitors, that block the final pathway for platelets aggregation, has become the standard of care for the higher-risk NSTE-ACS patients, mainly for those undergoing PCI. However, patients with CKD were also excluded from entry into most randomized trials investigating GPIIb/IIIa antagonists. Thus, it is not clear if they may derive the same therapeutic benefit with equivalent safety from these pharmacological agents, as do patients with normal renal function. Moreover, because agents such as tirofiban and eptifibatide are largely cleared through the kidneys, moderate to severe CKD would be expected to increase the mean plasma concentration of these drugs, producing a greater inhibition of platelet aggregation. Since platelet-bound abcximab is eliminated by the reticuloendothelial system[61], its use in patients with renal insufficiency should not be associated with greater impairment of platelet function. Thus, unlike tirofiban and eptifibatide, abciximab does not require dosing adjustment in CKD patients. Although all major randomized GPIIb/IIIa trials excluded CKD patients, particularly those with severe CKD, some data about the impact of these agents in this difficult population may be derived from retrospective trial analysis. Data extrapolated from the GUSTO-IV ACS study indicate that, in patients with a sCr over 2 mg/dL, abciximab bolus with infusion was associated with a reduction of the combined end-point of death or myocardial infarction when compared to placebo (15% vs 27%)[61]. However, the relationships between renal function and ischemic and hemorrhagic complications among ACS patients receiving an intracoronary stent and treated with either tirofiban or abciximab in the Do Tirofiban and ReoPro Give Similar Efficacy trial were assessed[62]. Although patients with a sCr > 2.5 mg/dL were excluded, those with the lowest CrCl quartile (< 70 mL/min) had a higher bleeding complication rate. Significant differences between patients receiving tirofiban and those receiving abcximab were not detected for major bleeding or transfusions, but minor bleeding rates were lower for patients who received tirofiban treatment (2.8% vs 4.3%, P = 0.006).
In a retrospective subanalysis of the Second Randomized Evaluation in PCI Linking Bivalirudin to Reduced Clinical Events trial[63], within the total randomized population of 5710 patients undergoing PCI (43% of whom because of NSTE-ACS), 886 patients with moderate or severe CKD showed, as expected, an increased burden of early and late morbidity and mortality. However, bivalirudin (a direct thrombin inhibitor) use among those with CKD proved no inferiority when compared to heparin and GPIIb/IIIa inhibition, with respect to ischemic events. In addition, in the overall population, as well as in patients who had a CrCl < 60 mL/min, bivalirudin was associated with fewer bleeding events. These results are in line with a previous meta-analysis of trials evaluating bivalirudin administration in patients undergoing PCI that showed a reduction of bleeding and ischemic events, with a greater absolute benefit among patients with CKD[64], and with data from the Acute Catheterization and Urgent Intervention Triage strategy trial in which, compared with UFH plus a GP IIb/IIIa inhibitor, the use of bivaluridin monotherapy in patients with CKD results in a nonstatistically different ischemic outcomes, but significantly less 30-d major bleeding (6.2% vs 9.8%, P = 0.008)[65]. In consideration of the important clinical implications of antithrombotic therapy in this clinical setting, additional randomized trials are warranted to further evaluate the effect of bivalirudin, in terms of ischemic and bleeding complications prevention, in ACS patients with CKD.
Statins in ACS patients with CKD
Acute coronary syndrome patients with CKD are less likely to be treated with statins, despite the evidence of reduced mortality with statins use in patients with or at risk for coronary events[8,23,25,66]. In addition to the long-term benefit of statin therapy in ACS patients, data collected on 300 823 patients of the National Registry of Myocardial Infarction 4, suggest that administration of statins within the first 24 h of hospitalization for acute myocardial infarction significantly lowers the rate of early complications and in-hospital mortality, possibly due to their pleiotropic effects[67]. Indeed, acute myocardial infarction is associated with a number of abnormalities, including inflammation, endothelial dysfunction, and coagulation disorders, all of which appear to be dampened by statins[68]. The reasons for this “therapeutic nihilism” in ACS patients with advanced CKD are not clear. Concern about further impairment of renal function and toxic side effects are potential explanations. Furthermore, patients with CKD have more co-morbidities and, as a consequence, more contraindications to these medications.
Statins are primarily eliminated by the liver, while the renal route is usually a minor elimination pathway. However, reluctance to prescribe statins in patients with CKD is likely due to remaining uncertainty regarding their clinical effects in patients with CKD. In particular, no clear evidence of a positive relationship between blood cholesterol and cardiovascular events has been found in these patients. As they approach end-stage renal disease, there appears to be increased oxidation of low-density lipoprotein, with progressive lowering of total cholesterol levels. The influence of dyslipidemia upon cardiovascular outcomes shows a “U”-shaped relationship, with increasing cardiovascular event rate seen among patients with severe CKD having low cholesterol levels. This “low-cholesterol paradox” has been attributed to the effects of chronic malnutrition and inflammation, which become increasingly important in severe CKD[69]. This paradox has raised the question of the utility of lipid-lowering therapy, although previous evidence that statin therapy may delay progression of renal dysfunction[70]. Moreover, some concerns about drug toxicity related to high statin doses exist in CKD patients. Indeed, high statin doses have been associated with an increased risk of myopathy in CKD patients[71]. This problem could be overcome by the combination of ezetimibe plus low-dose statins, as recently demonstrated in the Study of Heart and Renal Protection trial, in which lowering LDL cholesterol with this combination safely reduced the risk of major atherosclerotic events in a wide range of patients with CKD, including patients treated with dialysis[72].
CONCLUSION
CKD - of any degree - is present in a substantial proportion of patients with ACS, and represents a potent and independent risk factor for adverse outcome. Unfortunately, data are still limited regarding the value of most therapeutic interventions, because CKD patients with ACS have typically been excluded from randomized trials. Thus, our current challenge is to further study these high-risk patients in prospective randomized trials in order to identify adjunctive pharmacological therapies and newer interventional strategies that may favorably affect their otherwise poor prognosis. Nevertheless, as long as evidence-based data are not provided to guide clinical practice, all attempts must be made to promote the use of more aggressive therapies, when they can be applied with an acceptable level of safety.
Footnotes
Peer reviewers: Andrzej Jozef Jaroszynski, MD, PhD, Associate Professor, Department of Family Medicine, Medical University of Lublin, Staszica 11, 20-081 Lublin, Poland; Pornanong Aramwit, PhD, Department of Pharmacy Practice, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Payathai Road, Bangkok 10330, Thailand
S- Editor Wang JL L- Editor A E- Editor Zheng XM
References
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Herzog CA, Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998;339:799–805. doi: 10.1056/NEJM199809173391203. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Normand SL, Silva LR, McNeil BJ. Survival after acute myocardial infarction in patients with end-stage renal disease: results from the cooperative cardiovascular project. Am J Kidney Dis. 2000;35:1044–1051. doi: 10.1016/s0272-6386(00)70038-2. [DOI] [PubMed] [Google Scholar]
- 5.Beattie JN, Soman SS, Sandberg KR, Yee J, Borzak S, Garg M, McCullough PA. Determinants of mortality after myocardial infarction in patients with advanced renal dysfunction. Am J Kidney Dis. 2001;37:1191–1200. doi: 10.1053/ajkd.2001.24522. [DOI] [PubMed] [Google Scholar]
- 6.Wright RS, Reeder GS, Herzog CA, Albright RC, Williams BA, Dvorak DL, Miller WL, Murphy JG, Kopecky SL, Jaffe AS. Acute myocardial infarction and renal dysfunction: a high-risk combination. Ann Intern Med. 2002;137:563–570. doi: 10.7326/0003-4819-137-7-200210010-00007. [DOI] [PubMed] [Google Scholar]
- 7.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med. 2002;137:555–562. doi: 10.7326/0003-4819-137-7-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 8.Santopinto JJ, Fox KA, Goldberg RJ, Budaj A, Piñero G, Avezum A, Gulba D, Esteban J, Gore JM, Johnson J, et al. Creatinine clearance and adverse hospital outcomes in patients with acute coronary syndromes: findings from the global registry of acute coronary events (GRACE) Heart. 2003;89:1003–1008. doi: 10.1136/heart.89.9.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James SK, Lindahl B, Siegbahn A, Stridsberg M, Venge P, Armstrong P, Barnathan ES, Califf R, Topol EJ, Simoons ML, et al. N-terminal pro-brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: a Global Utilization of Strategies To Open occluded arteries (GUSTO)-IV substudy. Circulation. 2003;108:275–281. doi: 10.1161/01.CIR.0000079170.10579.DC. [DOI] [PubMed] [Google Scholar]
- 10.Keeley EC, Kadakia R, Soman S, Borzak S, McCullough PA. Analysis of long-term survival after revascularization in patients with chronic kidney disease presenting with acute coronary syndromes. Am J Cardiol. 2003;92:509–514. doi: 10.1016/s0002-9149(03)00716-1. [DOI] [PubMed] [Google Scholar]
- 11.Gibson CM, Dumaine RL, Gelfand EV, Murphy SA, Morrow DA, Wiviott SD, Giugliano RP, Cannon CP, Antman EM, Braunwald E. Association of glomerular filtration rate on presentation with subsequent mortality in non-ST-segment elevation acute coronary syndrome; observations in 13,307 patients in five TIMI trials. Eur Heart J. 2004;25:1998–2005. doi: 10.1016/j.ehj.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Masoudi FA, Plomondon ME, Magid DJ, Sales A, Rumsfeld JS. Renal insufficiency and mortality from acute coronary syndromes. Am Heart J. 2004;147:623–629. doi: 10.1016/j.ahj.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernández-Avilés F, Fox KA, Hasdai D, Ohman EM, Wallentin L, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 14.Wong JA, Goodman SG, Yan RT, Wald R, Bagnall AJ, Welsh RC, Wong GC, Kornder J, Eagle KA, Steg PG, et al. Temporal management patterns and outcomes of non-ST elevation acute coronary syndromes in patients with kidney dysfunction. Eur Heart J. 2009;30:549–557. doi: 10.1093/eurheartj/ehp014. [DOI] [PubMed] [Google Scholar]
- 15.Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121:357–365. doi: 10.1161/CIRCULATIONAHA.109.865352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baber U, Stone GW, Weisz G, Moreno P, Dangas G, Maehara A, Mintz GS, Cristea E, Fahy M, Xu K, et al. Coronary plaque composition, morphology, and outcomes in patients with and without chronic kidney disease presenting with acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5:S53–S61. doi: 10.1016/j.jcmg.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Pelisek J, Hahntow IN, Eckstein HH, Ockert S, Reeps C, Heider P, Luppa PB, Frank H. Impact of chronic kidney disease on carotid plaque vulnerability. J Vasc Surg. 2011;54:1643–1649. doi: 10.1016/j.jvs.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 18.Ponda MP, Barash I, Feig JE, Fisher EA, Skolnik EY. Moderate kidney disease inhibits atherosclerosis regression. Atherosclerosis. 2010;210:57–62. doi: 10.1016/j.atherosclerosis.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naito K, Anzai T, Yoshikawa T, Anzai A, Kaneko H, Kohno T, Takahashi T, Kawamura A, Ogawa S. Impact of chronic kidney disease on postinfarction inflammation, oxidative stress, and left ventricular remodeling. J Card Fail. 2008;14:831–838. doi: 10.1016/j.cardfail.2008.07.233. [DOI] [PubMed] [Google Scholar]
- 20.McCullough PA, Soman SS, Shah SS, Smith ST, Marks KR, Yee J, Borzak S. Risks associated with renal dysfunction in patients in the coronary care unit. J Am Coll Cardiol. 2000;36:679–684. doi: 10.1016/s0735-1097(00)00774-9. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Sørensen CR, Brendorp B, Rask-Madsen C, Køber L, Kjøller E, Torp-Pedersen C. The prognostic importance of creatinine clearance after acute myocardial infarction. Eur Heart J. 2002;23:948–952. doi: 10.1053/euhj.2001.2989. [DOI] [PubMed] [Google Scholar]
- 23.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 24.Januzzi JL, Cannon CP, DiBattiste PM, Murphy S, Weintraub W, Braunwald E. Effects of renal insufficiency on early invasive management in patients with acute coronary syndromes (The TACTICS-TIMI 18 Trial) Am J Cardiol. 2002;90:1246–1249. doi: 10.1016/s0002-9149(02)02844-8. [DOI] [PubMed] [Google Scholar]
- 25.Wison S, Foo K, Cunningham J, Cooper J, Deaner A, Knight C, Ranjadayalan K, Timmis AD. Renal function and risk stratification in acute coronary syndromes. Am J Cardiol. 2003;91:1051–1054. doi: 10.1016/s0002-9149(03)00147-4. [DOI] [PubMed] [Google Scholar]
- 26.Wallentin L, Lagerqvist B, Husted S, Kontny F, Ståhle E, Swahn E. Outcome at 1 year after an invasive compared with a non-invasive strategy in unstable coronary-artery disease: the FRISC II invasive randomised trial. FRISC II Investigators. Fast Revascularisation during Instability in Coronary artery disease. Lancet. 2000;356:9–16. doi: 10.1016/s0140-6736(00)02427-2. [DOI] [PubMed] [Google Scholar]
- 27.Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann FJ, Robertson DH, DeLucca PT, DiBattiste PM, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–1887. doi: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 28.Fox KA, Poole-Wilson PA, Henderson RA, Clayton TC, Chamberlain DA, Shaw TR, Wheatley DJ, Pocock SJ. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Randomized Intervention Trial of unstable Angina. Lancet. 2002;360:743–751. doi: 10.1016/s0140-6736(02)09894-x. [DOI] [PubMed] [Google Scholar]
- 29.Mueller C, Neumann FJ, Perruchoud AP, Buettner HJ. Renal function and long term mortality after unstable angina/non-ST segment elevation myocardial infarction treated very early and predominantly with percutaneous coronary intervention. Heart. 2004;90:902–907. doi: 10.1136/hrt.2003.021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szummer K, Lundman P, Jacobson SH, Schön S, Lindbäck J, Stenestrand U, Wallentin L, Jernberg T. Influence of renal function on the effects of early revascularization in non-ST-elevation myocardial infarction: data from the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) Circulation. 2009;120:851–858. doi: 10.1161/CIRCULATIONAHA.108.838169. [DOI] [PubMed] [Google Scholar]
- 31.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 32.Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, Grazi M, Veglia F, Bartorelli AL. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785. doi: 10.1016/j.jacc.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 33.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 34.Sadeghi HM, Stone GW, Grines CL, Mehran R, Dixon SR, Lansky AJ, Fahy M, Cox DA, Garcia E, Tcheng JE, et al. Impact of renal insufficiency in patients undergoing primary angioplasty for acute myocardial infarction. Circulation. 2003;108:2769–2775. doi: 10.1161/01.CIR.0000103623.63687.21. [DOI] [PubMed] [Google Scholar]
- 35.Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Gruppo Italiano per lo Studio della Streptochinasi nell'Infarto Miocardico (GISSI) Lancet. 1986;1:397–402. [PubMed] [Google Scholar]
- 36.Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349–360. [PubMed] [Google Scholar]
- 37.An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 38.Hobbach HP, Gibson CM, Giugliano RP, Hundertmark J, Schaeffer C, Tscherleniak W, Schuster P. The prognostic value of serum creatinine on admission in fibrinolytic-eligible patients with acute myocardial infarction. J Thromb Thrombolysis. 2003;16:167–174. doi: 10.1023/B:THRO.0000024055.13207.50. [DOI] [PubMed] [Google Scholar]
- 39.Gibson CM, Pinto DS, Murphy SA, Morrow DA, Hobbach HP, Wiviott SD, Giugliano RP, Cannon CP, Antman EM, Braunwald E. Association of creatinine and creatinine clearance on presentation in acute myocardial infarction with subsequent mortality. J Am Coll Cardiol. 2003;42:1535–1543. doi: 10.1016/j.jacc.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Marenzi G, Moltrasio M, Assanelli E, Lauri G, Marana I, Grazi M, Rubino M, De Metrio M, Veglia F, Bartorelli AL. Impact of cardiac and renal dysfunction on inhospital morbidity and mortality of patients with acute myocardial infarction undergoing primary angioplasty. Am Heart J. 2007;153:755–762. doi: 10.1016/j.ahj.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 41.Medi C, Montalescot G, Budaj A, Fox KA, López-Sendón J, FitzGerald G, Brieger DB. Reperfusion in patients with renal dysfunction after presentation with ST-segment elevation or left bundle branch block: GRACE (Global Registry of Acute Coronary Events) JACC Cardiovasc Interv. 2009;2:26–33. doi: 10.1016/j.jcin.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Hachinohe D, Jeong MH, Saito S, Ahmed K, Hwang SH, Lee MG, Sim DS, Park KH, Kim JH, Hong YJ, et al. Management of non-ST-segment elevation acute myocardial infarction in patients with chronic kidney disease (from the Korea Acute Myocardial Infarction Registry) Am J Cardiol. 2011;108:206–213. doi: 10.1016/j.amjcard.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 43.Berger AK, Duval S, Krumholz HM. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42:201–208. doi: 10.1016/s0735-1097(03)00572-2. [DOI] [PubMed] [Google Scholar]
- 44.Sagripanti A, Barsotti G. Bleeding and thrombosis in chronic uremia. Nephron. 1997;75:125–139. doi: 10.1159/000189522. [DOI] [PubMed] [Google Scholar]
- 45.Stam F, van Guldener C, Schalkwijk CG, ter Wee PM, Donker AJ, Stehouwer CD. Impaired renal function is associated with markers of endothelial dysfunction and increased inflammatory activity. Nephrol Dial Transplant. 2003;18:892–898. doi: 10.1093/ndt/gfg080. [DOI] [PubMed] [Google Scholar]
- 46.Baigent C, Landray M, Leaper C, Altmann P, Armitage J, Baxter A, Cairns HS, Collins R, Foley RN, Frighi V, et al. First United Kingdom Heart and Renal Protection (UK-HARP-I) study: biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. Am J Kidney Dis. 2005;45:473–484. doi: 10.1053/j.ajkd.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Ethier J, Bragg-Gresham JL, Piera L, Akizawa T, Asano Y, Mason N, Gillespie BW, Young EW. Aspirin prescription and outcomes in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2007;50:602–611. doi: 10.1053/j.ajkd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Best PJ, Steinhubl SR, Berger PB, Dasgupta A, Brennan DM, Szczech LA, Califf RM, Topol EJ. The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J. 2008;155:687–693. doi: 10.1016/j.ahj.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 50.James S, Budaj A, Aylward P, Buck KK, Cannon CP, Cornel JH, Harrington RA, Horrow J, Katus H, Keltai M, et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2010;122:1056–1067. doi: 10.1161/CIRCULATIONAHA.109.933796. [DOI] [PubMed] [Google Scholar]
- 51.Palmer SC, Di Micco L, Razavian M, Craig JC, Perkovic V, Pellegrini F, Copetti M, Graziano G, Tognoni G, Jardine M, et al. Effects of antiplatelet therapy on mortality and cardiovascular and bleeding outcomes in persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;156:445–459. doi: 10.7326/0003-4819-156-6-201203200-00007. [DOI] [PubMed] [Google Scholar]
- 52.Boneu B, Caranobe C, Cadroy Y, Dol F, Gabaig AM, Dupouy D, Sie P. Pharmacokinetic studies of standard unfractionated heparin, and low molecular weight heparins in the rabbit. Semin Thromb Hemost. 1988;14:18–27. doi: 10.1055/s-2007-1002751. [DOI] [PubMed] [Google Scholar]
- 53.Cadroy Y, Pourrat J, Baladre MF, Saivin S, Houin G, Montastruc JL, Vernier I, Boneu B. Delayed elimination of enoxaparin in patients with chronic renal insufficiency. Thromb Res. 1991;63:385–390. doi: 10.1016/0049-3848(91)90141-i. [DOI] [PubMed] [Google Scholar]
- 54.Goodman SG, Fitchett D, Armstrong PW, Tan M, Langer A. Randomized evaluation of the safety and efficacy of enoxaparin versus unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes receiving the glycoprotein IIb/IIIa inhibitor eptifibatide. Circulation. 2003;107:238–244. doi: 10.1161/01.cir.0000050144.67910.13. [DOI] [PubMed] [Google Scholar]
- 55.Petersen JL, Mahaffey KW, Hasselblad V, Antman EM, Cohen M, Goodman SG, Langer A, Blazing MA, Le-Moigne-Amrani A, de Lemos JA, et al. Efficacy and bleeding complications among patients randomized to enoxaparin or unfractionated heparin for antithrombin therapy in non-ST-Segment elevation acute coronary syndromes: a systematic overview. JAMA. 2004;292:89–96. doi: 10.1001/jama.292.1.89. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez JS, Sadaniantz BT, Sadaniantz A. Review of antithrombotic agents used for acute coronary syndromes in renal patients. Am J Kidney Dis. 2003;42:446–455. doi: 10.1016/s0272-6386(03)00800-x. [DOI] [PubMed] [Google Scholar]
- 57.Fox KA, Antman EM, Montalescot G, Agewall S, SomaRaju B, Verheugt FW, Lopez-Sendon J, Hod H, Murphy SA, Braunwald E. The impact of renal dysfunction on outcomes in the ExTRACT-TIMI 25 trial. J Am Coll Cardiol. 2007;49:2249–2255. doi: 10.1016/j.jacc.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 58.Januzzi JL, Snapinn SM, DiBattiste PM, Jang IK, Theroux P. Benefits and safety of tirofiban among acute coronary syndrome patients with mild to moderate renal insufficiency: results from the Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) trial. Circulation. 2002;105:2361–2366. doi: 10.1161/01.cir.0000016359.94919.16. [DOI] [PubMed] [Google Scholar]
- 59.Fox KA, Bassand JP, Mehta SR, Wallentin L, Theroux P, Piegas LS, Valentin V, Moccetti T, Chrolavicius S, Afzal R, et al. Influence of renal function on the efficacy and safety of fondaparinux relative to enoxaparin in non ST-segment elevation acute coronary syndromes. Ann Intern Med. 2007;147:304–310. doi: 10.7326/0003-4819-147-5-200709040-00005. [DOI] [PubMed] [Google Scholar]
- 60.Alexander KP, Chen AY, Roe MT, Newby LK, Gibson CM, Allen-LaPointe NM, Pollack C, Gibler WB, Ohman EM, Peterson ED. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. JAMA. 2005;294:3108–3116. doi: 10.1001/jama.294.24.3108. [DOI] [PubMed] [Google Scholar]
- 61.Freeman RV, Mehta RH, Al Badr W, Cooper JV, Kline-Rogers E, Eagle KA. Influence of concurrent renal dysfunction on outcomes of patients with acute coronary syndromes and implications of the use of glycoprotein IIb/IIIa inhibitors. J Am Coll Cardiol. 2003;41:718–724. doi: 10.1016/s0735-1097(02)02956-x. [DOI] [PubMed] [Google Scholar]
- 62.Berger PB, Best PJ, Topol EJ, White J, DiBattiste PM, Chan AW, Kristensen SD, Herrmann HC, Moliterno DJ. The relation of renal function to ischemic and bleeding outcomes with 2 different glycoprotein IIb/IIIa inhibitors: the do Tirofiban and ReoPro Give Similar Efficacy Outcome (TARGET) trial. Am Heart J. 2005;149:869–875. doi: 10.1016/j.ahj.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Chew DP, Lincoff AM, Gurm H, Wolski K, Cohen DJ, Henry T, Feit F, Topol EJ. Bivalirudin versus heparin and glycoprotein IIb/IIIa inhibition among patients with renal impairment undergoing percutaneous coronary intervention (a subanalysis of the REPLACE-2 trial) Am J Cardiol. 2005;95:581–585. doi: 10.1016/j.amjcard.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Chew DP, Bhatt DL, Kimball W, Henry TD, Berger P, McCullough PA, Feit F, Bittl JA, Lincoff AM. Bivalirudin provides increasing benefit with decreasing renal function: a meta-analysis of randomized trials. Am J Cardiol. 2003;92:919–923. doi: 10.1016/s0002-9149(03)00970-6. [DOI] [PubMed] [Google Scholar]
- 65.Mehran R, Nikolsky E, Lansky AJ, Kirtane AJ, Kim YH, Feit F, Manoukian S, Moses JW, Ebrahimi R, Ohman EM, et al. Impact of chronic kidney disease on early (30-day) and late (1-year) outcomes of patients with acute coronary syndromes treated with alternative antithrombotic treatment strategies: an ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) substudy. JACC Cardiovasc Interv. 2009;2:748–757. doi: 10.1016/j.jcin.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 66.Dumaine R, Collet JP, Tanguy ML, Mansencal N, Dubois-Randé JL, Henry P, Steg PG, Michel PL, Allouch P, Cohen A, et al. Prognostic significance of renal insufficiency in patients presenting with acute coronary syndrome (the Prospective Multicenter SYCOMORE study) Am J Cardiol. 2004;94:1543–1547. doi: 10.1016/j.amjcard.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 67.Fonarow GC, Wright RS, Spencer FA, Fredrick PD, Dong W, Every N, French WJ. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am J Cardiol. 2005;96:611–616. doi: 10.1016/j.amjcard.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 68.Ray KK, Cannon CP. Early time to benefit with intensive statin treatment: could it be the pleiotropic effects. Am J Cardiol. 2005;96:54F–60F. doi: 10.1016/j.amjcard.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 69.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–S23. [PubMed] [Google Scholar]
- 70.Tonelli M, Isles C, Craven T, Tonkin A, Pfeffer MA, Shepherd J, Sacks FM, Furberg C, Cobbe SM, Simes J, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation. 2005;112:171–178. doi: 10.1161/CIRCULATIONAHA.104.517565. [DOI] [PubMed] [Google Scholar]
- 71.Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Peto R, Collins R. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376:1658–1669. doi: 10.1016/S0140-6736(10)60310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldberg A, Hammerman H, Petcherski S, Zdorovyak A, Yalonetsky S, Kapeliovich M, Agmon Y, Markiewicz W, Aronson D. Inhospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am Heart J. 2005;150:330–337. doi: 10.1016/j.ahj.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 74.Han JH, Chandra A, Mulgund J, Roe MT, Peterson ED, Szczech LA, Patel U, Ohman EM, Lindsell CJ, Gibler WB. Chronic kidney disease in patients with non-ST-segment elevation acute coronary syndromes. Am J Med. 2006;119:248–254. doi: 10.1016/j.amjmed.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 75.Pitsavos C, Kourlaba G, Panagiotakos DB, Kogias Y, Mantas Y, Chrysohoou C, Stefanadis C. Association of creatinine clearance and in-hospital mortality in patients with acute coronary syndromes: the GREECS study. Circ J. 2007;71:9–14. doi: 10.1253/circj.71.9. [DOI] [PubMed] [Google Scholar]
- 76.Inrig JK, Patel UD, Briley LP, She L, Gillespie BS, Easton JD, Topol EJ, Szczech LA. Mortality, kidney disease and cardiac procedures following acute coronary syndrome. Nephrol Dial Transplant. 2008;23:934–940. doi: 10.1093/ndt/gfm689. [DOI] [PubMed] [Google Scholar]
- 77.Hanna EB, Chen AY, Roe MT, Wiviott SD, Fox CS, Saucedo JF. Characteristics and in-hospital outcomes of patients with non-ST-segment elevation myocardial infarction and chronic kidney disease undergoing percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4:1002–1008. doi: 10.1016/j.jcin.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


