Summary
Candida albicans hypha formation which has been stimulated via the Ras1-cAMP-Efg1 signalling cascade is inhibited by farnesol, a C. albicans autoregulatory factor, and small molecules such as dodecanol. In cultures containing farnesol or dodecanol, hypha formation was restored upon addition of dibutyryl-cAMP. The CAI4-Ras1G13V strain, which carries a dominant-active variant of Ras1 and forms hyphae in the absence of inducing stimuli, grew as yeast in medium with farnesol or dodecanol; the heat shock sensitivity of the CAI4-Ras1G13V strain was also suppressed by these compounds. Neither Pde1 nor Pde2 was necessary for the repression of hyphal growth by farnesol or dodecanol. Two transcripts, CTA1 and HSP12, which are at higher levels upon mutation of Ras1 or Cdc35, were increased in abundance in cells grown with farnesol or dodecanol. Microscopic analysis of strains carrying CTA1 and HWP1 promoter fusions grown with intermediate concentrations of farnesol or dodecanol indicated a link between cells with the increased expression of cAMP-repressed genes and cells repressed for hypha formation. Because several cAMP-controlled outputs are affected by farnesol and dodecanol, our findings suggest that these compounds impact activity of the Ras1-Cdc35 pathway, thus leading to an alteration of C. albicans morphology.
Introduction
Candida albicans is the most common causative agent of fungal infection in immunocompromised patients with high mortality rates associated with systemic candidiasis (Fridkin and Jarvis, 1996; Viudes et al., 2002). Several studies indicate that the ability to switch between hyphal and yeast form growth is critical for C. albicans virulence (Lo et al., 1997; Mitchell, 1998; Saville et al., 2003) by promoting invasion and dissemination (Gow et al., 2002). Furthermore, many virulence-related genes are coordinately regulated with morphology (Fu et al., 2002; Sundstrom et al., 2002).
Several small molecules have been identified that repress hypha formation (Hornby et al., 2001; Oh et al., 2001; Shchepin et al., 2003; Hogan et al., 2004). Farnesol, a sesquiterpene produced by C. albicans itself, acts as a quorum-sensing molecule that represses hyphal growth and biofilm formation in dense populations (Hornby et al., 2001; Ramage et al., 2002). Such regulation of morphology by an extracellular signal may allow for diversification within a microbial population by promoting dissemination or the formation of structure within a biofilm community. Another class of molecules includes 3-oxo-C12-homoserine lactone (3OC12HSL), a signalling molecule produced by Pseudomonas aeruginosa, and dodecanol, an alcohol with activities similar to that of 3OC12HSL (Hogan et al., 2004). These two compounds as well as farnesol share a 12-carbon backbone length in common, and they all repress hypha formation, but not growth, at micromolar concentrations.
Most of the studies on the effects of farnesol, dodecanol, 3OC12HSL, and chemical analogues of these molecules on hypha induction have been performed under conditions that stimulate hyphal growth by the Ras1-Cdc35 (adenylate cyclase)-PKA-Efg1 pathway. This pathway controls the induction of hypha formation in response to multiple stimuli including GlcNAc (Cho et al., 1992), serum (Feng et al., 1999) and glucose (Maidan et al., 2005). The regulation of hyphal growth by this pathway appears to be particularly important for C. albicans virulence in animal models for disseminated candidiasis (Lo et al., 1997; Rocha et al., 2001). In this pathway, the small GTPase Ras1, when activated, stimulates adenylate cyclase (Cdc35) leading to the generation of a cAMP signal that promotes PKA-mediated activation of transcription factor(s), including Efg1, that mediate the yeast-to-hypha transition (Castilla et al., 1998; Feng et al., 1999; Leberer et al., 2001). The addition of db-cAMP, a non-hydrolysable functional analogue of cAMP, rescues hypha formation in C. albicans ras1/ras1 and cdc35/ cdc35 cells which cannot induce the appropriate cAMP signal in hypha-inducing medium (Leberer et al., 2001; Rocha et al., 2001). The presence of a dominant-active Ras1 variant (Ras1G13V) leads to hypha formation under conditions that normally promote yeast-form growth, and Cdc35 and Efg1 are required for this hyperfilamentation phenotype on solid medium containing serum (Leberer et al., 2001). Other regulatory factors, such as components of the mitogen-activated protein (MAP) kinase regulatory pathway, are also required for maximal hyphal growth on solid medium with serum suggesting that the Ras1-Cdc35 signalling pathway can interact with other regulatory networks. Thus, between the production of autoregulatory molecules and interwoven regulatory pathways, C. albicans has multiple mechanisms by which it can precisely regulate its morphological state in response to environmental cues (Leberer et al., 2001).
Here, we report data suggesting that farnesol and dodecanol, two compounds that inhibit hyphal growth, affect the activity of the Ras1-Cdc35-PKA-Efg1-dependent signalling pathway that controls hypha formation. Hyphal growth can be rescued in cultures containing either farnesol or dodecanol by the addition of db-cAMP which artificially stimulates the downstream portion of this pathway. Furthermore, we provide evidence that farnesol and dodecanol impact cAMP-controlled genes involved in processes other than morphogenesis such as the heat shock response and the transcriptional control of stress response genes. In cultures grown with concentrations of farnesol or dodecanol that inhibit hypha formation in only a subpopulation of cells, we observe a link between the induction of cAMP-repressed genes and the repression of hypha formation using reporter gene fusion strains. These data strongly support the hypothesis that inhibition of the Ras1-Cdc35-dependent signalling pathway leads to the inhibition of hyphal growth caused by C. albicans-produced farnesol and dodecanol.
Results
Hyphal growth induced by the Ras1-Cdc35-PKA-Efg1 pathway is repressed by farnesol and dodecanol, and the repression is rescued by the addition of db-cAMP
To determine if farnesol and dodecanol repress hyphal growth by affecting the cAMP-mediated regulatory cascade (Fig. 1A), we performed experiments to determine if artificial stimulation of hypha formation by db-cAMP could rescue filamentation in cultures grown with dodecanol and farnesol. Prior to performing these experiments, it was confirmed that the hyphal growth stimuli used in these experiments (glucose and GlcNAc) induced hypha and pseudohypha formation via the Ras1-Cdc35-Efg1 pathway as has been described previously (Cho et al., 1992; Maidan et al., 2005). Quantitative microtiter dish-based microscopic assays were developed to assess the percentages of hyphae, pseudohyphae and yeast. As predicted, the C. albicans wild-type CAF2 cultures formed abundant hyphae after 3 h (92.7 ± 4.7% of total cells grew as hyphae), while the ras1/ras1, cdc35/ cdc35 and efg1/efg1 mutants grew exclusively as yeast (data not shown). The percentage of CAF2 cells growing as hyphae drastically reduced to 5.2 ± 0.1% in dodecanol-treated cultures, and to 25.9 ± 1.2% in farnesol-treated cultures. As reported previously, the growth rate of C. albicans was not affected by the addition of farnesol or dodecanol (Hogan et al., 2004). While the per cent of cells present as hyphae in control cultures did vary between experiments, dodecanol and farnesol always reduced hypha formation by more than 75%. In morphology experiments performed in phosphate buffer at 37°C, it was also shown that glucose and GlcNAc could serve as single stimuli to trigger hypha formation, and that dodecanol and farnesol suppress hyphal growth in response to either stimulus (Table S1). Because there is evidence that both the cAMP signalling pathway and the MAP kinase pathway can contribute to hyphal growth in some conditions (Leberer et al., 2001), the cph1/cph1 mutant, which is defective in hypha formation in response to stimulation of the MAP kinase pathway (Csank et al., 1998), was also analysed in YNBNP medium at 37°C. The cph1/cph1 mutant strain exhibited hypha formation similar to that of the wild-type strain with 90.6 ± 0.9% of cells growing as hyphae, indicating that Cph1 was not required for hypha formation under these conditions. Furthermore, dodecanol and farnesol both repressed hypha formation in cph1/cph1 mutant cultures with kinetics indistinguishable from those for the wild type (data not shown).
Fig. 1.
Effects of db-cAMP on CAF2, ras1/ras1 and efg1/efg1 cells treated with dodecanol and farnesol. Black bars represent the percentage of cells present as hyphae and white bars represent the percentage of cells present as pseudohyphae. The remainder of cells were yeast. The error bars represent the standard deviation between three replicate cultures. The experiments were performed with similar results on more than three different days.
A. A simplified model of Efg1-dependent C. albicans hyphal induction pathway.
B. The morphology of C. albicans CAF2, ras1/ras1 and efg1/efg1 cells grown in YNBNP for 5 h with and without db-cAMP (10 mM).
C. The morphology of cells grown in medium with vehicle alone, 200 µM dodecanol, or 200 µM farnesol in the presence or absence of 10 mM db-cAMP. The asterisks represent cultures in which no hyphae or pseudohyphae were detected.
Consistent with previously published reports (Leberer et al., 2001), quantitative microscopic counts were used to demonstrate that the addition of an exogenous cAMP signal rescued hyphal growth in a ras1/ras1 strain (Fig. 1B). Cultures of ras1/ras1 cells that were amended with 10 mM db-cAMP contained 34 ± 6% and 41 ± 9% of cells in the hyphal and pseudohyphal morphology, respectively, in comparison with the ras1/ras1 controls in which no hyphae and only 1% pseudohyphae were observed (Fig. 1B). The morphologies detected in efg1/efg1 mutant cultures were unaffected by the addition of db-cAMP; no hyphae and low levels of pseudohyphae (7.8 ± 4.4%) were observed in both controls and db-cAMP-amended cultures (Fig. 1B). CAF2 wild-type cultures exhibited only slightly enhanced percentages of hyphae and pseudohyphae in medium containing db-cAMP (Fig. 1B).
To determine if db-cAMP could rescue hypha formation in CAF2 cultures grown in hypha-inducing YNBNP medium with dodecanol or farnesol, similar morphological analyses to those described above were performed. While CAF2 cells grown with either dodecanol or farnesol were almost entirely in the yeast morphology, the addition of db-cAMP to the growth medium restored true hyphae and pseudohyphae formation to levels comparable with those in vehicle control cultures in the absence of dodecanol or farnesol (Fig. 1C). Under these conditions, farnesol was consistently less effective than dodecanol as an inhibitor of hypha formation, and db-cAMP addition caused a greater stimulation of hyphal growth in dodecanol-grown cultures. The addition of db-cAMP to the medium of ras1/ras1 cultures that contained either dodecanol or farnesol also resulted in hyphal and pseudohyphal growth at levels comparable with those observed in the wild-type control cultures (Fig. 1C). In contrast, the morphologies of cells within efg1/efg1 cultures grown with dodecanol and farnesol were unaffected by db-cAMP with only yeast and low levels of pseudohyphae detected (Fig. 1C). Representative differential interference contrast microscopic (DIC) images of cells from CAF2, ras1/ras1 and efg1/efg1 cultures with and without cAMP grown with vehicle alone, farnesol or dodecanol are shown in Fig. S1 A–C respectively. These data indicate that exogenously added cAMP can rescue filamentation in wild-type (CAF2) cultures, and promote hypha and pseudohypha formation by ras1/ras1 cells, in the presence of dodecanol and farnesol. The hyphal growth stimulated by db-cAMP was dependent on Efg1.
Farnesol and dodecanol repress hypha formation in C. albicans bearing the dominant-active Ras1G13V
Because exogenous db-cAMP could restore hypha formation in CAF2 cultures grown with farnesol and dodecanol, experiments were performed to determine if the artificial hyperactivation of the Ras1-Cdc35-PKA pathway due to the presence of a dominant-active Ras1 variant could promote hypha formation in cultures containing farnesol or dodecanol. To test this, a comparative microscopic analyses of the strains CAI4-Ras1G13V, carrying a dominant-active Ras1 variant and CAI4-Ras1, which carries a third copy of the non-mutated RAS1 gene, was performed. To specifically focus on the hyphal growth phenotypes that could be attributed to the RAS1G13V allele, a microscopic assay for hypha formation was developed using phosphate buffer at 37°C with no added inducer; under these conditions, 83 ± 6% of CAI4-Ras1G13V cells formed hyphae within 6 h (Fig. 2A and E). Cells of the isogenic control strain, CAI4-Ras1, doubled several times, but formed less than 6 ± 1.9% hyphae (Fig. 2B and E). The addition of GlcNAc to SC5314 cells under these conditions stimulated hypha formation in 80.9 ± 16.6% of cells, and this hypha formation was repressed by farnesol and dodecanol (Table S1); similar results were obtained with the CAI4-Ras1 strain (data not shown).
Fig. 2.
Farnesol and dodecanol repress hypha formation in cells containing dominant-active Ras1G13V.
A–D. DIC images of cells grown in phosphate buffer at 37°C for 6 h.
A. CAI4-Ras1G13V grown in phosphate buffer.
B. CAI4-Ras1 cells grown in phosphate buffer.
C. The effects of 200 µM farnesol or (D) 200 µM dodecanol on Ras1G13V cells in phosphate buffer.
E. Quantitative morphological assessment was determined by microscopy for both CAI4-Ras1 (white bars) and Ras1G13V (grey bars). Cells were grown in phosphate buffer alone, buffer with 200 µM farnesol or buffer with 200 µM dodecanol for 6 h at 37°C. Each bar represents the percentage of hyphae out of the total number of cells counted. The asterisk indicates that there were no hyphae observed in those cultures.
When CAI4-Ras1G13V was incubated in phosphate buffer containing either farnesol (Fig. 2C) or dodecanol (Fig. 2D), hypha formation was reduced by more than 97% (Fig. 2E) with no obvious defects in growth (data not shown). These data indicate that farnesol and dodecanol can repress the hyphal growth stimulated by the dominant-active Ras1 variant protein.
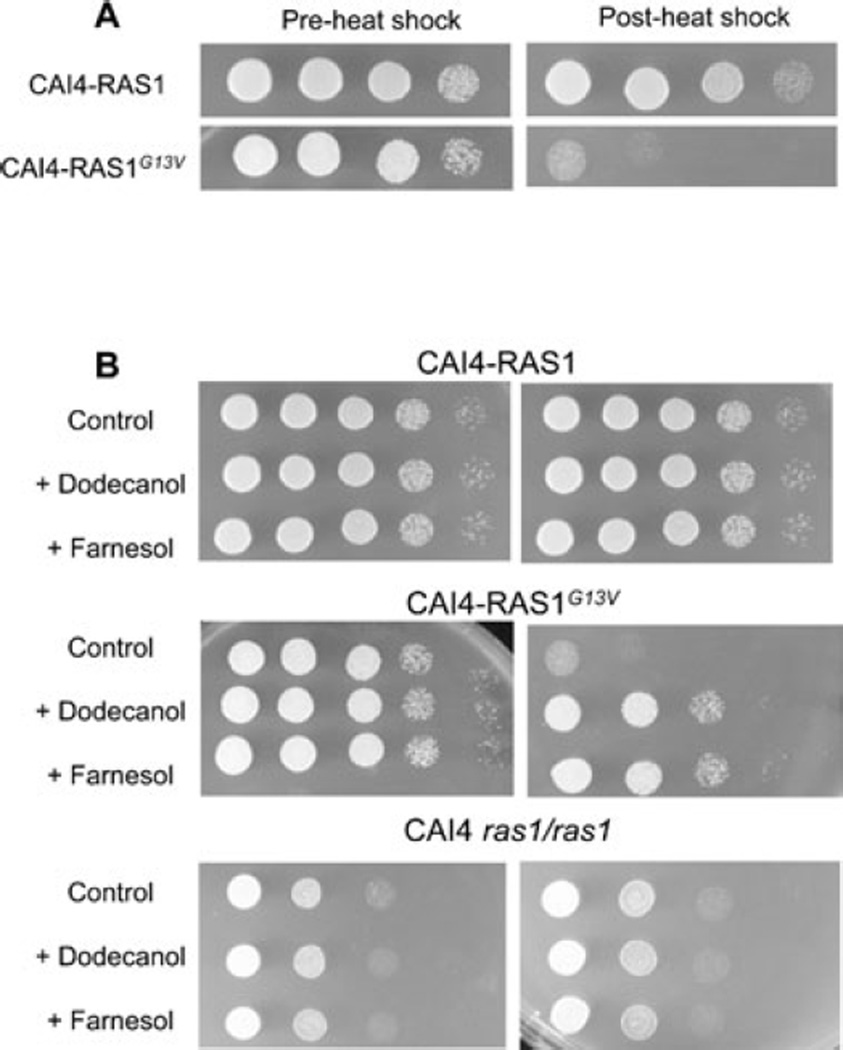
Farnesol and dodecanol rescue Ras1G13V-induced heat shock sensitivity
In addition to hypha formation under non-inducing conditions, the hyperactivation of the Ras1-Cdc35-PKA pathway in the CAI4-Ras1G13V strain confers hypersensitivity to certain stresses due to the inability to induce the appropriate stress responses. As reported previously (Feng et al., 1999), strains carrying the dominant-active RAS1G13V allele were severely impaired in the survival of heat shock in comparison with the reference strain CAI4-Ras1 (Fig. 3A), while ras1/ras1 mutants showed heat shock survival similar to that observed for wild-type (data not shown) and CAI4-Ras1 strains (Fig. 3B).
Fig. 3.
Effects of dodecanol and farnesol on heat shock survival. Titers of cultures grown in YPD for 6 h at 30°C prior to and after heat shock at 48°C for 6 min are shown.
A. Heat shock survival of CAI4-Ras1 and Ras1G13V cultures.
B. Heat shock survival of CAI4-Ras1, Ras1G13V, and ras1/ras1 cultures grown in YPD for 6 h at 30°C with either 75 µM dodecanol or farnesol.
Because farnesol and dodecanol could suppress the hyperfilamentation phenotype of the CAI4-Ras1G13V strain, we sought to determine if farnesol and dodecanol could also repress the heat shock sensitivity phenotype of CAI4-Ras1G13V cells. To facilitate the quantification of cell survival after heat shock by colony-forming units counts, these experiments were performed under non-inducing conditions. Heat shock survival of cultures grown with the vehicle alone, or with dodecanol or farnesol was determined. While addition of dodecanol or farnesol did not affect heat shock survival of C. albicans CAF2 (wild-type), CAI4-Ras1, or ras1/ras1 cultures, dodecanol and farnesol increased the survival of the CAI4-Ras1G13V by more than two orders of magnitude relative to vehicle control cultures (Fig. 3B). There were no differences in growth rate between CAI4-Ras1G13V cultures that received dodecanol or farnesol compared with vehicle controls (Fig. S2). The observations that both dodecanol and farnesol inhibited two cAMP signalling mediated outputs in the CAI4-Ras1G13V strain, hypha formation and heat shock sensitivity, are consistent with a model in which these compounds impact the Ras1-cAMP signalling cascade.
The cAMP phosphodiesterases Pde1 and Pde2 are not required for repression of hypha formation by farnesol or dodecanol
Because both hypha formation in wild-type strains and heat shock sensitivity in a strain producing a dominant-active Ras1 variant were impacted by farnesol and dodecanol, we hypothesized that increased cAMP phosphodiesterase activity may be responsible for the effects of these compounds on cAMP signalling and C. albicans morphology. C. albicans has two cAMP phosphodiesterases, Pde1, a low-affinity phosphodiesterase, and Pde2, a high-affinity phosphodiesterase involved in the modulation of cAMP levels during the induction of hyphal growth (Hoyer et al., 1994; Bahn et al., 2003; Jung and Stateva, 2003). To determine if farnesol and dodecanol are impacting cAMP-induced hypha formation by stimulating the activity of Pde1 or Pde2, the responses of C. albicans strains lacking Pde1 (pde1/pde1), Pde2 (pde2/pde2), or both phosphodiesterases (pde1/pde1 pde2/pde2) to dodecanol and farnesol were analysed in YNBNP at 37°C. The C. albicans pde1/pde1 mutant and its complemented derivative formed hyphae with similar kinetics to the CAF2 reference strain at 3 h, and hypha formation was repressed in pde1/pde1 cultures grown with farnesol or dodecanol (Table 1). Consistent with published results (Jung and Stateva, 2003), pde2/pde2 vehicle control cultures were largely pseudohyphal with ∼13% true hyphae at 3 h. Farnesol or dodecanol completely repressed both hypha and pseudohypha formation in this strain (Table 1). The complemented Pde2 mutant (pde2/pde2 PDE2) displayed a similar hyphal profile and sensitivity to dodecanol and farnesol to that seen in CAF2 cultures. An examination of the pde1/pde1 pde2/pde2 mutant, which lacks both phosphodiesterases, found 44 ± 2.3% true hyphae in control cultures, and this percentage decreased to 0% and 2 ± 0.7% respectively, yielding cultures that were mainly yeast when grown with farnesol or dodecanol (Table 1). These data indicate that neither Pde1 nor Pde2 is required for farnesol or dodecanol effects on hypha formation.
Table 1.
Effects of farnesol and dodecanol on hypha formation in phosphodiesterase mutants grown in YNBNP at 37°C for 3 h.
| % hyphae |
|||
|---|---|---|---|
| Strain | Control | Farnesol | Dodecanol |
| CAF2 | 92.6 (± 4.7) | 25.9 (± 1.2) | 5.2 (± 0.1) |
| pde1/pde1 | 91.4 (± 0.3) | 27.6 (± 7.8) | 15.9 (± 0.1) |
| pde1/pde1 PDE1 | 91.4 (± 3.1) | 1.3 (± 0.2) | 1.5 (± 0.1) |
| pde2/pde2 | 12.8 (± 1.9) | 0 | 0 |
| pde2/pde2 PDE2 | 93.9 (± 1.5) | 15.4 (± 1.7) | 4.3 (± 0.9) |
| pde1/pde1 pde2/pde2 | 44.3 (± 2.3) | 0 | 1.7 (± 0.7) |
The standard deviation between the three replicate cultures is reported in parenthesis.
Intracellular cAMP levels
To further test the hypothesis that farnesol and dodecanol impacted cAMP signalling, based on the observations that db-cAMP rescued hyphal growth in cultures containing either dodecanol or farnesol, intracellular C. albicans cAMP levels in CAF2 cultures grown with vehicle alone, dodecanol or farnesol were measured. As controls, analyses of cAMP levels in ras1/ras1 and cdc35/cdc35 mutants were also performed. CAF2 control cultures contained 2.05 ± 0.24 pmol of cAMP per 106 cells; while ras1/ras1 cultures contained 0.56 ± 0.16 pmol of cAMP per 106 cells. The cdc35/cdc5 mutant had no detectable levels of cAMP Numerous comparisons were made between the vehicle control CAF2 cultures and CAF2 cultures grown with dodecanol or farnesol, and no consistent, statistically significant differences in bulk intracellular cAMP levels were observed in YNBNP medium at early time points (1–5 min after induction of hyphal growth) or in exponential-phase cultures (15 min to 4 h after transfer to inducing medium) (data not shown).
Because the CAI4-Ras1G13V cells exhibit phenotypes attributed to hyperactivated cAMP signalling, which could be suppressed by either farnesol or dodecanol, intracellular cAMP levels were also determined in cultures of CAI4-Ras1G13V and CAI4-Ras1 reference strain in exponential-phase cultures grown with farnesol, dodecanol or vehicle alone. Intracellular cAMP levels in cells from either CAI4-Ras1G13V or CAI4-Ras1 cultures grown with dodecanol or farnesol did not show any statistically significant differences from their corresponding vehicle controls. Interestingly, however, in three independent experiments, the comparison of cAMP levels in CAI4-Ras1G13V cells with those in CAI4-Ras1 or wild-type cultures found that intracellular cAMP levels were 2- to 2.5-fold lower in the CAI4-Ras1G13V strain (2.0 ± 0.20 vs. 0.96 ± 0.50 pmol of cAMP per 106 cells) suggesting that, like in S. cerevisiae (Ma et al., 1999), the hyperactivation of Ras leads to lower levels of intracellular cAMP due to increased PDE activity. These data suggest that whole cell cAMP levels may not always reflect the status of the Ras1-Cdc35-PKA pathway, as the pathway is subject to a strong feedback regulation.
The abundance of CTA1 and HSP12, cAMP-responsive transcripts, increases in farnesol- and dodecanol-grown cells
As an alternative approach to determine the effects of farnesol and dodecanol on the Ras1-Cdc35-dependent signalling pathway, the levels of two transcripts responsive to cAMP-mediated signalling were monitored in cultures grown with farnesol or dodecanol relative to control cultures. S. cerevisiae CTT1, which encodes catalase, and HSP12 are two genes negatively regulated by cAMP (Bissinger et al., 1989; Enjalbert et al., 2003; Ferguson et al., 2005), and there is evidence for similar regulation of the C. albicans homologues, CTA1 and HSP12 (Enjalbert et al., 2003). Consistent with the array data published by Harcus et al. (2004), real-time RT-PCR analyses showed that CTA1 and HSP12 transcript levels are inversely correlated with cAMP levels in C. albicans mutants that lack RAS1 or CDC35. CTA1 levels were 6.1- and 5.8-fold higher in ras1/ras1 and cdc35/cdc35 mutant cells, respectively, than in the wild-type CAF2 strain (Fig. 4A), and HSP12 levels were 62- and 102-fold higher than CAF2 controls in ras1/ras1 and cdc35/cdc35 cultures respectively (Fig. 4C). The levels of CTA1 and HSP12 were not different when RNA from wild-type cells grown under hypha-inducing and non-inducing conditions were compared (Harcus et al., 2004 and data not shown).
Fig. 4.
Real-time RT-PCR analysis of CTA1, HSP12 and HWP1 transcripts normalized to GPD1 control in CAF2, ras1/ras1 and cdc35/cdc35 cultures grown in YNBNP medium for 2 h at 37°C. Levels are expressed relative to the maximum value among the samples analysed within each graph to represent fold differences. The maximal values are not necessarily the same between graphs for a given transcript.
A. Normalized levels of CTA1 transcript in CAF2, ras1/ras1 and cdc35/cdc35 cultures.
B. Normalized levels of CTA1 transcript in CAF2 cells grown with vehicle alone, 75 µM dodecanol or 75 µM farnesol.
C. Normalized levels of HSP12 transcript in CAF2, ras1/ras1 and cdc35/cdc35 cells.
D. Normalized levels of HSP12 transcript in CAF2 cells grown with vehicle alone, 75 µM dodecanol or 75 µM farnesol.
E. Normalized levels of HWP1 transcript in CAF2, ras1/ras1 and cdc35/cdc35 cultures.
F. Normalized levels of HWP1 transcript in CAF2 cells grown with vehicle alone, 75 µM dodecanol or 75 µM farnesol.
To determine if CTA1 and HSP12 levels are altered in C. albicans CAF2 cells upon growth in inducing conditions (YNBNP at 37°C for 2 h) in the presence of farnesol or dodecanol, RNA was isolated from cells and relative transcript levels were measured. Levels of CTA1 in cells grown with either farnesol or dodecanol were increased by 9- and 8.1-fold respectively (Fig. 4B), and those of HSP12 by 37-fold in cultures grown with dodecanol and 10-fold in cultures grown with farnesol (Fig. 4D). As a control, levels of HWP1 transcript, a well-characterized, hypha-specific marker were examined in CAF2, ras1/ras1, and cdc35/ cdc35 cells and in CAF2 cultures grown with farnesol or dodecanol (Fig. 4E and F). Consistent with the observation that the ras1/ras1 and cdc35/cdc35 mutant strains do not form hyphae in YNBNP medium, RT-PCR analysis showed that HWP1 transcript levels were more than 100-fold higher in the CAF2 strain compared with ras1/ras1 or cdc35/cdc35 cells (Fig. 4E).In the dodecanol and farnesol-grown cultures that exhibited increased CTA1 and HSP12 expression (Fig. 4B and D), HWP1 levels were lower by 114- and 20-fold respectively (Fig. 4F).
To determine if the increase in expression levels of CTA1 and HSP12 in CAF2 cells upon growth with dodecanol and farnesol required an intact cAMP signalling pathway, the effects of dodecanol and farnesol on CTA1 and HSP12 transcript levels were also determined in the C. albicans ras1/ras1 and cdc35/cdc35 mutants. In the ras1/ras1 mutant strain, growth in the presence of farnesol led to only a twofold increase in CTA1 levels and a threefold increase in HSP12 levels, relative to vehicle controls, and growth with dodecanol led to a threefold increase in CTA1 levels and a fourfold increase in HSP12 levels (data not shown). In the cdc35/cdc35 mutant, CTA1 levels changed by less than threefold and HSP12 levels were not different between cultures grown with vehicle alone or with farnesol or dodecanol (data not shown).
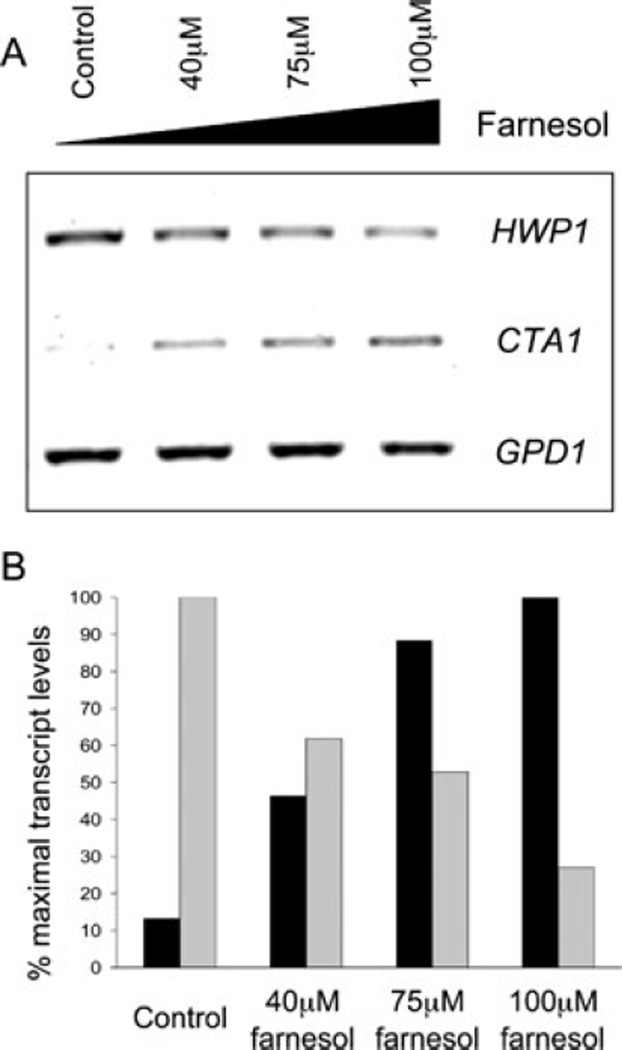
Previously published experiments have shown that increasing concentrations of dodecanol and farnesol led to an increased percentage of cells in the yeast morphology along with corresponding decreases in HWP1 promoter activity (Hogan et al., 2004). To determine if there is a correlation between the decreased expression of HWP1 and the increased expression of Ras1- and Cdc35-repressed genes, levels of the cAMP-responsive gene, CTA1 were determined in C. albicans SC5314 cultures grown with 0, 40, 75 and 100 µM farnesol or dodecanol. As the concentration of farnesol or dodecanol increased, the levels of CTA1 transcript increased, while those of HWP1 decreased (Fig. 5A and data not shown). Quantification of the transcript levels confirmed the dose-dependent changes in RNA levels (Fig. 5B).
Fig. 5.
RT-PCR analysis of HWP1 and CTA1 transcripts in C. albicans CAF2 cultures grown in YNBNP at 37°C for 2 h.
A. RT-PCR analysis of C. albicans HWP1 CTA1 and GPD1 transcripts in cultures treated with increasing concentrations of farnesol.
B. Quantification of transcripts treated with increasing concentrations of farnesol using ImageJ. CTA1 (black bars) and HWP1 (grey bars) transcripts were normalized to the GPD1 control transcript and expressed as a percentage of the maximal transcript level.
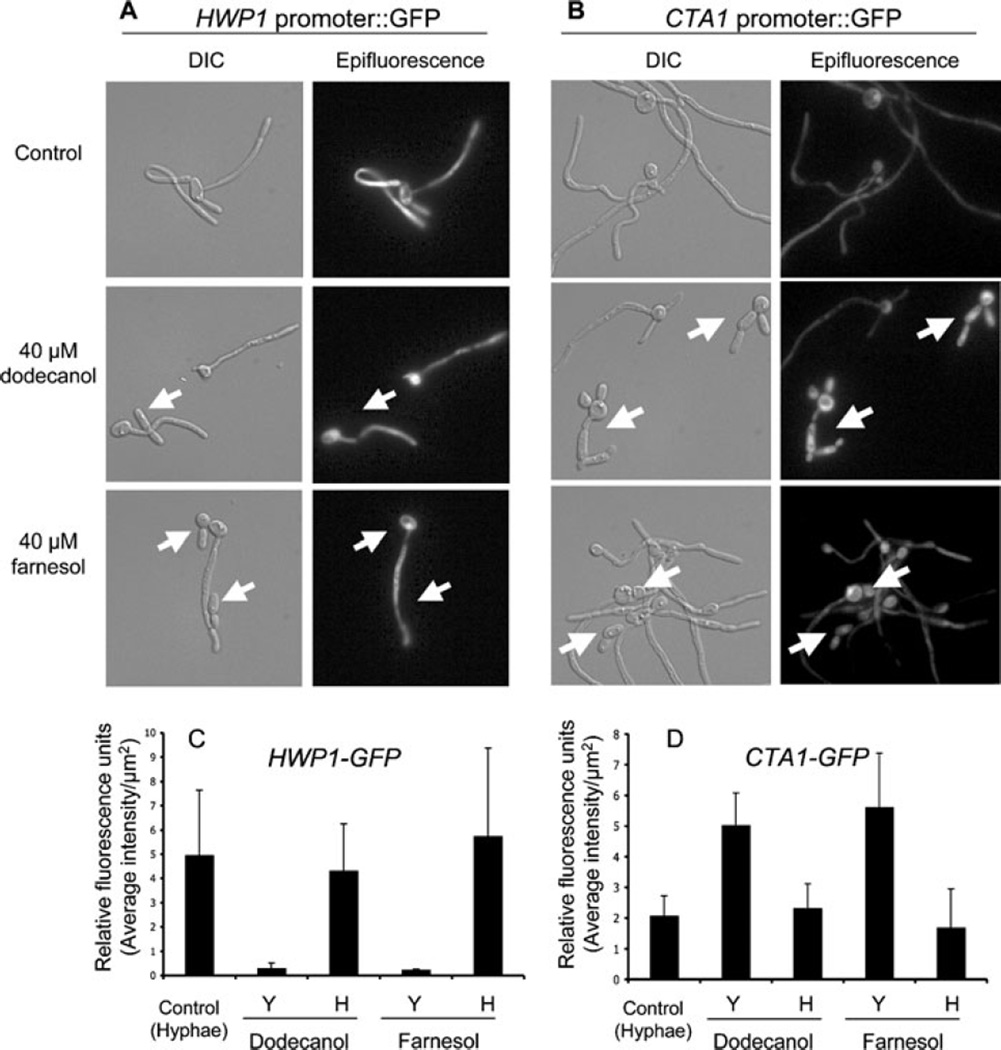
Microscopic examination of CTA1 and HWP1 promoter activity in cultures containing farnesol and dodecanol
To further assess the potential link between depressed cAMP signalling and the suppression of hyphal growth, the promoter activities of HWP1 and CTA1 were observed in single cells from cultures grown with concentrations of farnesol or dodecanol that inhibited hypha formation in only a portion of the population. Cells that contain either an HWP1- or CTA1-promoter fusion to GFP (Staab et al., 2003; Enjalbert et al., 2007) and a control strain expressing GFP from a constitutive promoter (Enjalbert et al., 2007) were examined. While greater than 99% of cells grew as hyphae in control cultures, cultures grown with 40 µM farnesol or 40 µM dodecanol contained approximately 50% hyphae with the remaining cells present as pseudohyphae or yeast (data not shown). At concentrations of dodecanol or farnesol that gave rise to mixed populations of hyphae, pseudohyphae and yeast, a comparison of DIC and fluorescent images showed that yeast and pseudohyphae of the HWP1-GFP strain exhibited almost no fluorescence compared with the levels visualized within hyphae (Fig. 6A) (Staab et al., 2003). Measurement of the average fluorescence density per area using Axiovision software found a more than 10-fold decrease in fluorescence intensity when hyphal and non-hyphal cells were compared (Fig. 6C). In contrast, the experiments performed with the CTA1 promoter fusion strain found that those cells growing as yeast or pseudohyphae in dodecanol- and farnesol-treated cultures exhibited increased GFP fluorescence compared with hyphal cells within the same culture (Fig. 6B). Quantification of pixel intensity found levels of yeast cells within dodecanol and farnesol-grown cultures to be 2.2- and threefold greater, respectively, than those of hyphal cells (Fig. 6D). The constitutively expressing GFP strain showed only a slight increase (< 1.5-fold) in GFP fluorescence in yeast relative to hyphal cells (data not shown). For both HWP1 and CTA1, the fluorescence intensities in the vehicle control cultures, in which cells grew mainly as hyphae, were similar to levels observed within the hyphae present in cultures containing dodecanol or farnesol (Fig. 6C–D). These data indicate that cells within dodecanol- and farnesol-grown cultures are not uniformly expressing CTA1 (Fig. 6B), and that there is a correlation between CTA1 promoter activity, which is higher in the absence of Ras1 or Cdc35 activity (Fig. 4A), and repression of hyphal growth.
Fig. 6.
DIC and epifluorescent microscopic analyses of GFP-fusion strains grown with dodecanol and farnesol for 5 h in YNBNP at 37°C.
A. HWP1-GFP grown with vehicle alone, 40 µM dodecanol or 40 µM farnesol. White arrows represent yeast-form cells with decreased HWP1-GFP promoter activity.
B. CTA1-GFP grown with vehicle alone, 40 µM dodecanol or 40 µM farnesol. White arrows represent yeast-form cells with increased CTA1-GFP promoter activity.
C and D. Relative fluorescence, as measured by average fluorescence intensity per µm2 using Axiovision software, for strains carrying an HWP1-GFP(C) and a CTA1-GFP promoter fusion (D) in yeast (Y) and hyphal (H) cells within the same cultures grown with dodecanol or farnesol. Only hyphal cells were detected in the control cultures.
Discussion
Previous studies from our lab and others have shown that a range of compounds with basic structural similarities, including farnesol and dodecanol, repress hypha formation under conditions that normally stimulate the Ras1-Cdc35-PKA-Efg1 hyphal induction pathway (Hornby et al., 2001; Oh et al., 2001; Kim et al., 2002; Shchepin et al., 2003; Hogan et al., 2004). Here, we provide evidence that these compounds impact the Ras1-Cdc35 pathway which affects both hypha formation and other properties controlled by cAMP regulation.
Three lines of evidence support the hypothesis that both farnesol and dodecanol impact the Ras1-Cdc35-Efg1 signalling pathway. First, CAF2 cells under hypha inducing conditions grew as yeast in the presence of dodecanol or farnesol unless db-cAMP was added exogenously; the addition of db-cAMP restores robust hypha and pseudohypha formation in cultures that contain dodecanol or farnesol, and this filamentation is dependent on the Efg1 transcription factor as rescue does not occur in the efg1/efg1 background (Fig. 1C).
The second line of evidence that suggests that cAMP signalling is impacted by dodecanol and farnesol is derived from experiments with the strain carrying Ras1G13V, a dominant-active variant of Ras1. This strain exhibits hypha formation in the absence of a chemical stimulus, and farnesol and dodecanol can repress this hyperfilamentation phenotype. Furthermore, CAI4-Ras1G13V is highly sensitive to heat shock due to its hyper-activation of adenylate cyclase and repression of stress response genes. This heat shock-sensitive phenotype attributed to the RAS1G13V allele was also alleviated by growth with dodecanol or farnesol. Importantly, because these experiments were performed under conditions that did not stimulate hypha formation, the results suggest that both of these compounds are capable of affecting cAMP-controlled processes even in the absence of exogenous signals that induce hypha formation in wild-type strains.
The third line of evidence indicating that Ras1-Cdc35 signalling is impacted in cultures with dodecanol and farnesol stems from quantitative analysis of transcript levels and promoter activity of genes that are differentially expressed upon decreased cAMP signalling. Consistent with the results of Harcus et al. (2004), we showed that the absence of RAS1 or CDC35 led to elevated levels of CTA1 (Fig. 4A) and HSP12 (Fig. 4C), two transcripts predicted to be repressed by cAMP due to the presence of promoter STRE elements, and based on their expression patterns (Enjalbert et al., 2003). In contrast, levels of HWP1, a transcript at elevated levels in hyphal cells, were much lower in ras1/ras1 and cdc35/cdc35 cells than in isogenic wild-type control cultures consistent with their afilamentous phenotypes. Increasing concentrations of either farnesol or dodecanol caused dose-dependent decreases in the per cent of cells present as hyphae (data not shown) concomitant with decreases in HWP1 and corresponding increases in CTA1 (Fig. 5) transcript levels. Microscopic analyses of CTA1- and HWP1-promoter fusions to GFP showed that the cells that were growing as yeast in cultures treated with low concentrations of dodecanol or farnesol were those that exhibited decreased HWP1 and increased CTA1 expression respectively (Fig. 6). These data indicate the cells within the culture are not uniformly expressing stress response genes upon addition of dodecanol or farnesol, but rather those cells that have responded by alteration of cellular morphology are those expressing the markers indicative of decreased Ras1-Cdc35-PKA signalling.
While these studies provide the first evidence that cAMP signalling is impacted by small molecules that repress C. albicans hyphal growth, the precise mechanism of action is not yet known. Dodecanol and farnesol do not appear to solely act by inhibiting Ras1 conversion to the GTP-bound state as cells that carry the dominant-active Ras1 variant are no more resistant to repression of hyphal growth by dodecanol or farnesol than are wild-type reference strains. This conclusion is consistent with the data that farnesol and dodecanol block hyphal growth in response to different stimuli. For example, hypha formation in response to glucose and GlcNAc, which both stimulate hypha formation through the Ras1-cAMP-Efg1 pathway (though likely through different upstream effectors) is in both cases repressed by farnesol and dodecanol (Table S1). Others have reported that farnesol and dodecanol inhibit hypha formation in response to a number of different stimuli (Hornby et al., 2001; Hogan et al., 2004; Mosel et al., 2005). Because cAMP can rescue hypha formation in farnesol- and dodecanol-grown cultures, these compounds do not disrupt the ability to form hyphae. Our data also show that dodecanol and farnesol do not require PDE activity to exert their effects on morphogenesis in C. albicans as hypha formation in mutants that lack PDE1, PDE2, or both genes exhibited near complete repression of hypha formation upon growth with either compound albeit from a lower starting level of filamentation (Table 1). The observation that the pde1/ pde1 pde2/pde2 double mutant, which has more than fourfold higher basal levels of cAMP (D. Wilson and L.I. Stateva, unpublished), is not more resistant to dodecanol or farnesol suggests that these compounds impact the dynamics of the cAMP signalling, rather than simply changing the total cellular pools of cAMP. These data are consistent with a model where either Ras1, Cdc35, or the interaction between the two proteins, is affected by dodecanol and farnesol, although other factors that interact at this point in the pathway may certainly also be important (Rocha et al., 2001; Fang and Wang, 2006).
Kruppa et al. (2004) found that a C. albicans chk1/chk1 mutant, which lacks a histidine kinase, germinates in the presence of farnesol whereas cells of the CAF2 reference strain largely did not. One model for the phenotype of the chk1/chk1 mutant based on the results presented here is that deletion of CHK1 impacts cAMP signalling or leads to activation of downstream components of the pathway that stimulate hypha formation even in the presence of farnesol and dodecanol. Further evidence that Chk1 may impact cAMP signalling is the finding that chk1/chk1 mutant cells are more susceptible to oxidative stress (Li et al., 2004) similar to the increased sensitivity to oxidative stress exhibited by mutants defective in PDE2 (Wilson et al., 2007). Based on our findings that hypha formation was reduced even further in mutants lacking Pde activity by farnesol and dodecanol, Chk1 would be predicted to act downstream of the cAMP signal. Thus, the Chk1 kinase may act by stimulating PKA or other elements downstream of PKA, such as Efg1, that can restore hypha formation in the absence of these compounds, similar to the restoration of filamentation observed in db-cAMP-treated cultures. As an alternative hypothesis, the chk1/chk1 mutant may have altered cell surface properties which may impact the intracellular concentrations of farnesol when it is supplied exogenously (Li et al., 2002; Kruppa et al., 2003). Last, the Chk1 kinase may participate in the response to farnesol by a mechanism that remains to be determined.
A report by Sato et al. (2004) suggests that farnesol inhibits the MAP kinase cascade based on their findings that relative transcript levels of CPH1 (which encodes the MAP kinase controlled transcriptional regulator of hypha formation) and HST7 (which encodes the MAP kinase kinase) were reduced by twofold and sixfold, respectively, in C. albicans strain NIH A-207 upon addition of 30 or 300 mM farnesol to cultures growing with serum. One important difference between our results and those of Sato et al. is that we have observed large (> 100-fold) reductions in HWP1 levels (Fig. 4F) and those of the coregulated transcripts ECE1 and RBT1 while Sato et al. did not observe a decrease in hyphal growth markers HWP1, ALS1 and HYR1 known to be highly induced during Efg1-dependent pathway-mediated hypha formation (Braun and Johnson, 2000), suggesting that there are some significant unknown differences between the two experimental systems (Hogan et al., 2004). Other pub-lished reports that have examined changes in expression levels in response to farnesol have reported farnesol-induced decreases in HWP1 expression (Ramage et al., 2002; Enjalbert and Whiteway, 2005). In our experiments, decreased HWP1 levels upon addition of farnesol or dodecanol were not accompanied by changes in HST7 levels (data not shown). The finding that both the MAP kinase- and the cAMP-Efg1 pathways are repressed by farnesol might indicate that Ras1 activity is impacted by dodecanol and farnesol because Ras1 is a common element of both pathways (Feng et al., 1999; Leberer et al., 2001). Furthermore, there is evidence that the absence of Cdc35 can impact Hst7-dependent regulation or hyphal growth by an unknown mechanism (Leberer et al., 2001). Thus, these findings may lend further support to the model that Ras1-cAMP signalling is affected by farnesol and dodecanol.
Analysis of the effects of farnesol and dodecanol on hypha formation that has been stimulated by other pathways will provide further insight into the mechanisms by which these molecules impact C. albicans morphology and physiology. Dumitru et al. (2004) found that in an anaerobic environment that promoted hypha formation by an unknown regulatory pathway, farnesol did not inhibit hypha formation. This observation along with our finding that db-cAMP rescued Efg1-dependent hyphal growth in medium containing either farnesol or dodecanol indicates that cells are still physically capable of forming hyphae in the presence of these compounds.
In a recent investigation (Jensen et al., 2006), C. albicans CAI4 mutants with a constitutively hyphal phenotype generated via chemical and UV mutagenesis were tested for their response to farnesol on solid medium at 28°C. In these experiments farnesol completely repressed or partially repressed hypha formation in large fractions (38% and 58% respectively) of mutants as assessed by colony morphology. Though the types of mutations that occurred to give rise to the constitutively hyphal phenotypes were not determined, our data would suggest that mutants with hyperactivation of the hypha-inducing, cAMP-dependent signalling pathway, would be completely inhibited by farnesol. The partial repression of hyphal growth phenotype exhibited by more than 50% of the mutants generated in the CAI4 background is also consistent with our observation that a subpopulation of cells within the entire population is more susceptible to repression of hyphal growth by farnesol and dodecanol (Fig. 6).
Because farnesol, dodecanol and their functional analogues are extremely hydrophobic and fluorescent derivatives localize to membranes (Shchepin et al., 2005), these molecules may impact the membrane environment where Ras1 and adenylate cyclase proteins interact (Mitts et al., 1990; Fang and Wang, 2006; Wang and Deschenes, 2006). These compounds could also inhibit specific enzyme activities that are essential for Ras1 post-translational modifications and localization (Farh et al., 1995; Lobo et al., 2002) as has been previously suggested (McGeady et al., 2002). As small molecules have been shown to modulate adenylate cyclase activity (Jain et al., 2003; Wu et al., 2006), adenylate cyclase itself may be a target of these compounds. Although farnesol and dodecanol have very similar effects in our assays, without knowing their specific target(s), we do not know that their mechanisms of action are the same.
We were unable to detect significant differences in intracellular cAMP upon addition of dodecanol or farnesol in spite of the evidence that these substances affect the status of the Ras-cAMP signal transduction pathway. However, Maidan et al. (2005) showed that despite the fact that glucose, serum, GlcNAc and proline all induce hypha formation via the Ras1-cAMP-Efg1 signalling pathway, changes in cAMP levels upon induction of hyphal growth were only observed in glucose and serum-induced cells. Thus, the analysis of changes in total cAMP pools may not necessarily be sensitive enough to detect changes in cAMP levels upon activation of the pathway. Furthermore, we observed that total cAMP levels were 2.5-fold lower in CAI4-Ras1G13V cells in which the Ras1-cAMP signalling pathway is hyperactivated than in the isogenic control strain. A report by Ma et al. (1999) similarly saw low levels of intracellular cAMP in S. cerevisiae carrying a dominant-active Ras2 variant unless both PDE1 and PDE2 were deleted. These data indicate that because of technical limitations of the current analysis methods and the strong and dynamic feedback mechanism that operates in the cAMP pathway, the intracellular levels of cAMP may not always reflect the activation status of the pathway. Powerful new methods that allow for spatial and temporal resolution of cAMP signals in mammalian cells have demonstrated that cAMP accumulates within specific microdomains within the cell rather than being uniformly distributed (Zaccolo and Pozzan, 2002 and Zaccolo et al., 2006, for review). The non-uniform distribution or gradients are formed by limited diffusion of cAMP after its synthesis (Rich et al., 2000), localization of phosphodiesterases (Barnes et al., 2005), or the channelling of cAMP via macromolecular structures to different effectors (Zhang et al., 2005) as a way to exert fine tuning on the cAMP response (Taskén and Aandahl, 2006). It is not known if similar subcellular localization of cAMP occurs within the fungal cytoplasm, but the potential certainly exists, as nuclear localization of Pde2 has been demonstrated in S. cerevisiae (Namy et al., 2002). If so, it might explain why a large, non-specific signal generated by an exogenous cAMP can rescue downstream components of a cAMP-controlled pathway by flooding the cell, whereas a mutation that leads to increased cAMP signalling, such as the RAS1G13V mutation, may not lead to increases in intracellular cAMP that can be easily measured. Because the cAMP signalling pathway impacts processes as diverse as morphology, carbon source utilization, and stress responses among others, it will not be surprising if there are multiple ways to direct and fine-tune cAMP signals to properly regulate cell physiology under different conditions.
The finding that farnesol and dodecanol can impact morphogenesis under inducing conditions and affect stress responses in the strains carrying the Ras1G13V variant protein under conditions that do not stimulate hyphal growth, suggests that multiple cAMP-mediated outputs can be impacted. The effects of farnesol and dodecanol on different cAMP-controlled responses will require further study under a variety of different conditions. Decreased cAMP signalling due to mutation of components in the Ras1-cAMP pathway has been shown to prevent entry into apoptosis (Phillips et al., 2006), increase starvation survival (Bahn et al., 2003) and decrease resistance to sterol-targeting antibiotics (Jain et al., 2003), thus downregulation of the cAMP signalling pathway may affect numerous phenotypes that will impact C. albicans survival. The evidence presented here linking decreased hypha formation to increased expression of CTA1 and HSP12 suggests that farnesol, dodecanol, and likely the bacterially produced 3OC12HSL molecule may be similarly multifactorial in their effects on C. albicans fitness (Fig. 6) (Hogan, 2006; Nickerson et al., 2006). Future studies on both the mechanism of action and the environmental control of production of and response to these small molecules will provide new and valuable insight into the physiology of fungi in single-species populations and mixed-species communities where these molecules accumulate. This understanding may lead to the development of new strategies for modulating fungal behaviour in beneficial ways.
Experimental procedures
Strains and growth conditions
For a list of all strains used in these studies, refer to Table 2. Strains CAI4-Ras1 and CAI4-Ras1G13V were generated using plasmids (pDH240 and pLJ57) using the lithium acetate method (Leberer et al., 2001). Strains were streaked from freezer stocks stored at −80°C onto YNB (0.67% yeast nitrogen base and 1% glucose), or YPD (2% peptone, 1% yeast extract and 2% glucose) plates every 10 days. Phosphodiesterase mutant strains were streaked onto YNB plates every 3 days. Overnight cultures were grown in 5 ml of YNB 1% glucose or YPD 2% glucose at 30°C in a roller drum for 16–18 h. Cells were induced to grow as hyphae in either YNBNP, which contains YNB salts with 0.2% glucose, 5 mM N-acetylglucosamine (GlcNAc) and 25 mM potassium phosphate buffer (pH 7), or 100 mM phosphate buffer containing either 1% glucose or 5 mM GlcNAc (pH 7). Hypha induction for microscopic quantification was carried out in 6, 12 or 24 well flat bottom non-tissue culture-treated plastic plates that were incubated at 37°C without shaking. Cells were generally grown for 2–5 h under inducing conditions and then counted unless otherwise stated. In all of the experiments performed, the initial cell concentration was 5 × 105 cells ml−1.
Table 2.
Strains used in these studies.
| Strain # | Genotype | Reference | |
|---|---|---|---|
| C. albicans strains | |||
| SC5314 | DH35 | Prototrophic clinical isolate | Gillum et al. (1984) |
| CAF2 | DH331 | Ura+ derivative of CAI4 | Leberer et al. (2001) |
| CAI-4 | DH332 | ura3::λimm434/ura3 | Fonzi and Irwin (1993) |
| CAI-4 HWP1-lacZ | DH322 | Constructed by transformation of DH332 with pAU95 | Hogan et al. (2004) |
| CAI4-Ras1G13V | DH409 | Constructed by transformation of DH332 with pDH240 | This study |
| CAI4-Ras1 | DH545 | Constructed by transformation of DH326 with pLJ57 | This study |
| CR216 (cdc35/cdc35) | DH346 |
ura3::λimm434/ura3::λimm434 cdc35::hisG-URA3-hisG/cdc35::hisG |
Rocha et al. (2001) |
| CDH107 (ras1/ras1) | DH483 |
ura3/ura3 ras1::hisG-URA3-hisG/ras1::hisG |
Leberer et al. (2001) |
| HLC52 (efg1/efg1) | DH116 |
ura3::λimm434/ura3::λimm434 efg1::hisG/efg1::hisG-URA3-hisG |
Lo et al. (1997) |
| JCK19 (cph1/cph1) | DH118 |
ura3::λimm434/ura3::λimm434 cph1::hisG/cph1::hisG-URA3-hisG |
Liu et al. (1994) |
| DP42 (pde1/pde1) | DH827 |
ura3::λimm434/ura3::λimm434 pde1::hisG/pde1::hisG-URA3-hisG |
Wilson et al. (2007) |
| WDR1 (pde1/pde1PDE1) | DH828 | ura3::λimm434/ura3::λimm434 pde1::hisG/pde1::hisG-PDE1-URA3 |
Wilson et al. (2007) |
| WH3-2U (pde2/pde2) | DH829 |
ura3::λimm434/ura3::λimm434 pde2::hisG/pde2::hisG-URA3-hisG |
Jung and Stateva (2003) |
| WH2-1U (pde2/pde2PDE2) | DH830 |
ura3::λimm434/ura3::λimm434 pde2::hisG/pde2::hisG::PDE2-URA3 |
Jung and Stateva (2003) |
| WD7 (pde1/pde1 pde2/pde2) | DH831 |
ura3::λimm434/ura3::λimm434 pde1::hisG/pde1::hisG pde2::hisG/pde2::hisG-URA3-hisG |
Wilson et al. (2007) |
| HWP1GFP3 (HWP1-GFP) | DH271 | As CAI4 but with plasmid pHWP1GFP3 integrated at the ENO1 locus |
Staab et al. (2003) |
| CTA1-GFP | DH939 | As CAI4 but with plasmid pCTA1-GFP-URA3 integrated at the RSP10 locus |
Enjalbert et al. (2007) |
| GFP | DH937 | As CAI4 but with plasmid pGFP-URA3 integrated at the RSP10 locus |
Enjalbert et al. (2007) |
| Plasmids | |||
| pDH240 | DH326 | pYPB1-ADHpL-CaRAS1 (pDH240) | Rocha et al. (2001) |
| pLJ57 | DH327 | pYPB1-ADHpL-CaRAS1G13V (pLJ57) | Rocha et al. (2001) |
Assessment of C. albicans morphology
Candida albicans strains were grown in 5 ml YNB 1% glucose overnight at 30°C in a roller drum and then centrifuged for 2 min and washed once with 100 mM phosphate buffer (pH 7) before resuspension to a concentration of 5 × 105 cells ml−1 in the specified hypha-inducing medium. Acidified ethyl acetate (0.01% glacial acetic acid) was used to make 50 mM stock solutions of trans,trans-farnesol and 1-dodecanol (Sigma-Aldrich). Acidified ethyl acetate was added to vehicle control wells. Stock solutions were made fresh before each experiment and added to appropriate wells of microtiter plates at a final concentration of 200 µM unless otherwise specified. Lower concentrations of farnesol and dodecanol (75 µM) were needed to repress hypha formation in borosilicate glass tubes. Each experimental condition was tested in triplicate on a given day, and each experiment was repeated on multiple days. Cell morphology was determined following published procedures (Merson-Davies and Odds, 1989; Warenda and Konopka, 2002) using a Zeiss Axiovert inverted microscope equipped with a 63× long-working distance objective and Axiovision software. For each replicate, over 200 cells per well were counted, and the percentage of cells growing as yeast, pseudohyphae and hyphae was determined from an average of three replicate wells.
cAMP rescue experiments
In experiments using db-cAMP (Sigma), the db-cAMP was prepared as a 100 mM stock solution in water. C. albicans strains were grown overnight in YNB 1% glucose. Cells were diluted into YNBNP at an initial concentration of 5 × 105 cells ml−1 and aliquotted into wells of 24 well plates with 1 ml per well. Plates were incubated at 37°C. Dibutyryl-cAMP was added to the cultures to a final concentration of 10 mM immediately following the addition of 200 µM farnesol, dodecanol, or vehicle alone. Cells were visualized by microscopy at 5 h using a long-working distance 63× objective and morphology was assessed using the methods described above.
Heat shock experiments
Candida albicans strains CAI4-Ras1 and CAI4-Ras1G13V were grown in 5 ml YPD 2% glucose overnight at 30°C in a roller drum. Cultures were diluted 15-fold into fresh YPD 0.4% glucose and distributed into glass tubes containing 3 ml of the cell suspensions. Ethyl acetate (control), 75 µM dodecanol, or 75 µM farnesol was added prior to incubation for 6 h at 30°C in a roller drum. To assess sensitivity to heat shock, a 500 µl aliquot was removed and incubated at 48°C for 6 min before being diluted in a 10-fold dilution series and plated (5 µl per spot) on YPD. Plates were incubated for 24 h at 30°C. Similar results were obtained from four experiments performed four separate days.
Intracellular cAMP measurements
From C. albicans CAI4-Ras1 and CAI4-Ras1G13V overnight cultures grown in YPD 2% glucose at 30°C, 1 ml containing 1 × 108 cells was centrifuged, washed in 100 mM phosphate buffer and resuspended in 15 ml of YPD 0.4% glucose. The cell suspension was divided into three borosilicate glass tubes, 5 ml tube−1, and each was treated with either ethyl acetate alone, 75 µM dodecanol or 75 µM farnesol. Cultures were incubated at 30°C on a roller drum and aliquots were taken at various time points for growth curve analysis at OD600.At 6 h, 350 µl aliquots were removed and frozen immediately in liquid nitrogen for intracellular cAMP analysis. For cAMP extraction, samples were thawed on ice, then centrifuged at 13 000 r.p.m. for 1 min at 4°C to recover the cell pellet. cAMP was extracted from cells with 1 ml ice cold 1 M formic acid saturated with N-butanol by vortexing, followed by a 5 min incubation on ice. Cell debris was removed by centrifugation, and 150 µl of the supernatant was removed and dried under vacuum. Samples were assayed using the non-acetylation procedure provided by Amersham cAMP EIA system (RPN225 GE Healthcare).
Real-time RT-PCR analysis of C. albicans transcripts
Candida albicans CAF2 and ras1/ras1 cultures were grown overnight in YNB 1% glucose and cdc35/cdc35 cultures were grown in YPD 2% glucose. Cultures were diluted to an initial concentration of 1 × 106 cells ml−1 in 5 ml prewarmed YNBNP 0.2% glucose in borosilicate glass tubes. Each tube received either 75 µM dodecanol, farnesol or equal amount of vehicle control and was incubated at 37°C in a roller drum for 2 h. RNA was isolated using the RNeasy Mini Kit (Qiagen) and DNase treated with DNA-free (Ambion). For each reaction 450 ng of RNA was used in cDNA synthesis. Real-time RT-PCR was carried out using the 7500 Fast Real-time PCR System (Applied Biosystems) and designed primers to delete HWP1 orf19.1321 (HWP1F 5′-CCA CTA CTA CTG AAG CCA AAT C-3′ and HWP1R 5′-AAG TGG ATA CTG TAC CAG TTG G-3′), CTA1 orf19.6229 (CAT1RTF 5′-ACT CCA GTG TTT TTC ATT AGA G-3′ and CAT1RTR 5′-AGA GTA ACC ATT CAT TTC TCT G-3′), HSP12 orf19.3160 (HSP12F 5′-TGT TGG CTC AAA TGT TCC AG-3′ and HSP12R 5′-TTC AGC AGC CTT TCC AAT TT-3′) and GPD1 orf19. 1756 (GPD1RTF 5′-AGT ATG TGG AGC TTT ACT GGG A-3′ and GPD1RTR 5′-CAG AAA CAC CAG CAA CAT CTT C-3′) which was used as a control transcript. The following conditions were used for amplification: 50°C for 2 min, 95°C for 10 min, 95°C for 15 s, 56°C for 1 min, 72°C for 1 min for 40 cycles followed by dissociation 95°C for 15 s, 60°C for 1 min, 95°C for 15 s.
RT-PCR analysis of C. albicans transcripts
Candida albicans CAF2 cultures were grown overnight in 5 ml YNB 1% glucose and diluted to an initial concentration of 1 × 106 cells ml−1 in 25 ml prewarmed YNBNP 0.2% glucose in 125 ml plastic flasks. To each flask, 40, 75 or 100 µM farnesol or dodecanol, or equal volumes of vehicle alone, was added. At 2 h, 2 ml aliquots were removed, centrifuged for 1 min and pellets were frozen in dry ice-ethanol. Cells were lysed by mechanical disruption of the frozen pellets using 0.5 mm silica beads, and total RNA was isolated using the RNeasy Mini Kit (Qiagen) and DNase treated using DNA-free (Ambion). Pre-made random hexamer (IDT) and Superscript III (Invitrogen) were used in cDNA synthesis, run at 52°C for 60 min, 70°C for 15 min and 4°C. To verify there was no DNA contamination, a control without Superscript III was included. PCR reactions were performed using the same primers (CTA1, HWP1, GPD1) as in real-time RT-PCR experiments. The following conditions were used for amplification: 94°C for 5 min, 94°C for 30 s, 56°C for 30 s, 72°C for 30 s and 72°C for 30 s for 30 cycles. Transcripts were quantified using ImageJ (Abramoff et al., 2004).
Microscopic analysis of GFP fusion strains
Candida albicans cultures were grown in YNBNP medium (5 ml cultures in borosilicate glass tubes) with 40 µM dodecanol and 40 µM farnesol and incubated at 37°C in a roller drum for 5–6 h. Cultures were examined by epifluorscence microscopy and quantification of fluorescence was performed using Axiovision software. Values were obtained by dividing pixel intensity by area within yeast and hyphae from more than 10 fields of view (> 100 cells per replicate).
Supplementary Material
Acknowledgements
Strains and plasmids were kindly provided by Paula Sundstrom, Ekkehard Leberer, Doreen Harcus, Alistair Brown and Gerald Fink. This research was supported by the NIH (K22 DE016542) (D.A.H.).
Footnotes
Supplementary material
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1365-2958.2007.06013.x
(This link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- Bahn YS, Staab J, Sundstrom P. Increased high-affinity phosphodiesterase PDE2 gene expression in germ tubes counteracts CAP1-dependent synthesis of cyclic AMP, limits hypha production and promotes virulence of Candida albicans. Mol Microbiol. 2003;50:391–409. doi: 10.1046/j.1365-2958.2003.03692.x. [DOI] [PubMed] [Google Scholar]
- Barnes A, Livera G, Huang P, Sun C, O’Neal W, Conti M, et al. Phosphodiesterase 4D forms a cAMP diffusion barrier at the apical membrane of the airway epithelium. J Biol Chem. 2005;280:7997–8003. doi: 10.1074/jbc.M407521200. [DOI] [PubMed] [Google Scholar]
- Bissinger PH, Wieser R, Hamilton B, Ruis H. Control of Saccharomyces cerevisiae catalase T gene (CTT1) expression by nutrient supply via the RAS-cyclic AMP pathway. Mol Cell Biol. 1989;9:1309–1315. doi: 10.1128/mcb.9.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun BR, Johnson AD. TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics. 2000;155:57–67. doi: 10.1093/genetics/155.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla R, Passeron S, Cantore ML. N-acetyl-D-glucosamine induces germination in Candida albicans through a mechanism sensitive to inhibitors of cAMP-dependent protein kinase. Cell Signal. 1998;10:713–719. doi: 10.1016/s0898-6568(98)00015-1. [DOI] [PubMed] [Google Scholar]
- Cho T, Hamatake H, Kaminishi H, Hagihara Y, Watanabe K. The relationship between cyclic adenosine 3′,5′-monophosphate and morphology in exponential phase Candida albicans. J Med Vet Mycol. 1992;30:35–42. [PubMed] [Google Scholar]
- Csank C, Schroppel K, Leberer E, Harcus D, Mohamed O, Meloche S, et al. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun. 1998;66:2713–2721. doi: 10.1128/iai.66.6.2713-2721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru R, Hornby JM, Nickerson KW. Defined anaerobic growth medium for studying Candida albicans basic biology and resistance to eight antifungal drugs. Antimicrob Agents Chemother. 2004;48:2350–2354. doi: 10.1128/AAC.48.7.2350-2354.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert B, Whiteway M. Release from quorum-sensing molecules triggers hyphal formation during Candida albicans resumption of growth. Eukaryot Cell. 2005;4:1203–1210. doi: 10.1128/EC.4.7.1203-1210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert B, Nantel A, Whiteway M. Stress-induced gene expression in Candida albicans: absence of a general stress response. Mol Biol Cell. 2003;14:1460–1467. doi: 10.1091/mbc.E02-08-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert B, MacCallum DM, Odds FC, Brown AJ. Niche-specific activation of the oxidative stress response by the pathogenic fungus Candida albicans. Infect Immun. 2007;75:2143–2151. doi: 10.1128/IAI.01680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang HM, Wang Y. RA domain-mediated interaction of Cdc35 with Ras1 is essential for increasing cellular cAMP level for Candida albicans hyphal development. Mol Microbiol. 2006;61:484–496. doi: 10.1111/j.1365-2958.2006.05248.x. [DOI] [PubMed] [Google Scholar]
- Farh L, Mitchell DA, Deschenes RJ. Farne-sylation and proteolysis are sequential, but distinct steps in the CaaX box modification pathway. Arch Biochem Biophys. 1995;318:113–121. doi: 10.1006/abbi.1995.1211. [DOI] [PubMed] [Google Scholar]
- Feng Q, Summers E, Guo B, Fink G. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J Bacteriol. 1999;181:6339–6346. doi: 10.1128/jb.181.20.6339-6346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SB, Anderson ES, Harshaw RB, Thate T, Craig NL, Nelson HC. Protein kinase A regulates constitutive expression of small heat-shock genes in an Msn2/4p-independent and Hsf1p-dependent manner in Saccharomyces cerevisiae. Genetics. 2005;169:1203–1214. doi: 10.1534/genetics.104.034256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9:499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Ibrahim AS, Sheppard DC, Chen YC, French SW, Cutler JE, et al. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol Microbiol. 2002;44:61–72. doi: 10.1046/j.1365-2958.2002.02873.x. [DOI] [PubMed] [Google Scholar]
- Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Gow NA, Brown AJ, Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002;5:366–371. doi: 10.1016/s1369-5274(02)00338-7. [DOI] [PubMed] [Google Scholar]
- Harcus D, Nantel A, Marcil A, Rigby T, Whiteway M. Transcription profiling of cyclic AMP signaling in Candida albicans. Mol Biol Cell. 2004;15:4490–4499. doi: 10.1091/mbc.E04-02-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DA. Talking to themselves: autoregulation and quorum sensing in fungi. Eukaryot Cell. 2006;5:613–619. doi: 10.1128/EC.5.4.613-619.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DA, Vik A, Kolter R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol. 2004;54:1212–1223. doi: 10.1111/j.1365-2958.2004.04349.x. [DOI] [PubMed] [Google Scholar]
- Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001;67:2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer LL, Cieslinski LB, McLaughlin MM, Torphy TJ, Shatzman AR, Livi GP. A Candida albicans cyclic nucleotide phosphodiesterase: cloning and expression in Saccharomyces cerevisiae and biochemical characterization of the recombinant enzyme. Microbiology. 1994;140:1533–1542. doi: 10.1099/13500872-140-7-1533. [DOI] [PubMed] [Google Scholar]
- Jain P, Akula I, Edlind T. Cyclic AMP signaling pathway modulates susceptibility of Candida species and Saccharomyces cerevisiae to antifungal azoles and other sterol biosynthesis inhibitors. Antimicrob Agents Chemother. 2003;47:3195–3201. doi: 10.1128/AAC.47.10.3195-3201.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EC, Hornby JM, Pagliaccetti NE, Wolter CM, Nickerson KW, Atkin AL. Farnesol restores wild-type colony morphology to 96% of Candida albicans colony morphology variants recovered following treatment with mutagens. Genome. 2006;49:346–353. doi: 10.1139/g05-117. [DOI] [PubMed] [Google Scholar]
- Jung WH, Stateva LI. The cAMP phosphodiesterase encoded by CaPDE2 is required for hyphal development in Candida albicans. Microbiology. 2003;149:2961–2976. doi: 10.1099/mic.0.26517-0. [DOI] [PubMed] [Google Scholar]
- Kim S, Kim E, Shin DS, Kang H, Oh KB. Evaluation of morphogenic regulatory activity of farnesoic acid and its derivatives against Candida albicans dimorphism. Bioorg Med Chem Lett. 2002;12:895–898. doi: 10.1016/s0960-894x(02)00038-0. [DOI] [PubMed] [Google Scholar]
- Kruppa M, Goins T, Cutler JE, Lowman D, Williams D, Chauhan N, et al. The role of the Candida albicans histidine kinase (CHK1) gene in the regulation of cell wall mannan and glucan biosynthesis. FEMS Yeast Res. 2003;3:289–299. doi: 10.1111/j.1567-1364.2003.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Kruppa M, Krom BP, Chauhan N, Bambach AV, Cihlar RL, Calderone RA. The two-component signal transduction protein Chk1p regulates quorum sensing in Candida albicans. Eukaryot Cell. 2004;3:1062–1065. doi: 10.1128/EC.3.4.1062-1065.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E, Harcus D, Dignard D, Johnson L, Ushinsky S, Thomas DY, Schroppel K. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol Microbiol. 2001;42:673–687. doi: 10.1046/j.1365-2958.2001.02672.x. [DOI] [PubMed] [Google Scholar]
- Li D, Bernhardt J, Calderone R. Temporal expression of the Candida albicans genes CHK1 and CSSK1, adherence, and morphogenesis in a model of reconstituted human esophageal epithelial candidiasis. Infect Immun. 2002;70:1558–1565. doi: 10.1128/IAI.70.3.1558-1565.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Gurkovska V, Sheridan M, Calderone R, Chauhan N. Studies on the regulation of the two-component histidine kinase gene CHK1 in Candida albicans using the heterologous lacZ reporter gene. Microbiology. 2004;150:3305–3313. doi: 10.1099/mic.0.27237-0. [DOI] [PubMed] [Google Scholar]
- Liu H, Kohler J, Fink GR. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous Candida albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Lobo S, Greentree WK, Linder ME, Deschenes RJ. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem. 2002;277:41268–41273. doi: 10.1074/jbc.M206573200. [DOI] [PubMed] [Google Scholar]
- Ma P, Wera S, Van Dijck P, Thevelein JM. The PDE1-encoded low-affinity phosphodiesterase in the yeast Saccharomyces cerevisiae has a specific function in controlling agonist-induced cAMP signaling. Mol Biol Cell. 1999;10:91–104. doi: 10.1091/mbc.10.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeady P, Logan DA, Wansley DL. A protein-farnesyl transferase inhibitor interferes with the serum-induced conversion of Candida albicans from a cellular yeast form to a filamentous form. FEMS Microbiol Lett. 2002;213:41–44. doi: 10.1111/j.1574-6968.2002.tb11283.x. [DOI] [PubMed] [Google Scholar]
- Maidan MM, De Rop L, Serneels J, Exler S, Rupp S, Tournu H, et al. The G protein-coupled receptor Gpr1 and the Galpha protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol Biol Cell. 2005;16:1971–1986. doi: 10.1091/mbc.E04-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merson-Davies LA, Odds FC. A morphology index for characterization of cell shape in Candida albicans. J Gen Microbiol. 1989;135:3143–3152. doi: 10.1099/00221287-135-11-3143. [DOI] [PubMed] [Google Scholar]
- Mitchell AP. Dimorphism and virulence in Candida albicans. Curr Opin Microbiol. 1998;1:687–692. doi: 10.1016/s1369-5274(98)80116-1. [DOI] [PubMed] [Google Scholar]
- Mitts MR, Grant DB, Heideman W. Adenylate cyclase in Saccharomyces cerevisiae is a peripheral membrane protein. Mol Cell Biol. 1990;10:3873–3883. doi: 10.1128/mcb.10.8.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosel DD, Dumitru R, Hornby JM, Atkin AL, Nickerson KW. Farnesol concentrations required to block germ tube formation in Candida albicans in the presence and absence of serum. Appl Environ Microbiol. 2005;71:4938–4940. doi: 10.1128/AEM.71.8.4938-4940.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namy O, Duchateau-Nguyen G, Rousset JP. Translation readthrough of the PDE2 stop codon modulates cAMP levels in Saccharomyces cerevisiae. Mol Microbiol. 2002;43:641–652. doi: 10.1046/j.1365-2958.2002.02770.x. [DOI] [PubMed] [Google Scholar]
- Nickerson KW, Atkin AL, Hornby JM. Quorum sensing in dimorphic fungi: farnesol and beyond. Appl Environ Microbiol. 2006;72:3805–3813. doi: 10.1128/AEM.02765-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KB, Miyazawa H, Naito T, Matsuoka H. Purification and characterization of an autoregulatory substance capable of regulating the morphological transition in Candida albicans. Proc Natl Acad Sci USA. 2001;98:4664–4668. doi: 10.1073/pnas.071404698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJ, Crowe JD, Ramsdale M. Ras pathway signaling accelerates programmed cell death in the pathogenic fungus Candida albicans. Proc Natl Acad Sci USA. 2006;103:726–731. doi: 10.1073/pnas.0506405103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol. 2000;116:143–145. doi: 10.1085/jgp.116.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha CR, Schroppel K, Harcus D, Marcil A, Dignard D, Taylor BN, et al. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol Biol Cell. 2001;12:3631–3643. doi: 10.1091/mbc.12.11.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Watanabe T, Mikami T, Matsumoto T. Farnesol, a morphogenetic autoregulatory substance in the dimorphic fungus Candida albicans, inhibits hyphae growth through suppression of a mitogen-activated protein kinase cascade. Biol Pharm Bull. 2004;27:751–752. doi: 10.1248/bpb.27.751. [DOI] [PubMed] [Google Scholar]
- Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell. 2003;2:1053–1060. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchepin R, Hornby JM, Burger E, Niessen T, Dussault P, Nickerson KW. Quorum sensing in Candida albicans: probing farnesol’s mode of action with 40 natural and synthetic farnesol analogs. Chem Biol. 2003;10:743–750. doi: 10.1016/s1074-5521(03)00158-3. [DOI] [PubMed] [Google Scholar]
- Shchepin R, Dumitru R, Nickerson KW, Lund M, Dussault PH. Biologically active fluorescent farnesol analogs. Chem Biol. 2005;12:639–641. doi: 10.1016/j.chembiol.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Staab JF, Bahn YS, Sundstrom P. Integrative, multifunctional plasmids for hypha-specific or constitutive expression of green fluorescent protein in Candida albicans. Microbiology. 2003;149:2977–2986. doi: 10.1099/mic.0.26445-0. [DOI] [PubMed] [Google Scholar]
- Sundstrom P, Balish E, Allen CM. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J Infect Dis. 2002;185:521–530. doi: 10.1086/338836. [DOI] [PubMed] [Google Scholar]
- Taskén K, Aandahl E. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2006;84:137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- Viudes A, Peman J, Canton E, Ubeda P, Lopez-Ribot JL, Gobernado M. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur J Clin Microbiol Infect Dis. 2002;21:767–774. doi: 10.1007/s10096-002-0822-1. [DOI] [PubMed] [Google Scholar]
- Wang G, Deschenes RJ. Plasma membrane localization of Ras requires class C Vps proteins and functional mitochondria in Saccharomyces cerevisiae. Mol Cell Biol. 2006;26:3243–3255. doi: 10.1128/MCB.26.8.3243-3255.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warenda AJ, Konopka JB. Septin function in Candida albicans morphogenesis. Mol Biol Cell. 2002;13:2732–2746. doi: 10.1091/mbc.E02-01-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D, Tutulan-Cunita A, Jung W, Hauser NC, Hernandez R, Williamson T, et al. Deletion of the high-affinity cAMP phosphodiesterase encoded by PDE2 affects stress responses and virulence in Candida albicans. Mol Microbiol. 2007;65:841–856. doi: 10.1111/j.1365-2958.2007.05788.x. [DOI] [PubMed] [Google Scholar]
- Wu KY, Zippin JH, Huron DR, Kamenetsky M, Hengst U, Buck J, et al. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat Neurosci. 2006;9:1257–1264. doi: 10.1038/nn1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Di Benedetto G, Lissandron V, Mancuso L, Terrin A, Zamparo I. Restricted diffusion of a freely diffusible second messenger: mechanisms underlying compartmentalized cAMP signalling. Biochem Soc Trans. 2006;34:495–497. doi: 10.1042/BST0340495. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts β-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005;437:569–573. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.