Abstract
Increasing numbers of adults with congenital heart disease are referred for cardiac magnetic resonance imaging. Knowledge of the congenital heart anatomy, prior surgical interventions, and the development of an imaging focus for each individual patient plays a crucial role when performing a successful cardiac magnetic resonance imaging examination. The following manuscript focuses on cardiac magnetic resonance imaging considerations of three specific conotruncal congenital heart lesions: tetralogy of Fallot, transposition of the great arteries (TGA), and physiologically corrected TGA (c-TGA).
Keywords: adult congenital heart disease, cardiac magnetic resonance imaging, cardiac imaging, tetralogy of Fallot, transposition of the great arteries, conotruncal congenital heart lesions

Introduction
There are growing numbers of adults with congenital heart disease (CHD), and the role of cardiac magnetic resonance (CMR) imaging is continually expanding in this patient population.1 The majority of these patients have undergone surgical repairs in childhood, and lifelong follow-up is recommended.2 Serial imaging of adults with CHD is important to monitor for interval changes, as many adults with CHD do not recognize subtle changes in exercise capacity.3 CMR is a particularly attractive imaging technique due to its excellent tissue border delineation, tissue characterization, and quantification of ventricular volumes and valvular regurgitation that allows for serial comparisons without the need for ionizing radiation.
The 2008 American College of Cardiology/American Heart Association Guidelines for the Management of Adults with Congenital Heart Disease (ACHD) recommends CMR imaging for a variety of CHD patients.4 This review will focus on three specific conotruncal congenital heart lesions, including tetralogy of Fallot (TOF), transposition of the great arteries (TGA), and physiologically corrected TGA (c-TGA). For each diagnosis, we will develop an imaging focus of important findings to consider and suggest potential imaging protocols; we also recognize that a key feature of CHD is anatomic variation, and individualization of protocols is often required. Many of these adults will undergo CMR imaging at regular intervals, and knowledge of the patient’s anatomy, surgical interventions, and prior imaging findings is critical to focus the protocol so that the essential information is obtained within a reasonable amount of time. The majority of these protocols should be completed within an hour of scanning time.
Tetralogy of Fallot
One of the most common ACHD referrals for CMR is the patient with repaired tetralogy of Fallot (TOF). TOF represents the most common form of cyanotic congenital heart disease, affecting up to 0.5 per 1,000 live births.5 Although survival following TOF repair is excellent, there is a three-fold increase in mortality in the third postoperative decade of life,6 and 14% of patients develop markedly impaired functional status late after surgical repair.6, 7 This congenital anomaly results from the anterior deviation of the conal septum, resulting in a ventricular septal defect (VSD), varying degrees of right ventricular outflow tract obstruction (RVOTO), an overriding aorta, and right ventricular hypertrophy. Importantly, the degree of RVOTO can range from only mild subpulmonary stenosis to the most severe form involving complete absence of the main pulmonary artery (TOF with pulmonary atresia).
Presently, the majority of patients undergo surgical repair in infancy or childhood, although older adults may have first undergone a palliative shunt (Blalock-Taussig, Waterston, or Potts shunt) and then returned for a complete repair at a later date. Strategies to repair TOF have evolved over time. Whereas in the early experience a transannular or right ventricular patch was performed to eliminate the outflow tract obstruction, current strategies have been modified to help preserve the integrity of the pulmonary valve. Patients with TOF/pulmonary atresia and those with anomalous left coronary artery from the right sinus may undergo a right ventricular-to-pulmonary artery (RV-PA) conduit. A detailed knowledge of the patient’s surgical history is critical as it will determine the specific CMR protocol to employ that will focus on potential residual lesions. The following list provides an imaging focus for adults with repaired TOF:
Right Ventricular Outflow Tract
A goal of TOF surgery is to relieve the RVOT obstruction, yet many patients are left with varying degrees of obstruction that may be located in the subpulmonary area, at the level of the pulmonary valve, or more distally in the main or branch pulmonary arteries (PA). Visualization of the entire RVOT is important using specific RVOT long-axis (Figure 1A) and two-chamber RV cine views (Figure 1B). The RV two-chamber view allows visualization of the right atrium, tricuspid valve, right ventricle, and main pulmonary artery all in one plane. Regardless of the type of repair and whether it involves a transannular or right ventricular patch, RVOT regional wall motion abnormalities and aneurysms are common. RVOT aneurysms are not only associated with decreased right ventricular (RV) ejection fraction (EF) but are also associated with unfavorable ventricular interactions, resulting in a reduced left ventricular (LV) EF.8 Assessing for downstream stenosis in the branch PAs can be performed with either a steady-state free precession (SSFP) stack in an axial plane or a magnetic resonance angiogram (MRA).
Figure 1.
Steady-state free precession images of a patient with tetralogy of Fallot and a transannular patch demonstrating (A) a large right ventricular outflow tract aneurysm (*) and thrombus in the pulmonary artery (+) in the right ventricular outflow tract view, and (B) right ventricular two-chamber view. RA: right atrium; RV: right ventricle; LV: left ventricle; Ao: aorta; PA; pulmonary artery.

Ventricular Size and Function
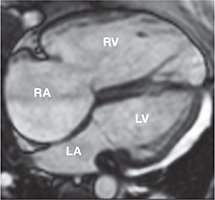
Accurate quantification of RV size and function is particularly important in repaired TOF patients, as longstanding pulmonary regurgitation (PR) contributes to RV dilation (Figure 2). RV dilatation and dysfunction are associated with adverse outcomes in this group.9 In adults with repaired CHD, echocardiographic windows are often limited by body habitus and surgical scar tissue. By contrast, CMR provides excellent image quality and is highly reproducible for quantifying RV size and function.10 LV dysfunction is present in >20% of adults with TOF repair, particularly those who were repaired later in life, had prior palliative shunts, and concomitant RV dysfunction.7, 11 LV dysfunction (LVEF <40%) has been associated with sudden cardiac death in this patient population.12
Figure 2.
Steady-state free precession four-chamber view of a patient with tetralogy of Fallot and severe pulmonary and tricuspid regurgitation with marked right atrial and right ventricular dilation. RA: right atrium; RV: right ventricle; LA: left atrium and LV: left ventricle.
Pulmonary Regurgitation
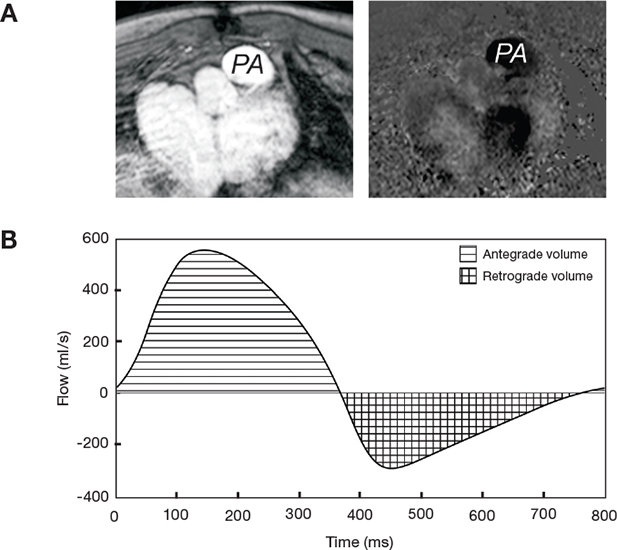
Pulmonary regurgitation (PR) is a common consequence of prior RVOT surgery and may be associated with RV dilation, predisposing to RV dysfunction, arrhythmias, and death. CMR phase contrast sequences assess antegrade and retrograde flow through the main pulmonary artery (Figure 3), and studies demonstrate this to be a highly reproducible technique for quantifying pulmonary regurgitation. In our laboratory, a PR fraction greater than 40% is considered severe.
Figure 3.
Pulmonary valve flow quantification in a patient with tetralogy of Fallot. (A) Magnitude and phase contrast images are obtained in the proximal main pulmonary artery oblique to the pulmonary valve. (B) Volume and direction of flow is determined, and the pulmonary regurgitation fraction can be calculated by dividing the retrograde volume (checks) by the antegrade volume (lines). This patient has severe pulmonary regurgitation with a regurgitation fraction of 47%. PA: pulmonary artery.
Pulmonary valve replacement (PVR) usually eliminates significant PR, however, optimal timing of PVR to prevent the adverse sequelae of RV dilation and dysfunction remains unclear.13 PVR usually results in dramatic decreases in RV volumes and improvement in functional status,14 but studies have demonstrated mixed results on whether PVR improves RV systolic function.15
Newer catheter-based pulmonary valves are promising developments in patients with CHD affecting the right heart. At the current time, most percutaneous valves are placed inside existing RV-PA conduits or dysfunctional bioprosthetic valves. However, a few patients may have small enough outflow tracts and high enough surgical risk that a percutaneous pulmonary valve could be considered in an off-label use. CMR can help determine the size of the outflow tract to identify if the patient may be a potential candidate. Delineation of the coronary artery course is essential prior to any RVOT intervention, as 5% to 10% of patients with TOF have an anomalous left coronary artery that may course across the RVOT, which could complicate possible interventions.
Tricuspid Regurgitation
There are several mechanisms that lead to tricuspid regurgitation (TR) in repaired TOF patients, including annular dilation and structural valve abnormalities.16 The TR fraction may be assessed at the time of CMR and should be considered in surgical plans and the time of PVR.17
Aortic Root Dilation
Patients with TOF have larger aortic roots compared to healthy controls, likely due to increased aortic flow prior to repair from shunting through the VSD and/or aorta pulmonary collateral flow that may cause volume loading of the LV. Some patients develop progressive dilation of the aortic root that can lead to significant aortic regurgitation.18
Myocardial Fibrosis
Late gadolinium enhancement (LGE) has predicted increased arrhythmic events in patients with multiple forms of acquired heart diseases.19, 20 LGE occurs commonly in myocardial locations of prior surgery (RVOT, VSD patch). Repaired TOF patients with greater degrees of LGE are at a higher risk of sustained symptomatic arrhythmia; however, it is unclear if LGE is associated with increased mortality in this patient population.21
With these components of the imaging focus in mind, here is a suggested imaging protocol for adults after TOF repair:
- ECG-gated cine steady-state free precession (SSFP)
- LV two- and four-chamber views
- Ventricle short-axis stack from the base to the apex for quantitative assessment of ventricular size, function, and mass
- RVOT outflow view parallel to the RVOT
- RV two-chamber view to assess RV and tricuspid valve
- Branch pulmonary artery axial stack to assess for pulmonary artery stenosis
- Gadolinium enhanced three-dimensional (3D) magnetic resonance angiogram (MRA)
ECG-gated phase contrast images perpendicular to the main pulmonary artery, aorta, and atrioventricular valves (and branch pulmonary arteries if there is concern about branch pulmonary artery stenosis resulting in unequal pulmonary blood flow)
LGE to assess for myocardial fibrosis
Coronary artery imaging with ECG and respiratory navigator 3D SSFP
Transposition of the Great Arteries
Transposition of the great arteries (TGA), also referred to as D-loop TGA and {S,D,D} TGA, is a congenital abnormality resulting from ventricular-arterial discordance. In TGA, the aorta connects to the RV, pumping deoxygenated blood systemically, and the pulmonary artery connects to the LV, pumping oxygenated blood back to the lungs. This abnormality is incompatible with life without mixing of blood between the two circulations through a septal defect or patent ductus arteriosus. The vast majority of children born with TGA undergo surgical correction to relieve cyanosis and the long-term sequelae depend on the type of prior surgical repair. Older adults with TGA most frequently have undergone an atrial switch procedure (Mustard or Senning operation), whereas the younger adult with TGA may have undergone an arterial switch operation (ASO) (Figure 4).
Figure 4.
Illustration of transposition of the great arteries repaired with (A) atrial switch and (B) arterial switch. RV: right ventricle; LV: left ventricle; Ao: aorta; PA: pulmonary artery

Atrial Switch
The first surgical repairs for TGA were pioneered by Mustard in 1958 and Senning in 1963. These atrial switch operations directed deoxygenated blood via baffles to the LV and out the pulmonary artery and directed oxygenated blood to the RV and out the aorta. These procedures relieved the cyanosis yet resulted in the RV ejecting to systemic pressure (systemic RV). Again, detailed knowledge of the patient’s surgical history is critical as it will determine the specific CMR protocol needed to focus on potential residual lesions. The following list provides an imaging focus for adults with TGA who have undergone an atrial switch procedure:
a. Atrial Baffle Obstruction and/or Leaks
Patients with these intra-atrial baffle repairs may develop baffle stenosis or leaks, and attention should be paid to optimal visualization of these baffles during CMR exams. SSFP cine images of the baffles can be obtained with a set of axial images of the atria to view the baffles in short axis, an oblique coronal view to visualize the superior vena cava and inferior vena cava baffles in long axis, and occasionally extra views are required to optimally visualize the pulmonary venous baffles. Baffle stenosis can often be directly visualized by cine imaging, and it most often occurs in the superior vena cava baffle.22 Indirect signs of baffle obstruction can also be detected by CMR, such as superior vena cava or azygous vein dilation.23
SSFP images of the short- and long-axis views of the atrial baffles can also demonstrate baffle leak through visualizing a deficiency in the baffle wall or flow dephasing between two chambers (Figure 5). The flow direction from a baffle leak in an atrial switch patient is usually left-to-right, similar to an atrial septal defect, such that blood from the pulmonary venous baffle (oxygenated blood) flows to the systemic venous baffle (deoxygenated blood). The amount of shunt can be quantified by comparing volume of flow by phase contrast imaging of the proximal pulmonary artery and ascending aorta and calculating a Qp:Qs ratio.24 A Qp:Qs >1.2 suggests significant left-to-right shunt, and a Qp:Qs <0.8 suggests right-to-left shunt that may occur in the setting of elevated pulmonary artery resistance and result in cyanosis.
Figure 5.
Steady-state free precession oblique sagittal image to optimize visualization of the inferior vena cava baffle in a patient with transposition of the great arteries with an atrial switch surgery. An inferior vena cava baffle to left atrium (pulmonary venous baffle) leak (*) is identified. Ao: aorta; RV: right ventricle; LA: left atrium (pulmonary venous baffle); IVC: inferior vena cava baffle.

b. Systemic Right Ventricular Size and Function
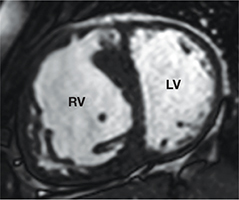
RV dysfunction is common following atrial switch, occurring in 8% to 48% of patients depending on imaging modality used and length of follow-up.25 The mechanisms for systemic RV dysfunction are incompletely understood but may include suboptimal myofiber arrangement,26 myocardial ischemia from supply-demand mismatch, and a less robust conduction system. CMR can quantify the amount of systemic RV hypertrophy and the systemic ventricular size and function (Figure 6).27
Figure 6.
Steady-state free precession basal short-axis image in a patient with transposition of the great arteries and an atrial switch procedure with systemic right ventricular hypertrophy, dilation, and pressure overload indicated by flattening of the intraventricular septum. RV: right ventricle; LV: left ventricle.
c. Tricuspid Regurgitation
Tricuspid regurgitation is common in patients with a systemic RV and often coexists with systemic RV failure. TR tends to progressively worsen with time.28 The structure of the tricuspid valve can be assessed with cine imaging, and the regurgitation fraction can be calculated using phase contrast imaging.
d. Left Ventricular Outflow Tract Obstruction
Patients with TGA atrial switch may have some dynamic LVOT obstruction, often due to systolic anterior motion (SAM) of the mitral valve. Since the PA originates from the LV, SAM results in dynamic subpulmonary LV obstruction. LV obstruction may actually lead to more favorable outcomes due to an increase in the subpulmonary LV pressure that results in rightward deviation of the ventricular septum. The rightward ventricular septal deviation may prevent excessive dilation of the systemic RV and allow for improved geometry of the ventricular septal position and more effective biventricular interaction.29
e. Myocardial Fibrosis
Several small single center studies have reported varying degrees of LGE in adults following atrial switch procedures and correlated greater degrees of LGE with ventricular dysfunction, electrophysiological parameters, and clinical events.30
With these components of the imaging focus in mind, here is a suggested imaging protocol for adults with TGA after atrial switch (Mustard or Senning) repair:
- ECG-gated cine SSFP
- Axial images from the transverse aortic arch to the diaphragm to provide dynamic imaging of the venous pathways, ventricles, atrioventricular (AV) valves and great arteries
- Superior and inferior vena cava pathway long-axis views with oblique coronal planes parallel to the vena cava baffles
- Ventricle short-axis stack from the base to the apex for quantitative assessment of ventricular size, function and mass
- LV and RV outflow tract views
Gadolinium enhanced 3D MRA
ECG-gated phase contrast sequences perpendicular to the main pulmonary artery, ascending aorta, AV valves
LGE to assess for myocardial fibrosis
Arterial Switch
By the early 1980s, the ASO largely replaced the atrial switch procedure. Most commonly, the ASO is performed by transecting the anterior-positioned aorta and the posterior-pulmonary artery above the sinuses, draping the pulmonary artery anteriorly over the aorta, and connecting the pulmonary artery to the aortic (neo-pulmonary) root. The aorta is connected to the pulmonary (neo-aortic) root, and the coronary arteries are reimplanted into the neoaortic root. Survival rates for patients with TGA after ASO are excellent; however, patients with associated VSD or aortic arch obstruction require reintervention more often.31 The following list provides an imaging focus for adults with TGA who have undergone an ASO:
a. Supravalvar Pulmonary Artery Stenosis
Stenosis of the main or branch PAs at either the anastomotic site or the location where the proximal PA branches are stretched over the aorta was common, particularly in the early surgical experience. CMR black blood imaging can detect most cases of PA stenosis following arterial switch,32 and more recently, SSFP axial images and MRA techniques (Figure 7) can be used to identify this complication.
Figure 7.
Three-dimensional volume-rendered magnetic resonance angiogram image of a patient with transposition of the great arteries and an arterial switch surgery. The surgery involves transecting the proximal aorta and pulmonary artery and draping the pulmonary artery over the aorta. PA: pulmonary artery; Ao: aorta.

b. Neo-Aortic Root Dilation
Nearly half of patients with TGA after ASO develop neo-aortic root dilation 10 years following an arterial switch procedure, defined as an aortic root dimension greater than or equal to 3 standard deviations above the mean. Approximately 10% of patients have moderate aortic regurgitation and 5% require aortic valve or aortic root surgery.33 SSFP images of the LVOT and short-axis images of the aortic root can be used to measure the aortic root dimensions, and aortic regurgitation can be quantified by phase contrast sequences of the ascending aorta.
c. Coronary Artery Abnormalities
Coronary artery complications can occur acutely or late after ASO34 since coronary artery reimplantation increases the risk for coronary stenosis due to ostial fibrosis at the suture line, intimal thickening, and mechanical kinking or stretching of the coronary arteries.35, 36 The coronary artery origins can be assessed with 3D isotropic resolution images that are gated for both cardiac cycle and respiration.37
d. Ventricular Size and Function
The majority of adults who have undergone ASO have normal biventricular size and function. However, special attention should be paid to any regional wall motion abnormality, which may indicate a coronary artery problem.
e. Myocardial Fibrosis
If coronary artery occlusion results in myocardial infarction, LGE in a coronary artery territory may represent irreversible myocardial damage.19 LGE assessed by CMR can differentiate myocardial infarct from other causes of systolic myocardial dysfunction.
With these components of the imaging focus in mind, here is a suggested imaging protocol for adults with TGA after an arterial switch operation (ASO):
- ECG-gated cine SSFP sequences
- LV two- and four-chamber views
- Ventricle short-axis stack from the base to the apex for quantitative assessment of ventricular size, function, and mass
- RV and LV outflow tract views
- RV two-chamber view
- Branch pulmonary artery axial stack to assess for pulmonary artery stenosis
- Aortic root short axis
Gadolinium enhanced 3D MRA
ECG-gated phase contrast sequences perpendicular to the main pulmonary artery, ascending aorta (and branch pulmonary arteries if there is concern of branch pulmonary stenosis resulting in unequal pulmonary blood flow)
LGE to assess for myocardial fibrosis
Coronary artery imaging with ECG and respiratory navigator 3D SSFP
Physiologically Corrected TGA
Physiologically corrected transposition of the great arteries (c-TGA), also referred to as congenitally corrected TGA, L-loop TGA, or {S,L,L} TGA, is a congenital abnormality that may not be diagnosed until later in life. Patients with c-TGA have atrioventricular discordance and ventricular arterial discordance such that deoxygenated blood passes thru a LV and out the PA, and oxygenated blood passes to a systemic RV and then is pumped out the aorta; therefore, these patients are not cyanotic. They are at significant risk for systemic RV dysfunction similar to patients with TGA with an atrial switch procedure, and the current adult congenital heart disease guidelines recommend imaging every year or at least every other year to assess systemic RV function.4 Additionally, many patients with c-TGA have associated cardiac anomalies including VSD, pulmonary stenosis, Ebstein anomaly, or dysplastic tricuspid valves that may have required surgery in the past. Dextrocardia may be present in up to 20% of patients with c-TGA. The following list provides an imaging focus for adults with c-TGA:
Right Ventricular Size and Function
Adults with c-TGA have dilated, hypertrophied, systemic right ventricles (Figure 8). Similar to patients with atrial switch procedures for TGA, the prevalence of systemic RV dysfunction varies based on associated anomalies. In one large multicenter study of adults with c-TGA, systemic RV dysfunction and heart failure were higher with increasing age, the presence of significant associated cardiac lesions, a history of arrhythmia, pacemaker implantation, and prior cardiac surgery.38
Figure 8.
(A) Illustration of physiologically corrected transposition of the great arteries. (B) Steady-state free precession four-chamber image of a patient with physiologically corrected transposition of the great arteries with a dilated, hypertrophied, systemic right ventricle. RA: right atrium; RV: right ventricle; LA: left atrium; LV: left ventricle.

Tricuspid Valve Regurgitation
Malformations of the morphological tricuspid valve (systemic atrioventricular valve) are common, including Ebstein anomaly. However, the valve appears distinctly different from classic Ebstein anomaly as it does not exhibit the large, sail-like anterior leaflet and little, if any, atrialized portion of the RV. Progressive TR begets more dilation of the systemic RV, which in turn contributes to more regurgitation.39
Left Ventricular Outflow Tract Obstruction
In c-TGA, the incidence of LVOTO ranges from 35% to 50%. Pulmonary stenosis can be valvar and/or subvalvar due to accessory AV valve tissue or a fibrous ridge.
Myocardial Fibrosis
The significance of myocardial fibrosis in patients with c-TGA has not been thoroughly investigated, as the several small studies that have been reported often include both TGA atrial switch patients and c-TGA patients. However, the presence of LGE in patients with a systemic RV is associated with RV dysfunction, poor exercise tolerance, arrhythmia, and progressive clinical deterioration.40
With these components of the imaging focus in mind, here is a suggested imaging protocol for adults with c-TGA:
- ECG-gated cine SSFP sequences
- LV two- and four-chamber views
- Ventricle short-axis stack from the base to the apex for quantitative assessment of ventricular size, function, and mass
- RV and LV outflow tract views
- RV two-chamber view to assess tricuspid valve
Gadolinium enhanced 3D MRA
ECG-gated phase contrast sequences perpendicular to the main PA, ascending aorta, AV valves
LGE enhancement to assess for myocardial fibrosis
Summary
In conclusion, an increasing number of adults with CHD will undergo CMR imaging in the future. Knowledge of the congenital heart anatomy, prior surgical interventions, and development of an imaging focus for each individual patient is crucial to perform a successful CMR examination. The information provided by the CMR may identify factors contributing to current symptomatology and provide some prognostic information regarding future risk for adverse outcomes in this unique set of patients.
Funding Statement
Funding/Support: The authors have no funding disclosures.
Footnotes
Conflict of Interest Disclosure: All authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Contributor Information
Sara L. Partington, Boston Children’s Hospital, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts
Anne Marie Valente, Boston Children’s Hospital, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts
References
- 1.Kilner PJ, Geva T, Kaemmerer H, Trindade PT, Schwitter J, Webb GD. Recommendations for cardiovascular magnetic resonance in adults with congenital heart disease from the respective working groups of the European Society of Cardiology. Eur Heart J. 2010 Apr;31(7):794–805. doi: 10.1093/eurheartj/ehp586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackie AS, Ionescu-Ittu R, Therrien J, Pilote L, Abrahamowicz M, Marelli AJ. Children and adults with congenital heart disease lost to follow-up: who and when? Circulation. 2009 Jul 28;120(4):302–9. doi: 10.1161/CIRCULATIONAHA.108.839464. [DOI] [PubMed] [Google Scholar]
- 3.Diller GP, Dimopoulos K, Okonko D, Li W, Babu-Narayan SV, Broberg CS, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005 Aug 9;112(6):828–35. doi: 10.1161/CIRCULATIONAHA.104.529800. [DOI] [PubMed] [Google Scholar]
- 4.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease). Circulation. 2008 Dec 2;118(23):2395–451. doi: 10.1161/CIRCULATIONAHA.108.190811. [DOI] [PubMed] [Google Scholar]
- 5.Murphy JG, Gersh BJ, Mair DD, Fuster V, McGoon MD, Ilstrup DM, et al. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med. 1993 Aug 26;329(9):593–9. doi: 10.1056/NEJM199308263290901. [DOI] [PubMed] [Google Scholar]
- 6.Nollert G, Fischlein T, Bouterwek S, Böhmer C, Dewald O, Kreuzer E, et al. Long-term results of total repair of tetralogy of Fallot in adulthood: 35 years follow-up in 104 patients corrected at the age of 18 or older. Thorac Cardiovasc Surg. 1997 Aug;45(4):178–81. doi: 10.1055/s-2007-1013719. [DOI] [PubMed] [Google Scholar]
- 7.Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004 Mar 17;43(6):1068–74. doi: 10.1016/j.jacc.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 8.Davlouros PA, Kilner PJ, Hornung TS, Li W, Francis JM, Moon JC, et al. Right ventricular function in adults with repaired tetralogy of Fallot assessed with cardiovascular magnetic resonance imaging: detrimental role of right ventricular outflow aneurysms or akinesia and adverse right-to-left ventricular interaction. J Am Coll Cardiol. 2002 Dec 4;40(11):2044–52. doi: 10.1016/s0735-1097(02)02566-4. [DOI] [PubMed] [Google Scholar]
- 9.Knauth AL, Gauvreau K, Powell AJ, Landzberg MJ, Walsh EP, Lock JE, et al. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart. 2008 Feb;94(2):211–6. doi: 10.1136/hrt.2006.104745. [DOI] [PubMed] [Google Scholar]
- 10.Mooij CF, de Wit CJ, Graham DA, Powell AJ, Geva T. Reproducibility of MRI measurements of right ventricular size and function in patients with normal and dilated ventricles. J Magn Reson Imaging. 2008 Jul;28(1):67–73. doi: 10.1002/jmri.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broberg CS, Aboulhosn J, Mongeon FP, Kay J, Valente AM, Khairy P, et al. Prevalence of left ventricular systolic dysfunction in adults with repaired tetralogy of fallot. Am J Cardiol. 2011 Apr 15;107(8):1215–20. doi: 10.1016/j.amjcard.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Ghai A, Silversides C, Harris L, Webb GD, Siu SC, Therrien J. Left ventricular dysfunction is a risk factor for sudden cardiac death in adults late after repair of tetralogy of Fallot. J Am Coll Cardiol. 2002 Nov 6;40(9):1675–80. doi: 10.1016/s0735-1097(02)02344-6. [DOI] [PubMed] [Google Scholar]
- 13.Harrild DM, Berul CI, Cecchin F, Geva T, Gauvreau K, Pigula F, et al. Pulmonary valve replacement in tetralogy of Fallot: impact on survival and ventricular tachycardia. Circulation. 2009 Jan 27;119(3):445–51. doi: 10.1161/CIRCULATIONAHA.108.775221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vliegen HW, van Straten A, de Roos A, Roest AA, Schoof PH, Zwinderman AH, et al. Magnetic resonance imaging to assess the hemodynamic effects of pulmonary valve replacement in adults late after repair of tetralogy of fallot. Circulation. 2002 Sep 24;106(13):1703–7. doi: 10.1161/01.cir.0000030995.59403.f8. [DOI] [PubMed] [Google Scholar]
- 15.Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson. 2011 Jan 20;13:9. doi: 10.1186/1532-429X-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahle WT, Parks WJ, Fyfe DA, Sallee D. Tricuspid regurgitation in patients with repaired Tetralogy of Fallot and its relation to right ventricular dilatation. Am J Cardiol. 2003 Sep 1;92(5):643–5. doi: 10.1016/s0002-9149(03)00746-x. [DOI] [PubMed] [Google Scholar]
- 17.Srichai MB, Lim RP, Wong S, Lee VS. Cardiovascular applications of phase-contrast MRI. AJR Am J Roentgenol. 2009 Mar;192(3):662–75. doi: 10.2214/AJR.07.3744. [DOI] [PubMed] [Google Scholar]
- 18.Niwa K, Siu SC, Webb GD, Gatzoulis MA. Progressive aortic root dilatation in adults late after repair of tetralogy of Fallot. Circulation. 2002 Sep 10;106(11):1374–8. doi: 10.1161/01.cir.0000028462.88597.ad. [DOI] [PubMed] [Google Scholar]
- 19.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999 Nov 9;100(19):1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 20.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006 Nov 21;48(10):1977–85. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 21.Babu-Narayan SV, Kilner PJ, Li W, Moon JC, Goktekin O, Davlouros PA, et al. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006 Jan 24;113(3):405–13. doi: 10.1161/CIRCULATIONAHA.105.548727. [DOI] [PubMed] [Google Scholar]
- 22.Chung KJ, Simpson IA, Glass RF, Sahn DJ, Hesselink JR. Cine magnetic resonance imaging after surgical repair in patient with transposition of the great arteries. Circulation. 1988 Jan;77(1):104–9. doi: 10.1161/01.cir.77.1.104. [DOI] [PubMed] [Google Scholar]
- 23.Campbell RM, Moreau GA, Johns JA, Burger JD, Mazer M, Graham TP, Jr, et al. Detection of caval obstruction by magnetic resonance imaging after intraatrial repair of transposition of the great arteries. Am J Cardiol. 1987 Sep 15;60(8):688–91. doi: 10.1016/0002-9149(87)90383-3. [DOI] [PubMed] [Google Scholar]
- 24.Powell AJ, Tsai-Goodman B, Prakash A, Greil GF, Geva T. Comparison between phase-velocity cine magnetic resonance imaging and invasive oximetry for quantification of atrial shunts. Am J Cardiol. 2003 Jun 15;91(12):1523–5 A9. doi: 10.1016/s0002-9149(03)00417-x. [DOI] [PubMed] [Google Scholar]
- 25.Kirjavainen M, Happonen JM, Louhimo I. Late results of Senning operation. J Thorac Cardiovasc Surg. 1999 Mar;117(3):488–95. doi: 10.1016/s0022-5223(99)70329-6. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen E, Smerup M, Agger P, Frandsen J, Ringgard S, Pedersen M, et al. Normal right ventricular architecture as assessed with diffusion tensor magnetic resonance imaging is preserved during experimentally induced right ventricular hypertrophy. Anat Rec (Hoboken). 2009 May;292(5):640–51. doi: 10.1002/ar.20873. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz CH, Walker ES, Graham TP, Jr, Powers TA. Right ventricular performance and mass by use of cine MRI late after atrial repair of transposition of the great arteries. Circulation. 1995 Nov 1;92(9 Suppl):II233–9. doi: 10.1161/01.cir.92.9.233. [DOI] [PubMed] [Google Scholar]
- 28.Warnes CA. Transposition of the great arteries. Circulation. 2006 Dec 12;114(24):2699–709. doi: 10.1161/CIRCULATIONAHA.105.592352. [DOI] [PubMed] [Google Scholar]
- 29.Winlaw DS, McGuirk SP, Balmer C, Langley SM, Griselli M, Stümper O, et al. Intention-to-treat analysis of pulmonary artery banding in conditions with a morphological right ventricle in the systemic circulation with a view to anatomic biventricular repair. Circulation. 2005 Feb 1;111(4):405–11. doi: 10.1161/01.CIR.0000153355.92687.FA. [DOI] [PubMed] [Google Scholar]
- 30.Babu-Narayan SV, Goktekin O, Moon JC, Broberg CS, Pantely GA, Pennell DJ, et al. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation. 2005 Apr 26;111(16):2091–8. doi: 10.1161/01.CIR.0000162463.61626.3B. [DOI] [PubMed] [Google Scholar]
- 31.Fricke TA, d’Udekem Y, Richardson M, Thuys C, Dronavalli M, Ramsay JM, et al. Outcomes of the arterial switch operation for transposition of the great arteries: 25 years of experience. Ann Thorac Surg. 2012 Jul;94(1):139–45. doi: 10.1016/j.athoracsur.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Beek FJ, Beekman RP, Dillon EH, Mali WP, Meiners LC, Kramer PP, et al. MRI of the pulmonary artery after arterial switch operation for transposition of the great arteries. Pediatr Radiol. 1993;23(5):335–40. doi: 10.1007/BF02011950. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz ML, Gauvreau K, del Nido P, Mayer JE, Colan SD. Long-term predictors of aortic root dilation and aortic regurgitation after arterial switch operation. Circulation. 2004 Sep 14;110(11 Suppl 1):II128–32. doi: 10.1161/01.CIR.0000138392.68841.d3. [DOI] [PubMed] [Google Scholar]
- 34.Legendre A, Losay J, Touchot-Koné A, Serraf A, Belli E, Piot JD, et al. Coronary events after arterial switch operation for transposition of the great arteries. Circulation. 2003 Sep 9;108 Suppl 1:II186–90. doi: 10.1161/01.cir.0000087902.67220.2b. [DOI] [PubMed] [Google Scholar]
- 35.Bonnet D, Bonhoeffer P, Piéchaud JF, Aggoun Y, Sidi D, Planché C, et al. Long-term fate of the coronary arteries after the arterial switch operation in newborns with transposition of the great arteries. Heart. 1996 Sep;76(3):274–9. doi: 10.1136/hrt.76.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanel RE, Wernovsky G, Landzberg MJ, Perry SB, Burke RP. Coronary artery abnormalities detected at cardiac catheterization following the arterial switch operation for transposition of the great arteries. Am J Cardiol. 1995 Jul 15;76(3):153–7. doi: 10.1016/s0002-9149(99)80048-4. [DOI] [PubMed] [Google Scholar]
- 37.Botnar RM, Stuber M, Kissinger KV, Manning WJ. Free-breathing 3D coronary MRA: the impact of “isotropic” image resolution. J Magn Reson Imaging. 2000 Apr;11(4):389–93. doi: 10.1002/(sici)1522-2586(200004)11:4<389::aid-jmri6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 38.Graham TP, Jr, Bernard YD, Mellen BG, Celermajer D, Baumgartner H, Cetta F, et al. Long-term outcome in congenitally corrected transposition of the great arteries: a multi-institutional study. J Am Coll Cardiol. 2000 Jul;36(1):255–61. doi: 10.1016/s0735-1097(00)00682-3. [DOI] [PubMed] [Google Scholar]
- 39.Mongeon FP, Connolly HM, Dearani JA, Li Z, Warnes CA. Congenitally corrected transposition of the great arteries ventricular function at the time of systemic atrioventricular valve replacement predicts long-term ventricular function. J Am Coll Cardiol. 2011 May 17;57(20):2008–17. doi: 10.1016/j.jacc.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 40.Giardini A, Lovato L, Donti A, Formigari R, Oppido G, Gargiulo G, et al. Relation between right ventricular structural alterations and markers of adverse clinical outcome in adults with systemic right ventricle and either congenital complete (after Senning operation) or congenitally corrected transposition of the great arteries. Am J Cardiol. 2006 Nov 1;98(9):1277–82. doi: 10.1016/j.amjcard.2006.05.062. [DOI] [PubMed] [Google Scholar]


