Abstract
Myocardial infarction (MI) is a major cause of mortality and morbidity worldwide. Each year, an estimated 785,000 persons will have a new MI in the United States alone, and approximately every minute an American will succumb to one.1 In addition, MI has major psychological and legal implications for patients and the society and is an important outcome measure in research studies. The prevalence of MI provides useful data regarding the burden of coronary artery disease and offers insight into health care planning, policy, and resource allocation. The importance of accurately and reproducibly defining MI is therefore self-evident. The Third Universal Definition of Myocardial Infarction (MI) expert consensus document was published in October 2012 by the global Myocardial Infarction Task Force.2 This landmark document was cosponsored by multiple cardiovascular societies and included both updated definitions and a modified classification of MI that have important clinical, epidemiological, and research implications. We hereby present a critical overview of this important document and summarize its key recommendations.
Keywords: acute coronary syndrome, myocardial infarction, revascularization, Third Universal Definition of Myocardial Infarction, cardiac troponin biomarkers

Background
Since the 1971 publication of the first standardized definition of MI by the World Health Organization (WHO), there was a persistent need for a better definition of MI for diagnostic, epidemiological, and research purposes. At that time, the WHO definition did not include biomarkers of cardiac necrosis because of their lack of specificity and reproducibility, and its definition was therefore open to biased interpretation. The highly specific and sensitive cardiac troponin biomarkers of myonecrosis were subsequently introduced in the late 1980s and early 1990s and became the backbone of the first universal definition of MI and its subsequent update.3, 4 These universal definitions of MI were published in 2000 and 2007, and they included more standardized and reproducible definitions and a new classification of MI.3 The first global MI task force classified any degree of myocardial necrosis in the setting of myocardial ischemia as MI and provided qualifications to characterize the MI (size, trigger, timing, etc).3 The second global MI task force updated the first MI definition and included a new five-category classification.4 Significant developments in the diagnosis of cardiac necrosis (i.e., high-sensitivity assays) and revised definitions of myocardial necrosis, particularly in the settings of critical illnesses and post-revascularization, resulted in the publication of the Third Universal Definition of Myocardial Infarction.2 Last December, the American College of Cardiology Foundation5 published the 2012 expert consensus document on the practical clinical considerations in the interpretation of troponin elevations.5
The Third Universal Definition of Myocardial Infarction
The detection of a rise and/or fall of cardiac biomarkers, with at least one of the values being elevated (>99th percentile upper reference limit, or URL), is central to the third universal definition of MI.2 The highly sensitive and specific cardiac troponin (cTn) is the preferred biomarker of myocardial necrosis. In addition, one of the five following predefined criteria should be satisfied before a diagnosis of MI is made: (1) symptoms of myocardial ischemia; (2) new (or presumably new) significant ST-segment/T-wave changes or left bundle branch block; (3) development of pathological Q waves on ECG; (4) new loss of viable myocardium or regional wall motion abnormality by imaging; (5) identification of intracoronary thrombus by angiography or autopsy.
The third global MI task force maintains that the electrocardiogram (ECG) is an integral part of the diagnostic work-up in patients with suspected MI and should be obtained and interpreted in a timely manner.2 It also advocates the use of serial recordings to detect dynamic ECG changes, and it adopts ECG criteria similar to the 2007 expert consensus document for the diagnosis of acute myocardial injury/ischemia and prior MI (criteria pertaining to the ST-segment shift and Q waves/QS complexes, respectively).2 Additionally, the third global MI task force summarizes the ECG abnormalities that mimic myocardial ischemia or MI (e.g., left bundle branch block, pre-excitation). It also includes brief discussions on the utility of various imaging modalities and highlights their improved capabilities in assessing myocardial thickness, wall motion, perfusion, and fibrosis.2
This task force updated the universal classification of MI with a few notable modifications (Table 1).2 Type 1 MI is spontaneous MI induced by plaque disruption (e.g., rupture, erosion, fissuring) with overlying coronary thrombosis. Type 2 MI is induced by an imbalance in myocardial oxygen demand and supply (severe anemia, thyrotoxicosis, hypertension urgency, etc). An MI resulting in cardiac death is classified as type 3 (in the absence of available biomarker data), while types 4 and 5 are PCI- and CABG-related MI, respectively.
Table 1.
MI classification from the Third Universal Definition of Myocardial Infarction.
| The Third Universal Definition of Myocardial Infarction | |
| Type 1: | Spontaneous myocardial infarction |
| Type 2: | Myocardial infarction secondary to an ischemic imbalance |
| Type 3: | Myocardial infarction resulting in death when biomarker values are unavailable |
| Type 4a: | Myocardial infarction related to percutaneous coronary intervention (PCI) |
| Type 4b: | Myocardial infarction related to stent thrombosis |
| Type 4c: | Myocardial infarction related to restenosis |
| Type 5: | Myocardial infarction related to coronary artery bypass grafting (CABG) |
Adapted with modifications from Thygesen et al.2
Biomarkers of Cardiac Necrosis
Cardiac troponins are part of the myocyte contractile apparatus and are the cardiac necrosis biomarkers of choice for diagnosing MI. Myocardial necrosis results in myocyte membrane damage and the release of myocyte-specific proteins into the circulation. Cardiac-specific isoforms of cTnI and cTnT can be measured with great accuracy using commercially available assays that employ monoclonal antibodies specific to epitopes of these isoforms. These assays provide superior discrimination of myocardial injury when creatine kinase MB (CK-MB) levels are normal or minimally increased; they also impart additional prognostic information in patients who have elevated troponin levels despite normal CK-MB levels. In these patients, elevated cTn levels are associated with a higher risk of recurrent cardiac events.6 Even the most cardio-specific CK-MB isoform constitutes 1–3% of the CK in skeletal muscle and is present in minor quantities in other organs (e.g., intestine, diaphragm, uterus, prostate). The specificity of CK-MB is therefore impaired in the setting of major injury to these organs.
Elevated levels of serum cTn indicate myocardial necrosis regardless of the underlying pathophysiology. The third global MI task force has delineated various conditions associated with myocardial necrosis2: (A) injury related to primary myocardial ischemia/infarction (plaque rupture/erosion/fissuring with superimposed thrombus formation); (B) injury related to supply-demand ischemia imbalance (vasospasm, tachyarrhythmias, severe anemia, hypotension); (C) injury unrelated to ischemia (myocarditis, cardiac contusion, cardiotoxic agents); (D) multifactorial or indeterminate injury (heart failure, renal failure, stress cardiomyopathy).
A cTn level >99th percentile of the URL is considered elevated and is the cut-off level for a diagnosis of MI. This threshold value is determined for each specific assay in each laboratory and should be characterized by optimal precision, described by a coefficient of variation (CV) ≤10%. Blood samples for measuring cTn levels should be drawn serially: on initial assessment and 3–6 hours later, when further ischemic episodes occur, or when the timing of the initial symptoms is unclear. To establish the diagnosis of MI, a rise and/or fall in values with at least one value above the decision level is required, coupled with a strong clinical suspicion.2
The third global task force reduced the emphasis on the use of other cardiac biomarkers. Use of cTn is preferred over CK-MB, and the latter is to be used only when cTn assays are not available.2 The use of older nonspecific biomarkers (including total CK, CK-MB activity, lactate dehydrogenase, aspartate aminotransferase, etc.) was not mentioned in this document. These markers are largely considered to be historical and should no longer be used alone to diagnose MI.2, 7
Troponin Elevations Related to Non-ACS Ischemic and Non-Ischemic Clinical Conditions
Many demand-mediated ischemic conditions unrelated to acute coronary syndrome (ACS) can result in cTn elevation (Figure 1). Although disruption of epicardial blood supply (e.g., emboli) can result in ischemic ECG changes and serial troponin changes similar to a spontaneous MI, the other causes of non-ACS ischemic troponin elevations may result in a more subtle increase, with less change evident on serial determinations (Figure 1). Nonischemic conditions may present with chest discomfort or other symptoms that create diagnostic uncertainty for the treating physician. Elevated cTn levels also have been detected in many entities unrelated to primary cardiac conditions. In some instances, the mechanism of cardiac involvement is obvious. Prolonged secondary subendocardial ischemia resulting from right ventricular pressure overload following a pulmonary embolus is one of many examples. In many instances, however, cTn release appears to represent a nonspecific response to systemic illness.
Figure 1.
Conceptual model for clinical distribution of elevated troponin. Adapted from Newby et al.6 ACS: acute coronary syndrome; AMI: acute myocardial infarction; CAD: coronary artery disease; CHF: congestive heart failure; CM: cardiomyopathy; CT: cardiothoracic; PCI: percutaneous coronary intervention; PE: pulmonary embolism; STEMI: ST-segment elevation myocardial infarction.
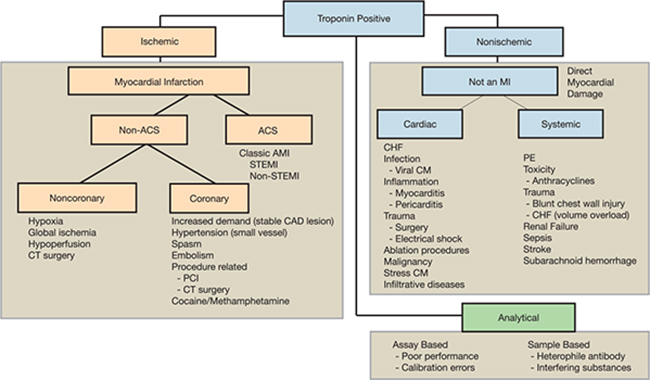
The 2012 task force emphasizes that measurable cTn levels are present in nearly all patients with heart failure, with a significant percentage of these patients having levels >99th percentile URL (especially in severe/acutely decompensated HF).2 The task force recognizes that type 1 MI may be an important cause of acutely decompensated HF, and that other pathophysiologic mechanisms may be implicated including MI type 2, apoptosis secondary to excessive wall stretch, and direct cellular toxicity (e.g., inflammation, circulating neurohormones). Irrespective of its associated etiology, the magnitude and persistence of cTn elevation in HF is an independent predictor of adverse outcomes in both acute and chronic HF patients.2
As troponin assays become more sensitive, there will be more conditions discovered that are associated with low-level troponin elevations.5 Even a small proportion of apparently healthy individuals will have elevated high-sensitivity troponin levels. Clinical judgment should be exercised as to the timing and extent of CAD evaluation after cTn elevation.
Myocardial Infarction Related to Revascularization
The use of higher cTn cutoff values in the third MI definition reflects the recognition that while very small amounts of myocardial injury are detected by highly sensitive biomarkers and imaging modalities, not all of them necessarily constitute revascularization-related MIs.
The third global MI task force has modified the cTn threshold levels for the diagnosis of PCI-related MI. A cTn level elevation >5 x 99th percentile URL within 48 hours of PCI (in patients with normal baseline values) is now used to classify Type 4a MI.2 Moreover, the 2012 task force has also specified an increase in cTn levels of >20% to characterize PCI-related MI in patients with elevated but stable or falling baseline levels. It has also been recognized that up to 6% of patients with stable CAD have elevated pre-PCI cTn levels.8 The new cTn cutoff was arbitrarily defined and should be associated with one of the following for an event to be labeled as a PCI-related MI: (A) symptoms of myocardial ischemia; (B) new ECG changes of ischemia; (C) new loss of viable myocardium or new regional wall motion abnormality detected by imaging; or (D) angiographic findings consistent with a procedural complication. Using the 2007 definition (>3 x 99th percentile URL), ≥15% of patients undergoing PCI would be defined as having a PCI-related MI.9 The adoption of higher biomarker thresholds and more stringent criteria for revascularization-related MI resulted in widespread implications in the interventional cardiology community, especially with the increased performance of complex and aggressive multivessel coronary interventions.2,10 MI associated with stent thrombosis remains an important subcategory (type 4b) in the current classification of MI.2 Restenosis after PCI and coronary stenting is an important shortcoming of percutaneous revascularization and may be associated with MI in up to 10% of patients.11, 12 The significance of in-stent restenosis is emphasized in the third universal definition of MI by the incorporation of PCI-related MI associated with restenosis (type 4c MI).2 The new type 4c MI is defined by a rise and/or fall of cTn values in patients with ≥50% stenosis at coronary angiography (or a complex lesion) in the absence of more obstructive CAD following initially successful PCI.2
MI related to coronary artery bypass surgery (CABG) was redefined by the 2012 task force using an arbitrarily defined cutoff of an elevation of cardiac biomarker >10 x 99th percentile URL during the first 48 hours following CABG. CABG-related MI should also satisfy one of the following additional diagnostic criteria: (1) new pathological Q waves or LBBB, or (2) angiographically documented new graft or native coronary artery occlusion, or (3) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality (except perhaps a paradoxical septal motion, which is a common finding after cardiac surgery). The 2012 global MI task force emphasized that the aforementioned threshold is more robust for isolated on-pump CABG. Cardiac biomarker release is, however, considerably higher after valve replacement with CABG than with CABG alone, and with on- versus off-pump CABG.2 According to the 2011 ACCF/AHA CABG guidelines, the measurement of myonecrosis biomarkers (e.g., CK-MB, troponin) is reasonable in the first 24 hours after CABG, and cTn is preferred to CK-MB as the optimal indicator of myonecrosis.13
Additional Considerations
The 2012 task force included new sections pertinent to myocardial injury and MI in patients undergoing cardiac and non-cardiac procedures, in critically-ill patients, and in patients with heart failure.2 These sections emphasized the risk of myocardial necrosis due to regional ischemia or direct trauma in certain cardiovascular procedures, including transcatheter aortic valve replacement (TAVR) or mitral clip. In the absence of supporting evidence, the task force recommended using the same criteria for an MI diagnosis in patients undergoing TAVR. Caution is advised against mislabeling myocardial necrosis associated with the ablation of arrhythmias as MI. In accordance with the 2008-2009 revision of the WHO definition of MI,14 the third global MI task force also differentiated between recurrent MI and reinfarction.2 Reinfarction describes an acute MI occurring within 28 days of an incident or recurrent MI. The 2012 task force did not recommend CK-MB measurements in these patients but, rather, serial cTn measurements, with the reinfarction diagnosis established when a ≥20% increase in cTn values is observed. If characteristics of MI occur after 28 days following an incident MI, it is considered to be a recurrent MI. The 2012 task force also recommends the routine monitoring of cardiac biomarkers in high-risk patients both prior to and 48–72 hours after major noncardiac surgery, but it does not define high-risk surgical procedures.2 In general, major vascular surgery (aortic/peripheral vascular surgery with reported perioperative cardiac risk >5%) is considered a high- risk or major surgery.15
Conclusion
In summary, the Third Universal Definition of Myocardial Infarction consensus document incorporates patient symptoms, ECG changes, the highly sensitive cTn biochemical markers, and information gleaned from various imaging techniques into comprehensive, clinically oriented, and reproducible definitions of MI.
Funding Statement
Funding/Support: Dr Bozkurt receives grant funding from the National Institutes of Health and from Forest Pharmaceuticals.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012 Jan 3;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third Universal Definition of Myocardial Infarction. Circulation. 2012 Oct 16;126(16):2020–35. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 3.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000 Sep;36(3):959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 4.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani, et al. Universal definition of myocardial infarction. Circulation. 2007 Nov 27;116(22):2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 5.Newby LK, Jesse RL, Babb JD, Christenson RH, De Fer TM, Diamond GA, et al. ACCF 2012 Expert Consensus Document on Practical Clinical Considerations in the Interpretation of Troponin Elevations. J Am Coll Cardiol. 2012 Dec 11;60(23):2427–63. doi: 10.1016/j.jacc.2012.08.969. [DOI] [PubMed] [Google Scholar]
- 6.Kontos MC, de Lemos JA, Ou FS, Wiviott SD, Foody JM, Newby LK, et al. Troponin-positive, MB-negative patients with non-ST-elevation myocardial infarction: An undertreated but high-risk patient group: Results from the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network-Get With The Guidelines (NCDR ACTION-GWTG) Registry. Am Heart J. 2010 Nov;160(5):819–25. doi: 10.1016/j.ahj.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007 Apr 3;115(13):e356–75. doi: 10.1161/CIRCULATIONAHA.107.182882. [DOI] [PubMed] [Google Scholar]
- 8.Jeremias A, Kleiman NS, Nassif D, Hsieh WH, Pencia M, Maresh K, et al. Prevalence and prognostic significance of preprocedural cardiac troponin elevation among patients with stable coronary artery disease undergoing percutaneous coronary intervention: results from the evaluation of drug eluting stents and ischemic events registry. Circulation. 2008 Aug 5;118(6):632–8. doi: 10.1161/CIRCULATIONAHA.107.752428. [DOI] [PubMed] [Google Scholar]
- 9.Alcock RF, Roy P, Adorini K, Lau GT, Kritharides L, Lowe E, et al. Incidence and determinants of myocardial infarction following percutaneous coronary interventions according to the revised Joint Task Force definition of troponin T elevation. Int J Cardiol. 2010 Apr 1;140(1):66–72. doi: 10.1016/j.ijcard.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K, Mair J, Katus H, Plebani M, Venge P, Collinson P, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 2010 Sep;31(18):2197–204. doi: 10.1093/eurheartj/ehq251. [DOI] [PubMed] [Google Scholar]
- 11.Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006 Jun;151(6):1260–4. doi: 10.1016/j.ahj.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Lee MS, Pessegueiro A, Zimmer R, Jurewitz D, Tobis J. Clinical presentation of patients with in-stent restenosis in the drug-eluting stent era. J Invasive Cardiol. 2008 Aug;20(8):401–3. [PubMed] [Google Scholar]
- 13.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011 Dec 6;124(23):e652–735. doi: 10.1161/CIR.0b013e31823c074e. [DOI] [PubMed] [Google Scholar]
- 14.Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mahonen M, Ngu Blackett K, et al. World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol. 2011 Feb;40(1):139–46. doi: 10.1093/ije/dyq165. [DOI] [PubMed] [Google Scholar]
- 15.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation. 2009 Nov 24;120(21):e169–276. doi: 10.1161/CIRCULATIONAHA.109.192690. [DOI] [PubMed] [Google Scholar]


