Abstract
The mechanism of action of silver nanoparticles (AgNPs) is unclear due to the particles’ strong tendency to agglomerate. Preventing agglomeration could offer precise control of the physicochemical properties that drive biological response to AgNPs. In an attempt to control agglomeration, we exposed zebrafish embryos to AgNPs of 20 or 110 nm core size, and polypyrrolidone (PVP) or citrate surface coatings in media of varying ionic strength. AgNPs remained unagglomerated in 62.5 μM CaCl2 (CaCl2) and ultrapure water (UP), but not in standard zebrafish embryo medium (EM). Zebrafish embryos developed normally in the low ionic strength environments of CaCl2 and UP. Exposure of embryos to AgNPs suspended in UP and CaCl2 resulted in higher toxicity than suspensions in EM. 20 nm AgNPs were more toxic than 110 nm AgNPs, and the PVP coating was more toxic than the citrate coating at the same particle core size. The silver tissue burden correlated well with observed toxicity but only for those exposures where the AgNPs remained unagglomerated. Our results demonstrate that size- and surface coating-dependent toxicity is a result of AgNPs remaining unagglomerated, and thus a critical-design consideration for experiments to offer meaningful evaluations of AgNP toxicity.
1. Introduction
Health and safety concerns about engineered nanomaterials (ENMs) are growing. Silver nanoparticles (AgNPs) are attracting increasing regulatory concern due to their extensive use in consumer products and industrial applications [8, 31]. AgNPs are heavily used in anti-microbial products such as socks, washing machines, wet wipes, water filters, detergents and soaps, industrial textiles and bedding [9]. Multiple studies have documented the toxicity of AgNPs to non-target bacteria, fish, cell lines and rodents [1-3, 7, 10, 18, 21, 26, 27, 29]. However, apparent contradictions have produced contention over AgNP toxicity and mode of action. AgNP size, size distribution, dispersion and agglomeration state each appear to be drivers of AgNP toxicity, but the relative importance of each variable and their interaction is not fully understood [23]. For instance, smaller AgNPs caused more severe embryonic lethality and malformation than larger ones [3], but other studies reported no correlation between size and toxicity [5, 37]. PVP-coated nanosilver was shown to be four times less toxic than silver ions [11], but, by contrast, a study on juvenile Japanese medaka (Oryzias latipes) reported that nanosilver was more toxic than ionic silver after 24 hours of exposure and similar in toxicity after 96 hours [6]. Commonly used biocompatible surface-coatings like polyvinylpyrrolidone (PVP) and citrate that stabilize AgNPs in suspension also appeared to variably affect AgNP toxicity [25, 28, 33, 34, 37]. These results are not surprising as the current state of AgNPs characterization is often sufficiently cursory that nominally similar AgNP formulations appear to be quite different depending on the laboratory that performed the characterization. Thus, consistently applied metrics controlling basic characterization parameters for AgNPs will be quite beneficial to understand their toxicity dependent on physicochemical property.
Agglomeration state is a critical concern because it can change dramatically with changes in ionic strength of the medium and the presence of biological molecules or organic matter. This, in turn, profoundly affects the transport and biological activity [23, 39]. It has been reported previously that ionic composition, as well as ionic strength, can affect agglomeration and NP stability [15, 16, 24]. We examined AgNP agglomeration in suspension as the confounding element of divergent toxicity results. We observed that AgNPs in suspension quickly agglomerate in the higher ionic strength of standard zebrafish embryo medium, which was independent of PVP and citrate surface coatings. Agglomeration has the effect of making the AgNPs less bioavailable as the dramatic size increase inhibits particle uptake and promotes rapid settling out of the suspension. The nanotoxicology field has begun to see the limitations of toxicology data relied on the AgNP manufacturer’s characterization rather than characterizing the AgNPs in the actual experimental condition. Thus, some recent studies have characterized the physicochemical state of NPs during toxicity tests and interpreted their result accordingly [4, 17, 27, 28]. Toxicological data obtained from appropriately characterized AgNPs will at last yield a clear understanding of AgNP safety.
Our group has developed a robust and rapid toxicity testing method in the embryonic zebrafish to define nanoparticle-biological interactions (NBI) [13, 14, 24]. The zebrafish is an established model for in vivo toxicity testing that helps identify molecular mechanisms, which can be extended for ecotoxicology [30]. Application of the developing zebrafish to investigate nanotoxicology is resource-efficient due to their small size and high fecundity. Additionally, the small quantity of material required for developmental exposure of zebrafish is especially advantageous for compounds under development, for which sample quantity available for testing may be limited to only a few milligrams.
The objective of this study was to investigate the biological responses of different size and surface coated AgNPs where particle dispersion was controlled. We used 20 and 110 nm size AgNPs, with either PVP or citrate surface coatings, to expose embryonic zebrafish in media of varying ionic strength. These AgNPs were selected by the Nanotechnology Health Implications Research (NCNHIR) Consortium and fully characterized by the National Characterization Laboratory (NCL). The varying ionic strength test media chosen to encompass agglomeration differential of AgNPs were: standard zebrafish embryo medium (EM, high ionic strength), 62.5 μM calcium chloride solution (CaCl2, low ionic strength) and ultrapure water (UP, no ions). AgNP toxicity results were based on a battery of morphological, developmental endpoints. We examined the relationship between toxicity and uptake in the different ionic strength media by quantifying the silver tissue burdens with inductively coupled plasma mass spectrometry (ICP-MS).
2. Materials and methods
2.1. Zebrafish maintenance
Adult zebrafish (Danio rerio) are maintained at Oregon State University’s Sinnhuber Aquatic Research Laboratory in a water flow through system at 28 °C under a 14:10 h light-dark photoperiod. Embryos utilized in the assay were obtained from the Tropical 5D zebrafish line and raised in buffered embryo medium (pH 7 ± 0.2) prepared in reverse osmosis water.
2.2 Embryonic zebrafish assay
Embryos collected were staged [19] and dechorionated with machine assist at 4 hours post fertilization (hpf) as previously described [24]. A 10 × concentrated serial diluted concentration of AgNPs was prepared. Individual wells of a 96-well plate were filled with 90 μL of test media and 6 hpf dechorionated embryos were placed into each well using automated embryo placement systems (AEPS) [24]. After embryos were loaded into the plate, 10 μL of the 10 × nanoparticle solution was mixed into respective wells for a final concentrations of 0.8, 4, 20, 10, and 50 mg/L. Exposed embryos were evaluated at 24 and 120 hpf for a total of 22 endpoints. The embryonic mortality was recorded at 24 and 120 hpf, and the percentage of malformation was calculated by the number of affected embryos per living embryos at each treatment.
We exposed zebrafish embryos to 4 different AgNPs suspended with 3 test media, EM, CaCl2 and UP (Gibco). Standard EM consisted of 15 mM NaCl, 0.5 mM KCl, 1 mM CaCl2, 1 mM MgSO4, 0.15 mM KH2PO4, 0.05 mM Na2HPO4 and 0.7 mM NaHCO3 [36]. We first tested each component of EM to determine the conditions that maintained zebrafish embryo health and AgNPs stability. We exposed zebrafish embryos to silver nitrate (> 99% purity, Sigma-Aldrich, St. Louis, MO) as a positive control for exposure to silver ion.
2.3. AgNPs and characterization in different test media
The National Institute of Environmental Health Sciences (NIEHS) is addressing the increasing health and safety concerns of AgNPs via an interdisciplinary program, the NCNHIR Consortium. The NCNHIR Consortium consists of eight cooperative centers that have proposed to identify a set of AgNPs, cerium dioxide and multi-wall carbon nanotubes of varying shapes, sizes and surface coatings that are widely used in industrial applications with the potential for human exposure. The consortium will jointly develop computational models to predict potential health effects in their respective in vitro and in vivo models. The first set of ENMs identified and characterized by the NCL was a set of AgNPs synthesized by nanoComposix (San Diego, CA) that included 20 nm PVP-stabilized AgNPs (20AgNPs-P), 20 nm citrate-stabilized AgNPs (20AgNPs-C), 110 nm PVP-stabilized AgNPs (110AgNPs-P) and 110 nm citrate-coated AgNPs (110AgNPs-C). AgNPs-P and AgNPs-C were supplied in water and 2 mM citrate buffer, respectively. All materials were stored at 4 °C and protected from light exposure. We followed the NCL recommended guidelines for sample handling and storage.
The NCL evaluated sterility and endotoxin levels of AgNPs. Hydrodynamic diameter and zeta potential of AgNPs in an unfiltered 10 fold diluted aliquot of stock solution (1000 mg/L) with 2 mM NaCl were measured using dynamic light scattering (DLS, Malvern Zetasizer Nano ZS instrument, Southborough, MA) with a back scattering detector (173°). UV-Vis absorbance spectra (UV-vis) and core diameter by TEM were provided by the NCL. The NCL characterization data can be found in Figure S1 and S2; the details are provided in the NCL report (NCL-NIEHS201111A). In this study, we analyzed size and zeta potential of 4 different AgNPs in each test medium by using ZetaPALS (Brookhaven Instruments Corporation, Holtsville, NY). To characterize AgNPs, we used 2 mL of 10 fold diluted AgNPs (100 mg/L) with UP, CaCl2 and EM. We used a 2 mM NaCl solution to account for machine variability when measuring hydrodynamic diameter and zeta potential. We conducted UV-vis analysis with 100 μL of 5 mg/L AgNPs dispersed in EM, CaCl2 and UP placed into 96-well plate using microplate reader (BioTek Synergy Mx, Winooski, VT).
2.4. Quantification of silver by ICP-MS
Embryos exposed to 0, 10 and 50 mg/L of 4 different AgNPs suspended in 3 test media (i.e., EM, CaCl2 and UP) were collected at 24 hpf from individual wells of a 96-well plate. Only living embryos with no morphological malformations were chosen. For all treatments except 50 mg/L AgNPs-C, pools of 30 embryos per treatment with 3 replicates were rinsed 3 times with Milli-Q water. Pools of embryos were used to increase sensitivity for ICP-MS. For 50 mg/L of AgNPs-C treatment, only 10 embryos were pooled because of the limited numbers of viable embryos at that concentration and time point. Once rinsed, embryos were placed into 15 mL Falcon tubes. Excess water was removed and samples were stored at 4 °C until time for analysis. Prior to analysis, samples were thawed and transferred to Teflon tubes (Savillex, Eden Prairie, MN) for digestion. To digest the samples, trace metal grade nitric acid (VWR International, West Chester, PA) was added to each Teflon tube containing embryos, and then heated to a boil (100 °C) for 5-10 minutes on a hotplate to evaporate all liquid. Once all the nitric acid was evaporated, the samples were digested in 20 drops of concentrated nitric acid, and then dried down on the hot plate. This step was repeated twice. After digestion, the residue was dissolved in 100 μL of nitric acid while the Teflon tubes were still warm. The dissolved samples were transferred back into 15 mL Falcon tubes and the volume was brought up to 5 mL Milli-Q water for a final concentration of 2% nitric acid. A 1 μg/L internal standard (Indium) was added to each sample prior to analysis with ICP-MS. A five point calibration was used with a R2 value of 0.998. The ICP-MS values from each treatment were averaged (n = 3) with standard errors.
2.5. Statistics
Statistical analyses were compiled using SigmaStat/Plot (SPSS Inc, Chicago, IL) or through custom R scripts. The significance of dose response was determined using logistic regression. A three-way ANOVA (p < 0.05) and Tukey’s post hoc test with AgNPs, medium and concentration as factors were used to determine the significance of silver body burden.
3. Results and discussion
3.1. Optimization of test medium
In a previous study, we conducted the embryonic toxicity test by using diluted EM to evaluate the toxicity of 3-mercaptopropionic acid-functionalized gold nanoparticles (3-MPA-AuNPs) which precipitate in 100% EM [35]. The portion of 3-MPA-AuNPs unagglomerated in suspension was directly affected by the percentage of EM used as the test medium. 3-MPA-AuNPs remained unagglomerated in low ionic medium and the incidence of morbidity and mortality was significantly higher than when dispersed in EM where the agglomeration was also observed. Zebrafish embryos can develop normally in a wide range of ionic strength media. We adjusted the ionic strength of test medium to prevent AgNP agglomeration and optimize bioavailability as a precursor to measurement of toxicity endpoints. Lee et al achieved stable AgNPs using 1.2 mM NaCl egg water to image AgNPs crossing the zebrafish chorion and to evaluate toxicity endpoints [21]. We observed that incubation in 1 mM CaCl2 did not induce developmental morbidity or mortality at 120 hpf. We then exposed embryos to a titration of CaCl2 to ascertain the minimal concentration necessary for normal development (i.e., no obvious malformation and mortality) at 120 hpf, the latest time point when we used to record toxicological responses. We found that 62.5 μM CaCl2 was the minimal concentration needed for normal development and low enough to maintain AgNPs unagglomeration which was confirmed by UV-vis measurement. The embryonic zebrafish assay is flexible and versatile for using low ionic strength test media, making it useful for nanoparticle toxicity evaluations compared to in vitro cell-based testing. Cell culture medium has a high ionic strength and causes AgNPs and other to agglomerate in suspension, which has likely confounded the conclusions of some reports of the hazard potential and toxicity mechanism of NPs.
3.2. Characterization of AgNPs in test medium
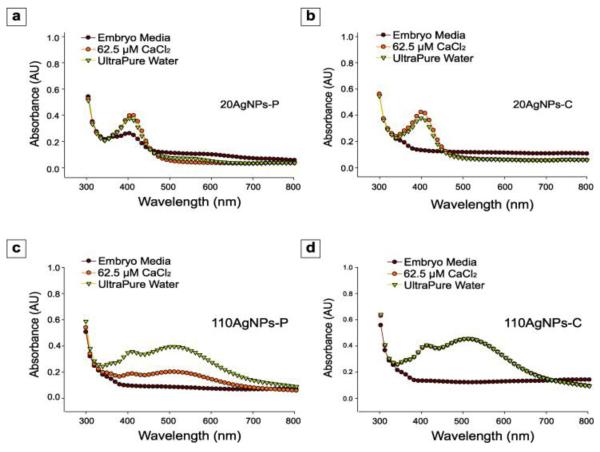
Prior to conducting the embryonic zebrafish assay, we identified potential changes in physicochemical properties of 4 different AgNPs in 3 test media of varying ionic strength. We measured UV-vis absorbance as a direct indicator of NP dispersal, hydrodynamic diameter with ZetaPALS and surface charge with zeta potential (Table 1, Figure 1). The characterization of AgNPs was also performed in 2 mM NaCl used by the NCL to originally characterize these particles and allowed independent comparison to the NCL reference data and correction for instrument differences. Notably, it is possible that the AgNP characteristics may vary at lower concentrations. Figure 1 illustrates that the medium significantly influence the degree of agglomeration. UV-vis spectra significantly decreased for all AgNPs tested in EM, indicative of agglomeration. The higher cation concentration of EM, relative to 62.5 μM CaCl2 or water, reduces the electrostatic repulsion between the negatively charged AgNPs resulting in agglomeration. When we measured the silver concentration of AgNP supernatant by ICP-MS, we found similar silver concentrations in CaCl2 and UP medium but significantly less silver in the EM supernatant (Figure S3) corroborating the drop in absorbance observed with agglomeration. UV-vis spectra analysis revealed that 20AgNPs-P remained more unagglomerated than 20AgNPs-C in EM, but 110AgNPs-C remained more unagglomerated than 110AgNPs-P in CaCl2. Huynh and Chen reported that AgNPs-P was more stable than AgNPs-C in the presence of divalent electrolytes such Ca2+ and Mg2+ due to steric repulsion imparted by the large uncharged polymers [15]. Our results suggest that the dispersion of AgNPs was influenced by the size of the particle in the different electrolytes.
Table 1.
The hydrodynamic diameter of 4 different AgNPs in 3 different test media.
| Diameter (± S.D) (nm)a |
20AgNPs-P | 20AgNPs-C | 110AgNPs-P | 110AgNPs-C |
|---|---|---|---|---|
| NCL b | 26.0 | 24.0 | 112.3 | 104.2 |
| 2mM NaCl | 29.3 ± 1.4 | 25.2 ± 0.5 | 104.9 ± 2.6 | 110.2 ± 6.5 |
| Embryo media | 69.7 ± 2.7 | 3078.4 ± 400.7 | 691.8 ± 40.3 | 1400.5 ± 91.2 |
| 62.5 μM CaCl2 | 30.5 ± 1.2 | 25.3 ± 0.3 | 107.8 ± 3.9 | 104.1 ± 3.2 |
| Ultrapure water | 31.4 ± 0.8 | 26.1 ± 0.8 | 123.2 ± 0.9 | 100.4 ± 2.1 |
These values are averaged on the intensity basis over all size populations.
Taken from table in the overview of the NCL report. These values are of the average of multiple experiments.
Figure 1.
The UV-vis absorbance spectra of (a) 20AgNPs-P, (b) 20AgNPs-C, (c) 110AgNPs-P and (d) 110AgNPs-C suspended in embryo media, 62.5 μM CaCl2 and ultrapure water. The concentration of each sample is 20 mg/L.
We next determined the hydrodynamic diameter of each AgNPs in 3 test media. AgNP diameters in each medium corresponded with the agglomeration level by UV-vis spectra (Table 1). Hydrodynamic diameters were first measured by diluting with 2 mM NaCl, similar to the NCL protocol. All AgNPs were stable (i.e., no significant size increase) over 5 exposure days when dispersed in CaCl2 and UP, but hydrodynamic diameter increased to hundreds nm for 110AgNPs-P and > 1000 nm for 20AgNPs-P and 110AgNPs-C in EM, indicative of agglomeration. The ability of the smaller 20AgNPs-P to remain more unagglomerated was evident by the suspension having only an attenuated absorbance peak at 380 nm (Figure 1). Zeta potentials were negative among the different particle sizes and coatings (data not shown) in all three media. There was no correlation between zeta potential and particle size, suggesting that the interfacial forces play more dominant roles in AgNPs stability rather than electrostatic repulsion [16]. Taken together, Figure 1 and Table 1 demonstrate that the higher stability of AgNPs in CaCl2 and UP compared to EM in which UV-vis was essentially non-existent and the sizes were significantly increased in EM, indicating that test media directly impacts AgNPs stability.
3.3. Developmental toxicity profiles
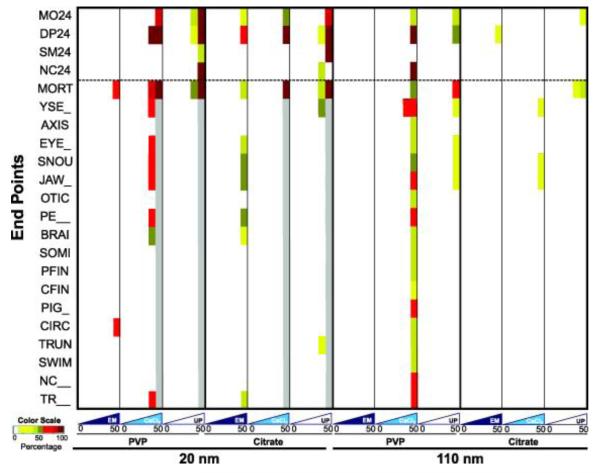
We generate heat maps to display the embryonic toxicity profiles of AgNPs in different test media to efficiently view the spectrum of multivariate biological responses. Colors represent the percent incidence of each evaluated endpoint (a total of 22) over the range of AgNP exposure concentrations (0, 0.08, 0.4, 2, 10 and 50 mg/L). The colors white, green and red indicate zero incidence of an endpoint above background, intermediate and high incidence, respectively. Gray indicates no viable embryos for further analysis. Increasing color intensity designates a greater incidence of an endpoint (refer to the Color Scale of Figure 2). To eliminate background and low incidence effects, a filter was added to convert incidence occurrences less than 30% to be white. Therefore, any white in the heatmap signifies <30% affected. AgNPs suspended in CaCl2 and UP caused higher toxicity. At 120 hpf, 20AgNPs-P, 20AgNPs-C and 110AgNPs-P induced 100% mortality at 50 mg/L when suspended in both CaCl2 and UP, not in EM, visualized as the absence of any coloration in their heatmap entries. Most toxicity occurred at the highest concentration of 50 mg/L even in CaCl2 and UP, employed to maximize dispersion. At exposures less than 50 mg/L, only 10 mg/L of 20AgNPs-P induced an increased incidence of morbidity and mortality in CaCl2 and UP. We note that ppm (mg/L) levels are much higher than predicted environmental concentrations (PEC) of AgNPs in various environmental compartments such as soil, sludge, surface water and sediment [10] and that the data suggest that AgNPs are not acutely toxic to the embryonic zebrafish. We calculated EC50 values (the concentration which causes a 50% incidence of adverse effects) for comparison of AgNP formulations (Table S1). EC50 values for AgNPs suspended in CaCl2 and UP were significantly lower than for suspensions in EM. According to the heatmap and EC50 values, we ranked the embryonic toxicity of AgNPs in EM as: 20AgNPs-C > 20AgNPs-P > 110AgNPs-C ≈ 110AgNPs-P. As expected, essentially no toxicity was observed from AgNPs suspended in EM due to strong agglomeration, with the exception of 20AgNPs-P where a low incidence of mortality and smaller agglomerate size were observed (69.7 nm as shown above). 110AgNPs-P showed some color gradients in EM, but these are not concentration-dependent and the percentage was below 30%. Embryonic toxicity in CaCl2 (and similarly UP) was ranked: 20AgNPs-P > 20AgNPs-C > 110AgNPs-P > 110AgNPs-C. AgNPs suspended in CaCl2 and UP caused higher incidence of mortality at 24 hpf, but only suspension in UP induced adverse responses at concentrations less than 50 mg/L. The mortality of 20AgNPs-P at 10 mg/L was 87.5 and 50 % in CaCl2 and UP, respectively. Eighty percentage of living embryos exposed to 10mg/L 20AgNPs-P exhibited the malformations such as yolk sac edema (YSE), eye defects (EYE) and snout defect (SNOU) etc.
Figure 2.
Heatmap of biological responses of 4 types of AgNPs suspended in embryo media, 62.5 μM CaCl2 and ultrapure water. The colors represent the percentage of effected embryos. Embryos were exposed to 5 concentrations of AgNPs (0, 0.08, 0.4, 2, 10, 50 mg/L) from 6-120 hpf and evaluated for mortality and adverse effects (including malformations). Mortality was assessed at both 24 and 120 hpf, while only the surviving embryos at 120 hpf were evaluated for 16 morphological malformations (n = 16, two replicates). When 100% mortality was observed, the endpoints were grayed out to illustrate there were no survivors at that concentration. Endpoint evaluated are defined as follows: MO24 = mortality observed at 24 hpf; DP24 = developmental progression at 24 hpf; SM24 = spontaneous movement at 24 hpf; NC24 = notochord malformation at 24 hpf. Endpoints evaluated at 120 hpf were: MORT = cumulative mortality; YSE = yolk sac edema; AXIS = axis defects; EYE = eye defects; SNOU = snout defect; JAW = jaw defect, OTIC = otic (ear) defect; PE = pericardial edema; BRAI = brain defect; SOMI = somite defect; PFIN and CFIN = pectoral and cadual fin defect; PIG = pigmentation abnormalities; CIRC = circulation defects; TRUN = trunk defect; SWIM = swim bladder abnormalities; NC = notochord defect at 120 hpf and TR = touch response abnormality.
3.4. Size and surface coating dependent toxicity
We demonstrated that smaller 20 nm AgNPs were more toxic than 110 nm AgNPs regardless of the surface coating. Size influence on AgNPs toxicity has been demonstrated in fish, fruit fly and cells in culture [3, 5, 12]. These toxicity results were driven by the altered hydrodynamic diameter of AgNPs due to the test medium [12, 32, 33]. As a confounding effect, consider that, dry particle size of 10 and 50 nm AgNPs-P increased to several hundred nm when in suspension [27], and the hydrodynamic diameter of 8 nm AgNPs-P was > 4 fold larger in a high ionic suspension [37]. Moreover, the effect of test medium on AgNPs hydrodynamic diameter is rarely considered or reported in published toxicity reports. Our approach was novel and importantly precise because we controlled the desired size of AgNPs, with different surface coatings, and by manipulating the electrolyte concentration of the test medium. The developmental toxicity profile we have generated for 4 different AgNPs is the most accurate ever made for this class of ENMs.
Our results indicated that PVP coating was more toxic than the citrate coating, a departure from some published reports. Suresh et al demonstrated that AgNPs-C were more toxic than AgNPs-P [33], and Powers et al reported that the exposure of zebrafish embryos to 10 nm AgNPs-C caused some mortality and malformations whereas neither 10 nor 50 nm AgNPs-P were toxic [28]. These studies did not consider agglomeration of AgNPs in suspension thus, comparative conclusions regarding the influence of coating material are difficult to draw. A similar limitation was evident in another study reporting surface-coating dependent cytotoxicity of AgNPs despite uncontrolled agglomeration in cell culture medium [34]. Some reports have indicated awareness of the agglomeration effect. Yang et al systematically investigated coating effects on AgNPs toxicity in Caenorhabditis elegans demonstrating that AgNPs-P was more toxic than AgNPs-C, consistent with this study [37]. They attributed the lower toxicity of AgNPs-C to agglomeration. In conclusion, when agglomeration is controlled for, the governing trend is for smaller ANPs to be more developmentally toxic than larger particles. Importantly, the contribution of surface coating to the toxicity profile is revealed when the confounding effect of agglomeration is controlled.
3.5. Quantification of silver
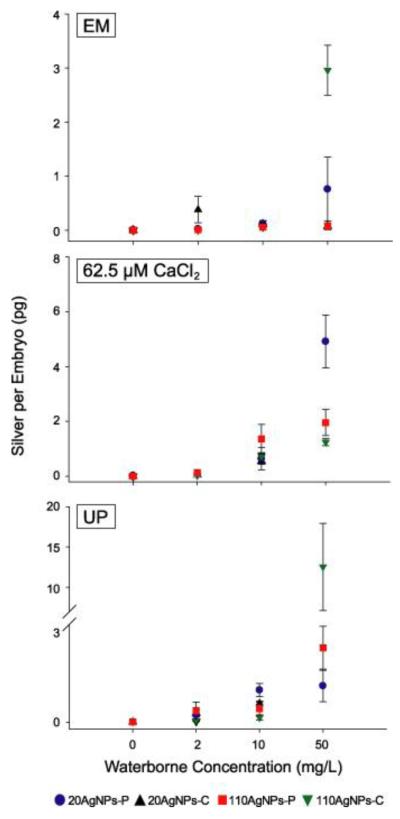
We analyzed the silver tissue burden of embryos exposed to AgNP by using ICP-MS. We could not discern whether silver was internalized by the embryos, remained attached to the embryo surface, or both. Nor could we establish whether the particulate or ionized forms were present in the bulk suspension or were generated by interacting with the embryos. Despite these limitations, we measured a higher level of silver body burden in embryos exposed to AgNPs suspended in CaCl2 and UP, compared to EM with the exception at a few concentrations of 20AgNPs-C (2 and 10 mg/L), which suggested that the unagglomerated AgNPs were more bioavailable. The silver body burden detected in embryos exposed to 110AgNPs-C (micron size when suspended in EM) was probably due to uptake, but to AgNPs attached to the epithelial surface. A positive correlative relationship existed between silver body burden of all AgNPs at 50 mg/L and induced toxicity when suspended in CaCl2 (Figure 3, Figure S3). These data were not plotted because the incidence of mortality was close to 100% and the surviving embryos showed malformations after 24 hpf which was inappropriate for ICP-MS analysis. We hypothesized that similar amounts of silver released from AgNPs or silver nitrate are equally toxic, thus silver ion is the toxic driver. We have analyzed silver body burden of embryos exposed to silver nitrate dissolved in EM, CaCl2 and UP and found that the toxicity of silver nitrate was associated with silver body burden. Silver nitrate dissolved in CaCl2 and UP was more toxic and embryos had a higher body burden of silver than when suspended in EM. The greater concentration of cations in EM reduced the amount of bioavailable free silver ion. The LC50 (lethal concentration where 50% mortality of test species was observed) of silver ion was 1.5 mg/L. There have been studies on the kinetics of silver ion release from AgNPs demonstrating that smaller AgNPs released more silver ions [22, 38], and the soluble fraction of silver ions was not affected by surface coating [20, 37]. Thus, we speculated that the particulate form played a certain role in uptake and toxicity along with silver ions. Future studies will focus on careful investigation for the effect of silver ion released from these selected AgNPs in different test media.
Figure 3.
Silver tissue burden of zebrafish embryos exposed to 4 types of AgNPs suspended in embryo media, 62.5 μM CaCl2 and ultrapure water. Error bars represent mean ± SE of three replicates.
On the other hand, the degree of toxicity in UP was the same as in CaCl2 which resulted from AgNPs stability; but silver body burden varied in UP. The body burden difference from embryos exposed to AgNPs in CaCl2 and UP is probably due to embryos physiological status. Even though the morphological effects of embryos exposed in UP was indistinguishable from CaCl2, some effects on osmosis may occur, which would influence the uptake of silver ions and the particulate form. In addition, the complexation of Ag+ with Cl− could affect the bioavailability of AgNPs.
4. Conclusions
In conclusion, the toxicity of AgNPs and tissue burden was strongly correlated to their stability in the selected media, and that the biological responses varied depending on size and surface coating. Although the NCL thoroughly characterized these NPs, the experimental physicochemical properties and behavior of AgNPs are greatly influenced by the test medium, and no other model but the embryonic zebrafish offered the experimental power and flexibility to make this discovery. Attempts at hazard identification of ENMs toxicology must take carefully into regards the ionic environment and NP agglomeration state to generate accurate assessments of NP hazard.
Supplementary Material
Acknowledgments
The authors would like to thank the staff of the Sinnhuber Aquatic Research Laboratory for the embryos, Chapell Miller for his assistance in evaluating the toxicity of the nanoparticles, Dr. Betts J EPA for the use of ZetaPALS and the Keck WM Collaboratory for Plasma Spectrometry for the use of ICP-MS. These studies were partially supported by National Institute of Environmental Health Sciences (NIEHS), R01 ES016896, F31 ES019445and P30 ES000210. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of NIEHS.
Footnotes
Supplementary data One table for LC50 and EC50 values and supplemental figures for the NCL characterization data, silver concentration of the supernatant in different test media and the relationship silver body burden toxicity.
References
- [1].Asharani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–90. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- [2].Asharani PV, Wu YL, Gong Z, Valiyaveettil S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology. 2008;25:255102. doi: 10.1088/0957-4484/19/25/255102. [DOI] [PubMed] [Google Scholar]
- [3].Bar-Ilan O, Albrecht RM, Fako VE, Furgeson DY. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small. 2009;5:1897–910. doi: 10.1002/smll.200801716. [DOI] [PubMed] [Google Scholar]
- [4].Bilberg K, Hovgaard MB, Besenbacher F, Baatrup E. In Vivo Toxicity of Silver Nanoparticles and Silver Ions in Zebrafish (Danio rerio) J. Toxicol. 2012:293784. doi: 10.1155/2012/293784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J. Phys. Chem. B. 2008;112:13608–19. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- [6].Chae YJ, Pham CH, Lee J, Bae E, Gu MB. Evaluation of the toxic impact of silver nanoparticles on Japanese medaka (Oryzias latipes) Aquat. Toxicol. 2009;94:320–7. doi: 10.1016/j.aquatox.2009.07.019. [DOI] [PubMed] [Google Scholar]
- [7].Chen D, Xi T, Bai J. Biological effects induced by nanosilver particles: in vivo study. Biomed. Mater. 2007;2:S126–8. doi: 10.1088/1748-6041/2/3/S08. [DOI] [PubMed] [Google Scholar]
- [8].de Lima R, Seabra AB, Duran N. Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012;32:867–79. doi: 10.1002/jat.2780. [DOI] [PubMed] [Google Scholar]
- [9].El-Badawy A, Feldhake D, Venkatapathy R. State of the Science Literature Review: Everything Nanosilver and More. United States Environmental Protection Agency; Wahsington DC: 2010. [Google Scholar]
- [10].Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR. Silver nanoparticles: behaviour and effects in the aquatic environment. Environ. Int. 2011;37:517–31. doi: 10.1016/j.envint.2010.10.012. [DOI] [PubMed] [Google Scholar]
- [11].Foldbjerg R, Olesen P, Hougaard M, Dang DA, Hoffmann HJ, Autrup H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 2009;190:156–62. doi: 10.1016/j.toxlet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- [12].Gorth DJ, Rand DM, Webster TJ. Silver nanoparticle toxicity in Drosophila: size does matter. Int. J. Nanomed. 2011;6:343–50. doi: 10.2147/IJN.S16881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Harper SL, Carriere JL, Miller JM, Hutchison JE, Maddux BL, Tanguay RL. Systematic Evaluation of Nanomaterial Toxicity: Utility of Standardized Materials and Rapid Assays. ACS Nano. 2011;5:4688–97. doi: 10.1021/nn200546k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harper SL, Usenko C, Hutchinson JE, Maddux BLS, Tanguay RL. In vivo biodistribution and toxicity depends on nanomaterial composition, size, surface functionalization and route of exposure. J. Exp. Nanosci. 2008;3:195–206. [Google Scholar]
- [15].Huynh KA, Chen KL. Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions. Environ. Sci. Technol. 2011;45:5564–71. doi: 10.1021/es200157h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jin X, Li M, Wang J, Marambio-Jones C, Peng F, Huang X, Damoiseaux R, Hoek EM. High-throughput screening of silver nanoparticle stability and bacterial inactivation in aquatic media: influence of specific ions. Environ. Sci. Technol. 2010;44:7321–8. doi: 10.1021/es100854g. [DOI] [PubMed] [Google Scholar]
- [17].Keene AM, Peters D, Rouse R, Stewart S, Rosen ET, Tyner KM. Toxicity testing of nanomaterials. Nanomedicine. 2012;7:199–209. doi: 10.2217/nnm.11.125. [DOI] [PubMed] [Google Scholar]
- [18].Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- [19].Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- [20].Kittler S, Greulich C, Diendorf J, Koller M, Epple M. Toxicity of Silver Nanoparticles Increases during Storage Because of Slow Dissolution under Release of Silver Ions. Chem. Mater. 2010;22:4548–54. [Google Scholar]
- [21].Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XH. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano. 2007;1:133–43. doi: 10.1021/nn700048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu J, Sonshine DA, Shervani S, Hurt RH. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano. 2010;4:6903–13. doi: 10.1021/nn102272n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].MacCuspie RI, Rogers K, Patra M, Suo Z, Allen AJ, Martin MN. Toxicity testing of nanomaterials. J. Environ. Monit. 2011;13:1212–26. doi: 10.1039/c1em10024f. [DOI] [PubMed] [Google Scholar]
- [24].Mandrell D, Truong L, Jephson C, Sarker MR, Moore A, Lang C, Simonich MT, Tanguay RL. Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. J. Lab. Autom. 2012;17:66–74. doi: 10.1177/2211068211432197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Meyer JN, Lord CA, Yang XY, Turner EA, Badireddy AR, Marinakos SM, Chilkoti A, Wiesner MR, Auffan M. Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans. Aquat. Toxicol. 2010;100:140–50. doi: 10.1016/j.aquatox.2010.07.016. [DOI] [PubMed] [Google Scholar]
- [26].Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- [27].Powers CM, Badireddy AR, Ryde IT, Seidler FJ, Slotkin TA. Silver nanoparticles compromise neurodevelopment in PC12 cells: critical contributions of silver ion, particle size, coating, and composition. Environ. Health Perspect. 2011;119:37–44. doi: 10.1289/ehp.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Powers CM, Slotkin TA, Seidler FJ, Badireddy AR, Padilla S. Silver nanoparticles alter zebrafish development and larval behavior: distinct roles for particle size, coating and composition. Neurotoxicol. Teratol. 2011;33:708–14. doi: 10.1016/j.ntt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rahman MF, Wang J, Patterson TA, Saini UT, Robinson BL, Newport GD, Murdock RC, Schlager JJ, Hussain SM, Ali SF. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 2009;187:15–21. doi: 10.1016/j.toxlet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- [30].Scholz S, Fischer S, Gundel U, Kuster E, Luckenbach T, Voelker D. The zebrafish embryo model in environmental risk assessment-applications beyond acute toxicity testing. Environ. Sci. Pollut. Res. Int. 2008;15:394–404. doi: 10.1007/s11356-008-0018-z. [DOI] [PubMed] [Google Scholar]
- [31].Schrand AM, Dai K, Schlager JJ, Hussain SM. Toxicity testing of nanomaterials. Adv. Exp. Med. Biol. 2012;745:58–75. doi: 10.1007/978-1-4614-3055-1_5. [DOI] [PubMed] [Google Scholar]
- [32].Scown TM, Santos EM, Johnston BD, Gaiser B, Baalousha M, Mitov S, Lead JR, Stone V, Fernandes TF, Jepson M, van Aerle R, Tyler CR. Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout. Toxicol. Sci. 2010;115:521–34. doi: 10.1093/toxsci/kfq076. [DOI] [PubMed] [Google Scholar]
- [33].Suresh AK, Pelletier DA, Wang W, Moon JW, Gu B, Mortensen NP, Allison DP, Joy DC, Phelps TJ, Doktycz MJ. Silver nanocrystallites: biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity on gram-negative and gram-positive bacteria. Environ. Sci. Technol. 2010;44:5210–5. doi: 10.1021/es903684r. [DOI] [PubMed] [Google Scholar]
- [34].Suresh AK, Pelletier DA, Wang W, Morrell-Falvey JL, Gu B, Doktycz MJ. Cytotoxicity induced by engineered silver nanocrystallites is dependent on surface coatings and cell types. Langmuir. 2012;28:2727–35. doi: 10.1021/la2042058. [DOI] [PubMed] [Google Scholar]
- [35].Truong L, Zaikova T, Richman EK, Hutchison JE, Tanguay RL. Media ionic strength impacts embryonic responses to engineered nanoparticle exposure. Nanotoxicology. 2012;6:691–9. doi: 10.3109/17435390.2011.604440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Westerfield M. The Zebrafish Book. University of Oregon Press; Eugene, OR: 2000. [Google Scholar]
- [37].Yang X, Gondikas AP, Marinakos SM, Auffan M, Liu J, Hsu-Kim H, Meyer JN. Mechanism of silver nanoparticle toxicity is dependent on dissolved silver and surface coating in Caenorhabditis elegans. Environ. Sci. Technol. 2012;46:1119–27. doi: 10.1021/es202417t. [DOI] [PubMed] [Google Scholar]
- [38].Zhang W, Yao Y, Sullivan N, Chen Y. Modeling the primary size effects of citrate-coated silver nanoparticles on their ion release kinetics. Environ. Sci. Technol. 2011;45:4422–8. doi: 10.1021/es104205a. [DOI] [PubMed] [Google Scholar]
- [39].Zook JM, Long SE, Cleveland D, Geronimo CL, MacCuspie RI. Measuring silver nanoparticle dissolution in complex biological and environmental matrices using UV-visible absorbance. Anal. Bioanal. Chem. 2011;401:1993–2002. doi: 10.1007/s00216-011-5266-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.