Abstract
The purpose of this study was to evaluate whether treatment time and concentration of these reagents have an effect on the resulting gliding resistance. Forty peroneus longus (PL) tendons were used, from 20 adult mongrel dogs, along with the A2 pulley obtained from the ipsilateral hind paw. After the baseline gliding resistance was measured, the PL tendons were treated with one of three concentrations of hyaluronic acid (HA) and 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) or N-hydroxysuccinimide (NHS) mixed with 10% gelatin for various times (5, 30, and 60 min). Tendon friction was measured over 1000 cycles of simulated flexion/extension motion. Gliding resistance of the untreated PL tendons had no significant difference among the groups. After surface treatment with low concentration of HA and EDC/NHS for 5-min cure, the gliding resistance was similar to that of the untreated PL tendon and significantly higher than its 30- and 60-min treatment. For the rest of high concentration of HA and EDC/NHS groups, the gliding resistance was lower than that of untreated PL tendon. However, there was no significant difference among the timing points. It is possible to optimize the effect of surface treatment on friction and durability by regulating cure time and concentration of reagents in a canine extrasynovial tendon in vitro.
Keywords: hyaluronic acid, flexor tendon, extrasynovial tendon, friction, chemical modification
INTRODUCTION
Adhesion formation after tendon suture and grafting is a common postoperative complication.1 Clinically, most tendon grafts are obtained from extrasynovial tendon sources and are used in a synovial environment, that is, a tendon system including a synovially lined sheath. Tendon grafts from extrasynovial sources are known to develop adhesions to a greater extent than grafts from intrasynovial sources.2,3 However, the availability of intrasynovial tendon for a donor graft is limited.
The carbodiimide derivatization (CD) reaction can extend hyaluronic acid (HA) half-life and can strengthen HA binding ability on the tendon surface.4–6 The reaction involves the activation of the carboxyl groups by a water-soluble carbodiimide such as 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC), which activates the carboxyl groups of the HA molecule and forms the intermediate O-acylisourea. This intermediary can then bind chemically to any exposed amino groups, such as those that exist in the collagenous tendon matrix, forming a new collagen-HA polymer, fixed to the tendon surface. Agents such as N-hydroxysuccinimide (NHS) can enhance the coupling efficiencies of this reaction.7
Studies of peroneus longus (PL) tendon treated with CD–gelatin HA have shown that this modification can reduce friction after repeated motion to a level similar to that of normal intrasynovial tendon.8 However, the effect of reaction time was not studied. It is important to minimize the impact of any innovation on operating time. In addition, there have been no systematic studies of the effectiveness of differing combinations of the concentrations of these reagents on the friction-reducing ability of the resulting compound.
The purpose of this study was to find the minimal interval to effectively modify a tendon graft to reduce friction in an in vitro canine model of simulated tendon grafting. The results will be important for surgical procedures that use this methodology in the future.
MATERIALS AND METHODS
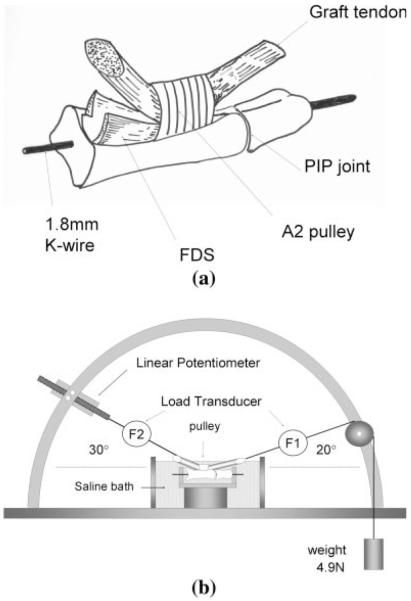
Peroneus longus (PL) tendons (n = 40) were harvested from 20 adult mongrel dogs. The dogs had been killed for other IACUC-approved projects. The superficial aspect of the paratenon was carefully removed, as recommended clinically when extrasynovial tendons are used for tendon grafting.9,10 Care was taken not to injure the tendon surface. The second digit was dissected from each hind paw. The proximal and middle phalanges were obtained by disarticulating the metacarpophalangeal joint and distal interphalangeal joint. The A2 pulley was carefully preserved. The flexor digitorum superficialis was cut just at the proximal edge of the proximal phalanx to ensure that only the FDP would glide [Fig. 1(A)]. To simplify the measurement of its interaction with the FDS,11,12 a 1.8-mm Kirschner wire was inserted through the PIP joint to maintain it in an extended position.11,13
Figure 1.
The schema of the specimen. The flexor digitorum superficialis was cut at the proximal edge of proximal phalanx to ensure a smooth gliding bed for the FDP. B: Testing apparatus for the measurement of gliding resistance between the tendon and pulley. The measurement was carried out in a saline bath at 37°C. The tendon was pulled proximally by the actuator against the weight at a rate of 2.0 mm/s.
Treatment of the extrasynovial tendon by CD-HA
The reaction components included sodium hyaluronate (HA), 95%, >1.5 × 106 Mw, from the fermentation of Streptococcus equi zooepidemicus (Acros Organics, Pittsburgh, PA), gelatin (from porcine skin, Sigma Chemical, St. Louis, MO), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC; Sigma), and N-hydroxysuccinimide (NHS; Pierce, Rockford, IL) in a saline solution [0.9% NaCl, 0.1 M 2-(N-Morpholion) ethanesulfonic acid (MES, pH 6.0; Sigma)]. MES was used to control the pH of the saline.8 HA, EDC, and NHS were first mixed and put into a syringe, and the 10% gelatin was put into another syringe. These two syringes were connected with one common needle and were injected simultaneously into one dish; the tendons were then dipped into this mixture.8 All tendons were immersed for 30 s in their respective HA-gelatin combinations at 37°C, were allowed to ‘cure’ for the respective time (5, 30, or 60 min) in a smooth rubber sheet (a section of the elastic bandage used to exsanguinate the limb), within a saline moistened towel to keep the tendon hydrated until grafting, and were then tested. During the cure time, the carbodiimide derivatization reaction was progressing in the moist environment. At the end of that time, excess reagent was washed off and the tendon was tested.
The gliding resistance was first measured for the normal PL tendons. The PL tendons were then randomly divided into 10 treatment groups of four tendons each (Table I).
TABLE I.
Testing Group
| Group | Gelatin (%) |
HA (%) |
EDC (%) |
NHS (%) |
Exposure (s) |
Cure (min) |
|---|---|---|---|---|---|---|
| A5 | 10 | 0.5 | 0.25 | 0.25 | 30 | 5 |
| A30 | 10 | 0.5 | 0.25 | 0.25 | 30 | 30 |
| A60 | 10 | 0.5 | 0.25 | 0.25 | 30 | 60 |
| B5 | 10 | 0.5 | 1 | 1 | 30 | 5 |
| B30 | 10 | 0.5 | 1 | 1 | 30 | 30 |
| B60 | 10 | 0.5 | 1 | 1 | 30 | 60 |
| C5 | 10 | 1 | 1 | 1 | 30 | 5 |
| C30 | 10 | 1 | 1 | 1 | 30 | 30 |
| C60 | 10 | 1 | 1 | 1 | 30 | 60 |
| D | 0 | 0 | 0 | 0 | 0 | 60 |
“Exposure” is the time that the tendons were immersed in one of the HA-gelatin combinations at 37°C. “Cure” is the time after treatment that these tendons were kept at 37°C before implantation.
Measurement of frictional force
The gliding resistance test was performed between the PL tendon and the ipsilateral proximal pulley of the 2nd digit in a custom testing jig, as described previously8,12,14 [Fig. 1(B)], with the proximal tendon at an angle of 30° from the horizontal, and the distal tendon at an angle of 20° from the horizontal, and a distal load of 4.9 N. Immediately after treatment, the tendon was placed in a saline bath (ISOTEMP 202, Fisher Scientific, Houston, TX) at 37°C for testing. Testing time was ~20 min. The excursion distance was 14 mm, an average distance for canine digital flexor tendon excursion.12 The rate of movement was 2 mm/s. Gliding resistance between the tendon and proximal pulley was recorded until 200 cycles, after every 25 cycles from 200 to 1000. The reading from the load transducers and the corresponding excursion were recorded by a computer workstation at a sampling rate of 20 Hz. The tendon gliding resistance over the total excursion was summarized by averaging the results from flexion and extension and was calculated as (F2flexion – F2extension)/2.
Statistical analysis
Two-way ANOVA was used to compare the friction data among the nine groups excluding group D (control) after 1000 cycles. For effects of interest, difference in means and 95% confidence intervals were determined. A p-value of less than 0.05 was considered to indicate statistical significance in all cases. The analyses were performed with JMP 4 software (SAS Institute).
RESULTS
The gliding resistance of the tendons before surface treatment was not significantly different among the 10 groups of tendons (Fig. 2).
Figure 2.
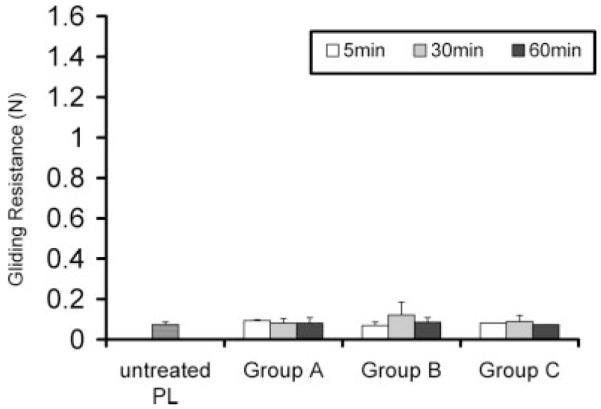
Gliding resistance of PL tendon before surface treatment.
After treatment, the physical appearance of the tendons changed somewhat, as the coating provided a slippery surface that was initially a few millimeters thick on removing the tendon from the saline soaked towel. However, this appeared to be simply excess reaction materials, as the thick coating was removed by the first few repetitions, during which gliding resistance varied somewhat (Fig. 3). After that, the resistance stabilized for the remainder of testing. Tendon stiffness was grossly similar before and after treatment, and there was no visible swelling or shrinking of the tendon itself.
Figure 3.
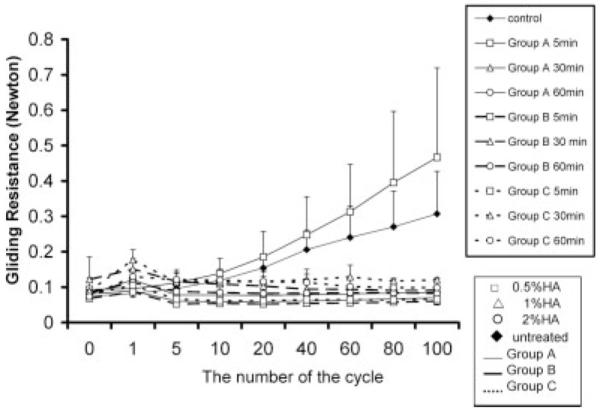
Gliding resistance from 0 to 100 cycles. The x-axis is not equally spaced to show detail at the earlier cycles.
After 1000 cycles, gliding resistance of group A5 (0.91 N) was similar to the untreated PL tendon (0.81 N) (group D). The gliding resistances for groups A30 and A60 were less than 0.31 N and significantly less than the values for groups A5 (p < 0.05) (Fig. 4). However, in groups A30 and A60, the gliding resistance gradually increased over the 1000 cycles of motion. In group B, the gliding resistances of B5, B30, and B60 remained stably low (<0.14 N) up to 1000 cycles (Fig. 5). In group C, C5, and C60 was stably low (<0.15 N) but the gliding resistance of C30 gradually increased to 0.21 N (Fig. 6).
Figure 4.
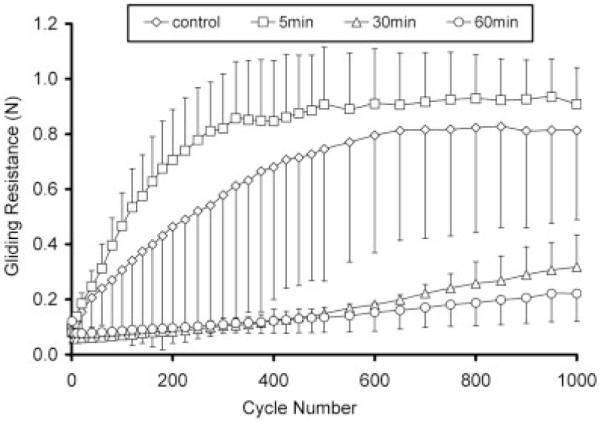
Group A: Gliding resistance of PL tendon treated (0.5% HA, 0.25% EDC/NHS) under various times and control.
Figure 5.
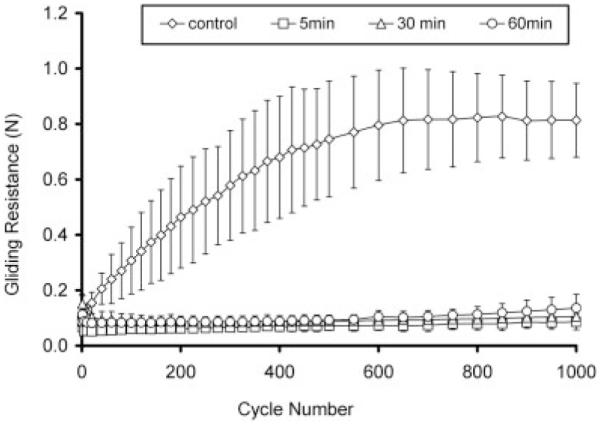
Group B: Gliding resistance of PL tendon treated (0.5% HA, 1% EDC/NHS) under various times and control.
Figure 6.
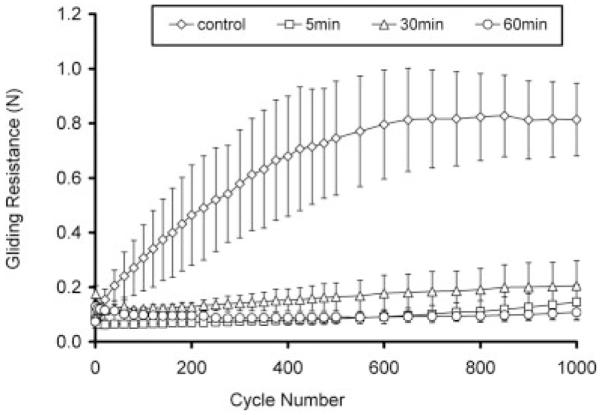
Group C: Gliding resistance of PL tendon treated (1% HA, 1% EDC/NHS) under various times and control.
There were significant differences in gliding resistance due to treatment time and surface treatment. There was also a significant interaction between the two main effects (Fig. 7) after 1000 cycles.
Figure 7.
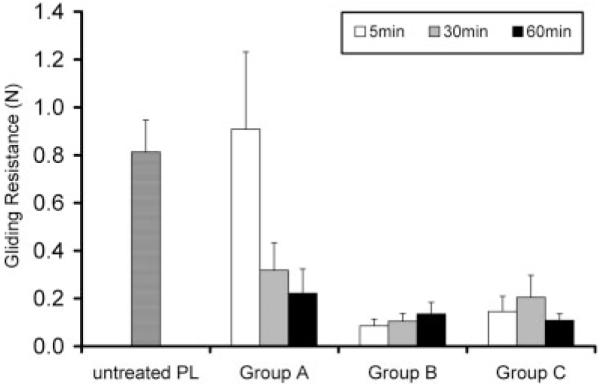
Gliding resistance of PL tendon after treatment at 1000 cycles. There were significant differences in friction due to time and treatment group and the interaction between time and treatment group was significant (p < 0.05).
DISCUSSION
The carbodiimide derivatization, a chemical modification of HA, has been used clinically for many years, usually in the form of prefabricated films. More recently, the use of this reaction in situ has been investigated in vitro, as a treatment to improve the gliding of a tendon graft.8,12,15 Momose et al. reported that treated (1% HA, 1% EDC, 1.5% NHS) tendons had a significantly lower gliding resistance than the untreated and unmodified HA-treated tendons.12 However, their experiment was only for 100 cycles of flexion/extension and the treatment time was 2 h, which is rather long for clinical use. Sun et al. added gelatin to the reaction mixture (1% HA, 0.25% EDC/NHS, 10% gelatin) and showed that treated tendons had significantly lower gliding resistance than untreated or carbodimide derivatized HA treated tendons after 500 cycles in a canine model,8 with a 1 h treatment time.
Since it has been shown that the carbodiimide-driven cross-linking reaction of amino and carboxyl groups progresses for 12 h,16 it is pertinent to know at what point sufficient crosslinking has occurred to prevent abrasion of the linked compounds with clinically relevant motion. The answer to this question will determine how long the ex vivo reaction must proceed before implantation. After implantation, the reaction may continue, but if the lubricating compound is not sufficiently fixed, in vivo motion will remove it prematurely. We have found that in this tendon surface treatment, a lower EDC/NHS concentration requires a longer reaction time for initial fixation, while with higher concentrations of EDC and NHS even a 5-min fixation time is sufficient to improve gliding resistance and to protect from subsequent abrasion with 1000 repetitions of simulated motion.
Sun et al. obtained a significantly lower gliding resistance than that noted in previous reports.8 While previous studies had used hyaluronic acid from rooster comb, Sun and coworkers used hyaluronic acid from Streptococcus equi zooepidemicus and added gelatin to the reaction mixture.8,17 However, Sun and coworkers only tested one combination of reagents (1% HA, 0.25% EDC/NHS, 10% gelatin).17 On the basis of the current in vitro study, we believe that the concentration of HA and any reactive agents are critical factors in determining the ultimate HA persistence after repetitive motion, and that the effect of the concentration of the EDC and NHS has a more important role than that of the HA in this regard. On the basis of our data, 0.5% HA, 0.25% EDC/NHS, and 10% gelatin (group A), 0.5% HA, 1% EDC/NHS, and 10% gelatin (group B), and 1% HA, 1% NHS/EDC, and 10% gelatin (group C) were the most effective combinations to reduce friction, enhance binding strength, and increase the residual HA on the tendon surface after 1000 flexion–extension cycles in vitro. This study showed that groups B and C were not dependent upon the reaction time.
We wished to select a number of repetitions large enough to provide an opportunity to abrade away the test preparation, while being mindful of the fact that extended simulations in vitro may not be relevant to in vivo conditions. 1000 cycles is a round number, and the testing could be completed in roughly 20 min. The rate, less than 1 Hz, is in the physiological range. As most postoperative tendon therapy regimens suggest 10 or more hourly repetitions, this number of cycles would be experienced in the critical first week after surgery, when adhesions begin to form.12
Repetitive cycling alters the viscoelasticity of the tendon, may affect the swelling/shrinking or other tendon properties, and could thereby affect our resistance measures. Indeed, we noted changes in the first five cycles which may well represent viscoelastic conditioning. After that, the results were very consistent, suggesting that further viscoelastic changes did not take place.
The principal limitation of this study is that it was performed in vitro. Thus, no effect of enzymatic digestion of our preparation could be observed, and the effect of this surface treatment on tendon viability and healing could not be assessed. The strength of this study is that we have studied a clinically relevant treatment which can improve the gliding resistance of tendon grafts and have shown that as little as 5 min ex situ is sufficient set up time to avoid abrasion of the curing surface treatment in situ. This is important, as it makes clinical application of intra-operative surface treatment practical. We plan to evaluate this possibility in vivo in future studies.
Acknowledgments
Contract grant sponsor: National Institutes of Health; contract grant number: AR49407
References
- 1.Komurcu M, Akkus O, Basbozkurt M, Gur E, Akkas N. Reduction of restrictive adhesions by local aprotinin application and primary sheath repair in surgically traumatized flexor tendons of the rabbit. J Hand Surg [Am] 1997;22:826–832. doi: 10.1016/S0363-5023(97)80076-7. [DOI] [PubMed] [Google Scholar]
- 2.Gelberman RH, Seiler JG, III, Rosenberg AE, Heyman P, Amiel D. Intercalary flexor tendon grafts. A morphological study of intrasynovial and extrasynovial donor tendons. Scand J Plast Reconstr Surg Hand Surg. 1992;26:257–264. doi: 10.3109/02844319209015268. [DOI] [PubMed] [Google Scholar]
- 3.Seiler JG, III, Chu CR, Amiel D, Woo SL, Gelberman RH. The Marshall R. Urist Young Investigator Award. Autogenous flexor tendon grafts. Biologic mechanisms for incorporation. Clin Orthop Relat Res. 1997;345:239–247. [PubMed] [Google Scholar]
- 4.Kuo JW, Swann DA, Prestwich GD. Chemical modification of hyaluronic acid by carbodiimides. Bioconjugate Chem. 1991;2:232–241. doi: 10.1021/bc00010a007. [DOI] [PubMed] [Google Scholar]
- 5.Hanthamrongwit M, Reid WH, Grant MH. Chondroitin-6-sulphate incorporated into collagen gels for the growth of human keratinocytes: The effect of cross-linking agents and diamines. Biomaterials. 1996;17:775–780. doi: 10.1016/0142-9612(96)81414-1. [DOI] [PubMed] [Google Scholar]
- 6.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152–169. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Staros JV, Wright RW, Swingle DM. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986;156:220–222. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- 8.Sun YL, Yang C, Amadio PC, Zhao C, Zobitz ME, An KN. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res. 2004;22:984–989. doi: 10.1016/j.orthres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Stark HH, Boyes JH, Johnson L, Ashworth CR. The use of paratenon, polyethylene film, or silastic sheeting to prevent restricting adhesions to tendons in the hand. J Bone Joint Surg Am. 1977;59:908–913. [PubMed] [Google Scholar]
- 10.Schneider L. Flexor tendons-late reconstruction. In: Green D, Hotchkiss R, Pederson W, editors. Green’s Operative Hand Surgery. Churchill Livingstone; Philadelphia: 1999. pp. 1898–1949. [Google Scholar]
- 11.Momose T, Amadio PC, Zhao C, Zobitz ME, Couvreur PJ, An KN. Suture techniques with high breaking strength and low gliding resistance: Experiments in the dog flexor digitorum profundus tendon. Acta Orthop Scand. 2001;72:635–641. doi: 10.1080/000164701317269085. [DOI] [PubMed] [Google Scholar]
- 12.Momose T, Amadio PC, Sun YL, Zhao C, Zobitz ME, Harrington JR, An KN. Surface modification of extrasynovial tendon by chemically modified hyaluronic acid coating. J Biomed Mater Res. 2002;59:219–224. doi: 10.1002/jbm.1235. [DOI] [PubMed] [Google Scholar]
- 13.Uchiyama S, Amadio PC, Coert JH, Berglund LJ, An KN. Gliding resistance of extrasynovial and intrasynovial tendons through the A2 pulley. J Bone Joint Surg Am. 1997;79:219–224. doi: 10.2106/00004623-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Coert JH, Uchiyama S, Amadio PC, Berglund LJ, An KN. Flexor tendon-pulley interaction after tendon repair. A biomechanical study. J Hand Surg [Br] 1995;20:573–577. doi: 10.1016/s0266-7681(05)80113-5. [DOI] [PubMed] [Google Scholar]
- 15.Menderes A, Mola F, Tayfur V, Vayvada H, Barutcu A. Prevention of peritendinous adhesions following flexor tendon injury with seprafilm. Ann Plast Surg. 2004;53:560–564. doi: 10.1097/01.sap.0000134507.00053.1a. [DOI] [PubMed] [Google Scholar]
- 16.Sehgal D, Vijay IK. A method for the high efficiency of water-soluble carbodiimide-mediated amidation. Anal Biochem. 1994;218:87–91. doi: 10.1006/abio.1994.1144. [DOI] [PubMed] [Google Scholar]
- 17.Yang C, Amadio PC, Sun YL, Zhao C, Zobitz ME, An KN. Tendon surface modification by chemically modified HA coating after flexor digitorum profundus tendon repair. J Biomed Mater Res B: Appl Biomater. 2004;68:15–20. doi: 10.1002/jbm.b.10074. [DOI] [PubMed] [Google Scholar]