Abstract
Background
Clinical and experimental studies have demonstrated that restrictive adhesions and poor digital motion are common complications after extrasynovial tendon grafting in an intrasynovial environment. The purpose of this study was to test the hypothesis that surface modification of an extrasynovial tendon with use of a carbodiimide-derivatized hyaluronic acid-gelatin polymer (cd-HA) improves gliding ability and digital function after tendon grafting in a canine model in vivo.
Methods
The peroneus longus tendons from both hindpaws of twenty-four dogs were harvested and transplanted to replace the flexor digitorum profundus tendons in the second and fifth digits of one forepaw. Prior to grafting, one of the peroneus longus tendons was coated with cd-HA, which consists of 1% hyaluronic acid, 10% gelatin, 0.25% 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), and 0.25% N-hydroxysuccinimide (NHS), while the other was immersed in saline solution only. Eight dogs were killed at one, three, and six weeks. Digital normalized work of flexion, tendon gliding resistance, and hyaluronic acid quantification (with the hyaluronic acid-binding-protein staining technique) were the outcome measures.
Results
The normalized work of flexion of the tendons treated with cd-HA was significantly lower than that of the saline-solution-treated controls at each time- point (p < 0.05). The gliding resistance of the cd-HA group was significantly lower than that of the saline-solution group at three and six weeks (p < 0.05). The ratio between the intensity of staining of the cd-HA treated tendons with that of the saline solution treated controls was significantly greater at time-0 than at three or six weeks (p < 0.05), but there was no significant difference between time-0 and one-week values.
Conclusions
Treating the surface of an extrasynovial tendon autograft with a carbodiimide-derivatized hyaluronic acid-gelatin polymer decreases digital work of flexion and tendon gliding resistance in this flexor tendon graft model in vivo.
Clinical Relevance
cd-HA gelatin may provide surgeons with a new and useful method to improve the quality of tendon graft surgery.
Although there has been a marked improvement in the outcomes after flexor tendon laceration with new regimens of direct repair and postoperative controlled mobilization1-5, adhesion formation continues to be a difficult problem after flexor tendon repair, especially in zone II6-11. A tendon graft is still indicated when a flexor tendon repair fails or when tendon rupture or tendon transfer requires elongation of the muscle-tendon unit10,12-14. While the tendon graft plays a very important role in reconstructions performed to restore finger function10,15-17, clinical studies have demonstrated that restrictive adhesions and poor digital motion are frequent sequelae of tendon grafting5,18-20. Although flexor tendons are intrasynovial in zone II (i.e., the tendon system includes a synovially lined sheath21,22), most donor tendons come from extrasynovial sources. Studies of animal models have shown that extrasynovial tendon grafts are associated with more adhesions to the surrounding tissue than are intrasynovial tendon grafts22-24. Unfortunately, potential sources of intrasynovial tendons available for use as tendon grafts are limited.
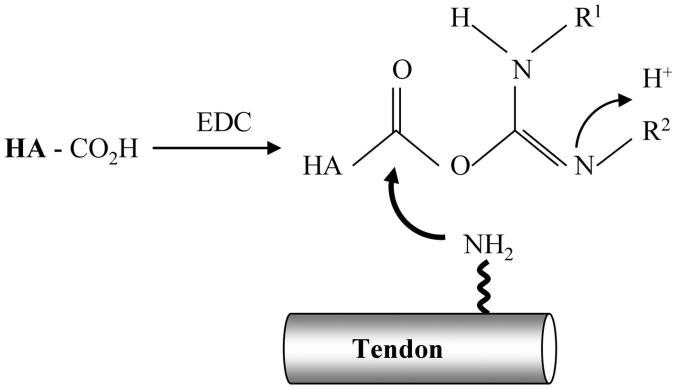
The effect of hyaluronic acid on flexor tendon repair has been investigated in experimental and clinical studies25-29. Some studies have suggested that exogenously applied hyaluronic acid may prevent formation of adhesions between the flexor tendon and the surrounding tissue following tendon repair without affecting healing30-33. However, other in vivo studies have demonstrated contradictory results26,34,35. As the half-life of hyaluronic acid in tissues is short, native hyaluronic acid is probably eliminated too rapidly to maintain a long-lasting physical barrier between opposing tissues36. In addition, the flexor sheath usually cannot be closed after tendon injury, repair, or grafting, thereby limiting the volume of exogenous hyaluronic acid that remains in contact with the tendon. Maintaining a strong attachment between hyaluronic acid and the tendon surface may actually be more important than the absolute concentration of the hyaluronic acid, as abrasion during tendon gliding constantly threatens to remove hyaluronic acid from the tendon surface. Recently, carbodiimide derivatization has been developed to modify hyaluronic acid (cd-HA) for clinical use37-39. Modification of glucuronides requires activation of the carboxyl groups, which can be accomplished with use of a water-soluble carbodiimide such as 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) as the condensing agent. EDC activates carboxyl groups of the hyaluronic acid molecule and forms the intermediate O-acylisourea, which can chemically bind to exposed amino groups, such as those in the collagenous tendon matrix, forming a new collagen-hyaluronic acid polymer, fixed to the tendon surface (Fig. 1). This modification of hyaluronic acid is believed to decrease water solubility, increase binding strength, and therefore increase tissue residence time40. Recent reports on carbodiimide-modified hyaluronic acid combined with gelatin on the surface of extrasynovial tendons noted decreased gliding resistance between the tendon and its pulley system during repeated flexion-extension motion over 500 cycles in a canine model in vitro41,42. The purpose of the current study was to investigate whether such a modified extrasynovial tendon, treated with cd-HA, would show improved performance in vivo, as demonstrated by increased tendon gliding ability, decreased digital work of flexion, and reduced adhesion formation.
Fig. 1.
Carboxyl groups in the hyaluronic acid (HA) are activated by EDC to form O-acylisoureas, which bind to the amino groups on the tendon surface.
Materials and Methods
Study Design
Twenty-four dogs were used for this study, which was approved by our Institutional Animal Care and Use Committee. The dogs were evenly divided into three groups depending on whether they were killed at one, three, or six weeks. The peroneus longus tendons of the two hindlegs (extrasynovial tendons) were grafted into the second and fifth digits of one forepaw in each dog. One tendon was treated with cd-HA (described below) prior to grafting, while the other was immersed in saline solution as a control. The digits were randomly assigned to the treatment and control groups. On the day on which the animal was killed, the function of the operatively treated digits was assessed by measuring the work of flexion and frictional force of the grafted tendon. Following the mechanical evaluation, the grafts were stained to evaluate the residual surface hyaluronic acid concentration with use of biotinylated hyaluronic acid-binding-protein staining.
Surgical Procedures and Tendon Modification
The dogs were anesthetized with intravenous ketamine and diazepam. The selected forelimb and both hindlimbs were shaved, scrubbed with povidone iodine, and sterilely draped. The peroneus longus tendons were taken from the musculotendinous junction to the ankle level, a length of approximately 10 to 15 cm. One of the two peroneus longus tendons was randomly selected for the treatment group and was immersed for thirty seconds in cd-HA solution, consisting of 1% sodium hyaluronate (95%; Acros, Geel, Belgium), 10% gelatin (from porcine skin; Sigma Chemical, St. Louis, Missouri), 0.25% 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) (Sigma), and 0.25% N-hydroxysuccinimide (NHS) (Sigma) in 0.1-M NaCl (pH 6.0) and 0.9% phosphate-buffered saline solution41. When the hyaluronic acid is mixed with the EDC, the reaction quickly forms a gel-like mixture. The tendon should be put into this mixture as the gel begins to form in order to achieve strong binding to the tendon surface. To keep it hydrated until grafting, the treated tendon was wrapped with a smooth rubber sheet (a section of the elastic bandage used to exsanguinate the limb) and a towel moistened with saline solution was wrapped around the rubber sheet. The control tendon was immersed in 0.9% NaCl solution and similarly wrapped.
After the peroneus longus tendons were harvested and treated, a proximal radial neurectomy was performed through a lateral humeral incision on the selected recipient forelimb, in order to denervate the triceps muscle and prevent elbow extension and thus weight-bearing. An elastic bandage was then used to exsanguinate the forelimb and to act as a tourniquet for the distal part of the procedure.
The flexor tendons were approached through a midlateral incision at the level of the distal interphalangeal joint. The flexor digitorum profundus tendon was sharply divided at a level 5 mm proximal to the tendon-bone insertion site. The distal portion of the flexor digitorum profundus tendon was connected to the proximal end of the tendon graft by a towing suture approximately 20 cm in length. A second incision was then made at the middle metacarpal level to expose the flexor digitorum profundus tendon proximal to the flexor sheath. By means of traction on the proximal part of the flexor digitorum profundus in the proximal incision, the flexor digitorum profundus tendon was carefully delivered from the sheath, with the towing suture left within the sheath. Then 50 mm of the flexor digitorum profundus tendon was excised with a transverse cut. The peroneus longus tendon graft was cut transversely to approximately 80 mm in length and was pulled through the flexor sheath by the towing suture, again by means of traction from the proximal incision. Approximately 10 mm of the distal part of the tendon graft was left to overlap the distal stump of the flexor digitorum profundus tendon (5 mm was left to insert into the osseous tunnel and 5 mm, to overlap the flexor digitorum profundus tendon stump), while 20 mm of the proximal graft was used to overlap the proximal end of the flexor digitorum profundus tendon, to create the proximal graft junction tendon weave. In this way, the length of the flexor digitorum profundus tendon that was removed (approximately 50 mm) and the interpositional length of the graft were kept the same. A 3-mm-diameter drill was then used to make a tunnel in the distal phalanx. A 20-gauge needle was driven through this tunnel, emerging dorsally through the middle third of the nail. The distal end of the peroneus longus tendon was sutured with a Pennington loop of 3-0 nylon suture. The suture was passed through the needle and the needle was withdrawn, bringing the suture out through the nail. The end of the graft was fed into the canal. The suture was tied over a button on the nail. For additional reinforcement, the juncture of the overlapping graft and the stump of the flexor digitorum profundus tendon was sutured with a 4-0 Ethibond (Ethicon, Somerville, New Jersey).
The proximal repair of the graft with the flexor digitorum profundus recipient was done with an interlace suture technique. The flexor digitorum profundus tendon was slit at its end, and the graft was threaded into the slit. Four corners of the interface of two tendons were sutured with 4-0 Ethibond suture (Fig. 2). The graft was then threaded transversely in a different plane of the slit with the same technique of suture reinforcement. The fish-mouth thus created was closed with 4-0 Ethibond suture to embrace the graft. Finally, the skin was closed with 3-0-Vicryl suture (polyglactin; Ethicon, Somerville, New Jersey).
Fig. 2.
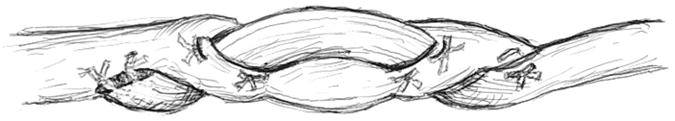
Illustration of the proximal suture technique.
Postoperative Care and Rehabilitation
Following recovery from the anesthesia, the dogs were fitted with a custom-made sling-jacket to support the operatively treated forelimb on the front of the chest with the elbow and wrist in flexion. The wounds were checked and cleaned beginning at three days postoperatively. Five days after the surgery, rehabilitation commenced with use of a synergistic motion protocol43 consisting of twenty repeated motions twice daily, seven days per week. Following the therapy, the sling-jacket was repositioned in order to keep the limb on the front of the chest. This regimen continued until the animal was killed at the designated time.
Measurement of Digital Work of flexion
After the animal was killed with an overdose of pentobarbital, both forepaws were amputated and the second and fifth digits of both the surgically treated and the non-surgically treated paws were harvested. The grafted tendons were carefully exposed at the proximal junction level. The proximal grafttendon junction was dissected free from the surrounding tissues. The proximal end of the tendon graft was sutured to a cable that was connected to a load transducer. The tendon graft within zone II, including the distal attachment, which was kept enclosed by skin, was all preserved. A Kirschner wire was inserted longitudinally through the metacarpal bone to fix the metacarpophalangeal joint in extension. Two small metal beads (approximately 0.5 mm in diameter) were inserted into the middle phalanx and a short Kirschner wire was inserted into the distal part of the nail to serve as markers for the phalanges.
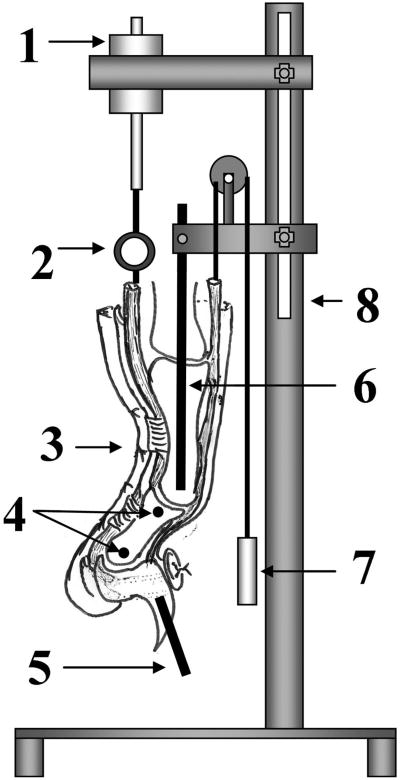
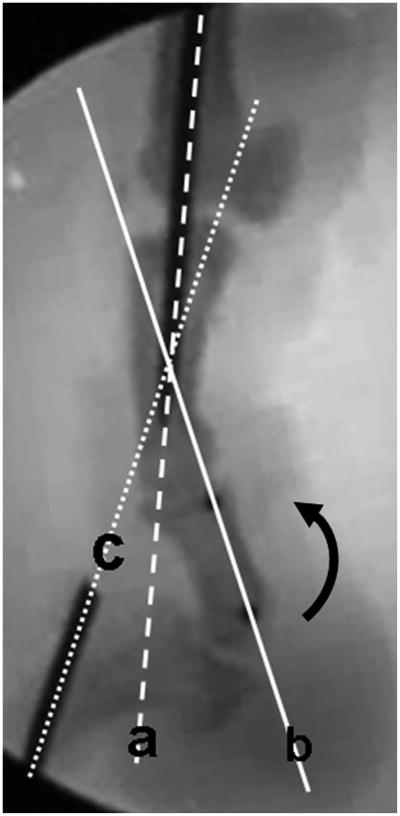
The prepared digit was mounted on the testing device by fixing the proximal Kirschner wire to a custom jig. The testing device consisted of a testing frame, actuator, linear potentiometer, and one load transducer. A 0.5-N weight was attached to the extensor tendon to ensure full extension of the digit as a starting position and to apply an initial tension to the graft (Fig. 3). A 0.1-N preload was applied to the proximal part of the graft before testing. The actuator pulled the tendon proximally at a rate of 2 mm/sec, causing digital flexion. Data from the linear potentiometer and the proximal load transducer were recorded at 20 Hz. During the testing, digital motion (from extension to flexion) was recorded simultaneously by digital fluoroscopy. The video images were digitized with use of Analyze Software (Biomedical Imaging Resource, Mayo Clinic, Rochester, Minnesota). Then, the range of motion of the proximal and distal interphalangeal joints was determined by calibrating the digitized data of the metal markers (a metal pin in the proximal phalanx, two metal beads in the middle phalanx, and a metal pin in the distal phalanx) (Fig. 4). The measurement of work of flexion continued until the distal interphalangeal joint angle reached 40° of flexion, on the basis of a previously published study44. Work-of-flexion data were calculated from the tendon displacement versus loading curve during digital flexion and then were normalized (divided) by the total proximal interphalangeal and distal interphalangeal joint motion angle at the point where the distal interphaangeal joint reached 40°44.
Fig. 3.
Work-of-flexion tester. 1 = actuator, 2 = load transducer, 3 = specimen, 4 = two metal beads in the middle phalanx, 5 = metal pin in the distal phalanx, 6 = metal pin in the proximal phalanx, 7 = weight, and 8 = frame.
Fig. 4.
Image obtained with fluoroscopy during testing. The curved arrow shows the direction of digital flexion. The angle between line a (the pin in the proximal phalanx) and line b (the line along the two metal beads in the middle phalanx) represents the proximal interphalangeal joint. The angle between line b and line c (the pin in the distal phalanx) represents the distal interphalangeal joint.
Measurement of Tendon Frictional Force
Following the measurement of work of flexion, the tendon graft was further dissected, with the proximal pulley kept intact. The gliding resistance between the tendon graft and the proximal pulley was measured with use of a custom tendon-pulley friction testing device, as previously described45,46.
Quantification of Hyaluronic Acid on the Tendon Graft Surface
After measurement of the frictional force, a 10-mm segment of the central portion of the tendon graft was excised and was stained with biotinylated hyaluronic acid-binding-protein. For the purpose of comparison, four peroneus longus tendons treated with cd-HA and four untreated peroneus longus tendons were used for time-0 staining. For this analysis, the graft segment was incubated in 1% hydrogen peroxide for five minutes after a wash in phosphate-buffered saline solution. Then the tendon was blocked with 1% bovine serum albumin in phosphate-buffered saline solution for five minutes and was incubated in biotinylated hyaluronic acid-binding protein (Calbiochem; EMD Biosciences, San Diego, California) in 1% bovine serum albumin in phosphate-buffered saline solution overnight at 4°C. An ABC Vectastain kit (Vector Laboratories, Burlingame, California) was prepared according to the manufacturer's instructions, and the tendon graft was incubated with the ABC Vectastain for thirty minutes at room temperature. After washing with phosphate-buffered saline solution for five minutes, the sample was incubated with diaminobenzidine (DAB) for five minutes and then washed in distilled water three times. The paired tendons (cd-HA and saline-solution groups) from each dog were placed together on a white background and were photographed with an Olympus C-7070 digital camera (Olympus America, Melville, New York), with use of a constant background, lighting, distance, and camera settings. The color images were converted to gray-scale images with use of Adobe Photoshop 6.0 (Adobe Systems, San Jose, California). The intensity of the gray scale was analyzed with use of Scion Image Software (Scion, Frederick, Maryland).
Scanning Electron Microscopy
In order to better observe the tendon surface, two tendons from each of the cd-HA and saline-solution-treated groups were examined with scanning electron microscopy. The tendons were fixed in 1% glutaraldehyde and 4% formaldehyde in 0.1-M phosphate-buffered saline solution and were dehydrated through a graded series of ethanol solutions in a critical point dryer. The specimens were then attached to stubs, sputter-coated with a gold-palladium mixture, and viewed in a Hitachi 4700 scanning electron microscope (Hitachi Scientific Instruments, Pleasanton, California) at 5 kV.
Statistical Methods
The data obtained from the measurements of work of flexion and frictional force were analyzed with use of two-factor (time and treatment) repeated analysis of variance, followed by the Tukey Studentized range (HSD [honestly significant difference]) post hoc test to compare control, cd-HA , and normal digits at three time points (one, three, and six weeks). For the comparison of the hyaluronic acid concentrations at different time points, the intensity ratio between the cd-HA and saline-solution groups at each time point was evaluated with one-way analysis of variance. In all cases, a level of p < 0.05 was considered to be significant.
Results
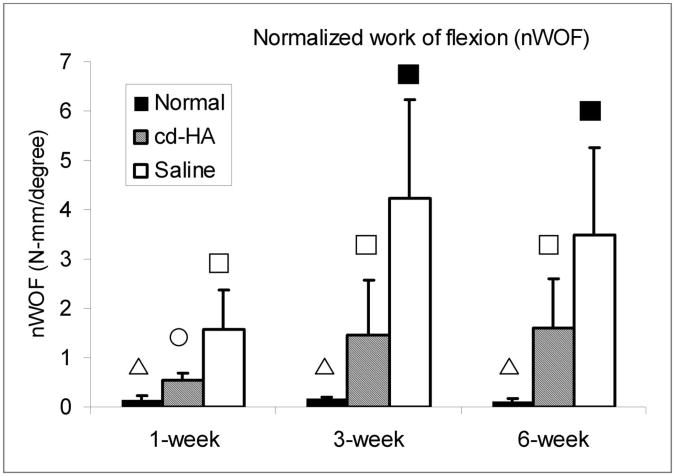
None of the grafted digits showed infection or rupture. The normalized work of flexion of the grafted digits in both the cd-HA-treated and the control group were significantly higher than that of the normal digits at one, three, and six weeks (p < 0.05). The normalized work of flexion of the cd-HA-treated grafts was significantly lower than that of the saline-solution-treated grafts at all three time points (p < 0.05). There was no significant difference among the findings at one, three, and six weeks in the normal digits. However, the normalized work of flexion in the cd-HA-treated and saline-solution-treated groups at three weeks and six weeks was significantly increased compared with the normalized work of flexion at one week in the respective groups (p < 0.05). There was no significant difference in the normalized work of flexion between the three and six-week grafts in either the cd-HA or the saline-solution treated group (Fig. 5).
Fig. 5.
The work of flexion normalized according to the proximal and distal interphalangeal joint angle (normalized work of flexion) in the normal, cd-HA-treated, and saline-solution-treated groups at one, three, and six weeks. A difference in symbols denotes a significant difference between values (p < 0.05), with the triangle being significantly less than the circle, which is significantly less than the open square, which is significantly less than the solid square.
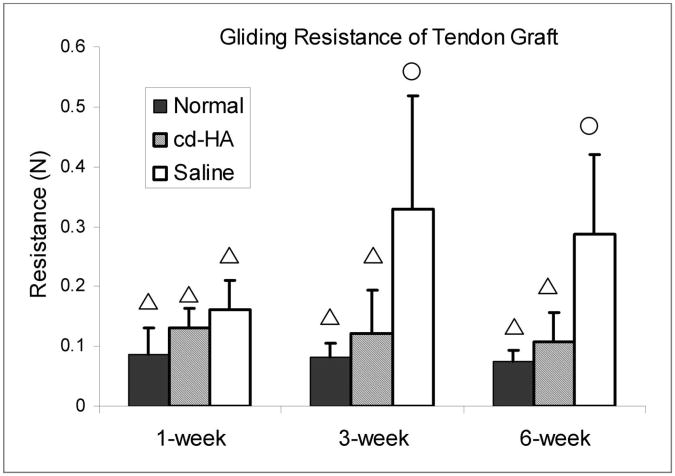
The gliding resistance of the saline-solution-treated grafts was significantly higher than that of the normal flexor digitorum profundus tendons as well as the cd-HA-treated tendon grafts at three and six weeks (p < 0.05). There was no significant difference in the gliding resistance between the normal flexor digitorum profundus tendons and the cd-HA-treated tendon grafts at any time point. The gliding resistance of the saline-solution-treated tendon grafts at three and six weeks was significantly higher than that at one week (p < 0.05). There was no significant difference in the gliding resistance between the three and six week saline-solution-treated grafts (Fig. 6).
Fig. 6.
The gliding resistance in the normal, cd-HA-treated, and saline-solution-treated groups at one, three, and six weeks. A difference in symbols denotes a significant difference between values (p < 0.05), with the triangle being significantly less than the circle.
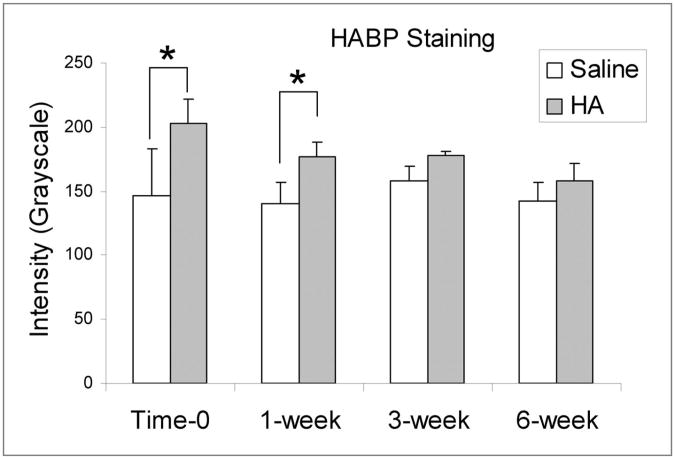
The intensity of the hyaluronic acid staining of the cd-HA-treated tendons was significantly higher than that of the saline-solution-treated tendons group at time-0 and at one week (p < 0.05). However, there was no significant difference between these two groups at three or six weeks (p > 0.05) (Fig. 7). The intensity ratio between the cd-HA and saline-solution-treated tendons at time-0 was significantly higher than that at three or six weeks (p < 0.05). There was no significant difference between the time-0 and one-week tendons.
Fig. 7.
The staining intensity of the cd-HA and saline-solution-treated tendon grafts at one, three, and six weeks. The asterisk denotes a significant difference (p < 0.05). HABP = hyaluronic acid binding protein.
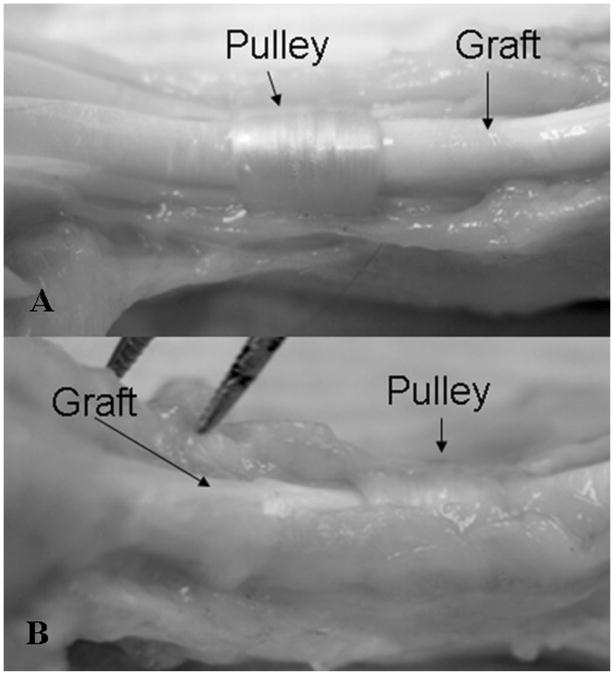
As observed during dissection, adhesions occurred mainly at the proximal and distal repair sites in all grafts. The cd-HA-treated grafts seemed to have less adhesion along the graft itself than did the saline-solution-treated grafts. The tendon graft surface within the flexor sheath was smooth and shining in the cd-HA group but not in the control group, in which the surface was rougher because of the greater number of adhesions. It was difficult to separate the tendon graft from the surrounding soft tissue, especially in the case of the six-week specimens, in the control group (Fig. 8). Evaluation with scanning electron microscopy confirmed that cd-HA treatment resulted in a smooth surface, whereas the control tendons presented a rough surface (Fig. 9). However, we did not evaluate adhesions quantitatively because these observations were made after the work-of-flexion test, which broke some of the adhesions before they could be dissected and examined.
Fig. 8.
A: cd-HA-treated tendon graft harvested at six weeks, showing a smooth surface without adhesions to the flexor sheath. B: Saline-solution-treated tendon graft harvested at six weeks, showing severe adhesions between the graft and the flexor sheath. It was difficult to separate the tendon from the sheath.
Fig. 9.
A: cd-HA-treated tendon graft harvested at six weeks, showing a smooth surface under scanning electron microscopy (×30). B: Saline-solution-treated tendon graft harvested at six weeks, showing a rough surface under scanning electron microscopy (×30).
Discussion
Extrasynovial tendons, such as the palmaris longus47, the plantaris48, and the long-toe extensors49, are the most commonly available tendons for flexor tendon grafting. However, experimental studies have demonstrated that extrasynovial tendon grafts are associated with more adhesions to the surrounding tissue than are intrasynovial tendon grafts22,50. Alternatives intended to improve the results of tendon grafting, such as staged tendon reconstruction19,51,52, use of free vascularized tendon grafts53, or use of tendon graft substitutes54, remain controversial. The ideal tendon graft would be one that allows a single-stage reconstruction with a simple surgical procedure, that can be obtained from a readily available autologous source, and that has the performance of the native intrasynovial tendon. The cd-HA that was developed in this study provides the possibility of achieving this goal, although additional studies are clearly needed and the formulation must be further optimized.
In the current study, the tendon grafts treated with cd-HA exhibited decreased digital work of flexion compared with the untreated grafts. While the reason for this improvement cannot be definitely ascribed to the cd-HA treatment, these in vivo findings are consistent with observations of improved gliding ability after cd-HA treatment in in vitro studies41,55. It is possible that the cd-HA also serves as a mechanical barrier that inhibits adhesion formation in vivo, as an antiadhesive effect of hyaluronic acid has been described41,55. However, treatment of the grafts with cd-HA did not achieve normal function at any time postoperatively. Compared with the saline-solution-treated grafts, the cd-HA-treated tendon grafts had little adhesion within the flexor sheath, but adhesions remained prominent at the proximal and distal repair sites. These are areas where additional measures to reduce adhesions and improve motion might be fruitful. The tendon graft surface was improved after cd-HA treatment, as indicated by decreased frictional force and by the appearance on scanning electron microscopy. This was true even though there was no difference in the intensity of the hyaluronic acid staining between the cd-HA and control groups at three or six weeks. This finding suggests to us that the first three weeks after tendon grafting is a critical time with regard to adhesion formation. A similar observation has been made in other studies45,56.
The modification of hyaluronic acid with use of the carbodiimide (EDC) reaction to create new biopolymers, crosslinking with other biological molecules such as collagen, has been recently and widely investigated for applications such as anti-adhesion membranes40,57 and biodegradable scaffolds58,59, for tissue regeneration, or as timed-release drug-delivery vehicles60,61. However, to our knowledge, we are the first to report the use of cd-HA by direct bonding to the tendon surface to improve flexor tendon grafting in vivo. As the half-life of hyaluronic acid in the tissue is normally less than three days62, the exogenous hyaluronic acid concentration on the tendon surface should decrease to the normal level as a result of hyaluronic acid turnover after that point. However, our data showed that the intensity of the hyaluronic acid staining in the cd-HA group was significantly higher than that in the saline-solution group at one week, which suggests that this chemical modification of hyaluronic acid might prolong its resident time somewhat. It is not clear, however, whether this prolongation is caused by an increased resistance to enzymatic digestion or an increased binding strength of hyaluronic acid to the tendon surface, with delayed physical removal by abrasion.
The principal strength of this study is that it demonstrates that a lubricant, hyaluronic acid, can be bound to the surface of a tendon graft and persist in vivo for at least one week. This may have important clinical implications. However, the study had several limitations as well. First, the tendon graft surgery was performed under ideal conditions, which included a normal digit, a normal flexor digitorum profundus tendon, and a normal flexion sheath, that were quite different from the typical clinical situation of previous injury, a damaged flexor sheath, and, often, prior failed tendon surgery. Second, a follow-up period of longer than six weeks would be needed to observe the full evolution of the healing period and the resulting changes in mechanical and biological behavior. Third, we did not use a second control group consisting of tendons treated with hyaluronic acid and/or gelatin but without the cd modification. This would have required more animals. We based the decision not to include such a control group in part on previous clinical and animal studies of the use of unmodified hyaluronic acid with tendon surgery26,34,35 as well as published reports in which there was no difference in gliding resistance, after repetitive motion in vitro, between tendons treated with unmodified hyaluronic acid and untreated tendons41,55. Finally, while the hyaluronic acid-binding-protein technique has been well studied and accepted as a sensitive and specific hyaluronic acid staining method63-68, it is only semiquantitative as a result of a variety of factors including, but not limited to, sample preparation (frozen or paraffin), dilution of the antibody, staining techniques (for example, washing duration and frequency), varying photographic conditions and lighting, and selection of the analytic area. Using the ratio between the cd-HA and saline-solution groups may have eliminated some errors in photographic and color density analysis as paired tendons (one treated with cd-HA and one treated with saline solution) from the same dog were photographed and analyzed together. However, any comparison across time points was of necessity less precise.
In conclusion, we demonstrated that an extrasynovial tendon graft modified with carbodiimide-derivatized hyaluronic acid improved graft lubrication and resulted in lower digital work of flexion, decreased tendon gliding resistance, and reduced adhesions in a canine in vivo model. We believe that these findings have important clinical implications and that the bonding of lubricants, cytokines, and other bioactive compounds to the tendon surface may improve the results of tendon surgery in the future.
Acknowledgments
In support of their research for or preparation of this manuscript, one or more of the authors received grants or outside funding from the Orthopaedic Research and Education Foundation. None of the authors received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, educational institution, or other charitable or nonprofit organization with which the authors are affiliated or associated.
Footnotes
Supplementary material: Commentary and Perspective, data tables, additional images, video clips and/or translated abstracts are available for this article. This information can be accessed at http://www.ejbjs.org/cgi/content/full/88/10/2181/DC1
Reprints and Permissions: Click here to order reprints or request permission to use material from this article, or locate the article citation on jbjs.org and click on the [Reprints and Permissions] link.
References
- 1.Chow JA, Thomes LJ, Dovelle S, Milnor WH, Seyfer AE, Smith AC. A combined regimen of controlled motion following flexor tendon repair in “no man's land.”. Plast Reconstr Surg. 1987;79:447–55. doi: 10.1097/00006534-198703000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Kleinert HE, Kutz JE, Atasoy E, Stormo A. Primary repair of flexor tendons. Orthop Clin North Am. 1973;4:865–76. [PubMed] [Google Scholar]
- 3.Lister GD, Kleinert HE, Kutz JE, Atasoy E. Primary flexor tendon repair followed by immediate controlled mobilization. J Hand Surg [Am] 1977;2:441–51. doi: 10.1016/s0363-5023(77)80025-7. [DOI] [PubMed] [Google Scholar]
- 4.Small JO, Brennen MD, Colville J. Early active mobilisation following flexor tendon repair in zone 2. J Hand Surg [Br] 1989;14:383–91. doi: 10.1016/0266-7681_89_90152-6. [DOI] [PubMed] [Google Scholar]
- 5.Verdan CE. Half a century of flexor-tendon surgery. Current status and changing philosophies. J Bone Joint Surg Am. 1972;54:472–91. [PubMed] [Google Scholar]
- 6.Amadio PC, Wood MB, Cooney WP, 3rd, Bogard SD. Staged flexor tendon reconstruction in the fingers and hand. J Hand Surg [Am] 1988;13:559–62. doi: 10.1016/s0363-5023(88)80095-9. [DOI] [PubMed] [Google Scholar]
- 7.Jansen CW, Watson MG. Measurement of range of motion of the finger after flexor tendon repair in zone II of the hand. J Hand Surg [Am] 1993;18:411–7. doi: 10.1016/0363-5023(93)90083-f. [DOI] [PubMed] [Google Scholar]
- 8.Kessler I. The “grasping” technique for tendon repair. Hand. 1973;5:253–5. doi: 10.1016/0072-968x(73)90038-7. [DOI] [PubMed] [Google Scholar]
- 9.Lane JM, Black J, Bora FW., Jr Gliding function following flexor-tendon injury. A biomechanical study of rat tendon function. J Bone Joint Surg Am. 1976;58:985–90. [PubMed] [Google Scholar]
- 10.Silfverskiold KL, May EJ. Early active mobilization after tendon transfers using mesh reinforced suture techniques. J Hand Surg [Br] 1995;20:291–300. doi: 10.1016/s0266-7681(05)80081-6. [DOI] [PubMed] [Google Scholar]
- 11.Strickland JW. Flexor tendon repair. Hand Clin. 1985;1:55–68. [PubMed] [Google Scholar]
- 12.Pulvertaft RG. Tendon grafting for the isolated injury of flexor digitorum profundus. Bull Hosp Jt Dis Orthop Inst. 1984;44:424–34. [PubMed] [Google Scholar]
- 13.Strickland JW. Flexor tendon surgery. Part 2: Free tendon grafts and tenolysis. J Hand Surg [Br] 1989;14:368–82. doi: 10.1016/0266-7681_89_90151-4. [DOI] [PubMed] [Google Scholar]
- 14.White WL. Secondary restoration of finger flexion by digital tendon grafts; an evaluation of seventy-six cases. Am J Surg. 1956;91:662–8. doi: 10.1016/0002-9610(56)90301-4. [DOI] [PubMed] [Google Scholar]
- 15.Coenen L, Boeckx W, Gruwez JA. The treatment of flexor tendon lesions of the fingers. Acta Chir Belg. 1981;80:195–204. [PubMed] [Google Scholar]
- 16.Strickland JW. Flexor tendon injuries. Part 3. Free tendon grafts. Orthop Rev. 1987;16:18–26. [PubMed] [Google Scholar]
- 17.Wehbe MA, Mawr B, Hunter JM, Schneider LH, Goodwyn BL. Two-stage flexor tendon reconstruction. Ten-year experience. J Bone Joint Surg Am. 1986;68:752–63. [PubMed] [Google Scholar]
- 18.LaSalle WB, Strickland JW. An evaluation of the two-stage flexor tendon reconstruction technique. J Hand Surg [Am] 1983;8:263–7. doi: 10.1016/s0363-5023(83)80155-5. [DOI] [PubMed] [Google Scholar]
- 19.Strickland JW. Flexor tendon injuries. Part 4. Staged flexor tendon reconstruction and restoration of the flexor pulley. Orthop Rev. 1987;16:78–90. [PubMed] [Google Scholar]
- 20.Tonkin M, Hagberg L, Lister G, Kutz J. Post-operative management of flexor tendon grafting. J Hand Surg [Br] 1988;13:277–81. doi: 10.1016/0266-7681_88_90085-x. [DOI] [PubMed] [Google Scholar]
- 21.Abrahamsson SO, Gelberman RH, Amiel D, Winterton P, Harwood F. Autogenous flexor tendon grafts: fibroblast activity and matrix remodeling in dogs. J Orthop Res. 1995;13:58–66. doi: 10.1002/jor.1100130110. [DOI] [PubMed] [Google Scholar]
- 22.Gelberman RH, Seiler JG, 3rd, Rosenberg AE, Heyman P, Amiel D. Intercalary flexor tendon grafts. A morphological study of intrasynovial and extrasynovial donor tendons. Scand J Plast Reconstr Surg Hand Surg. 1992;26:257–64. doi: 10.3109/02844319209015268. [DOI] [PubMed] [Google Scholar]
- 23.Noguchi M, Seiler JG, 3rd, Boardman ND, 3rd, Tramaglini DM, Gelberman RH, Woo SL. Tensile properties of canine intrasynovial and extrasynovial flexor tendon autografts. J Hand Surg [Am] 1997;22:457–63. doi: 10.1016/S0363-5023(97)80013-5. [DOI] [PubMed] [Google Scholar]
- 24.Seiler JG, 3rd, Chu C, Abrahamsson SO, Gelberman RH. The fate of autogenous tendon grafts. Iowa Orthop J. 1993;13:56–62. [PMC free article] [PubMed] [Google Scholar]
- 25.Amiel D, Ishizue K, Billings E, Jr, Wiig M, Vande Berg J, Akeson WH, Gelberman R. Hyaluronan in flexor tendon repair. J Hand Surg [Am] 1989;14:837–43. doi: 10.1016/s0363-5023(89)80085-1. [DOI] [PubMed] [Google Scholar]
- 26.Hagberg L. Exogenous hyaluronate as an adjunct in the prevention of adhesions after flexor tendon surgery: a controlled clinical trial. J Hand Surg [Am] 1992;17:132–6. doi: 10.1016/0363-5023(92)90128-c. [DOI] [PubMed] [Google Scholar]
- 27.Meyers SA, Seaber AV, Glisson RR, Nunley JA. Effect of hyaluronic acid/chondroitin sulfate on healing of full-thickness tendon lacerations in rabbits. J Orthop Res. 1989;7:683–9. doi: 10.1002/jor.1100070508. [DOI] [PubMed] [Google Scholar]
- 28.Miller JA, Ferguson PL, Powers DL, Burns JW, Shalaby SW. Efficacy of hyaluronic acid/nonsteroidal anti-inflammatory drug systems in preventing postsurgical tendon adhesions. J Biomed Mater Res. 1997;38:25–33. doi: 10.1002/(sici)1097-4636(199721)38:1<25::aid-jbm4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 29.Thomas SC, Jones LC, Hungerford DS. Hyaluronic acid and its effect on post-operative adhesions in the rabbit flexor tendon. A preliminary look. Clin Orthop Relat Res. 1986;206:281–9. [PubMed] [Google Scholar]
- 30.Gaughan EM, Nixon AJ, Krook LP, Yeager AE, Mann KA, Mohammed H, Bartel DL. Effects of sodium hyaluronate on tendon healing and adhesion formation in horses. Am J Vet Res. 1991;52:764–73. [PubMed] [Google Scholar]
- 31.Salti NI, Tuel RJ, Mass DP. Effect of hyaluronic acid on rabbit profundus flexor tendon healing in vitro. J Surg Res. 1993;55:411–5. doi: 10.1006/jsre.1993.1161. [DOI] [PubMed] [Google Scholar]
- 32.St Onge R, Weiss C, Denlinger JL, Balazs EA. A preliminary assessment of Na-hyaluronate injection into “no man's land” for primary flexor tendon repair. Clin Orthop Relat Res. 1980;146:269–75. [PubMed] [Google Scholar]
- 33.Weiss C, Suros JM, Michalow A, Denlinger J, Moore M, Tejeiro W. The role of Na-hylan in reducing postsurgical tendon adhesions: part 2. Bull Hosp Jt Dis Orthop Inst. 1987;47:31–9. [PubMed] [Google Scholar]
- 34.Foland JW, Trotter GW, Powers BE, Wrigley RH, Smith FW. Effect of sodium hyaluronate in collagenase induced superficial digital flexor tendinitis in horses. Am J Vet Res. 1992;53:2371–6. [PubMed] [Google Scholar]
- 35.Wiig M, Abrahamsson SO, Lundborg G. Tendon repair—cellular activities in rabbit deep flexor tendons and surrounding synovial sheaths and the effects of hyaluronan: an experimental study in vivo and in vitro. J Hand Surg [Am] 1997;22:818–25. doi: 10.1016/S0363-5023(97)80075-5. [DOI] [PubMed] [Google Scholar]
- 36.Laurent TC. The chemistry, biology and medical applications of hyaluronan and its derivatives. London: Portland Press; 1998. pp. 33–42. [Google Scholar]
- 37.Kuo JW, Swann DA, Prestwich GD. Chemical modification of hyaluronic acid by carbodiimides. Bioconjug Chem. 1991;2:232–41. doi: 10.1021/bc00010a007. [DOI] [PubMed] [Google Scholar]
- 38.Hanthamrongwit M, Reid WH, Grant MH. Chondroitin-6-sulphate incorporated into collagen gels for the growth of human keratinocytes: the effect of crosslinking agents and diamines. Biomaterials. 1996;17:775–80. doi: 10.1016/0142-9612(96)81414-1. [DOI] [PubMed] [Google Scholar]
- 39.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152–69. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 40.Tzianabos AO, Cisneros RL, Gershkovich J, Johnson J, Miller RJ, Burns JW, Onderdonk AB. Effect of surgical adhesion reduction devices on the propagation of experimental intra-abdominal infection. Arch Surg. 1999;134:1254–9. doi: 10.1001/archsurg.134.11.1254. [DOI] [PubMed] [Google Scholar]
- 41.Sun YL, Yang C, Amadio PC, Zhao C, Zobitz ME, An KN. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res. 2004;22:984–9. doi: 10.1016/j.orthres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Yang C, Amadio PC, Sun YL, Zhao C, Zobitz ME, An KN. Tendon surface modification by chemically modified HA coating after flexor digitorum profundus tendon repair. J Biomed Mater Res B Appl Biomater. 2004;68:15–20. doi: 10.1002/jbm.b.10074. [DOI] [PubMed] [Google Scholar]
- 43.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. Effect of synergistic wrist motion on adhesion formation after repair of partial flexor digitorum profundus tendon lacerations in a canine model in vivo. J Bone Joint Surg Am. 2002;84:78–84. [PubMed] [Google Scholar]
- 44.Zhao C, Amadio PC, Paillard P, Tanaka T, Zobitz ME, Larson DR, An KN. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Joint Surg Am. 2004;86:320–7. doi: 10.2106/00004623-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 45.An KN, Berglund L, Uchiyama S, Coert JH. Measurement of friction between pulley and flexor tendon. Biomed Sci Instrum. 1993;29:1–7. [PubMed] [Google Scholar]
- 46.Uchiyama S, Coert JH, Berglund L, Amadio PC, An KN. Method for the measurement of friction between tendon and pulley. J Orthop Res. 1995;13:83–9. doi: 10.1002/jor.1100130113. [DOI] [PubMed] [Google Scholar]
- 47.Silfverskiold KL, May EJ. Early active mobilization of tendon grafts using mesh reinforced suture techniques. J Hand Surg [Br] 1995;20:301–7. doi: 10.1016/s0266-7681(05)80082-8. [DOI] [PubMed] [Google Scholar]
- 48.Carlson GD, Botte MJ, Josephs MS, Newton PO, Davis JL, Woo SL. Morphologic and biomechanical comparison of tendons used as free grafts. J Hand Surg [Am] 1993;18:76–82. doi: 10.1016/0363-5023(93)90249-3. [DOI] [PubMed] [Google Scholar]
- 49.White WL. Tendon grafts: a consideration of their source, procurement and suitability. Surg Clin North Am. 1960;40:403–13. doi: 10.1016/s0039-6109(16)36047-9. [DOI] [PubMed] [Google Scholar]
- 50.Seiler JG, 3rd, Chu CR, Amiel D, Woo SL, Gelberman RH. Autogenous flexor tendon grafts. Biologic mechanisms for incorporation. Clin Orthop Relat Res. 1997;345:239–47. [PubMed] [Google Scholar]
- 51.Hunter JM, Singer DI, Jaeger SH, Mackin EJ. Active tendon implants in flexor tendon reconstruction. J Hand Surg [Am] 1988;13:849–59. doi: 10.1016/0363-5023(88)90259-6. [DOI] [PubMed] [Google Scholar]
- 52.Amadio PC, Wood MB, Cooney WP, Bogard SD. Staged tendon grafting: experience with 100 cases, 1972 to 1984. Orthop Trans. 1986;10:212. [Google Scholar]
- 53.Guimberteau JC, Panconi B, Boileau R. Mesovascularized island flexor tendon: new concepts and techniques for flexor tendon salvage surgery. Plast Reconstr Surg. 1993;92:888–903. [PubMed] [Google Scholar]
- 54.Derwin K, Androjna C, Spencer E, Safran O, Bauer TW, Hunt T, Caplan A, Iannotti J. Porcine small intestine submucosa as a flexor tendon graft. Clin Orthop Relat Res. 2004;423:245–52. doi: 10.1097/01.blo.0000131235.91264.d7. [DOI] [PubMed] [Google Scholar]
- 55.Momose T, Amadio PC, Sun YL, Zhao C, Zobitz ME, Harrington JR, An KN. Surface modification of extrasynovial tendon by chemically modified hyaluronic acid coating. J Biomed Mater Res. 2002;59:219–24. doi: 10.1002/jbm.1235. [DOI] [PubMed] [Google Scholar]
- 56.Zhao C, Amadio PC, Tanaka T, Yang C, Ettema AM, Zobitz ME, An KN. Shortterm assessment of optimal timing for postoperative rehabilitation after flexor digitorum profundus tendon repair in a canine model. J Hand Ther. 2005;18:322–9. doi: 10.1197/j.jht.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shih HN, Fang JF, Chen JH, Yang CL, Chen YH, Sung TH, Shih LY. Reduction in experimental peridural adhesion with the use of a crosslinked hyaluronate/collagen membrane. J Biomed Mater Res B Appl Biomater. 2004;71:421–8. doi: 10.1002/jbm.b.30106. [DOI] [PubMed] [Google Scholar]
- 58.Mao JS, Liu HF, Yin YJ, Yao KD. The properties of chitosangelatin membranes and scaffolds modified with hyaluronic acid by different methods. Biomaterials. 2003;24:1621–9. doi: 10.1016/s0142-9612(02)00549-5. [DOI] [PubMed] [Google Scholar]
- 59.Park SN, Park JC, Kim HO, Song MJ, Suh H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide cross-linking. Biomaterials. 2002;23:1205–12. doi: 10.1016/s0142-9612(01)00235-6. [DOI] [PubMed] [Google Scholar]
- 60.Park SN, Kim JK, Suh H. Evaluation of antibiotic-loaded collagen-hyaluronic acid matrix as a skin substitute. Biomaterials. 2004;25:3689–98. doi: 10.1016/j.biomaterials.2003.10.072. [DOI] [PubMed] [Google Scholar]
- 61.Cashman J, Burt HM, Springate C, Gleave J, Jackson JK. Camptothecinloaded films for the prevention of postsurgical adhesions. Inflamm Res. 2004;53:355–62. doi: 10.1007/s00011-004-1272-2. [DOI] [PubMed] [Google Scholar]
- 62.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 63.Asari A, Miyauchi S, Kuriyama S, Machida A, Kohno K, Uchiyama Y. Localization of hyaluronic acid in human articular cartilage. J Histochem Cytochem. 1994;42:513–22. doi: 10.1177/42.4.8126377. [DOI] [PubMed] [Google Scholar]
- 64.Bertheim U, Hellstrom S. The distribution of hyaluronan in human skin and mature, hypertrophic and keloid scars. Br J Plast Surg. 1994;47:483–9. doi: 10.1016/0007-1226(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 65.Laurent C, Hellstrom S, Engstrom-Laurent A, Wells AF, Bergh A. Localization and quantity of hyaluronan in urogenital organs of male and female rats. Cell Tissue Res. 1995;279:241–8. doi: 10.1007/BF00318480. [DOI] [PubMed] [Google Scholar]
- 66.Ichida T, Sugitani S, Satoh T, Matsuda Y, Sugiyama M, Yonekura K, Ishikawa T, Asakura H. Localization of hyaluronan in human liver sinusoids: a histochemical study using hyaluronan-binding protein. Liver. 1996;16:365–71. doi: 10.1111/j.1600-0676.1996.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 67.Matthiessen ME, Garbarsch C, Engelbrecht Olsen B, Hellstrom S, EngstromLaurent A. Hyaluronan in human deciduous tooth germs in the bell stage. Histochemistry and immunohistochemistry. Acta Anat (Basel) 1997;159:1–7. doi: 10.1159/000147958. [DOI] [PubMed] [Google Scholar]
- 68.Shibutani T, Imai K, Kanazawa A, Iwayama Y. Use of hyaluronic acid binding protein for detection of hyaluronan in ligature induced periodontitis tissue. J Periodontal Res. 1998;33:265–73. doi: 10.1111/j.1600-0765.1998.tb02199.x. [DOI] [PubMed] [Google Scholar]