Abstract
Antiviral treatment is the only option to prevent or defer the occurrence of hepatocellular carcinoma (HCC) in patients chronically infected with hepatitis B virus (HBV) or hepatitis C virus (HCV). The approved medication for the treatment of chronic HBV infection is interferon-α (IFNα) and nucleos(t)ide analogues (NAs), including lamivudine, adefovir dipivoxil, telbivudine, entecavir and tenofovir disoproxil fumarate. IFNα is the most suitable for young patients with less advanced liver diseases and those infected with HBV genotype A. IFNα treatment significantly decreases the overall incidence of HBV-related HCC in sustained responders. However, side effects may limit its long-term clinical application. Orally administered NAs are typically implemented for patients with more advanced liver diseases. NA treatment significantly reduces disease progression of cirrhosis and therefore HCC incidence, especially in HBV e antigen-positive patients. NA-resistance due to the mutations in HBV polymerase is a major limiting factor. Of the NA resistance-associated mutants, A181T mutant significantly increases the risk of HCC development during the subsequent course of NA therapy. It is important to initiate treatment with NAs that have a high genetic barrier to resistance, to counsel patients on medication adherence and to monitor virological breakthroughs. The recommended treatment for patients with chronic HCV infection is peg-IFN plus ribavirin that can decrease the occurrence of HCC in those who achieve a sustained virological response and have not yet progressed to cirrhosis. IFN-based treatment is reserved for patients with decompensated cirrhosis who are under evaluation of liver transplantation to reduce post-transplant recurrence of HCV. More effective therapeutic options such as direct acting antiviral agents will hopefully increase the response rate in difficult-to-treat patients with HCV genotype 1. However, the risk of HCC remains in cirrhotic patients (both chronic HBV and HCV infection) if treatment is initiated after cirrhosis is established. Future research should focus on investigating new agents, especially for those patients with hepatic decompensation or post-transplantation.
Keywords: Hepatitis B virus, Hepatitis C virus, Hepatocellular carcinoma, Antiviral therapy, Interferon, Nucleos(t)ide analogues, Virological response
INTRODUCTION
Hepatocellular carcinoma (HCC), one of the most common and aggressive malignancies, is the third leading cause of cancer-related deaths worldwide[1]. Chronic infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) affect over 400 million and 170 million individuals respectively, together contributing to 75%-80% of global HCC[2]. The incidence of HCC has a wide geographical variation due to the heterogeneous penetration of the main causal factors within a given population[3]. In HCC-endemic areas, such as Asia and Africa, chronic HBV infection is the principal etiological factor. While in the West and Japan, chronic HCV infection plays a predominant role. Chronic HBV and HCV are progressive diseases; the dynamic process involving the interplay between the hepatitis viruses and host inflammatory factors contributes to the development of advanced liver diseases such as HCC. The most effective measure to avert HCC is to prevent HBV and HCV infection. Hepatitis B vaccination for newborns has led to a substantial reduction in the incidence of HCC in HBV endemic regions, while no vaccine is currently available for HCV. For individuals who are chronically infected with HBV or HCV, antiviral therapy is the only option for the prevention of HCC. In chronic hepatitis B or C patients without cirrhosis, antiviral therapy may prevent the occurrence of HCC by slowing the progression of liver diseases and possibly reversing liver damage[4]. In patients with advanced fibrosis or cirrhosis, eradication or oppression of HBV or HCV does not remove this risk, but can control the complications and gain the time to prepare for liver transplantation[5]. Postoperative antiviral therapy also improves the prognosis of HBV/HCV-related HCC[6,7]. Therefore, the treatment might be of greater benefit if patients are treated earlier and adhere to medications during the course of chronic HBV or HCV infection[8]. Several safe and effective medications have been approved. Decision to start or defer treatment should take into consideration the stage of liver disease, initial virus replication status, adverse effects, drug resistance and costs of the treatment. Moreover, the response should be closely monitored so that the treatment can be modified in a timely fashion.
ANTIVIRAL THERAPY AND PREVENTION OF HBV-RELATED HCC
The natural course of chronic HBV infection consists of 4 phases, namely immune tolerant, immune clearance, inactive (carrier) and reactivation phases. The immune tolerance phase [or hepatitis B e antigen (HBeAg)-positive chronic hepatitis B] is characterized by the presence of HBeAg, normal serum alanine aminotransferase (ALT) and high HBV DNA levels. Most patients in this phase have minimal liver injury and little or no fibrosis[9]. The immune clearance phase is characterized by the presence of HBeAg, high serum HBV DNA levels, persistent or intermittent elevation of ALT and active inflammation in the liver. During this phase, some patients undergo spontaneous HBeAg seroconversion which occurs at a rate of 10%-20% per year and others may experience recurrent hepatitis flares and even progress to cirrhosis or hepatic decompensation. The inactive (carrier) phase is characterized by the absence of HBeAg, presence of HBe antibody (anti-HBe), persistently normal ALT levels and low or undetectable levels of serum HBV DNA. Patients in this phase have a favorable prognosis[10]. The reactivation phase (or HBeAg-negative chronic hepatitis B) is characterized by the absence of HBeAg, presence of anti-HBe, intermittently or persistently elevated serum HBV DNA and ALT levels, and active inflammation in the liver. Patients in this phase are usually older and have more advanced liver disease than those in other phases[11]. However, patients vary regarding which phases they go through. This is largely influenced by the HBV genotype and host immune status. Previous studies have demonstrated that the presence of HBeAg and persistently high serum HBV DNA levels are risk factors for the occurrence of cirrhosis and HCC[12-14].
Moreover, chronic infection with HBV genotype C is more likely to cause liver cirrhosis than genotype B[15]. Chronic infection with genotype C (C2) is related to HBV-associated HCC, especially in cirrhotic patients aged > 50 years, whereas HBV B2 infection is related to high prevalence of HCC in non-cirrhotic young patients and HCC recurrence after resection[16]. In addition, precore or core promoter HBV variants that occur in most patients can prevent or decrease the production of HBeAg[11]. Different HBV subgenotypes have distinct patterns of mutations. We and others have found that serum HBV load (> 104 copies/mL) and viral mutations in the enhancer II/basal core promoter (EnhII/BCP) regions (such as C1653T, T1753V, A1762T/G1764A, T1674C/G and C1766T/T1768A) and in the precore/core gene (such as G1899A, C2002T, A2159G, A2189C and G2203A/T), as well as in the preS region (such as T53C, preS2 start codon mutation, preS1 deletion, C2964A, A2962G, C3116T and C7A) are significantly associated with the occurrence of HCC[17-22]. Reduction of CD8+ T cell epitopes in HBV to evade immune clearance is one of the most common ways of these mutations. It remains to be evaluated if the HCC-associated HBV mutants are still sensitive to the antiviral treatment.
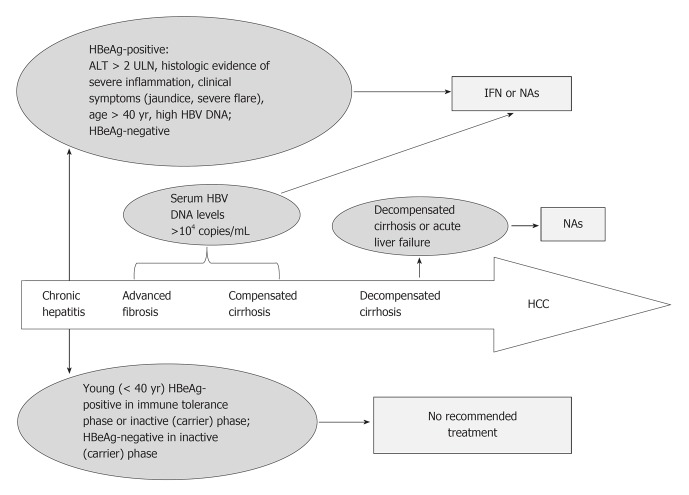
Clinical assessment of the treatment response relies on intermediate outcomes, including a decrease in levels of serum HBV DNA, HBeAg seroconversion, loss of HBsAg, normalization of ALT levels and a decrease in hepatic inflammation. The ultimate goal of the antiviral therapy is to obtain clinical benefits by reducing complications, including HCC. However, treatment choices mainly depend on the degree of viral replication and disease progression. Other factors, such as the patient’s age, HBeAg status, family history of HCC, occupational requirement and need for immunosuppressive or cancer chemotherapy, could also influence the decision to start or defer antiviral treatment. In patients with life-threatening liver diseases, such as acute liver failure, decompensated cirrhosis or severe exacerbation of chronic hepatitis B, antiviral treatment should be initiated as soon as possible in order to stabilize liver function and prepare for liver transplantation[23]. In patients with advanced fibrosis or compensated cirrhosis (those whose laboratory tests indicate normal hepatic function and no evidence of portal hypertension), antiviral treatment should be initiated when serum HBV DNA levels > 104 copies/mL because the risks of cirrhosis and HCC increase when serum HBV DNA reaches or exceeds this level[13,24]. HBeAg-positive chronic hepatitis patients who have ALT levels persistently twice the normal upper limit or with clinical and/or histological evidence of severe inflammation should be considered for treatment. Patients older than 40 years should be treated if HBeAg remains positive and the serum HBV DNA level is still high, regardless of ALT levels. Given that sustained spontaneous remission is rare, patients who have HBeAg-negative chronic hepatitis should also be considered for treatment. However, it is not recommended to initiate antiviral therapy for young HBeAg-positive patients (< 40 years) in the immune tolerance phase because most have little or no fibrosis and a favorable prognosis during follow-up of up to 10 years[25]. Another reason for deferring treatment is that antiviral treatment is less efficacious during this phase due to the low likelihood of treatment-related HBeAg seroconversion. Continued monitoring is necessary for timely initiation of treatment if patients fail to undergo spontaneous HBeAg seroconversion. Treatment may also be deferred in the inactive (carrier) phase because no evidence supports the hypothesis that antiviral therapy will alter the outcome of patients who are truly in this phase. However, the decision should only be made on the premise that patients have been observed for at least one year of HBV DNA and ALT levels tested on three or four occasions (Figure 1).
Figure 1.
Flowchart of therapy choice for patients with chronic hepatitis B virus infection. ALT: Alanine aminotransferase; HBeAg: Hepatitis B e antigen; HCC: Hepatocellular carcinoma; HBV: Hepatitis B virus; IFN: Interferon; NAs: Nucleos(t)ide analogues; ULN: Upper limit of normal.
Currently, interferon-α (IFNα) and nucleos(t)ide analogues (NAs) are the two main categories of medications approved for the treatment of chronic hepatitis B. IFNα has an immunomodulatory activity that can lead to a higher rate of HBeAg seroconversion and HBsAg loss[12,13]. Treatment with IFNα may significantly decrease overall HCC incidence in sustained responders, especially in Asians[26-28]. IFNα may be used in carefully selected patients with compensated cirrhosis. It is most appropriate for young patients, particularly among HBeAg-positive patients who have a genotype A infection. However, it is contraindicated in patients with decompensated cirrhosis to avoid sepsis and liver failure. It is also not used in patients with severe exacerbations of chronic hepatitis B or acute liver failure, and in those undergoing immunosuppressive or cancer chemotherapy. The major advantages of IFNα-based treatment include short treatment duration and a sustained off-treatment response once achieved. However, low probability of achieving a response and high costs as well as side-effects may limit its long-time clinical use.
Treatment with NAs can also result in a significantly lower incidence of HCC compared to untreated patients but it does not completely eliminate the risk of HCC, particularly in patients with pre-existing cirrhosis. Hence, patients with chronic hepatitis B require careful surveillance for HCC, even when they are undergoing antiviral therapy[29,30]. Another disadvantage of NA treatment is the long-term continuous treatment course, resulting in antiviral drug resistance[31]. The choice of treatment should consider the initial HBV-DNA levels, HBV genotype/subgenotype, age of the patient and any contraindication to NA treatment. NAs are most appropriate for patients who have decompensated liver diseases or contraindications to IFN, and those who are willing to commit to a long duration of treatment. The treatment should be initiated with NAs with a high genetic barrier to resistance (that is, a low potential for drug resistance).
IFNα
Two forms of IFNα are currently available: conventional IFNα and the pegylated, long-acting formulation (PEG-IFN). The introduction of PEG-IFN mainly impacts tolerability. It allows for weekly injections compared to the daily or three times/week schedules of conventional IFNα administration, while maintaining similar antiviral efficacy. Long-term follow-up of patients treated with conventional IFNα therapy shows that responders have a decreased incidence of decompensated cirrhosis or HCC and improved overall survival compared with non-responders[32]. A retrospective analysis of PEG-IFN in patients with HBeAg-positive hepatitis showed that factors associated with response to treatment included high ALT, low HBV DNA, female sex, older age and the absence of previous IFN therapy. Patients with the best outcomes were those with genotype A and high ALT or low HBV DNA, and those with genotypes B or C and both high ALT and low HBV DNA[33]. A recent meta-analysis of 14 trials suggested that PEG-IFN facilitated HBsAg clearance or seroconversion in chronic hepatitis B patients. According to an assessment conducted in 24 wk after completion of a 1 year course of PEG-IFN, approximately 30% of HBeAg-positive patients achieved HBeAg seroconversion and undetectable serum HBV DNA, and 15% of HBeAg-negative patients had normalized ALT levels[34]. Compared with the placebo, a 1 year course of PEG-IFN therapy resulted in a greater HBV DNA decline in HBeAg-seropositive patients (32% vs 11%)[35]. A large study evaluated long-term outcomes of IFNα therapy in HBeAg seropositive patients by comparing 233 IFNα-treated patients with 233 well-matched untreated controls and the cumulative incidences at the end of 15 years of follow-up (median 6.8 years, range 1.1-16.5 years) in the IFNα-treated patients vs the controls were: HBeAg seroconversion 74.6% vs 51.7% (P = 0.031); HBsAg seroclearance 3% vs 0.4% (P = 0.03); cirrhosis 17.8% vs 33.7% (P = 0.041); and HCC 2.7% vs 12.5% (P = 0.011)[26]. Another meta-analysis with a total of 2742 subjects pooled from 12 studies has shown that the risk of HCC in patients treated by IFNα is reduced by 34% (RR = 0.66, 95%CI: 0.48-0.89) and the benefit is more significant among patients with early cirrhosis than among those without cirrhosis[23]. These data indicated that the main advantages of IFN are durable administration course and a high rate of HBsAg loss and HBeAg seroconversion, particularly among HBeAg-positive patients who have a genotype A infection compared with patients who are infected with other HBV genotypes. However, the adverse effects of IFNα, including initial flu-like illness, fatigue, bone marrow suppression and exacerbation of autoimmune illnesses, should be closely monitored.
NAs
There are five NAs approved for the treatment of chronic hepatitis B: lamivudine, adefovir dipivoxil, telbivudine, entecavir and tenofovir disoproxil. We have summarized the pros and cons of each NA for the treatment of chronic HBV infection in Table 1.
Table 1.
Pros and cons of each nucleos(t)ide analogue therapy for the treatment of chronic hepatitis B infection
| Nucleos(t)ide analogue | Regimen | Pros | Cons |
| Lamivudine | 100 mg daily | First licensed agent | Highest incidence of resistant mutations of M204V/I substitution (20% at year 1, 70% at year 5) |
| Well established safety and efficacy record | |||
| Lowest cost | Adverse effects including hepatitis flare ups, hepatic decompensation and even death | ||
| Adefovir dipivoxil | 10 mg daily | Low drug resistance rate, and no cross resistance with other nucleos(t)ide analogs | Incidence of resistant mutations of N236T and/or A181V substitution (29% at year 5) |
| Adverse effects including renal tubular acidosis with hypophosphataemia when treatment is prolonged | |||
| Telbivudine | 600 mg daily | Higher seroconversion rate | Incidence of resistant mutations of M204I mutation (5% at year 1) |
| Adverse effects including myopathy and neuropathy | |||
| Entecavir | 1.0 mg daily | Anti-HBV effect | Incidence of resistant mutations of T184G or M250V (1.2% at year 5) (I169T and M250V, or T184G and S202I if also lamivudine-resistant) |
| Lowest rate of resistance | |||
| Most expensive | |||
| Tenofovir disoproxil | 300 mg daily | More potent in reducing HBV load in patients with prior failure or resistance to lamivudine and/or adefovir | No resistant mutations reported at year 3 |
HBV: Hepatitis B virus.
In patients who do not respond to IFNα, an oral sequential therapy with NAs is preferable because of its predictable efficacy and minimal side-effects. It has been demonstrated that a 1 year course of NA treatment results in high rates of undetectable serum HBV DNA, normalization of ALT levels and increase in liver function, but low rates of HBsAg loss in chronic hepatitis B patients with either positive or negative HBeAg[36,37]. Extending the duration of NA treatment to more than 1 year can increase rates of HBeAg seroconversion to 40%-50%, but rates of HBsAg loss remain below 10% in patients with HBeAg-positive chronic hepatitis B patients after 5 years of treatment[38,39]. In a randomized controlled trial, 651 HBsAg-positive patients with compensated liver diseases were allocated into two groups: receiving 100 mg/d lamivudine (n = 436) or receiving placebo (n = 215). HCC occurred in 3.9% of those in the lamivudine group and 7.4% of those in the placebo group (HR = 0.49, 95%CI: 0.25-0.99, P = 0.047) after a median treatment duration of 32.4 mo (ranging from 0 to 42 mo)[40]. A meta-analysis pooling 5 studies (n = 2289) compared the incidence of HCC in patients with and without NA treatment. It found that the incidence of HCC was reduced by 78% (RR = 0.22, 95%CI: 0.10-0.50) in the treatment arm, especially in HBeAg-positive patients[27]. Similarly, a recent meta analysis pooling 3881 patients with NA treatment and 534 untreated controls from 21 studies has concluded that NA treatment is associated with a lower incidence of HCC (2.8% vs 6.4%, P = 0.003)[29].
Current approved NAs act primarily by inhibiting the reverse transcription of the pregenomic HBV RNA to the first strand of HBV DNA rather than directly inhibiting cccDNA. Therefore, viral relapse is common after treatment. In addition, inadequate or slow decline in serum HBV DNA levels during the first 12-24 wk of NA treatment is associated with an increased risk of antiviral drug resistance during continued therapy. Patients receiving NAs with a low genetic barrier to resistance, such as lamivudine and telbivudine, should receive additional therapy if initial viral decline is inadequate, while patients who receive NAs that have a high genetic barrier to resistance, including entecavir and tenofovir disoproxil, may remain on the same drug if serum HBV DNA levels continue to decline[41,42].
Lamivudine 100 mg/d is the first approved NA to treat chronic hepatitis B and has been extensively used for more than a decade with an excellent safety record. Lamivudine treatment can reduce disease progression of HBV-related cirrhosis, resulting in approximately a 50% decrease in HCC incidence. Such efficacy is achieved despite emergence of drug resistance in approximately 50% of cases[28]. Long-term therapy with lamivudine leads to viral breakthrough in some patients, owing to the emergence of viral mutation harboring a M204V or I substitution in the YMDD motif[10]. M204V/I is the most frequently encountered lamivudine-resistant mutant. L180M mutation usually concurrently occurs with M204V mutation. Another mutation, A181T, exists in a substantial proportion of lamivudine-resistant patients. More importantly, the emergence of A181T mutant significantly increases the risk of HCC development in lamivudine-resistant patients during the subsequent course of antiviral therapy[43,44]. The rate of lamivudine resistance is 24% after 1 year and approximately 70% after 5 years[45]. Furthermore, lamivudine-resistance causes the attenuation of HBV suppression, hepatitis flare ups, hepatic decompensation and even death, thereby posing a serious clinical challenge[46]. Because of the overlap between the S and polymerase genes of HBV, a great proportion of patients carrying A181T mutation also possess sW172* nonsense mutation, resulting in truncation of the pre-S/S reading frames. This partially explains why chronic hepatitis B patients who fail to NA treatment at the late stage are more likely to develop HCC compared to those who respond to the treatment. Despite this, lamivudine is still widely used in several countries, mostly because of its low cost. Treatment of patients with lamivudine-resistance includes the addition of adefovir dipivoxil or tenofovir disoproxil and entecavir rescue treatment. A daily dose of 10 mg of adefovir dipivoxil is not an ideal first-line NA therapy because of its low potency. A proportion (20%-50%) of patients fails to achieve even a 102-fold reduction in serum HBV DNA. Although adefovir dipivoxil has been widely used in lamivudine-resistant HBV infection, up to 25% of patients fail to achieve a satisfactory response and 30% of naïve patients develop adefovir dipivoxil resistance in 5 years[47]. Furthermore, adefovir mutations harboring a N236T and/or A181V substitution emerge more frequently in lamivudine-resistant patients than in treatment-naïve patients[48-50]. Entecavir rescue monotherapy can be adopted as a treatment option for patients with resistance to both lamivudine and adefovir dipivoxil[51,52]. A clinical trial studying the long-term efficacy of entecavir therapy with 146 patients has shown that among patients with up to 5 years of continuous entecavir 0.5 or 1.0 mg therapy, 94% resulted in HBV DNA reduction to < 300 copies/mL and 80% achieve normalization of ALT levels, while the HBeAg seroconversion and decrease in HBsAg rates are only 23% and 1.4%, respectively[39]. Entecavir monotherapy may be efficacious in adefovir dipivoxil-refractory chronic hepatitis B patients with prior lamivudine-resistance if these patients have an early virological response to the monotherapy at 12 wk. Entecavir-resistance is rare in treatment-naïve patients, even with long-term therapy, but the cumulative probability of genotypic entecavir resistance with a combination of substitutions I169T and M250V, or T184G and S202I, in lamivudine-resistant patients increases up to 51% after 5 years of treatment[41,53]. A recent meta-analysis has demonstrated that a combination therapy with lamivudine and adefovir dipivoxil is more effective and produces long-lasting effects than switching to entecavir monotherapy in treating chronic hepatitis B patients with lamivudine resistance. However, taking into account the practical benefits and the limitations of adefovir dipivoxil, individualized therapy will be needed in patients with a prior history of lamivudine-resistant infections[54]. Entecavir rescue therapy for 96 wk is less efficacious in patients with lamivudine/adefovir dipivoxil-refractory HBV, particularly in those who have an initial HBV DNA of > 107 copies/mL. Patients who achieve a HBV DNA level of < 104 copies/mL and a normalized ALT level should continue, rather than stop, entecavir therapy[55]. Telbivudine is as potent as entecavir. The therapeutic response to telbivudine is superior to that of lamivudine in HBeAg-positive and HBeAg-negative patients. In HBeAg-positive patients, telbivudine has better outcomes compared to lamivudine in terms of nondetectable viremia, HBeAg loss and viral resistance[56]. Resistance to telbivudine is associated with a signature M204I mutation in viral polymerase[37,57]. Tenofovir disoproxil appears to be safe and effective in patients with prior resistance to lamivudine and adefovir dipivoxil and becomes the optimal choice of antiviral treatment[58,59]. The cost-effectiveness of switching to tenofovir disoproxil or adding tenofovir disoproxil to ongoing lamivudine in lamivudine-resistance patients is still debatable. Adefovir dipivoxil-resistant mutants are usually susceptible to lamivudine, telbivudine or entecavir and they may also be sensitive to tenofovir disoproxil, depending on the mutation pattern, while telbivudine or the rare entecavir resistance strains are usually sensitive to adefovir dipivoxil and tenofovir disoproxil[43,60]. Despite their high initial potency, 1 year of therapy with NAs does not usually lead to sustained off-therapy responses and therefore treatments usually last for several years or even longer. Nowadays, entecavir and tenofovir disoproxil are recommended as first-line treatments because of their higher potency and lower risk of resistance compared to lamivudine, adefovir or telbivudine[43,44]. Although it is difficult to compare entecavir and tenofovir disoproxil since no comparison studies have been conducted, tenofovir disoproxil monotherapy appears to be superior to entecavir monotherapy in multidrug-resistant HBV.
ANTIVIRAL THERAPY AND PREVENTION OF HCV-RELATED HCC
Cirrhosis is the strongest risk factor for HCC among patients with chronic HCV infection. Antiviral therapy to inhibit and even eradicate HCV can result in decreasing hepatic necroinflammation and, over time, causes reversal of fibrosis and eventually decreases the risk of HCC[61]. The standard of care in patients with chronic hepatitis C consists of a 24 to 48 wk course of PEG-IFNα2a or PEG-IFNα2b in combination with the guanosin analog ribavirin. This therapy leads to a sustained virological response (SVR) of 42%-52%, 65%-85% and 76%-82% of those infected with HCV genotype 1, HCV genotypes 4, 5 or 6, and HCV genotypes 2 or 3, respectively[62,63]. A clinical trial following-up 150 patients for 5 years has shown that the clinical, virological, biochemical and histological outcomes of patients with SVR are favorable and recovery of normal or nearly normal liver architecture is possible[64]. The standard of care can decrease the risk of HCC, although the effect is predominantly evident in patients who achieve SVR and in those who have not yet progressed to cirrhosis[65,66]. However, several studies have demonstrated that, for patients with advanced fibrosis or cirrhosis, the risk of developing HCC remains even if a SVR is achieved, highlighting the importance of continued surveillance in these patients[67,68]. A recent meta-analysis pooled data from 20 studies (4700 patients with HCV-related cirrhosis) and compared untreated patients with those given IFNα alone or combined with ribavirin treatment and it showed a reduced risk of HCC in the treatment group (RR = 0.43, 95%CI: 0.33-0.56). Another meta-analysis using data from 14 studies (n = 3310) indicated that patients achieving a SVR had a lower incidence of HCC (RR = 0.35, 95%CI: 0.26-0.46) compared with nonresponders and the maximum benefits were observed in those treated with ribavirin-based regimens (RR=0.25, 95%CI: 0.14-0.46)[65]. Due to the potential anti-tumoral, anti-angiogenic and anti-fibrotic roles of IFNα, maintaining IFNα therapy might decrease the risk of HCC in patients who fail to achieve SVR. However, several large-scale randomized controlled trials with long (3-4 years) and extended (up to 5 years) follow-up time have shown that low-dose PEG-IFN in patients with advanced fibrosis or cirrhosis have minimal or even no benefit on overall clinical outcomes[69-71]. Although the treatment of HCV chronic infection with the standard of care therapy can eradicate HCV in 40%-90% of patients, approximately 10%-15% of patients have to discontinue the treatment due to adverse effects. The adverse effects, ranging from mild to moderate in severity, impact most organ systems and can cause serious and even life-threatening toxicity, such as psychological disturbances, poor appetite, skin rash, infection, anemia and leukopenia[72]. Accordingly, patients should be closely monitored for adverse effects during treatment.
Although the standard of care therapy will probably continue for some years, more effective therapeutic options with shorter treatment durations are being introduced to increase the response rate in difficult-to-treat patients (mainly infected with genotype 1) and reduce the impact of HCV infection and related complications. So far, intensive efforts have been made to develop different compounds that specifically target the replication cycle of the virus. These direct-acting antiviral agents (DAAs) act by directly inhibiting the NS3/4A serine protease (which processes the HCV polyprotein to generate mature viral proteins), the NS5B polymerase (which replicates the viral RNA genome) and the NS5A phosphoprotein (which functions as a part of the replicase complex)[73-76]. The new standard of care for patients with chronic hepatitis C is to add nonstructural (NS) 3/4A protease inhibitors boceprevir or telaprevir to the Peg-IFNα plus ribavirin regimen. The recent sixty-first annual meeting of the American Association for the Study of Liver Diseases (AASLD) provided an overview of the pipeline of these novel drugs. Numerous other protease inhibitors, as well as nucleoside and non-nucleoside inhibitors of the RNA-dependent RNA NS5B polymerase and inhibitors of the NS5A protein, are also under evaluation currently. These can achieve higher SVR rates in previously untreated patients infected with HCV genotype 1 and provide successful medical care for those who have failed treatment under current standard of care[77]. In a recent phase II study, 465 chronic hepatitis C patients with poor response to PEG-IFNα plus ribavirin therapy were allocated to the triple therapy group and the control group. It was found that the triple therapy significantly increased SVR rates in these difficult-to-treat patients compared with the controls (53% vs 14%)[78]. Remarkably, in a group of HCV patients treated for 24 wk with this triple therapy followed by another 24 wk of PEG-IFNα2b plus ribavirin, the SVR rates for patients who had previously relapsed was 76% and up to 39% for those who did not response to the standard therapy. Another report of a phase II study indicated that adding boceprevir to the standard treatment leads to a notably increased SVR (75% vs 38%) in treatment-naive patients who were infected with HCV genotype 1[79]. The combination of three agents, PEG-IFNα, ribavirin and HCV protease inhibitor, is able to increase SVR rates substantially. The potency and safety of the two first generation HCV protease inhibitors are also confirmed in large phase III studies. In the treatment-naive patients infected with HCV genotype 1, the SVR rates are 75% with the addition of telaprevir vs 44% with the standard therapy and 68% with the addition of boceprevir vs 40% with the standard therapy. Although the upcoming triple therapy regimens including telaprevir or boceprevir may be different, this strategy means that in treatment-naive patients, treatment duration will be reduced to 24 or 28 wk for the patients with a rapid viral response, those with a negative result of serum HCV RNA after 4 wk of exposure to an HCV protease inhibitor. In addition, the single nucleotide polymorphisms around the gene encoding interleukin 28B (IL28B) have been identified as key predictive factors[80]. In treatment-naïve HCV-1 patients treated with PEG-IFN and ribavirin, among host and viral factors associated with SVR, combination of IL28B genotypes and rapid viral response monitoring seems to provide a high predictive value of treatment outcome, particularly in the context of emerging therapies and DDAs[81]. Furthermore, a study combining a nucleoside polymerase inhibitor (RG7128) and danoprevir led to an average of 5.1 log reduction of plasma HCV RNA levels within 14 d[82]. Overall, this treatment is not only an important step towards an IFN-free regimen, but also reflects the high genetic barrier to resistance associated with nucleoside polymerase inhibitors. Co-administration of different classes of DAAs, combined with or without PEG-IFNα and/or ribavirin, might make HCV RNA suppression possible in most individuals who are infected with HCV genotype 1, including those who are not responsive to PEG-IFNα[73]. However, it is still unclear whether the novel agents will be useful in the most difficult-to-treat patients, such as those with advanced or decompensated liver diseases or after liver transplantation. Furthermore, DAAs also need to be developed for other HCV genotypes. Therefore, characterizing resistance to DAAs and the combination of antiviral agents with different resistance profiles in clinical trials are the best strategies to prevent the emergence of drug-resistant mutants and thereby maximize SVR rate.
However, given the adverse effects, it remains uncertain whether patients with decompensated cirrhosis could tolerate HCV eradication treatment and therefore should be treated to prevent progression of decompensation. The AASLD recommends those patients to be referred for consideration of liver transplantation[83]. Eradication of HCV before liver transplantation could reduce post-transplant recurrence of HCV, especially in patients infected with HCV genotypes other than genotype 1[84]. Thus, treatment should be reserved for those patients awaiting liver transplantation. It is recommended that patients with decompensated cirrhosis initiate treatment at a low dose of IFN-based therapy. However, the treatment should be administered with caution since it is still unclear whether the novel agents will be effective in those most difficult-to-treat patients[79,85]. In addition, use of hematological growth factors can improve the life quality of treated patients and manage treatment-induced cytopenias (Figure 2).
Figure 2.
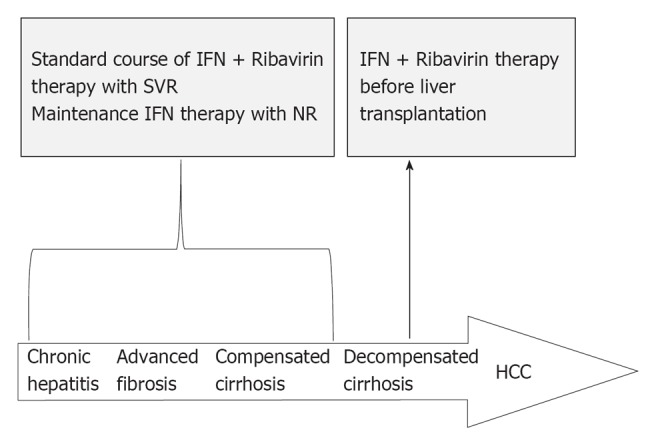
Flowchart of therapy choice for patients with chronic hepatitis C virus infection. HCC: Hepatocellular carcinoma; IFN: Interferon; NR: No response; SVR: Sustained virological response.
CONCLUSION
In summary, antiviral treatment of chronic hepatitis B or chronic hepatitis C is so far the only option to prevent HCC. Treatment of chronic hepatitis B with IFNα may significantly decrease overall incidence of HCC in sustained responders, while the adverse effects may limit its long-term application. Orally administered NAs significantly reduce disease progression of liver cirrhosis, resulting in up to a 78% decrease in HCC incidence, especially in HBeAg-positive patients. In patients with life-threatening liver diseases, antiviral treatment should be initiated as soon as possible in order to stabilize liver function and prepare for liver transplantation. Long-term continuous treatment with NAs results in antiviral drug resistance due to the mutations in HBV polymerase. Of the NA resistance-associated mutants, A181T mutant significantly increases the risk of HCC in lamivudine-resistant patients during the subsequent courses of antiviral therapy. In addition, it remains to be explored if the HCC-associated HBV mutants, whose emergence is likely to be selected by virus-host interaction during carcinogenesis, are sensitive to the antiviral therapy.
The recommended treatment for patients with chronic HCV infection is PEG-IFN plus ribavirin which can decrease HCC incidence in those who achieve SVR and have not yet progressed to cirrhosis. Patients with decompensated cirrhosis are under evaluation of liver transplantation, because achievement of a SVR is possible in these patients but does not forestall the disease progression. IFN and ribavirin therapy, therefore, should be reserved in these patients to prevent post-transplant recurrence of HCV. More effective therapeutic options such as DDAs are promising in increasing the response rate of difficult-to-treat patients with HCV genotype 1.
There is a great need to develop safer, more effective and affordable antiviral therapies. To optimize treatment responses, appropriate therapy should be initiated at the proper time. Patients must be educated about the importance of treatment compliance. The response to antiviral treatment should be closely monitored so that the therapy can be modified when the initial one fails. HCC risk remains in cirrhotic patients (both HBV and HCV infection) if treatment is initiated after cirrhosis is established and close monitoring is needed. Future research should focus on investigating the use of new agents, especially for patients with hepatic decompensation or after transplantation.
Footnotes
Supported by National Natural Scientific Foundation of China, No. 81025015 and 91129301
Peer reviewer: Gualtiero Alvisi, PhD, Department of Infectious Diseases, Heidelberg University, INF345, Heidelberg 69121, Germany
S- Editor Wang JL L- Editor Roemmele A E- Editor Zheng XM
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Lok AS. Does antiviral therapy for hepatitis B and C prevent hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:221–227. doi: 10.1111/j.1440-1746.2010.06576.x. [DOI] [PubMed] [Google Scholar]
- 5.Fink SA, Jacobson IM. Managing patients with hepatitis-B-related or hepatitis-C-related decompensated cirrhosis. Nat Rev Gastroenterol Hepatol. 2011;8:285–295. doi: 10.1038/nrgastro.2011.57. [DOI] [PubMed] [Google Scholar]
- 6.Chan AC, Chok KS, Yuen WK, Chan SC, Poon RT, Lo CM, Fan ST. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg. 2011;146:675–681. doi: 10.1001/archsurg.2011.125. [DOI] [PubMed] [Google Scholar]
- 7.Miao RY, Zhao HT, Yang HY, Mao YL, Lu X, Zhao Y, Liu CN, Zhong SX, Sang XT, Huang JF. Postoperative adjuvant antiviral therapy for hepatitis B/C virus-related hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2010;16:2931–2942. doi: 10.3748/wjg.v16.i23.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chotiyaputta W, Peterson C, Ditah FA, Goodwin D, Lok AS. Persistence and adherence to nucleos(t)ide analogue treatment for chronic hepatitis B. J Hepatol. 2011;54:12–18. doi: 10.1016/j.jhep.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Andreani T, Serfaty L, Mohand D, Dernaika S, Wendum D, Chazouillères O, Poupon R. Chronic hepatitis B virus carriers in the immunotolerant phase of infection: histologic findings and outcome. Clin Gastroenterol Hepatol. 2007;5:636–641. doi: 10.1016/j.cgh.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Manno M, Cammà C, Schepis F, Bassi F, Gelmini R, Giannini F, Miselli F, Grottola A, Ferretti I, Vecchi C, et al. Natural history of chronic HBV carriers in northern Italy: morbidity and mortality after 30 years. Gastroenterology. 2004;127:756–763. doi: 10.1053/j.gastro.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Chotiyaputta W, Lok AS. Hepatitis B virus variants. Nat Rev Gastroenterol Hepatol. 2009;6:453–462. doi: 10.1038/nrgastro.2009.107. [DOI] [PubMed] [Google Scholar]
- 12.Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168–174. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 13.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 14.Wu CF, Yu MW, Lin CL, Liu CJ, Shih WL, Tsai KS, Chen CJ. Long-term tracking of hepatitis B viral load and the relationship with risk for hepatocellular carcinoma in men. Carcinogenesis. 2008;29:106–112. doi: 10.1093/carcin/bgm252. [DOI] [PubMed] [Google Scholar]
- 15.Yin JH, Zhao J, Zhang HW, Xie JX, Li WP, Xu GZ, Shen J, Dong HJ, Zhang J, Wang L, et al. HBV genotype C is independently associated with cirrhosis in community-based population. World J Gastroenterol. 2010;16:379–383. doi: 10.3748/wjg.v16.i3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin J, Zhang H, Li C, Gao C, He Y, Zhai Y, Zhang P, Xu L, Tan X, Chen J, et al. Role of hepatitis B virus genotype mixture, subgenotypes C2 and B2 on hepatocellular carcinoma: compared with chronic hepatitis B and asymptomatic carrier state in the same area. Carcinogenesis. 2008;29:1685–1691. doi: 10.1093/carcin/bgm301. [DOI] [PubMed] [Google Scholar]
- 17.Yin J, Xie J, Liu S, Zhang H, Han L, Lu W, Shen Q, Xu G, Dong H, Shen J, et al. Association between the various mutations in viral core promoter region to different stages of hepatitis B, ranging of asymptomatic carrier state to hepatocellular carcinoma. Am J Gastroenterol. 2011;106:81–92. doi: 10.1038/ajg.2010.399. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Zhang H, Gu C, Yin J, He Y, Xie J, Cao G. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: a meta-analysis. J Natl Cancer Inst. 2009;101:1066–1082. doi: 10.1093/jnci/djp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin J, Xie J, Zhang H, Shen Q, Han L, Lu W, Han Y, Li C, Ni W, Wang H, et al. Significant association of different preS mutations with hepatitis B-related cirrhosis or hepatocellular carcinoma. J Gastroenterol. 2010;45:1063–1071. doi: 10.1007/s00535-010-0253-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Jin Y, Guo X, Bai X, Chen T, Wang J, Qian G, Groopman JD, Gu J, Li J, et al. Comparison study on the complete sequence of hepatitis B virus identifies new mutations in core gene associated with hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:2623–2630. doi: 10.1158/1055-9965.EPI-10-0469. [DOI] [PubMed] [Google Scholar]
- 21.Xie JX, Zhao J, Yin JH, Zhang Q, Pu R, Lu WY, Zhang HW, Wang HY, Cao GW. Association of novel mutations and haplotypes in the preS region of hepatitis B virus with hepatocellular carcinoma. Front Med China. 2010;4:419–429. doi: 10.1007/s11684-010-0160-0. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Xie J, Yin J, Zhang H, Zhang Q, Pu R, Li C, Ni W, Wang H, Cao G. A matched case-control study of hepatitis B virus mutations in the preS and core promoter regions associated independently with hepatocellular carcinoma. J Med Virol. 2011;83:45–53. doi: 10.1002/jmv.21829. [DOI] [PubMed] [Google Scholar]
- 23.Fontana RJ, Hann HW, Perrillo RP, Vierling JM, Wright T, Rakela J, Anschuetz G, Davis R, Gardner SD, Brown NA. Determinants of early mortality in patients with decompensated chronic hepatitis B treated with antiviral therapy. Gastroenterology. 2002;123:719–727. doi: 10.1053/gast.2002.35352. [DOI] [PubMed] [Google Scholar]
- 24.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Hui CK, Leung N, Yuen ST, Zhang HY, Leung KW, Lu L, Cheung SK, Wong WM, Lau GK. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology. 2007;46:395–401. doi: 10.1002/hep.21724. [DOI] [PubMed] [Google Scholar]
- 26.Lin SM, Yu ML, Lee CM, Chien RN, Sheen IS, Chu CM, Liaw YF. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol. 2007;46:45–52. doi: 10.1016/j.jhep.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008;28:1067–1077. doi: 10.1111/j.1365-2036.2008.03816.x. [DOI] [PubMed] [Google Scholar]
- 28.Lim SG, Mohammed R, Yuen MF, Kao JH. Prevention of hepatocellular carcinoma in hepatitis B virus infection. J Gastroenterol Hepatol. 2009;24:1352–1357. doi: 10.1111/j.1440-1746.2009.05985.x. [DOI] [PubMed] [Google Scholar]
- 29.Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348–356. doi: 10.1016/j.jhep.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 30.Thursz M, Brown A. Can antiviral therapy of chronic hepatitis B prevent the development of hepatocellular carcinoma. Gut. 2011;60:1025–1026. doi: 10.1136/gut.2010.236521. [DOI] [PubMed] [Google Scholar]
- 31.Sonneveld MJ, Janssen HL. Chronic hepatitis B: peginterferon or nucleos(t)ide analogues. Liver Int. 2011;31 Suppl 1:78–84. doi: 10.1111/j.1478-3231.2010.02384.x. [DOI] [PubMed] [Google Scholar]
- 32.van Zonneveld M, Honkoop P, Hansen BE, Niesters HG, Darwish Murad S, de Man RA, Schalm SW, Janssen HL. Long-term follow-up of alpha-interferon treatment of patients with chronic hepatitis B. Hepatology. 2004;39:804–810. doi: 10.1002/hep.20128. [DOI] [PubMed] [Google Scholar]
- 33.Buster EH, Hansen BE, Lau GK, Piratvisuth T, Zeuzem S, Steyerberg EW, Janssen HL. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology. 2009;137:2002–2009. doi: 10.1053/j.gastro.2009.08.061. [DOI] [PubMed] [Google Scholar]
- 34.Li WC, Wang MR, Kong LB, Ren WG, Zhang YG, Nan YM. Peginterferon alpha-based therapy for chronic hepatitis B focusing on HBsAg clearance or seroconversion: a meta-analysis of controlled clinical trials. BMC Infect Dis. 2011;11:165. doi: 10.1186/1471-2334-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen BE, Rijckborst V, Ter Borg MJ, Janssen HL. HBV DNA suppression in HBeAg-positive chronic hepatitis B patients treated with peginterferon or placebo. J Med Virol. 2011;83:1917–1923. doi: 10.1002/jmv.22208. [DOI] [PubMed] [Google Scholar]
- 36.Leung N. Treatment of HBeAg-positive chronic hepatitis B with nucleos(t)ide analogues. Liver Int. 2011;31 Suppl 1:85–89. doi: 10.1111/j.1478-3231.2010.02387.x. [DOI] [PubMed] [Google Scholar]
- 37.Papatheodoridis GV. Treatment of HBeAg-negative chronic hepatitis B patients with nucleos(t)ide analogues. Liver Int. 2011;31 Suppl 1:95–103. doi: 10.1111/j.1478-3231.2010.02392.x. [DOI] [PubMed] [Google Scholar]
- 38.Marcellin P, Chang TT, Lim SG, Sievert W, Tong M, Arterburn S, Borroto-Esoda K, Frederick D, Rousseau F. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2008;48:750–758. doi: 10.1002/hep.22414. [DOI] [PubMed] [Google Scholar]
- 39.Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422–430. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 40.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 41.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 42.Snow-Lampart A, Chappell B, Curtis M, Zhu Y, Myrick F, Schawalder J, Kitrinos K, Svarovskaia ES, Miller MD, Sorbel J, et al. No resistance to tenofovir disoproxil fumarate detected after up to 144 weeks of therapy in patients monoinfected with chronic hepatitis B virus. Hepatology. 2011;53:763–773. doi: 10.1002/hep.24078. [DOI] [PubMed] [Google Scholar]
- 43.Papatheodoridis GV, Deutsch M. Resistance issues in treating chronic hepatitis B. Future Microbiol. 2008;3:525–538. doi: 10.2217/17460913.3.5.525. [DOI] [PubMed] [Google Scholar]
- 44.European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, Brown N, Woessner M, Boehme R, Condreay L. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36:687–696. doi: 10.1086/368083. [DOI] [PubMed] [Google Scholar]
- 46.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 47.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–1751. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Yeon JE, Yoo W, Hong SP, Chang YJ, Yu SK, Kim JH, Seo YS, Chung HJ, Moon MS, Kim SO, et al. Resistance to adefovir dipivoxil in lamivudine resistant chronic hepatitis B patients treated with adefovir dipivoxil. Gut. 2006;55:1488–1495. doi: 10.1136/gut.2005.077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schildgen O, Sirma H, Funk A, Olotu C, Wend UC, Hartmann H, Helm M, Rockstroh JK, Willems WR, Will H, et al. Variant of hepatitis B virus with primary resistance to adefovir. N Engl J Med. 2006;354:1807–1812. doi: 10.1056/NEJMoa051214. [DOI] [PubMed] [Google Scholar]
- 50.Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, Chung YH, Lee YS, Yoo W, Kim SO. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43:1385–1391. doi: 10.1002/hep.21189. [DOI] [PubMed] [Google Scholar]
- 51.Sherman M, Yurdaydin C, Simsek H, Silva M, Liaw YF, Rustgi VK, Sette H, Tsai N, Tenney DJ, Vaughan J, et al. Entecavir therapy for lamivudine-refractory chronic hepatitis B: improved virologic, biochemical, and serology outcomes through 96 weeks. Hepatology. 2008;48:99–108. doi: 10.1002/hep.22323. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki F, Toyoda J, Katano Y, Sata M, Moriyama M, Imazeki F, Kage M, Seriu T, Omata M, Kumada H. Efficacy and safety of entecavir in lamivudine-refractory patients with chronic hepatitis B: randomized controlled trial in Japanese patients. J Gastroenterol Hepatol. 2008;23:1320–1326. doi: 10.1111/j.1440-1746.2008.05455.x. [DOI] [PubMed] [Google Scholar]
- 53.Kay A, Zoulim F. Hepatitis B virus genetic variability and evolution. Virus Res. 2007;127:164–176. doi: 10.1016/j.virusres.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Sheng YJ, Liu JY, Tong SW, Hu HD, Zhang DZ, Hu P, Ren H. Lamivudine plus adefovir combination therapy versus entecavir monotherapy for lamivudine-resistant chronic hepatitis B: a systematic review and meta-analysis. Virol J. 2011;8:393. doi: 10.1186/1743-422X-8-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwak MS, Choi JW, Lee JS, Kim KA, Suh JH, Cho YS, Won SY, Park BK, Lee CK. Long-term efficacy of entecavir therapy in chronic hepatitis B patients with antiviral resistance to lamivudine and adefovir. J Viral Hepat. 2011;18:e432–e438. doi: 10.1111/j.1365-2893.2011.01461.x. [DOI] [PubMed] [Google Scholar]
- 56.Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, Heathcote EJ, Manns M, Bzowej N, Niu J, et al. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486–495. doi: 10.1053/j.gastro.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 57.Tillmann HL, McHutchison JG. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2008;358:1517; author reply 1517–1518. doi: 10.1056/NEJMc080082. [DOI] [PubMed] [Google Scholar]
- 58.Patterson SJ, George J, Strasser SI, Lee AU, Sievert W, Nicoll AJ, Desmond PV, Roberts SK, Locarnini S, Bowden S, et al. Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut. 2011;60:247–254. doi: 10.1136/gut.2010.223206. [DOI] [PubMed] [Google Scholar]
- 59.van Bömmel F, de Man RA, Wedemeyer H, Deterding K, Petersen J, Buggisch P, Erhardt A, Hüppe D, Stein K, Trojan J, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51:73–80. doi: 10.1002/hep.23246. [DOI] [PubMed] [Google Scholar]
- 60.Locarnini S, Mason WS. Cellular and virological mechanisms of HBV drug resistance. J Hepatol. 2006;44:422–431. doi: 10.1016/j.jhep.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 61.Cammà C, Di Bona D, Schepis F, Heathcote EJ, Zeuzem S, Pockros PJ, Marcellin P, Balart L, Alberti A, Craxì A. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology. 2004;39:333–342. doi: 10.1002/hep.20073. [DOI] [PubMed] [Google Scholar]
- 62.Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444–2451. doi: 10.1056/NEJMct061675. [DOI] [PubMed] [Google Scholar]
- 63.Antaki N, Craxi A, Kamal S, Moucari R, Van der Merwe S, Haffar S, Gadano A, Zein N, Lai CL, Pawlotsky JM, et al. The neglected hepatitis C virus genotypes 4, 5 and 6: an international consensus report. Liver Int. 2010;30:342–355. doi: 10.1111/j.1478-3231.2009.02188.x. [DOI] [PubMed] [Google Scholar]
- 64.George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729–738. doi: 10.1002/hep.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singal AK, Singh A, Jaganmohan S, Guturu P, Mummadi R, Kuo YF, Sood GK. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2010;8:192–199. doi: 10.1016/j.cgh.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 66.Craxì A, Cammà C. Prevention of hepatocellular carcinoma. Clin Liver Dis. 2005;9:329–46, viii. doi: 10.1016/j.cld.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 67.Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnù L, Mazzella G, Ascione A, Santantonio T, Piccinino F, Andreone P, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–587. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 68.Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, Lee WM, Di Bisceglie AM, Bonkovsky HL, Dienstag JL, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–844. doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, Lee WM, Lok AS, Bonkovsky HL, Morgan TR, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429–2441. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lok AS, Everhart JE, Wright EC, Di Bisceglie AM, Kim HY, Sterling RK, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology. 2011;140:840–89; quiz e12. doi: 10.1053/j.gastro.2010.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sulkowski MS, Cooper C, Hunyady B, Jia J, Ogurtsov P, Peck-Radosavljevic M, Shiffman ML, Yurdaydin C, Dalgard O. Management of adverse effects of Peg-IFN and ribavirin therapy for hepatitis C. Nat Rev Gastroenterol Hepatol. 2011;8:212–223. doi: 10.1038/nrgastro.2011.21. [DOI] [PubMed] [Google Scholar]
- 73.Hofmann WP, Zeuzem S. A new standard of care for the treatment of chronic HCV infection. Nat Rev Gastroenterol Hepatol. 2011;8:257–264. doi: 10.1038/nrgastro.2011.49. [DOI] [PubMed] [Google Scholar]
- 74.Melnikova I. Hepatitis C--pipeline update. Nat Rev Drug Discov. 2011;10:93–94. doi: 10.1038/nrd3361. [DOI] [PubMed] [Google Scholar]
- 75.Bruno R, Cima S, Maiocchi L, Sacchi P. Forthcoming challenges in the management of direct-acting antiviral agents (DAAs) for hepatitis C. Dig Liver Dis. 2011;43:337–344. doi: 10.1016/j.dld.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 76.Ciesek S, Manns MP. Hepatitis in 2010: the dawn of a new era in HCV therapy. Nat Rev Gastroenterol Hepatol. 2011;8:69–71. doi: 10.1038/nrgastro.2010.219. [DOI] [PubMed] [Google Scholar]
- 77.Manns MP, Foster GR, Rockstroh JK, Zeuzem S, Zoulim F, Houghton M. The way forward in HCV treatment--finding the right path. Nat Rev Drug Discov. 2007;6:991–1000. doi: 10.1038/nrd2411. [DOI] [PubMed] [Google Scholar]
- 78.McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, Zeuzem S, Reesink HW, Garg J, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292–1303. doi: 10.1056/NEJMoa0908014. [DOI] [PubMed] [Google Scholar]
- 79.Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, Davis MN, Galati JS, Gordon SC, Ravendhran N, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705–716. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 80.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 81.Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, Urban T, Afdhal NH, Jacobson IM, Esteban R, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120–9.e18. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 82.Gane EJ, Roberts SK, Stedman CA, Angus PW, Ritchie B, Elston R, Ipe D, Morcos PN, Baher L, Najera I, et al. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2010;376:1467–1475. doi: 10.1016/S0140-6736(10)61384-0. [DOI] [PubMed] [Google Scholar]
- 83.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, Ray C. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42:255–262. doi: 10.1002/hep.20793. [DOI] [PubMed] [Google Scholar]
- 85.Sarrazin C, Rouzier R, Wagner F, Forestier N, Larrey D, Gupta SK, Hussain M, Shah A, Cutler D, Zhang J, et al. SCH 503034, a novel hepatitis C virus protease inhibitor, plus pegylated interferon alpha-2b for genotype 1 nonresponders. Gastroenterology. 2007;132:1270–1278. doi: 10.1053/j.gastro.2007.01.041. [DOI] [PubMed] [Google Scholar]