Abstract
Background
Current diagnostic strategies for detection of structural articular cartilage abnormalities, the earliest structural signs of osteoarthritis, often do not capture the condition until it is too far advanced for the most potential benefit of non-invasive interventions.
Purpose
Systematically review the literature relative to the following questions: (1) Is MRI a valid, sensitive, specific, accurate and reliable instrument to identify knee articular cartilage abnormalities compared to arthroscopy? (2) Is MRI a sensitive tool that can be utilized to identify early cartilage degeneration?
Study Design
Systematic Review
Methods
A systematic search was performed in November 2010 using PubMed MEDLINE (from 1966), CINAHL (from 1982), SPORTDiscus (from 1985), and SCOPUS (from 1996) databases.
Results
Fourteen level I and 13 level II studies were identified that met inclusion criteria and provided information related to diagnostic performance of MRI compared to arthroscopic evaluation. The diagnostic performance of MRI demonstrated a large range of sensitivities, specificities, and accuracies. The sensitivity for identifying articular cartilage abnormalities in the knee joint was reported between 26–96%. Specificity and accuracy was reported between 50–100% and 49–94%, respectively. The sensitivity, specificity, and accuracy for identifying early osteoarthritis were reported between 0–86%, 48–95%, and 5–94%, respectively. As a result of inconsistencies between imaging techniques and methodological shortcomings of many of the studies, a meta-analysis was not performed and it was difficult to fully synthesize the information to state firm conclusions about the diagnostic performance of MRI.
Conclusions
There is evidence in some MRI protocols that MRI is a relatively valid, sensitive, specific, accurate, and reliable clinical tool for identifying articular cartilage degeneration. Due to heterogeneity of MRI sequences it is not possible to make definitive conclusions regarding its global clinical utility for guiding diagnosis and treatment strategies.
Clinical Relevance
Traumatic sports injuries to the knee may be significant precursor events to early onset of posttraumatic osteoarthritis. MRI may aid in early identification of structural injuries to articular cartilage as evidenced by articular cartilage degeneration grading.
Keywords: magnetic resonance imaging, arthroscopy, chondromalacia, articular cartilage, osteoarthritis, knee
INTRODUCTION
The prevalence of osteoarthritis has increased almost 30% over the last decade and affects nearly 27 million adults in the United States.2, 45, 46 Knee osteoarthritis impacts an individual’s physical and psychological quality of life as well as has high societal economic costs associated with the diagnosis, treatment, and loss of work productivity related to the disease process.2 The lifetime cost of knee osteoarthritis is nearly $60,000 per person if a patient eventually goes on to total knee arthroplasty.53
Knee osteoarthritis is not just a condition that impacts older adults. The age of onset for articular pathology in athletes may be earlier than in the general population due to the high exposure risk to sports injuries in young athletes.20, 51, 52, 56 Participation in sports activities has important health and cardiovascular benefits for all age groups. However, articular cartilage pathology and, more specifically, osteoarthritis may significantly impact sports participation not only in master’s level athletes but in young adult athletes as well.30, 52 In addition to direct injury to articular cartilage, traumatic injury to the anterior cruciate ligament (ACL) or meniscus may be significant precursor events that accelerate the progression of degenerative changes in the knee joint.21, 51, 57 As a result of the high number of ACL and cartilage injuries that occur in adolescents and young adults, many athletes in their early 30s are at high risk of suffering significant knee pain and disability related to structural changes of osteoarthritis.52 Early identification of structural injuries to articular cartilage may provide opportunities for early treatment intervention, prediction, and monitoring of disease progression, and improve assessment capabilities for clinical outcomes measures.
Early intervention strategies such as pharmacologic management, patient education, weight loss, aerobic exercise, physical therapy, range of motion and strengthening exercises, bracing and joint protection may slow down or alleviate symptoms and potentially even delay articular cartilage disease progression.2 The success of these strategies is predicated on identifying early structural changes in the articular cartilage to both identify the individuals at risk for progressive degeneration and intervene at the time point when the benefits of therapeutic strategies would be maximized. Diagnostic strategies such as history, clinical exam, and radiographic imaging techniques have been utilized extensively over the past few decades to identify osteoarthritis in patients.1, 94 However, these methods may not be sensitive enough to detect early signs of articular cartilage degradation when many of these intervention strategies are most likely to offer the most benefit.
Arthroscopy is the only minimally invasive technique that allows direct visualization of articular cartilage and it is often utilized as a gold standard for identification and staging of articular cartilage disease.18, 67, 68 However, arthroscopy is limited as a diagnostic tool alone as a result of the high cost, invasive nature, and associated complications. A non-invasive technique such as magnetic resonance imaging (MRI) is needed to make accurate diagnoses of articular cartilage degeneration so that appropriate treatment choices can be made and further research can be conducted related to prevention, modification, and assessment of disease progression.
The diagnostic utility of MRI for identifying knee articular cartilage lesions has been extensively explored in the literature over the past two decades.18 As the imaging technology has improved in terms of magnet strength, sequence utilization, and spatial resolution, so has the diagnostic performance.34, 39, 41, 42, 61, 85, 87, 90 To date, no systematic reviews on MRI diagnosis and grading of osteoarthritis thoroughly summarize the literature as it relates to the sensitivity, specificity, accuracy, and reliability of MRI compared to arthroscopy. Without a comprehensive understanding of the diagnostic utility of MRI to characterize the severity of structural articular cartilage changes by articular cartilage degeneration grades, insight into its use as a non-invasive and evidence based tool to guide diagnostic and treatment practices remains limited.
The purpose of this study was to systematically review the literature relative to the following questions for a 1.5 and 3.0 Tesla (T) MRI: (1) Is MRI a valid, sensitive, specific, accurate, and reliable instrument to identify knee articular cartilage abnormalities compared to arthroscopy? (2) Is MRI a sensitive tool that can be utilized to identify early articular cartilage pathology? The aims of this systematic review included: 1) summarize relevant data, 2) identify strengths and weaknesses in the literature. The results of the systematic review are discussed relative to implications for the clinical utility of MRI as a tool to classify the extent of structural damage to articular cartilage for the knee.
METHODS
Search Strategy
Systematic searches were performed in November 2010 using PubMed MEDLINE (from 1966), CINAHL (from 1982), SPORTDiscus (from 1985), SCOPUS (from 1996), and EMBASE (from 1974) databases. The keyword selection was designed to capture all diagnostic studies that compared the diagnostic capabilities of MRI relative to arthroscopy for detection of articular cartilage degeneration of the tibiofemoral and patellofemoral joints. PubMed MEDLINE was searched using MeSH term selections for the keywords knee, arthroscopy, magnetic resonance imaging, chondromalacia, osteoarthritis, chondral defect, and articular cartilage (Figure 1). Similar search strategies were utilized for the other databases. The search was supplemented by a review of the bibliographies of retrieved articles and manual review of pertinent journals to identify additional studies.
Figure 1.
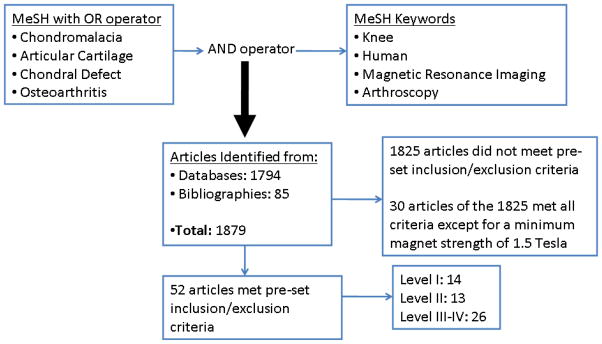
Keyword search strategy and results from systematic review
Study Selection
Two independent reviewers performed the first stage search and screen of abstracts identified through the database and bibliography searches. Any study identified by either reviewer was included. The first stage screen was utilized to identify articles that met the predetermined inclusion and exclusion criteria. Any article that met the inclusion and exclusion criteria was included in the second stage screen to identify the methodological quality and level of evidence.
Inclusion Criteria:
Human knee (patient population)
Both MRI and Arthroscopy were performed in the study
Diagnostic performance of MRI compared to arthroscopy is reported or can be calculated from the data provided in the article
Minimum of 10 subjects with articular cartilage involvement
Full manuscript provided in English or translated
MRI sequences and magnet strength reported
Minimum of 1.5 Tesla magnet used for MRI
Exclusion criteria:
Summary or clinical commentary articles
Case studies
Intervention studies (microfracture, autologous chondrocyte implantation, ACL reconstruction, osteoarticular transfer system procedure)
Cadaveric specimens
Pathology other than articular cartilage defects (inflammatory arthritis, infection, osteonecrosis, chondrocalcinosis, pigmented villonodular synovitis, chondral defects preceding a surgical treatment, osteochondritis dissecans)
Assessment of Methodologic Quality
Two independent reviewers evaluated each article based on the methodological criteria listed in Table 1 and determined a level of evidence (Level I–V). If there was a disagreement between the reviewers, a third reviewer was utilized to reconcile these differences. Levels of evidence for the diagnostic studies were determined by methods described by Wright et al.92 Level I studies included consecutive patients (as stated by the original authors), prospective data collection, and utilized established diagnostic criteria with gold standard comparison (arthroscopy). Level II studies included consecutive patients, retrospective data collection, and utilized established diagnostic criteria with gold standard comparison. If a study did not explicitly state that it was prospective or included consecutive patients it was considered a level II study if it utilized established diagnostic criteria with gold standard comparison. Level III studies included studies that included non-consecutive patients or studies that did not utilize established diagnostic criteria. Level IV studies included case-control studies, subjects selected or data pulled from larger clinical trials or cohorts, and studies without gold standard comparisons. Level V studies included expert opinion. The final comprehensive summary was limited to level I and II studies.
Table 1.
Methodological Quality Checklist modified from Jaesche R, Guyatt GH, Sackett DL, Users’ guides to the medical literature, VI. How to use an article about a diagnostic test. JAMA 1994; 271 (5): 389–391.
| Was there a clear question for the study to address with information about the test, setting, population, outcomes? |
| Was there a comparison with an appropriate reference standard? |
| Did all patients get the diagnostic test and reference standard? |
| Could the results of the test of interest have been influenced by the results of the reference standard? |
| Is the disease status of the tested population clearly described? |
| Were the methods for performing the test described in sufficient detail? |
| What are the results? (Sensitivity, Specificity, Accuracy) |
| How sure are we about these results? (Could they have occurred by chance) |
| Can the results be applied to patients/population of interest? |
| Can the test be applied to patients/population of interest? |
| Were all the outcomes important to the population of interest? (Will it change patient management) |
| What would be the impact of using this test on our population? |
RESULTS
The initial database and bibliography searches identified 1879 potential articles (Figure 1). The abstracts of all 1879 studies were reviewed and 52 articles met the pre-determined inclusion and exclusion criteria. Fourteen level I,7, 14, 16, 17, 27, 37, 40–43, 50, 72, 84, 85 13 level II4, 10, 26, 28, 38, 39, 47, 55, 59, 74, 78, 80, 90 and 26 level III–V5, 8, 12, 23, 29, 31–33, 35, 36, 44, 48, 49, 54, 60, 63–65, 69, 75, 81–83, 86, 93 studies were identified. For level I and II studies, Table 2 describes the breakdown of the number of articles that reported results regarding diagnostic performance of MRI compared to arthroscopy for grading of chondral involvement, reliability of grading between evaluators, MRI sequence performance, and involved articular cartilage surface or knee compartment. Tables 3 and 4 describe the level I and II studies identified and provide descriptions of the techniques used and overall diagnostic performance of MRI for each study. In level I and II studies identified, various grading systems were used to quantify articular cartilage involvement. Ten studies utilized Noyes and Stabler, 8 utilized the Outerbridge and Dunlop, and 8 utilized the Shahriaree grading systems (Tables 3 and 4).22, 28, 66, 70, 73, 79, 84 Table 1 in the appendix describes the grading classifications used by the different studies. One study described using the Ficat et al. grading system and the Shahriaree system; however, no information in the results was provided about how these systems compared to each other and it was not well described for which system was used for the final reporting of data. The Gluckert et al. grading system was used for two studies. Recht et al., Bachman et al., and Vallotton et al. were used in one study each (Tables 3 and 4, Appendix Table 1).
Table 2.
Breakdown of the number of articles that reported results regarding diagnostic performance of MRI compared to arthroscopy for different parameters for level I (14 studies identified) and level 2 (13 studies identified).
| Diagnostic Performance | Level I Studies (14) | Level II Studies (13) |
|---|---|---|
| Articular Cartilage Grading | 11 | 9 |
| Compartment/Surface Comparison | 7 | 8 |
| Evaluator Interrater Reliability | 7 | 5 |
| Magnet Strength 3.0 Tesla | 3 | 2 |
| Magnet Strength 1.5 Tesla | 11 | 13a |
Two studies compared 1.5 Tesla to 3.0 Tesla magnets.
Table 3.
Diagnostic performance for identifying articular cartilage abnormalities and information of level I studies identified by systematic search. Temporal refers to the time between MRI and arthroscopy. Sequence information is listed and sequence listed by a number in parentheses is used to describe the sequence order for the sensitivity, specificity, and accuracy. Sequence type abbreviations include: 3D fat suppressed spoiled gradient echo (FS SPGR); standard MRI is fast spin T1, T2, or proton weighted images in multiple planes; 3D water-excitation true fast imaging with steady-state precession (3D WE true FISP); 3D isotropic resolution fast spin echo (FSE-Cube); Fat with echo asymmetry and least-squares estimation gradient-recalled acquisition in steady-state imaging (IDEAL GRASS); Vastly undersampled isotropic projection steady-state free precession (VIPR-SSFP); Fast imaging employing steady-state acquisition (FIESTA); 3D T2 weighted gradient echo sequence (FISP); T1 weighted spin echo with intra-articular Gadolinium injection, FISP with intra-articular Gadolinium injection.
| Grading System | Magnet Strength | Sequence Information | Sensitivity | Specificity | Accuracy | Temporal | Compartments analyzed | Comments | |
|---|---|---|---|---|---|---|---|---|---|
| BREDELLA7 | Outerbridge | 1.5T | T2 Weighted Fast Spin Echo with Fat Suppression | 94% | 99% | 94% | Within 6 weeks | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Prospective, consecutive study of 130 patients out of 800 that had both and MRI and arthroscopy. Described Outerbridge technique but did not explicitly state that this was the technique used. Radiologists were blinded; surgeon had MRI report of lesion but no grading provided. |
| DISLER16 | Shahriaree | 1.5T | Standard MRI(1) FS SPGR(2) |
29–38% (1) 75–85% (2) |
97% (1) 97% (2) |
Not reported | Not reported | 8 articular surfaces assessed (medial and lateral patella, medial and lateral trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Prospective, consecutive study of 43 patients sent for MRI during a 4 month period; 12 had arthroscopic correlation. MRI report available to surgeons at arthroscopy. |
| DISLER15 | Shahriaree | 1.5T | Standard MRI(1) FS SPGR(2) |
29–38% (1) 75–85% (2) |
97% (1) 97% (2) |
Not reported | Within 112 days | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Consecutive, retrospective study that included 47 patients. MRI initially read by 2 radiologists provided to surgeon; retrospective grading done blinded by radiologists. Range of SN and SP due to multiple MRI evaluators. |
| DUC17 | Noyes | 1.5T | 3D WE true FISP | 52–74% | 78–95% | 71–78% | Within 9 days | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Prospective, consecutive, blinded (surgeons and radiologists) study that included 30 patients that could get a short notice MRI. Range of SN, SP, accuracy based on sequence type used. |
| GALEA27 | Outerbridge for arthroscopy; Vallotton for MRI | 1.5T | Fast Spin Echo | 83.2% | 94.3% | Not reported | Within 6 weeks | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Prospective study of 100 randomly selected patients with knee pain. Random selection not well described. Radiologists blinded, not stated if surgeons blinded to MRI reports. |
| IRIE37 | Gluckert | 1.5T | 59% Standard MRI | Not reported | Not reported | Not reported | Within 3 months | Tibia and femur surfaces | Prospective, consecutive study of 29 knees with MRI evaluated by radiologist prior to arthroscopy. Blinding of MRI and arthroscopy not reported. Utilizes grading system identical to Outerbridge. |
| KIJOWSKI42 | Noyes | 3.0T | Standard MRI (1) FSE-Cube (2) |
68.2% (1) 72.8% (2) |
92.8% (1) 88.2% (2) |
85.1% (1) 83.3% (2) |
Within 3 months | Not reported | Prospective, consecutive study of 100 patients. Radiologists blinded to arthroscopy results, surgeons had access to initial MRI reports. No significant difference was found in diagnostic performance between sequences. |
| KIJOWSKI41 | Noyes | 3.0T | Standard MRI (1) IDEAL GRASS (2) |
66.1% (1) 68.5% (2) |
92.9% 92.6% |
83.9% 84.5% |
Within 3 months | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Prospective, consecutive study of 257 patients, 95 of which had arthroscopy. One author had research support from GE (but author did not have control of data inclusion). Radiologists blinded and examined images two times, 4 months apart. Surgeons not blinded to MRI results. |
| KIJOWSKI40 | Noyes | 1.5T | Standard MRI (1) VIPR-SSFP (2) |
77.8% 76.5% |
88.4% 92.2% |
84.8% 86.8% |
Within 2 months | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Prospective, consecutive study of 95 patients. Radiologists blinded and reviewed images twice, 4 months apart. Surgeons not blinded to MRI results. |
| KRAMER43 | Outerbridge | 1.5T | FISP (1) T1+Gd (2) FISP+Gd (3) |
62% (1) 85% (2) 87% (3) |
50% (1) 100% (2) 100% (3) |
49% (1) 87% (2) 88% (3) |
Within 9 months | 5 articular surfaces (medial femur, lateral femur, medial tibia, lateral tibia, patella) | Prospective study of 60 knees in 58 patients. Not reported if consecutive. Surgeons and radiologists blinded. |
| LI50 | Noyes | 1.5T | FS SPGR (1) FIESTA (2) |
82% (1) 76–80% (2) |
90–92% (1) 94% (2) |
88–90% 90–92% |
Within 58 days | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Prospective, consecutive, 58 knees in 54 patients, radiologists blinded, orthopaedists given general result, but not specific grading. |
| POTTER72 | Outerbridge | 1.5T | Fast spin echo | 87% | 94% | 87% (calculated from data provided) | Within 377 days (mean 27 days) | 7 articular surfaces (lateral and medial femoral condyle, lateral and medial patellar surfaces, lateral and medial tibial plateau, trochlea) | Prospective, consecutive (enrolled, all eligible) study that included 90 patients. Surgeon not blinded to MRI report, but was not given grading sheet of chondral lesions. Prospective, blinded study off 33 |
| VALLOTTON84 | Noyes | 1.5T | Standard MRI | 84.7% | 97.2% | 91.6% | Not reported | Patella surface | consecutive patients by MRI before arthroscopy. |
| VON ENGELHARDT85 | Shahriaree arthroscopy; Bachman for MRI | 3.0T | Standard MRI | 91% | 85% | 88% | Within 16 days | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Prospective, consecutive study of 40 patients, MRI available for surgeons but grading of chondral lesions not provided. |
Table 4.
Diagnostic performance for identifying articular cartilage abnormalities and information of level II studies identified by systematic search. Temporal refers to the time between MRI and arthroscopy. Sequence information is listed and sequence listed by a number in parentheses is used to describe the sequence order for the sensitivity, specificity, and accuracy. Sequence type abbreviations include: Water excitation 3D FLASH (WE 3D FLASH) and fat saturated proton density weighted turbo spin echo (PDw TSE)
| Grading System | Magnet Strength | Sequence Information | Sensitivity | Specificity | Accuracy | Temporal | Compartments analyzed | Comments | |
|---|---|---|---|---|---|---|---|---|---|
| BACHMANN4 | Shahriaree | 1.5T | Spin Echo and FISP | 26–96% | 95% | 81% | Within 4 weeks | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Retrospective study of 320 patients after acute trauma. MRI evaluation blinded and read by 2 radiologists, no report of arthroscopic blinding. |
| BROWN10 | Shahriaree | 1.5T | Standard MRI | 91%* | 88%* | 89% | Not reported | Patellofemoral compartment | Retrospective study of 75 patients with complaints of anterior knee pain and subsequent arthroscopy. Grading of arthroscopy determined from operative note. Not stated if consecutive patients. *Only grades 3 and 4 reported. |
| GAGLIARDI26 | Shahriaree | 1.5T | Standard MRI | No overall reported | No overall reported | No overall reported | Not reported | Patella surface | Prospective study of 27 patients. MRI results blinded to one of 2 surgeons involved in surgery, not stated if consecutive. Only grade severity diagnostic performance provided. |
| GLUCKERT28 | Outerbridge | 1.5T | 3D gradient echo | 40.9% | 91.5% | Not reported | Median 12 days | 19 articular surfaces | Prospective, consecutive, blinded (surgeons and radiologists) study that included 30 patients that could get a short notice MRI. Range of SN, SP, accuracy based on sequence type used. |
| JUNGIUS38 | Outerbridge | 1.5T | Fast spin ECHO STIR | 76–77%$ | 89–95%$ | 85–91%$ | Within 15 weeks | Tibia and femur | Retrospective study of 2115 consecutive charts - 84 knees (83 patients) which had an arthroscopy and description of cartilage lesions in surgical report. Radiologists blinded, no IRB. |
| KIJOWSKI39 | Noyes | 1.5T and 3.0T | Standard MRI | 69.3% 1.5T 70.5% 3.0T |
78% 1.5T 85.9% 3.0T |
74.5% 1.5T 80.1% 3.0T |
Within 2 months | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Retrospective study of 200 symptomatic patients that underwent both MRI and arthroscopy. 100 consecutive patients that had 1.5T imaging and 100 different consecutive patients that had 3.0T. 3 fellowship trained musculoskeletal radiologists blinded to arthroscopic findings. Surgeons not blinded to MRI findings. |
| LEE47 | Outerbridge | 1.5T | Standard MRI | 75% early disease 80% advanced disease |
94% early disease 99% advanced disease |
94% early disease 98% advanced disease |
Within 55 days | Patella surface | 579 patients underwent MRI, 86 patients had MRI and subsequent arthroscopy. Not stated if consecutive or prospective. Radiologists blinded. |
| MACARINI55 | Noyes | 1.5T | Standard MRI | 63–64% | 74–90% | 69–79% | Within 1 month | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | 90 patients with MRI and arthroscopy. Not reported if it was consecutive, prospective or if authors blinded to data. Ranges of SN, SP, and accuracy based on location in patellofemoral or tibiofemoral compartments. |
| MOHR59 | Noyes | 1.5T | WE 3D FLASH (1) FS PDw TSE (2) |
46% (1) 91% (2) |
92% (1) 98% (2) |
74% (1) 95% (2) |
Within 2 months | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Retrospective study of 26 patients. Not reported if consecutive. Radiologist blinded to surgeon report and surgeon blinded to grade/size of lesion. |
| REISER74 | Shahriaree and Ficat | 1.5T | 3D Gradient Echo | 95% | 65% | 88% | Within 2 months | Patella surface | Blinded, retrospective analysis of MRI of 41 patients who were clinically suspected of having PF articular cartilage grading, 25 patients had arthroscopic correlation, 5 human cadaveric patellae. Some MRIs obtained after arthroscopy. |
| SCHMID78 | Outerbridge | 1.5T | 2D Multiple echo | 79%$ | 82%$ | 81%$ | Within 4 months | Patella surface | Blinded, retrospective analysis of MRI of 41 patients who were clinically suspected of having PF articular cartilage grading, 25 patients had arthroscopic correlation, 5 human cadaveric patellae. Some MRIs obtained after arthroscopy. |
| SONIN80 | Shahriaree | 1.5T | Standard MRI | 59–73.5% | 86.7–90.5% | 79.6–86.1% | Not reported | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Retrospective study of 54 patients, radiologists blinded to arthroscopy data, interpretation of wording on operative reports - converted to grading system. Not stated if consecutive. |
| WONG90 | Noyes for arthroscopy; Recht for MRI | 1.5T and 3.0T | Standard MRI | 70.6% 1.5T 75.7% 3.0T |
94.7% 1.5T 94.7% 3.0T |
86.4% 1.5T 88.2% 3.0T |
Within 180 days for arthroscopy and both MRIs | 6 articular surfaces assessed (patella, trochlear groove, medial and lateral femoral condyles, medial and lateral tibial plateaus) | Retrospective study of 19 patients with arthroscopy and 1.5T and 3.0T MRI. Radiologists blinded but surgeons were not blinded. Not reported if consecutive. |
Overall the diagnostic performance for MRI demonstrated a large range of sensitivities, specificities, and accuracies for level I and II studies. The sensitivity for identifying articular pathology in the knee joint was reported between 26–96%. Specificity and accuracy were reported between 50–100% and between 49–94%, respectively. Although there was a large range of reported performance capabilities, the majority of level I studies reported sensitivities over 80% (8 out of 14), specificities over 90% (11 out of 13 that reported specificity), and accuracies over 85% (9 out of 10 that reported accuracy).
Seventeen level I and II studies examined the diagnostic performance for different knee articular surfaces (Table 5). The reported sensitivities for each surface demonstrated a large range: medial tibial plateau (17–96%), lateral tibial plateau (0–58%), medial femoral condyle (28–100%), lateral femoral condyle (33–100%), trochlea (55–100%), patella (21–100%), patellofemoral compartment (44–95%), and tibiofemoral compartment (42–81%). All surfaces and compartments had greater than 85% reported specificities, other than one study that reported a patella specificity of 75% and one study that reported lateral femoral condyle specificity of 78% (Table 5). Accuracies for each surface and compartment ranged between 70–98%.
Table 5.
Diagnostic performance of knee compartments. Level of evidence designated by number in parentheses.
| Sensitivity | Specificity | Accuracy | Comment | |
|---|---|---|---|---|
|
Medial Tibial Compartment
| ||||
| BACHMANN (II)4 | 27–33% | Not reported | Not reported | Range due to reporting of condyles combined, no specification of medial or lateral. |
| DISLER (I)15 | 17–83% | 89–100% | Not reported | Range reported for different readers and same reader with different sequence type. |
| JUNGIUS(II)38 | 67% | 85–95% | 80–87% | Range due to two different readers. |
| KIJOWSKI(I)41 | 62.5–64% | 90.8–91.5% | 84.20% | Range reported for both standard MRI and IDEAL GRASS techniques. No significant differences reported between the two sequencing techniques. |
| LI(I)50 | 84–92% | 88–100% | 87–90% | Range reported for 2 readers and SPGR and FIESTA sequences. No significant differences reported between the two sequencing techniques. |
| POTTER(I)72 | 80% | 96% | 92% | Accuracy was calculated using data provided in manuscript. |
| SONIN (II)80 | 57% | 89% | 85% | All reviewers combined as provided by article. |
| VON ENGELHARDT (I)85 | 96% | 88% | 93% | |
|
| ||||
|
Lateral Tibial Compartment
| ||||
| BACHMANN (II)4 | 27–33% | Not reported | Not reported | Range due to reporting of condyles combined, no specification of medial or lateral. |
| DISLER (I)15 | 0–56% | 95–100% | Not reported | |
| JUNGIUS(II)38 | 58% | 93–96% | 88–91% | Range due to two different readers. |
| KIJOWSKI(I)41 | 34–42% | 92.1–94.4% | 76.8–81.6% | Range reported for both standard MRI and IDEAL GRASS techniques. No significant differences reported between the two sequencing techniques. |
| LI(I)50 | 43–52% | 80–86% | 74–80% | Range reported for 2 readers and SPGR and FIESTA sequences. No significant differences reported between the two sequencing techniques. |
| POTTER(I)72 | 58% | 93% | 88.60% | Accuracy was calculated using data provided in manuscript. |
| SONIN (II)80 | 44% | 95% | 78% | All reviewers combined as provided by article. |
| VON ENGELHARDT (I)85 | 64% | 86% | 80% | |
|
| ||||
|
Medial Femoral Compartment
| ||||
| BACHMANN (II)4 | 63–65% | Not reported | Not reported | Range due to reporting of tibial plateaus combined, no specification of medial or lateral. |
| DISLER (I)15 | 28–83% | 93–100% | Not reported | Range reported for different readers and same reader with different sequence type. |
| JUNGIUS(II)38 | 89% | 87–94% | 88–92% | Range due to two different readers. |
| KIJOWSKI(I)41 | 71.4–77.4% | 91.9–96.2% | 85.30% | Range reported for both standard MRI and IDEAL GRASS techniques. No significant differences reported between the two sequencing techniques. |
| LI(I)50 | 88–94% | 96–100% | 89–91% | Range reported for 2 readers and SPGR and FIESTA sequences. No significant differences reported between the two sequencing techniques. |
| POTTER(I)72 | 83% | 95% | 88.60% | Accuracy was calculated using data provided in manuscript. |
| SONIN (II)80 | 72% | 91% | 83% | All reviewers combined as provided by article. |
| VON ENGELHARDT (I)85 | 100% | 90% | 98% | |
|
| ||||
|
Lateral Femoral Compartment
| ||||
| BACHMANN (II)4 | 63–65% | Not reported | Not reported | Range due to reporting of tibial plateaus combined, no specification of medial or lateral. |
| DISLER (I)15 | 33–89% | 92–100% | Not reported | Range reported for different readers and same reader with different sequence type. |
| JUNGIUS(II)38 | 71–79% | 89–97% | 86–94% | Range due to two different readers. |
| KIJOWSKI(I)41 | 54.80% | 91.9–92.6% | 83.7–84.2% | Range reported for both standard MRI and IDEAL GRASS techniques. No significant differences reported between the two sequencing techniques. |
| LI(I)50 | 82–88% | 87–91% | 86–89% | Range reported for 2 readers and SPGR and FIESTA sequences. No significant differences reported between the two sequencing techniques. |
| POTTER(I)72 | 100% | 96% | 96.60% | Accuracy was calculated using data provided in manuscript. |
| SONIN (II)80 | 75% | 91% | 87% | All reviewers combined as provided by article. |
| VON ENGELHARDT (I)85 | 85% | 78% | 80% | |
|
| ||||
|
Trochlea
| ||||
| BACHMANN (II)4 | 55% | Not reported | Not reported | |
| DISLER (I)15 | 62–100% | 95–97% | Not reported | Range reported for different readers and same reader with different sequence type. |
| KIJOWSKI(I)41 | 73.5–76.5% | 94.9–93.4% | 87.4–87.9% | Range reported for both standard MRI and IDEAL GRASS techniques. No significant differences reported between the two sequencing techniques. |
| LI(I)50 | 63–72% | 82–93% | 81–84% | Range reported for 2 readers and SPGR and FIESTA sequences. No significant differences reported between the two sequencing techniques. |
| POTTER(I)72 | 92% | 95% | 94% | Accuracy was calculated using data provided in manuscript. |
| SONIN (II)80 | 62% | 94% | 89% | All reviewers combined as provided by article. |
| VON ENGELHARDT (I)85 | 100% | 86% | 93% | |
|
| ||||
|
Patella
| ||||
| BACHMANN (II)4 | 80% | Not reported | Not reported | |
| DISLER (I)15 | 21–100% | 88–100% | Not reported | Range reported for different readers and same reader with different sequence type. |
| KIJOWSKI(I)41 | 78.2–79.3% | 88.8–91.8% | 84.2–85.3% | Range reported for both standard MRI and IDEAL GRASS techniques. No significant differences reported between the two sequencing techniques. |
| LEE (II)47 | 75–80% | 94–99% | 94%–98% | Range due to reporting early and advanced disease. |
| LI(I)50 | 94–96% | 90–94% | 89–94% | Range reported for 2 readers and SPGR and FIESTA sequences. No significant differences reported between the two sequencing techniques. |
| POTTER(I)72 | 92–97% | 89–94% | 92–93% | Range reported for medial and lateral patella. Accuracy was calculated from data. |
| SCHMID (II)78 | 79% | 82% | 81% | Only reported diagnostic performance for grade II or higher. |
| SONIN (II)80 | 80% | 75% | 77% | All reviewers combined as provided by article. |
| VALLOTTON (I)84 | 84.70% | 97.20% | 91.60% | |
| VON ENGELHARDT (I)85 | 87% | 89% | 88% | |
|
| ||||
|
Patellofemoral Compartment
| ||||
| BROWN (II)10 | 63–91% | 78–88% | 71–89% | Range due to reporting of including all grades II–IV or only III–IV. |
| REISER(II)74 | 95% | 65% | 88% | |
| MACARINI (II)55 | 44–83% | 92–96% | 78–94% | Range due to reporting early and advanced disease. |
|
| ||||
|
Tibiofemoral Compartment
| ||||
| IRIE (I)37 | 59% | Not reported | Not reported | |
| MACARINI (II)55 | 42–81% | 80–97% | 70–94% | Range due to reporting early and advanced disease. |
Twelve studies provided information about interobserver reliability and one study provided information about intraobserver reliability (Table 6) for identifying articular abnormalities. The Kappa values reported were between 0 and 0.93 for interobserver agreement. All but one study reported moderate to excellent interobserver agreement (>0.40). Intraobserver reliability was reported to be moderate to excellent with Kappa values between 0.49–0.83.71
Table 6.
Diagnostic performance of MRI for early osteoarthritis (Grades I–II; IIA for Noyes scale). Level of evidence designated by number in parentheses.
| Article | Interobserver agreement | Intraobserver Agreement | Comment |
|---|---|---|---|
| DISLER (I)16 | Kappa Values 0–0.72 standard MRI | Kappa Values 0.49–0.83 standard MRI | Large range due to different compartment locations. |
| DUC(I)17 | Kappa Values 0.58–0.72 | Not reported | Range due to different sequences evaluated. |
| GLUCKERT (II)28 | 91.60% | Not reported | |
| JUNGIUS (II)38 | Kappa Value 0.63 | Not reported | |
| KIJOWSKI (I)42 | Kappa Values 0.68–0.69 | Not reported | Range due to different sequences evaluated. |
| KIJOWSKI (I)41 | Kappa Values 0.98–1.0 | Not reported | Range due to different sequences evaluated. |
| KIJOWSKI (I)40 | Kappa Values 0.69–0.75 | Not reported | Range due to different sequences evaluated. |
| KIJOWSKI (II)39 | Kappa Values 0.47–0.58 | Not reported | Range due to different magnet strengths. |
| LI (I)50 | Kappa Values 0.43–0.83 | Not reported | Large range due to different compartment locations. |
| POTTER(I)72 | Kappa Value 0.93 | Not reported | |
| SCHMID (II)78 | Kappa Value 0.68 | Not reported |
Diagnostic performance for severity grades was available for 20 of the level I and II studies (Table 7). Five of the 20 studies provided information about the agreement of grades between MRI and arthroscopy but did not provide sensitivity, specificity, or accuracy information. The sensitivity, specificity, and accuracy for identifying early osteoarthritis were reported between 0–86%, 48–95%, and 5–94%, respectively. The sensitivity, specificity, and accuracy for identifying advanced osteoarthritis were reported between 47–98%, 60–100%, and 57–98%, respectively. The agreement for MRI and arthroscopy grades demonstrated a large range of 0–76%. Six out of the 8 level I studies that reported agreement had identical grading for greater than 63% of the lesions. Many studies reported high agreement within one grade of the arthroscopic grading.
Table 7.
Interobserver and intraobserver agreement for identifying articular cartilage abnormalities. Level of evidence designated by number in parentheses.
| Article | Sensitivity | Specificity | Accuracy | Agreement of Grades |
|---|---|---|---|---|
| BACHMANN (II)4 | 17–31% | Not reported | Not reported | 75% of lesions on MRI graded identically to arthroscopy. |
| BREDELLA (I)7 | 74% | 85% | 80% | 64% of lesions on MRI graded identically to arthroscopy. |
| BROWN (II)10 | Could not be identified | Not reported | Not reported | Not reported |
| DISLER (I)16 | Not reported | Not reported | Not reported | 63% of lesions on MRI graded identically to arthroscopy, 96% within one grade. |
| GAGLIARDI (II)26 | 0–47% | 91–95% | 85–87% | Not reported |
| GLUCKERT (II)28 | Not reported | Not reported | 5–43.7% | Not reported |
| IRIE (I)37 | 23.1–50% | Not reported | Not reported | Not reported |
| JUNGIUS (II)38 | Not reported for grade ≤ II | Not reported for grade ≤ II | Not reported for grade ≤ II | Not reported |
| KIJOWSKI (I)42 | 25–51% | 88.2–92.8% | 86.7–91.2% | 28.8–33.9% identical grading, 68–72% within one grade |
| KIJOWSKI (I)41 | 9.1–48.6% | Not reported | Not reported | Cohen’s Kappa coefficient of agreement 0.65–0.67 |
| KIJOWSKI (I)40 | 0–67.6% | 65.5–72.9% | 64.8–71.1% | Not reported |
| KIJOWSKI (II)39 | 40.5–50.2% | 59–66.8% | 58.6–64.3% | 28.1% for 1.5T, 35.1% for 3.0T graded identically to arthroscopy. |
| KRAMER (I)43 | Not reported | Not reported | Not reported | 0–57% FISP; 0–93% T1+Gd; 0–97% FISP +Gd for identical grading on MRI and arthroscopy. |
| LEE (II)47 | 75% | 94% | 94% | 57.1% SN, 93.0% SP, 90.1% accuracy for correct grading |
| LI (I)50 | Not reported | Not reported | Not reported | 72–73% identical grading for MRI and arthroscopy. |
| MACARINI (II)55 | 42–44% | 80–92% | 70–78% | Cohen’s Kappa coefficient of agreement 0.63–0.71 |
| POTTER(I)72 | Not reported | Not reported | Not reported | 72–73% identical grading for MRI and arthroscopy. |
| SCHMID (II)78 | 79–86% | 48–82% | 62–81% | Not reported |
| VALLOTTON (I)84 | Not reported | Not reported | Not reported | 76.5% identical grading for MRI and arthroscopy. |
| VON ENGELHARDT (I)85 | 29–62% | 90–95% | 85–87% | Not reported |
DISCUSSION
The systematic search process utilized for this review identified nearly 1,800 studies that potentially provided information about the diagnostic performance of MRI and arthroscopy of the knee related to chondral involvement. Due to the wide variety of imaging techniques (sequences, slice size, plane of data collection, positioning of patients, and types of scanners utilized) and methodological differences between the studies, a meta-analysis was not performed. However, the rigorous search methodology identified 27 level I and II studies that provided unique and valuable diagnostic performance evidence.
Although all 27 of the level I and II studies utilized grading systems to evaluate the extent of of chondral involvement, several different types of grading systems were used and only 20 studies provided diagnostic performance information for MRI regarding the different grades of severity. The most common systems used for grading articular cartilage degeneration included Outerbridge, Noyes, and Shahriaree systems.66, 70, 79 Although all level I and II studies utilized established diagnostic criteria for arthroscopy, some studies described new modifications to the established grading systems in order to relate the MRI grades to the arthroscopic grading systems. Wong et al. used the Noyes system to quantify chondral involvement at arthroscopy and a system described by Recht et al. to compare MRI findings with arthroscopic findings.90 Likewise, Von Engelhardt et al. used the Shahriaree system to quantify arthroscopy findings and a similar MRI grading system described by Bachman et al.3, 85 The MRI grading systems used by Galea et al. (Vallotton grading system), Irie et al. and Gluckert et al. (Gluckert grading systems) had MRI grading “equivalents” to the Outerbridge arthroscopy grading system.27, 28, 37, 70, 84
Is MRI a valid, sensitive, specific, accurate, and reliable instrument to identify knee articular cartilage abnormalities compared to arthroscopy?
The diagnostic performance for MRI demonstrated a large range of sensitivities, specificities, accuracies, and reliability for level I and II studies. Comprehensively, the data indicates that MRI is highly specific and moderately sensitive and accurate for identifying articular cartilage degeneration of any severity. Interobserver and intraobserver agreement was moderate to high for the majority of studies. Although the range of sensitivities reported for different surfaces and compartments of the knee was quite large, several studies reported sensitivities over 85% for most of the surfaces and compartments.50, 72, 85 Disler et al. reported the lowest sensitivities for different surfaces; however, the reliability between MRI evaluators was reported to be low (Kappa value as low as 0.0).16 The lateral tibial plateau appeared to have the lowest sensitivity of all surfaces and interestingly, Li et al. reported the lowest interobserver agreement for grades in the lateral tibial compartment as well.50 Collectively, the specificity and accuracy of identifying articular cartilage degeneration was relatively high for all surfaces and compartments. The large differences in reported diagnostic performance of MRI for identifying articular cartilage degeneration may be attributable to the wide variety of different image slice sizes, surface coil sizes and shapes, types of scanners, sequencing techniques, and grading systems that were utilized for each study.
Is MRI a sensitive tool that can be utilized to identify early articular cartilage pathology?
The sensitivity for identifying early osteoarthritis articular cartilage degeneration (grades 1 and 2, or 2A on Noyes’ scale) was reported between 0–86%. Compared to identification of early osteoarthritis by lower grades of degeneration, advanced disease demonstrated a much higher range of sensitivities (47–98%). Although relatively low sensitivities were identified for grade 1 lesions and some studies only used a cut off score of “grade I articular cartilage degeneration” for early disease, the diagnostic performance of many studies was reportedly much higher (up to 60% greater sensitivity) for grade II disease.39–42, 55, 85 Bredella et al. and Lee et al. reported sensitivities >70% for identifying low grades of articular cartilage degeneration.7, 47 In contrast, Brown et al. could not identify early articular cartilage degeneration with MRI. The specificity was also reported over a wide range for early disease, but the majority of studies reported greater than 70% specificity. The relatively poor performance of some MRI studies in detecting early articular cartilage degeneration may be attributable to suboptimal spatial resolution, signal-to-noise ratio, artifacts, and tissue contrast as well as difficulty in capture and integration of cartilage in multiple planes.7, 24, 61, 76, 77 Approximately 0.3 mm in-plane spatial resolution is necessary to identify superficial articular cartilage changes, consistent with grade 1 articular cartilage degeneration, which is beyond the capabilities and time constraints of most MRI protocols utilized in clinical practice.77
Methodological Quality of the Studies
Despite meeting level I or II “criteria,” many of the studies had methodological limitations that may have affected their diagnostic results (Tables 1, 3, 4). Many studies failed to appropriately describe study methods such as the prospective or retrospective nature of data collection and consecutive versus non-consecutive identification of subjects. Several studies utilized varying magnet strengths with no breakdown of results based on the type of magnet used for data collection and therefore had to be excluded from the comprehensive analysis.25, 62 No studies had a control group of suspected “healthy” articular cartilage and many studies reported no blinding or did not describe blinding techniques of the surgical and radiologic evaluations. In addition, some studies did not report the temporal relationship between MRI and arthroscopy or had a considerable time delay between the studies (0–377 days). As a result of changes that can occur in the knee over time, large time delay may not only affect the reported diagnostic performance of MRI but also impact the agreement between arthroscopy and MRI for severity of articular cartilage degeneration grades. Finally, some studies used MRI studies that were performed after arthroscopy which may have altered the sensitivity, specificity, and accuracy of the results.
Clinical Relevance
Osteoarthritis, as structurally evidenced by higher articular cartilage degeneration grades (grades 3 and 4), often leads to significant pain, compromise of daily function and sports participation, and ultimately long-term health consequences from the restriction of physical activity. In addition to affecting the “aging” or master level athlete, many young adult athletes are at high risk of developing osteoarthritis at an early age as a result of traumatic knee injuries.30, 52 Early identification of superficial cartilage damage and osteoarthritic changes, may be the most critical time point to identify for these young athletes since it may be the most amendable to intervention and prevention of further degradation.2
The invasive nature and expense related to arthroscopy and the exposure to radiation and poor sensitivity of radiography for identifying articular cartilage pathology significantly limit their clinical utility. The results of this systematic review indicate that although MRI has relatively high specificity and moderate to high sensitivity for identifying advanced osteoarthritic structural changes (higher articular cartilage degeneration grades), MRI reportedly has up to a 70% chance of missing early chondral disease (lower grades of articular cartilage degeneration) when lower magnet strengths are used for the procedure.3, 16 However, radiologic investigations have even lower reported sensitivities (reported as low as 2–16% depending on technique) for identifying any stage of knee articular cartilage degeneration despite specificities of up to 90%.91 Higher MRI magnet strength and enhanced sequence techniques appear to improve MRI sensitivity. MRIs with 3.0 Tesla magnet strength demonstrate up to 91% sensitivity for identifying all articular cartilage degeneration grades and identify early stage disease with reported 48–94.7% sensitivity.39, 41, 42, 85, 90 Wong et al. found that the sensitivity and accuracy of 3.0 Tesla magnet strength MRI for cartilage lesions was significantly higher than 1.5 Tesla.90 Fat-suppressed-three-dimensional spoiled gradient-echo (SPGR) and fat saturated proton density-weighted turbo spin echo also demonstrate relatively higher sensitivity than standard MRI sequences.7, 16, 59 Thus, MRI may provide a superior clinical diagnostic tool for identifying early articular cartilage damage than radiographs due to its avoidance of exposure to radiation and higher diagnostic performance, especially if imaging technology continues to improve.39, 41, 42, 85, 90 Further, the non-invasive nature, lower costs, convenience and minimal complication risk of MRI offers an appealing alternative to arthroscopy for identifying early articular cartilage degeneration.
Future Studies
In recent years, there has been increased emphasis on clinical outcomes measures to aid in the implementation of evidence based treatment strategies and a socioeconomic push to develop performance based criteria for reimbursement practices. It is paramount that clinical tools that predict disease, monitor disease progression, and assess clinical outcomes of treatments of disease be developed and utilized. Ideally, MRI could be utilized as a non-invasive clinical tool to predict, monitor, and assess clinical outcomes of articular pathology. However, the systematic search strategies of this study failed to identify any studies that examined the validity of using MRI to predict or monitor disease progression of articular pathology. Thus future studies should focus on characterizing the clinical utility of MRI for these applications, especially utilizing 3.0 Tesla magnet strength studies.
New MRI techniques to assess the morphologic status of cartilage such as T2 mapping, delayed gadolinium-enhanced MR imaging of cartilage (dGemeric), T1ρ imaging, sodium imaging, and diffusion-weighted imaging have garnered interest in the radiologic literature and may provide promising results related to identification of early stage articular cartilage degeneration.6, 11, 19, 88, 89 However, none of these techniques were utilized in the studies that met the stringent inclusion and exclusion criteria of the current systematic review. Most of the evidence related to these techniques has only been evaluated in animal studies or do not have arthroscopic (“gold standard”) comparisons. There is a significant need for high level research that evaluates the diagnostic capabilities of these new techniques compared to arthroscopy in human pathologic populations.
This systematic review examined the diagnostic capabilities of 1.5 and 3.0 Tesla MRI as a tool to capture articular cartilage pathology in the human patient based population. The inclusion and exclusion criteria were specifically selected to identify the most relevant and current clinical information. Since most major medical facilities have access to 1.5T strength MRI scanners, only studies that utilized a 1.5T magnet or greater were included in the final analysis. Also, in order to target MRI diagnostic utility for identifying articular cartilage degeneration, studies that evaluated other articular pathology (i.e. rheumatoid, infection, osteonecrosis, osteochondritis dissecans) were excluded from the final analysis. Although many cadaveric and animal model studies were identified in the initial search that demonstrated high sensitivity, specificity, and accuracy of MRI, these studies were excluded from the current review because it is unclear how artificially created lesions in these simulated pathologic models relate to real world disease.9, 13, 58 Lower diagnostic performance than animal and cadaveric studies was expected due to the difficulty in positioning, relaxation, and comfort of patient. Moreover, the patient based population studies lack the consistent “clean cut” lesions such as those that are artificially created in cadaveric and animal models. This concept should be kept in mind as future studies for evaluating the diagnostic performance of clinical tools for articular cartilage pathology are developed.
Conclusions
In conclusion, although there is promising evidence that MRI is a relatively valid, sensitive, specific, accurate, and reliable clinical tool for identifying articular cartilage abnormalities, it is not possible to offer conclusive guidelines regarding its global clinical utility for guiding diagnosis and treatment strategies. However, technological improvements such as utilizing higher magnet strengths of 3.0T to MRI practices may provide superior diagnostic performance strategies in the future. There is a growing need for diagnostic techniques that identify the earliest structural changes in articular cartilage (articular cartilage degeneration) that predict future osteoarthritis in the field of sports medicine. Early identification of modifiable early structural changes to the articular cartilage of the knee is critical for the young adult athletes who are at high risk of developing osteoarthritis. Given the large discrepancies in diagnostic performance identified by this systematic review, there is clearly a significant need for a large clinical trial with rigorous methodology to evaluate the diagnostic performance of MRI as an instrument to identify and clinically grade articular cartilage pathology, particularly early chondral degeneration.
What is known about the subject
Magnetic resonance imaging is commonly used to evaluate articular cartilage injuries.
What this study adds to existing knowledge
This study provides comprehensive information about the sensitivity, specificity, accuracy, and reliability of MRI compared to arthroscopy for evaluating early and advanced articular cartilage degeneration, a structural sign of osteoarthritis changes in the knee.
Appendix: Table 1.
Description of the articular cartilage degeneration grading classifications used for each study.
| Author | Grade | Description | Defect Size |
|---|---|---|---|
|
| |||
| Bachman (MRI) | 0 | Normal intrinsic signal | |
| I | Focal intrinsic signal and normal surface contour | ||
| II | Fibrillation or erosion | <50% cartilage | |
| III | Fibrillation or erosion | >50% cartilage | |
| IV | Full thickness defect with denudation of bone | ||
|
| |||
| Ficat (MRI) | I | Closed chondromalacia, surface intact, softening | |
| II | Surface irregularities, superficial ulcerations | ||
| III | Ulceration with exposure of subchondral bone | ||
|
| |||
| Gluckert (Arthroscopy) | 0 | Smooth surface, no soft areas on probing | |
| I | Slight fibrillation | ||
| II | Moderate fibrillation | <50% depth | |
| III | Deep fibrillation, down to subchondral bone | >50% depth | |
| IV | Complete loss of cartilage, exposure of subchondral bone | ||
|
| |||
| Gluckert (Modification for MRI by Irie) | 0 | Continuous, smooth surface, homogenous signal | |
| I | Continuous, smooth, slight inhomogeneity | ||
| II | Surface irregularity | <50% thickness | |
| III | Marked surface irregularity, inhomogeneity | >50% thickness | |
| IV | Complete loss of cartilage layer | ||
| V | Inhomogeneity of subchondral bone | ||
|
| |||
| Noyes (Arthroscopy) | I | Intact cartilage | |
| IA | Softening | <1 cm | |
| IB | Softening with deformation | 1.5 cm | |
| II | Fibrillation/fissuring | ||
| IIA | Fibrillation/fissuring | <1/2 thickness | |
| IIB | Fibrillation/fissuring | >1/2 thickness | |
| III | Exposure of subchondral bone | ||
| IIIA | Exposure of subchondral bone, intact | ||
| IIIB | Exposure of subchondral bone, bony excavation | ||
|
| |||
| Outerbridge (Arthroscopy) | I | Softening and swelling | |
| II | Fragmentation/fissuring | <1/2 inch | |
| III | Fragmentation/fissuring | >1/2 inch | |
| IV | Erosion with exposed subchondral bone | ||
|
| |||
| Shahriaree (Arthroscopy) | I | Softening | |
| II | Blisterlike swelling | ||
| III | Surface irregularity, areas of thinning | ||
| IV | Ulceration, exposure of subchondral bone | ||
|
| |||
| Recht (MRI) | 0 | Intact cartilage surface | |
| 2A | Cartilage defect | <1/2 thickness | |
| 2B | Cartilage defect (not full thickness) | >1/2 thickness | |
| 3A | Exposed bone with normal bone contour | ||
| 3B | Exposed bone with cavitation or erosion | ||
|
| |||
| Vallotton (MRI) | 0 | Normal cartilage | |
| I | Surface intact, hypo or hyper signal | ||
| II | Mild surface irregularity | <50% thickness | |
| III | Severe surface irregularity, up to 100% thickness | >50% thickness | |
| IV | Bone reaction | 100% thickness | |
References
- 1.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 2.Altman RD. Early management of osteoarthritis. Am J Manag Care. 2010;16(Suppl Management):S41–47. [PubMed] [Google Scholar]
- 3.Bachmann G, Heinrichs C, Jurgensen I, Rominger M, Scheiter A, Rau WS. Comparison of different MRT techniques in the diagnosis of degenerative cartilage diseases. In vitro study of 50 joint specimens of the knee at T1.5. Rofo. 1997;166(5):429–436. doi: 10.1055/s-2007-1015453. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann GF, Basad E, Rauber K, Damian MS, Rau WS. Degenerative joint disease on MRI and physical activity: a clinical study of the knee joint in 320 patients. Eur Radiol. 1999;9(1):145–152. doi: 10.1007/s003300050646. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn WD, Jr, Bernreuter WK, Rominger M, Loose LL. Arthroscopic evaluation of knee articular cartilage: A comparison with plain radiographs and magnetic resonance imaging. Journal of Rheumatology. 1994;21(4):675–679. [PubMed] [Google Scholar]
- 6.Borthakur A, Shapiro EM, Beers J, Kudchodkar S, Kneeland JB, Reddy R. Sensitivity of MRI to proteoglycan depletion in cartilage: comparison of sodium and proton MRI. Osteoarthritis Cartilage. 2000;8(4):288–293. doi: 10.1053/joca.1999.0303. [DOI] [PubMed] [Google Scholar]
- 7.Bredella MA, Tirman PF, Peterfy CG, et al. Accuracy of T2-weighted fast spin-echo MR imaging with fat saturation in detecting cartilage defects in the knee: comparison with arthroscopy in 130 patients. AJR Am J Roentgenol. 1999;172(4):1073–1080. doi: 10.2214/ajr.172.4.10587150. [DOI] [PubMed] [Google Scholar]
- 8.Broderick LS, Turner DA, Renfrew DL, Schnitzer TJ, Huff JP, Harris C. Severity of articular cartilage abnormality in patients with osteoarthritis: evaluation with fast spin-echo MR vs arthroscopy. AJR Am J Roentgenol. 1994;162(1):99–103. doi: 10.2214/ajr.162.1.8273700. [DOI] [PubMed] [Google Scholar]
- 9.Brossmann J, Frank LR, Pauly JM, et al. Short echo time projection reconstruction MR imaging of cartilage: Comparison with fat-suppressed spoiled GRASS and magnetization transfer contrast MR imaging. Radiology. 1997;203(2):501–507. doi: 10.1148/radiology.203.2.9114112. [DOI] [PubMed] [Google Scholar]
- 10.Brown TR, Quinn SF. Evaluation of chondromalacia of the patellofemoral compartment with axial magnetic resonance imaging. Skeletal Radiol. 1993;22(5):325–328. doi: 10.1007/BF00198391. [DOI] [PubMed] [Google Scholar]
- 11.Crema MD, Roemer FW, Marra MD, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37–61. doi: 10.1148/rg.311105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Smet AA, Monu JU, Fisher DR, Keene JS, Graf BK. Signs of patellar chondromalacia on sagittal T2-weighted magnetic resonance imaging. Skeletal Radiol. 1992;21(2):103–105. doi: 10.1007/BF00241832. [DOI] [PubMed] [Google Scholar]
- 13.Disler DC, Raymond E, May DA, Wayne JS, McCauley TR. Articular cartilage defects: In vitro evaluation of accuracy and interobserver reliability for detection and grading with US. Radiology. 2000;215(3):846–851. doi: 10.1148/radiology.215.3.r00jn20846. [DOI] [PubMed] [Google Scholar]
- 14.Disler DG. Fat-suppressed three-dimensional spoiled gradient-recalled MR imaging: Assessment of articular and physeal hyaline cartilage. American Journal of Roentgenology. 1997;169(4):1117–1123. doi: 10.2214/ajr.169.4.9308475. [DOI] [PubMed] [Google Scholar]
- 15.Disler DG, McCauley TR, Kelman CG, et al. Fat-suppressed three-dimensional spoiled gradient-echo MR imaging of hyaline cartilage defects in the knee: comparison with standard MR imaging and arthroscopy. AJR Am J Roentgenol. 1996;167(1):127–132. doi: 10.2214/ajr.167.1.8659356. [DOI] [PubMed] [Google Scholar]
- 16.Disler DG, McCauley TR, Wirth CR, Fuchs MD. Detection of knee hyaline cartilage defects using fat-suppressed three-dimensional spoiled gradient-echo MR imaging: comparison with standard MR imaging and correlation with arthroscopy. AJR Am J Roentgenol. 1995;165(2):377–382. doi: 10.2214/ajr.165.2.7618561. [DOI] [PubMed] [Google Scholar]
- 17.Duc SR, Pfirrmann CW, Schmid MR, et al. Articular cartilage defects detected with 3D water-excitation true FISP: prospective comparison with sequences commonly used for knee imaging. Radiology. 2007;245(1):216–223. doi: 10.1148/radiol.2451060990. [DOI] [PubMed] [Google Scholar]
- 18.Duchateau F, Vande Berg BC. MR imaging of the articular cartilage of the knee with arthroscopy as gold standard: assessment of methodological quality of clinical studies. Eur Radiol. 2002;12(12):2977–2981. doi: 10.1007/s00330-002-1557-1. [DOI] [PubMed] [Google Scholar]
- 19.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232(2):592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50(9):2811–2819. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 21.Faber KJ, Dill JR, Amendola A, Thain L, Spouge A, Fowler PJ. Occult osteochondral lesions after anterior cruciate ligament rupture. Six-year magnetic resonance imaging follow-up study. Am J Sports Med. 1999;27(4):489–494. doi: 10.1177/03635465990270041301. [DOI] [PubMed] [Google Scholar]
- 22.Ficat RP, Philippe J, Hungerford DS. Chondromalacia patellae: a system of classification. Clin Orthop Relat Res. 1979;(144):55–62. [PubMed] [Google Scholar]
- 23.Figueroa D, Calvo R, Vaisman A, Carrasco MA, Moraga C, Delgado I. Knee chondral lesions: incidence and correlation between arthroscopic and magnetic resonance findings. Arthroscopy. 2007;23(3):312–315. doi: 10.1016/j.arthro.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Frank LR, Brossmann J, Buxton RB, Resnick D. MR imaging truncation artifacts can create a false laminar appearance in cartilage. American Journal of Roentgenology. 1997;168(2):547–554. doi: 10.2214/ajr.168.2.9016245. [DOI] [PubMed] [Google Scholar]
- 25.Friemert B, Oberländer Y, Schwarz W, et al. Diagnosis of chondral lesions of the knee joint: Can MRI replace arthroscopy?: A prospective study. Knee Surgery, Sports Traumatology, Arthroscopy. 2004;12(1):58–64. doi: 10.1007/s00167-003-0393-4. [DOI] [PubMed] [Google Scholar]
- 26.Gagliardi JA, Chung EM, Chandnani VP, et al. Detection and staging of chondromalacia patellae: relative efficacies of conventional MR imaging, MR arthrography, and CT arthrography. AJR Am J Roentgenol. 1994;163(3):629–636. doi: 10.2214/ajr.163.3.8079858. [DOI] [PubMed] [Google Scholar]
- 27.Galea A, Giuffre B, Dimmick S, Coolican MR, Parker DA. The accuracy of magnetic resonance imaging scanning and its influence on management decisions in knee surgery. Arthroscopy. 2009;25(5):473–480. doi: 10.1016/j.arthro.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Gluckert K, Kladny B, Blank-Schal A, Hofmann G. MRI of the knee joint with a 3-D gradient echo sequence. Equivalent to diagnostic arthroscopy? Arch Orthop Trauma Surg. 1992;112(1):5–14. doi: 10.1007/BF00431036. [DOI] [PubMed] [Google Scholar]
- 29.Gold GE, Fuller SE, Hargreaves BA, Stevens KJ, Beaulieu CF. Driven equilibrium magnetic resonance imaging of articular cartilage: Initial clinical experience. Journal of Magnetic Resonance Imaging. 2005;21(4):476–481. doi: 10.1002/jmri.20276. [DOI] [PubMed] [Google Scholar]
- 30.Golightly YM, Marshall SW, Callahan LF, Guskiewicz K. Early-onset arthritis in retired National Football League players. J Phys Act Health. 2009;6(5):638–643. doi: 10.1123/jpah.6.5.638. [DOI] [PubMed] [Google Scholar]
- 31.Guckel C, Jundt G, Schnabel K, Gachter A. Spin-echo and 3Dgradient -echo imaging of the knee joint: a clinical and histopathological comparison. Eur J Radiol. 1995;21(1):25–33. doi: 10.1016/0720-048x(95)00681-f. [DOI] [PubMed] [Google Scholar]
- 32.Halbrecht JL, Jackson DW. Office arthroscopy: a diagnostic alternative. Arthroscopy. 1992;8(3):320–326. doi: 10.1016/0749-8063(92)90062-g. [DOI] [PubMed] [Google Scholar]
- 33.Handelberg F, Shahabpour M, Casteleyn PP. Chondral lesions of the patella evaluated with computed tomography, magnetic resonance imaging, and arthroscopy. Arthroscopy. 1990;6(1):24–29. doi: 10.1016/0749-8063(90)90092-r. [DOI] [PubMed] [Google Scholar]
- 34.Hargreaves BA, Gold GE, Beaulieu CF, Vasanawala SS, Nishimura DG, Pauly JM. Comparison of new sequences for high-resolution cartilage imaging. Magn Reson Med. 2003;49(4):700–709. doi: 10.1002/mrm.10424. [DOI] [PubMed] [Google Scholar]
- 35.Heron CW, Calvert PT. Three-dimensional gradient-echo MR imaging of the knee: comparison with arthroscopy in 100 patients. Radiology. 1992;183(3):839–844. doi: 10.1148/radiology.183.3.1584944. [DOI] [PubMed] [Google Scholar]
- 36.Huegli RW, Moelleken SM, Stork A, et al. MR imaging of post-traumatic articular cartilage injuries confined to the femoral trochlea. Arthroscopic correlation and clinical significance. Eur J Radiol. 2005;53(1):90–95. doi: 10.1016/j.ejrad.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Irie K, Yamada T, Inoue K. A comparison of magnetic resonance imaging and arthroscopic evaluation of chondral lesions of the knee. Orthopedics. 2000;23(6):561–564. doi: 10.3928/0147-7447-20000601-13. [DOI] [PubMed] [Google Scholar]
- 38.Jungius KP, Schmid MR, Zanetti M, Hodler J, Koch P, Pfirrmann CW. Cartilaginous defects of the femorotibial joint: accuracy of coronal short inversion time inversion-recovery MR sequence. Radiology. 2006;240(2):482–488. doi: 10.1148/radiol.2401050077. [DOI] [PubMed] [Google Scholar]
- 39.Kijowski R, Blankenbaker DG, Davis KW, Shinki K, Kaplan LD, De Smet AA. Comparison of 1.5-and 3.0 -T MR imaging for evaluating the articular cartilage of the knee joint. Radiology. 2009;250(3):839–848. doi: 10.1148/radiol.2503080822. [DOI] [PubMed] [Google Scholar]
- 40.Kijowski R, Blankenbaker DG, Klaers JL, Shinki K, De Smet AA, Block WF. Vastly undersampled isotropic projection steady-state free precession imaging of the knee: diagnostic performance compared with conventional MR. Radiology. 2009;251(1):185–194. doi: 10.1148/radiol.2511081133. [DOI] [PubMed] [Google Scholar]
- 41.Kijowski R, Blankenbaker DG, Woods MA, Shinki K, De Smet AA, Reeder SB. 3.0-T evaluation of knee cartilage by using three-dimensional IDEAL GRASS imaging: comparison with fast spin-echo imaging. Radiology. 2010;255(1):117–127. doi: 10.1148/radiol.09091011. [DOI] [PubMed] [Google Scholar]
- 42.Kijowski R, Davis KW, Woods MA, et al. Knee joint: Comprehensive assessment with 3D isotropic resolution fast spin-echo MR imaging -Diagnostic performance compared with that of conventional MR imaging at 3.0 T. Radiology. 2009;252(2):486–495. doi: 10.1148/radiol.2523090028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramer J, Recht MP, Imhof H, Stiglbauer R, Engel A. Postcontrast MR arthrography in assessment of cartilage lesions. J Comput Assist Tomogr. 1994;18(2):218–224. doi: 10.1097/00004728-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Kuikka PI, Bostman OM, Kiuru MJ, Salminen ST, Mikkola S, Pihlajamaki HK. One screening magnetic resonance imaging sequence in evaluation of chondral and meniscal lesions of the knee -a pilot study. Open Orthop J. 2008;2:19–22. doi: 10.2174/1874325000802010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 47.Lee SH, Suh JS, Cho J, Kim SJ. Evaluation of chondromalacia of the patella with axial inversion recovery-fast spin-echo imaging. J Magn Reson Imaging. 2001;13(3):412–416. doi: 10.1002/jmri.1059. [DOI] [PubMed] [Google Scholar]
- 48.Lee SY, Jee WH, Kim SK, Koh IJ, Kim JM. Differentiation between grade 3 and grade 4 articular cartilage defects of the knee: fat-suppressed proton density-weighted versus fat-suppressed three-dimensional gradient-echo MRI. Acta Radiol. 2010;51(4):455–461. doi: 10.3109/02841851003662772. [DOI] [PubMed] [Google Scholar]
- 49.Levy AS, Lohnes J, Sculley S, LeCroy M, Garrett W. Chondral delamination of the knee in soccer players. Am J Sports Med. 1996;24(5):634–639. doi: 10.1177/036354659602400512. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Yu C, Wu H, et al. Prospective comparison of 3D FIESTA versus fat-suppressed 3D SPGR MRI in evaluating knee cartilage lesions. Clin Radiol. 2009;64(10):1000–1008. doi: 10.1016/j.crad.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 51.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: Osteoarthritis. American Journal of Sports Medicine. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 52.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 53.Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113–1121. doi: 10.1001/archinternmed.2009.136. discussion 1121–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lundberg M, Odensten M, Thuomas KA, Messner K. The diagnostic validity of magnetic resonance imaging in acute knee injuries with hemarthrosis. A single-blinded evaluation in 69 patients using high-field MRI before arthroscopy. Int J Sports Med. 1996;17(3):218–222. doi: 10.1055/s-2007-972835. [DOI] [PubMed] [Google Scholar]
- 55.Macarini L, Murrone M, Marini S, Mariano M, Zaccheo N, Moretti B. MR in the study of kneecartilage pathologies: influence of location and grade on the effectiveness of the method. Radiol Med. 2003;105(4):296–307. [PubMed] [Google Scholar]
- 56.Maffulli N, Longo UG, Gougoulias N, Loppini M, Denaro V. Long-term health outcomes of youth sports injuries. Br J Sports Med. 2010;44(1):21–25. doi: 10.1136/bjsm.2009.069526. [DOI] [PubMed] [Google Scholar]
- 57.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64(3):460–466. [PubMed] [Google Scholar]
- 58.Marshall KW, Mikulis DJ, Guthrie BM. Quantitation of articular cartilage using magnetic resonance imaging and three-dimensional reconstruction. J Orthop Res. 1995;13(6):814–823. doi: 10.1002/jor.1100130603. [DOI] [PubMed] [Google Scholar]
- 59.Mohr A. The value of water-excitation 3D FLASH and fat-saturated PDw TSE MR imaging for detecting and grading articular cartilage lesions of the knee. Skeletal Radiol. 2003;32(7):396–402. doi: 10.1007/s00256-003-0635-z. [DOI] [PubMed] [Google Scholar]
- 60.Mori R, Ochi M, Sakai Y, Adachi N, Uchio Y. Clinical significance of magnetic resonance imaging (MRI) for focal chondral lesions. Magn Reson Imaging. 1999;17(8):1135–1140. doi: 10.1016/s0730-725x(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 61.Mosher TJ, Pruett SW. Magnetic resonance imaging of superficial cartilage lesions: Role of contrast in lesion detection. Journal of Magnetic Resonance Imaging. 1999;10(2):178–182. doi: 10.1002/(sici)1522-2586(199908)10:2<178::aid-jmri11>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 62.Murphy BJ. Evaluation of grades 3 and 4 chondromalacia of the knee using T2*-weighted 3D gradient-echo articular cartilage imaging. Skeletal Radiol. 2001;30(6):305–311. doi: 10.1007/s002560100330. [DOI] [PubMed] [Google Scholar]
- 63.Nakanishi K, Inoue M, Harada K, et al. Subluxation of the patella: evaluation of patellar articular cartilage with MR imaging. Br J Radiol. 1992;65(776):662–667. doi: 10.1259/0007-1285-65-776-662. [DOI] [PubMed] [Google Scholar]
- 64.Nikolaou VS, Chronopoulos E, Savvidou C, et al. MRI efficacy in diagnosing internal lesions of the knee: a retrospective analysis. J Trauma Manag Outcomes. 2008;2(1):4. doi: 10.1186/1752-2897-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nojiri T, Watanabe N, Namura T, et al. Utility of delayed gadolinium-enhanced MRI (dGEMRIC) for qualitative evaluation of articular cartilage of patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):718–723. doi: 10.1007/s00167-005-0013-6. [DOI] [PubMed] [Google Scholar]
- 66.Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505–513. doi: 10.1177/036354658901700410. [DOI] [PubMed] [Google Scholar]
- 67.Oakley SP, Lassere MN. A critical appraisal of quantitative arthroscopy as an outcome measure in osteoarthritis of the knee. Semin Arthritis Rheum. 2003;33(2):83–105. doi: 10.1016/s0049-0172(03)00082-9. [DOI] [PubMed] [Google Scholar]
- 68.Oakley SP, Portek I, Szomor Z, et al. Arthroscopy -A potential “gold standard” for the diagnosis of the chondropathy of early osteoarthritis. Osteoarthritis and Cartilage. 2005;13(5):368–378. doi: 10.1016/j.joca.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Ochi M, Sumen Y, Kanda T, Ikuta Y, Itoh K. The diagnostic value and limitation of magnetic resonance imaging on chondral lesions in the knee joint. Arthroscopy. 1994;10(2):176–183. doi: 10.1016/s0749-8063(05)80090-8. [DOI] [PubMed] [Google Scholar]
- 70.Outerbridge RE, Dunlop JA. The problem of chondromalacia patellae. Clin Orthop Relat Res. 1975;(110):177–196. doi: 10.1097/00003086-197507000-00024. [DOI] [PubMed] [Google Scholar]
- 71.Portney LG, Watkins MP. Foundations of clinical research : applications to practice. 3. Upper Saddle River, N.J: Pearson/Prentice Hall; 2009. [Google Scholar]
- 72.Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80(9):1276–1284. doi: 10.2106/00004623-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Recht MP, Piraino DW, Paletta GA, Schils JP, Belhobek GH. Accuracy of fat-suppressed three-dimensional spoiled gradient-echo FLASH MR imaging in the detection of patellofemoral articular cartilage abnormalities. Radiology. 1996;198(1):209–212. doi: 10.1148/radiology.198.1.8539380. [DOI] [PubMed] [Google Scholar]
- 74.Reiser MF, Bongartz G, Erlemann R, et al. Magnetic resonance in cartilaginous lesions of the knee joint with three-dimensional gradient-echo imaging. Skeletal Radiol. 1988;17(7):465–471. doi: 10.1007/BF00364038. [DOI] [PubMed] [Google Scholar]
- 75.Rose PM, Demlow TA, Szumowski J, Quinn SF. Chondromalacia patellae: fat-suppressed MR imaging. Radiology. 1994;193(2):437–440. doi: 10.1148/radiology.193.2.7972759. [DOI] [PubMed] [Google Scholar]
- 76.Rubenstein JD, Kim JK, Henkelman RM. Effects of compression and recovery on bovine articular cartilage: Appearance on MR images. Radiology. 1996;201(3):843–850. doi: 10.1148/radiology.201.3.8939241. [DOI] [PubMed] [Google Scholar]
- 77.Rubenstein JD, Li JG, Majumdar S, Markhsnkelman R. Image resolution and signal-to-noise ratio requirements for MR imaging of degenerative cartilage. American Journal of Roentgenology. 1997;169(4):1089–1096. doi: 10.2214/ajr.169.4.9308470. [DOI] [PubMed] [Google Scholar]
- 78.Schmid MR, Pfirrmann CWA, Koch P, Zanetti M, Kuehn B, Hodler J. Imaging of patellar cartilage with a 2D multiple-echo data image combination sequence. American Journal of Roentgenology. 2005;184(6):1744–1748. doi: 10.2214/ajr.184.6.01841744. [DOI] [PubMed] [Google Scholar]
- 79.Shahriaree H. Chondromalacia. Contemporary Orthopaedics. 1985;11:27–39. [Google Scholar]
- 80.Sonin AH, Pensy RA, Mulligan ME, Hatem S. Grading articular cartilage of the knee using fast spin-echo proton density-weighted MR imaging without fat suppression. AJR Am J Roentgenol. 2002;179(5):1159–1166. doi: 10.2214/ajr.179.5.1791159. [DOI] [PubMed] [Google Scholar]
- 81.Speer KP, Spritzer CE, Goldner JL, Garrett WE., Jr Magnetic resonance imaging of traumatic knee articular cartilage injuries. Am J Sports Med. 1991;19(4):396–402. doi: 10.1177/036354659101900414. [DOI] [PubMed] [Google Scholar]
- 82.Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? A prospective study of 58 patients. J Bone Joint Surg Br. 1993;75(1):49–52. doi: 10.1302/0301-620X.75B1.8421033. [DOI] [PubMed] [Google Scholar]
- 83.Suh JS, Cho JH, Shin KH, Kim SJ. Chondromalacia of the knee: evaluation with a fat-suppression three-dimensional SPGR imaging after intravenous contrast injection. J Magn Reson Imaging. 1996;6(6):884–888. doi: 10.1002/jmri.1880060608. [DOI] [PubMed] [Google Scholar]
- 84.Vallotton JA, Meuli RA, Leyvraz PF, Landry M. Comparison between magnetic resonance imaging and arthroscopy in the diagnosis of patellar cartilage lesions: a prospective study. Knee Surg Sports Traumatol Arthrosc. 1995;3(3):157–162. doi: 10.1007/BF01565475. [DOI] [PubMed] [Google Scholar]
- 85.von Engelhardt LV, Kraft CN, Pennekamp PH, Schild HH, Schmitz A, von Falkenhausen M. The evaluation of articular cartilage lesions of the knee with a 3-Tesla magnet. Arthroscopy. 2007;23(5):496–502. doi: 10.1016/j.arthro.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 86.Von Engelhardt LV, Lahner M, Klussmann A, et al. Arthroscopy vs. MRI for a detailed assessment of cartilage disease in osteoarthritis: Diagnostic value of MRI in clinical practice. BMC Musculoskeletal Disorders. 2010:11. doi: 10.1186/1471-2474-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang YX. In vivo magnetic resonance imaging of animal models of knee osteoarthritis. Lab Anim. 2008;42(3):246–264. doi: 10.1258/la.2007.06041e. [DOI] [PubMed] [Google Scholar]
- 88.Welsch GH, Mamisch TC, Domayer SE, et al. Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures--initial experience. Radiology. 2008;247(1):154–161. doi: 10.1148/radiol.2471070688. [DOI] [PubMed] [Google Scholar]
- 89.Williams A, Sharma L, McKenzie CA, Prasad PV, Burstein D. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage in knee osteoarthritis: findings at different radiographic stages of disease and relationship to malalignment. Arthritis Rheum. 2005;52(11):3528–3535. doi: 10.1002/art.21388. [DOI] [PubMed] [Google Scholar]
- 90.Wong S, Steinbach L, Zhao J, Stehling C, Ma CB, Link TM. Comparative study of imaging at 3.0 T versus 1.5 T of the knee. Skeletal Radiology. 2009;38(8):761–769. doi: 10.1007/s00256-009-0683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wright RW, Boyce RH, Michener T, Shyr Y, McCarty EC, Spindler KP. Radiographs are not useful in detecting arthroscopically confirmed mild chondral damage. Clinical Orthopaedics and Related Research. 2006;(442):245–251. doi: 10.1097/01.blo.0000167670.03197.c2. [DOI] [PubMed] [Google Scholar]
- 92.Wright RW, Brand RA, Dunn W, Spindler KP. How to write a systematic review. Clin Orthop Relat Res. 2007;455:23–29. doi: 10.1097/BLO.0b013e31802c9098. [DOI] [PubMed] [Google Scholar]
- 93.Yoshioka H, Stevens K, Hargreaves BA, et al. Magnetic resonance imaging of articular cartilage of the knee: comparison between fat-suppressed three-dimensional SPGR imaging, fat-suppressed FSE imaging, and fat-suppressed three-dimensional DEFT imaging, and correlation with arthroscopy. J Magn Reson Imaging. 2004;20(5):857–864. doi: 10.1002/jmri.20193. [DOI] [PubMed] [Google Scholar]
- 94.Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69(3):483–489. doi: 10.1136/ard.2009.113100. [DOI] [PubMed] [Google Scholar]


