Abstract
The risk of incident hospitalized heart failure (HF) was evaluated for 23 electrocardiographic (ECG) variables in men and women free from cardiovascular disease. The hazard ratios with 95% confidence intervals were determined from Cox regression analysis for 13,428 participants 45 to 65 years old in the Atherosclerosis Risk in Communities (ARIC) study. New-onset HF during a 14-year follow-up period occurred in 695 men (11.9%) and 721 women (9.5%). Several ECG variables were significant predictors of incident HF when evaluated as single ECG variables. Predominant among them were spatial angles, reflecting deviations of the direction of the repolarization sequence from the normal reference direction. After controlling for collinearity among the ECG variables, the spatial angle between T peak and normal T reference vectors, Θ(Tp|Tref), was a significant independent predictor in men (HF risk increased 31%) and women (HF risk increased 46%). Other independent predictors in men included epicardial repolarization time (62% increased risk) and T wave peak to T wave end (TpTe) interval, reflecting global dispersion of repolarization (27% increased risk). The independent predictors in women, in addition to Θ(Tp|Tref), were Θ(R|STT) the spatial angle between the mean QRS and STT vectors (54% increased risk) and QRS nondipolar voltage (46% increased risk). In conclusion, wide Θ(Tp|Tref), wide Θ(R|STT), and increased QRS nondipolar voltage in women and wide Θ(Tp|Tref), increased epicardial repolarization time, prolonged TpTe interval and T wave complexity in men were independent predictors of incident HF, and the presence of these abnormal findings could warrant additional diagnostic evaluation for possible preventive action for HF.
Evaluation of the risk of adverse cardiac effects for QT prolongation has been the focal point of many clinical trials, particularly in the evaluation of arrhythmic events such as torsades de pointes as adverse effects of cardioactive agents.1 Of particular concern has been that 70% of torsades de pointes events occur in women.2 However, QT is known to have notable limitations.2–5 Earlier investigations have found several other electrocardiographic (ECG) variables to be valuable supplements to QT in the prediction of new-onset heart failure (HF), including a wide spatial angle between the mean QRS and T vectors [Θ(R|STT)], QRS nondipolar voltage, ST depression in V1 and increased T wave V1 amplitude.6,7 The main objective of the present study was to evaluate the risk of incident hospitalized HF for a comprehensive set of repolarization-related ECG parameters derived by a recently developed repolarization model8–10 and to evaluate gender differences in the predictors of HF.
Methods
The Atherosclerosis Risk in Communities (ARIC) study was designed as a prospective investigation of the cause and natural history of atherosclerosis, its clinical manifestations, and the community burden of coronary heart disease. The risk factors were measured and the outcomes evaluated in this population-based probability sample of adults aged 45 to 65 years at the 1987 to 1989 baseline examination; follow-up of the cohort is ongoing. The study population and definition of prevalent diseases at baseline and the outcomes have been previously described.11–14
The clinical outcomes were evaluated at the follow-up examinations through December 31, 2006. Deaths were classified as definite or possible coronary heart disease death, noncoronary heart disease death, and unclassified death. Coronary heart disease at baseline was classified as angina pectoris identified using the questionnaire from Rose et al.15 Myocardial infarction was defined by a self-reported episode requiring hospitalization for >1 week, myocardial infarct diagnosed by a physician, major Q waves at the baseline electrocardiogram (Minnesota Code 1.1),16 or previous coronary artery bypass grafting or coronary angioplasty. HF events were defined as a hospitalization discharge diagnosis code (“International Classification of Disease, Ninth Revision, Clinical Modification,” code 428). Prevalent (baseline) HF was determined on the basis of evidence of the use of HF-related medications and classified according to the Gothenburg criteria.17 Baseline cerebrovascular disease was defined as self-reported stroke or transient ischemic attack verified by a study physician's review of the reported symptoms.
The study group of 13,428 men and women was derived from a source file of 14,126 ARIC participants, excluding those with bundle branch block or Wolf-Parkinson-White patterns (QRS duration ≥120 ms), participants with cardiovascular disease at entry classified as coronary heart disease, stroke, or HF according to the criteria listed, and 193 participants with missing clinical data or ECG parameters from various ECG programs and special algorithms derived for the study. The mean follow-up period was 14 years.
Standardized procedures were used to record the 12-lead electrocardiograms using a MAC personal computer (Marquette Electronics, Milwaukee, Wisconsin) at each clinical center. The electrocardiograms were processed in a central ECG laboratory, initially using the Dalhousie ECG program.17,18 All electrocardiograms in the initial digital ECG file of 15,571 records were inspected visually. In addition, the ECG quality was graded using a computer algorithm. A total of 116 electrocardiograms was rejected because of poor quality or lead reversals, and electrocardiograms with a QRS duration of ≥ 120 ms were excluded. Occasional outliers in interval measurements by computer were corrected using an interactive computer graphics display system. All electrocardiograms were reprocessed using the GE Marquette 12-SL program (GE Marquette), and the global time points from that program were used to compute the derived ECG parameters used in the present study. However, the time point of the T wave end from the Dalhousie program was found to match more closely the visually verified T wave end, and the QT interval from Dalhousie program was used in the present study.
The schematic in Figure 1 illustrates the key variables of the repolarization model, defined in more explicit terms in Table 1. Because of the special importance of the QT peak interval (QTp) and QT end interval (QTe) for the parameters used in the repolarization model, special algorithms were used to detect the outlier measurements of QTp and QTe. Gender-specific predicted values were first computed for QTe as a linear function of the RR interval derived in cardiovascular disease-free men and women. QTe values above the 99th or below the 1st percentile limit of the distribution of the difference of each interval from the predicted value were replaced by the predicted value. The rate-adjusted QTe was computed using the formula also listed in Table 1. The rate-adjusted QTp was then derived as the difference of the rate-adjusted QTe-T wave peak (Tp)−T wave end interval. In 2% of the Tp−T wave end measurements, the values were >128 ms and were <50 ms in another 2%. Outlier measurements > 128 ms were constrained to 128 ms and those <50 ms to 50 ms. Similarly, the rate-adjusted QT onset was computed using the rate-adjusted QTp (QTpa) and T wave onset to Tp as the difference between the 2 (Table 1).
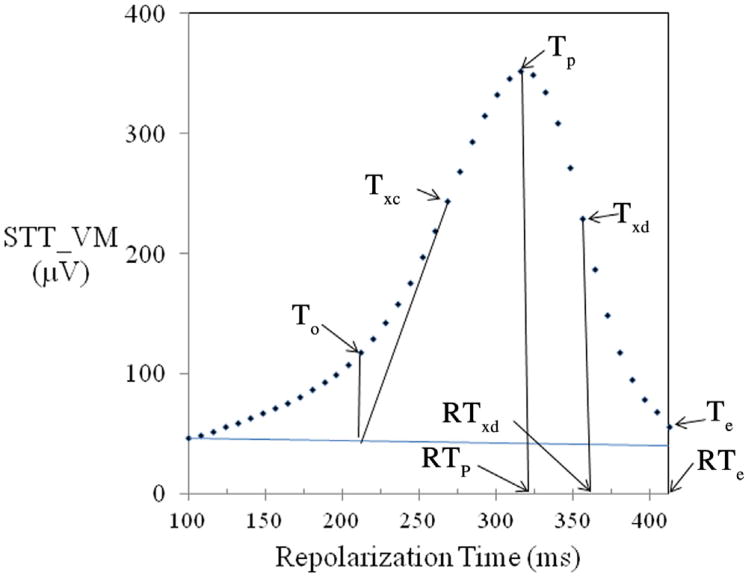
Figure 1.
A schematic illustrating key repolarization model's variables defined explicitly in Table 1. In normal repolarization sequence, RTp represents the RTepi. The TpTe interval is a measure of global temporal RT gradient or global RT dispersion, and the TpTxd interval is the local left ventricular free wall cross-mural RT gradient (XMRTgrad). A line extrapolated backward to the intersection of the horizontal line from the end of QRS identified time point of To. Te = T wave end; To = T wave onset; Txc = inflexion point (maximum slope) at T wave upstroke; Txd = inflexion point (minimum slope) at T wave downstroke. STT vector magnitude curve is shown at 40 sample points along RT from QRS end to Te. Arrows at RTp, RTxd, and RTend indicate RT at point of Tp, Txd, and Te, respectively.
Table 1. Definitions of electrocardiographic variables used in the repolarization model.
| Acronym | Description | Excel Algorithm |
|---|---|---|
| Pred. QTe | Predicted QT end interval (ms) | Pred. QTe = 183 × RR + 224 in men |
| Pred. QTe = 188 × RR + 228 in women | ||
| QTea* | Rate-adjusted QTe interval (ms) | QTea = QTe + 183 × (1 − RR) for men |
| QTea = QTe + 186 × (1 − RR) for women | ||
| QTpa | Rate-adjusted QT peak interval (ms) | QTpa = QTea − TpTe, where TpTe is the interval from T wave peak to T wave end |
| QToa | Rate-adjusted QT onset interval (ms) | QToa = QTpa − ToTp, where ToTp is the interval from T wave onset to T wave peak |
| Θ(Tp|Tref) | Tp vector deviation angle from normal reference direction (°) | Θ(Tp|Tref) = 57.3 × archcosine (Trefx × Tpx + Trefy × Tpy + Trefz × Tpz)/(TrefV × TpV)† |
| TpTxd | Left ventricular cross-mural repolarization time gradient (ms) | TpTxd = interval from Tp to Txd, where Txd is the inflection point at global T wave downstroke |
| RTepi | Epicardial repolarization time (ms) | RTepi = QTpa − (CosΘ(Tp|Tref) − 1) × TpTxd/2 |
| RTxd | Left ventricular repolarization time at time point Txd (ms) | RTxd = QTpa + (CosΘ(Tinit |Tref) + 1) × TpTxd/2 |
R-square 0.81 for men, 0.77 for women in regression of QTe on RR interval.
In Θ(Tp|Tref) algorithm, Trefx, Trefy, Trefz, Tpx, Tpy, and Tpz are the x, y, and z components of Tref and Tp vectors; Tref V is the vector magnitude of Tref Vector (=1); and TpV is the vector magnitude of Tp vector; Tref is the normal T reference vector in men and women free of cardiovascular disease with unit Vector xyz components (0.66, 0.48, −0.67). Spatial direction of repolarization is diametrically opposite to Tref vector.
Repolarization measurements were made using temporal reference points derived from the “global” T wave, the spatial T vector magnitude curve obtained from a transformation matrix used to derive the XYZ leads from the 8 linearly independent component leads of the 12-lead ECG signals.19 The QRS nondipolar voltage, QRS duration, and a set of 21 repolarization-related ECG variables from the repolarization model were chosen for evaluation because of their role in the generation of abnormal repolarization waveforms or because of previous data of their value as risk predictors.6–10 The QRS duration was included as the second depolarization-related parameter with the QRS nondipolar voltage, because even moderate QRS prolongation has been known to induce secondary repolarization abnormalities.
The conceptual model used to derive the repolarization parameters for the present study has been previously reported.8–10 The algorithms used to derive the epicardial repolarization time (RTepi) and other the key parameters of the repolarization model are described explicitly in Table 1. The RTepi was obtained from the QTpa, modified by the cosine of the T wave peak deviation angle [Θ(Tp|Tref)] from the normal reference direction of repolarization (Tref) in men and women free from cardiovascular disease. Thus, RTepi was assigned the value equal to QTpa when the Tp vector deviation angle was 0 (reverse repolarization sequence) and the value equal to QTpa plus left ventricular cross-mural RT gradient if the Tp vector deviation angle was 180° (concordant repolarization sequence). The left ventricular cross-mural RT gradient will be equal to the TpTxd interval, where Txd is the time point of the minimum slope (inflexion point) at the T wave downstroke. Θ(Tp|Tref) is a measure of the deviation of the repolarization sequence from the normal direction of repolarization. In addition to Θ(Tp|Tref) and Θ(R|STT), several other spatial angles between the T vectors from various repolarization subintervals and other interval and amplitude variables were used in various phases of the study. Their definitions are listed in the footnotes of the corresponding tabular data. Left ventricular hypertrophy was defined by the Cornell voltage.20
Descriptive statistics were used to determine the mean values, SDs, and upper and lower 20th percentiles (quintiles) for continuous variables and numbers and percentages for categorical variables. Cox proportional hazards regression analysis was used to compute hazard ratios (HR) and 95% confidence intervals (CIs) for the risk of HF. The ECG predictors were first evaluated as continuous variables and then stratified into quintiles. Quintiles 2 to 4 were first used as the reference group to evaluate the risk for the lowest and highest quintile to observe breakpoints at the high or low end of the distributions. The HRs were evaluated for increased values of the ECG parameters (quintile 5) as the test group, with quintiles 1 to 4 as the reference group. Finally, quintile 1, corresponding to decreased values, was used as the test group for T wave in lead aVL and Tp in lead V, with the remaining 4 quintiles as the reference group.
Gender interactions with HF risk for each ECG variable was evaluated, and the HRs for variables with a significant gender interaction (p <0.05) were listed separately for men and women; otherwise, both gender groups were combined. The HF risk data were summarized first by listing the unadjusted HRs and 95% CIs and then for multivariate-adjusted models with an adjustment for demographic (age, race, gender, education) and clinical (smoking status, diabetes, hypertension, family history of coronary heart disease or stroke, body mass index, systolic blood pressure, total cholesterol to high-density lipoprotein cholesterol ratio, glucose, creatinine, uric acid) factors. In addition to the single ECG variable models, the independent ECG predictors for incident HF were identified. When evaluating the collinearity among the ECG variables, it was observed that rate-adjusted QTe and rate-adjusted QT onset correlated highly with QTpa, which is functionally closely related to RTepi. From these 4 variables, it was decided to retain RTepi because of its central role in the repolarization model. The remaining set of ECG variables with low correlations (r <0.5) was chosen to evaluate the independent ECG predictors of incident HF. These variables were entered simultaneously into Cox regression model, first without additional adjustment and then after adjustment for demographic and clinical factors. Statistical analyses were performed using Statistical Analysis Systems, version 9.1.3 (SAS Institute, Cary, North Carolina) and Microsoft Excel 2007 version 5.0 (Microsoft, Redmond, Washington).
Results
The age range of the study population was 45 to 65 years (mean age 54; Table 2). The study population was predominantly white (73%). Some notable gender differences of clinical interest were present in the ECG parameters. The rate-adjusted QTe was 10 ms shorter and the rate-adjusted QTp 18 ms shorter in the men than in the women. The Θ(R|STT) was 10° wider in the men than in the women. Notable among the other gender differences were the lower T onset vector magnitude and Tp vector magnitude in women than in men and greater T wave onset/Tp vector magnitude ratio in the men than in the women.
Table 2. Characteristics of study population for key demographic, clinical, and electrocardiographic (ECG) variables stratified by gender.
| Variable | Men (n = 5,842) | Women (n = 7,596) |
|---|---|---|
| Demographic/clinical | ||
| Age (yrs) | 54.2 ± 5.8 | 53.6 ± 5.7* |
| Body mass index (kg/m2) | 27 ± 4.1 | 28 ± 6.0 |
| White | 4,563 (77%) | 5,450 (71%)* |
| Current smokers | 1,623 (27%) | 1,877 (25%)* |
| Systolic blood pressure (mm Hg) | 122 ± 17.5 | 120 ± 19.2* |
| Hypertension | 1,837 (31%) | 2,442 (32%) |
| Diabetes mellitus | 621 (11%) | 785 (10%) |
| Q wave myocardial infarction by Minnesota Code criteria | 179 (3.0%) | 107 (1.4%)* |
| LVH by Cornell Voltage | 91 (1.5%) | 297 (3.9%)* |
| LVH and strain | 9 (0.2%) | 34 (0.4%) |
| ECG parameters | ||
| Heart rate | 65 ± 10.2 | 67 ± 10.0* |
| PR interval (ms) | 166 ± 25.5 | 161 ± 25.4* |
| QRS duration (ms) | 95 ±9.1 | 87 ± 8.3* |
| RNDPV (μV) | 54 ± 22.6 | 43 ± 17.4* |
| QTea (ms) | 408 ± 12.9 | 415 ± 14.1* |
| QTpa (ms) | 316 ± 18.7 | 334 ± 18.2* |
| QToa (ms) | 226 ± 18.9 | 244 ± 19.3* |
| RTepi (ms) | 318 ± 19.3 | 336 ± 18.7* |
| RTendo (ms) | 351 ± 18.6 | 367 ± 18.2* |
| TpTxd (ms) | 37 ± 10.2 | 35 ± 11.4* |
| TpTe (ms) | 91 ± 14.9 | 81 ± 14.98* |
| Θ(R|STT) (°) | 58 ± 26.8 | 48 ± 24.5* |
| Θ(Rp|Tp) (°) | 51 ± 30.5 | 39 ± 19.4* |
| Θ(Tp|Tref) (°) | 21 ± 16.3 | 26 ± 18.4* |
| Θ(Tinit|Tterm) (°) | 18 ± 11.5 | 16 ± 11.0* |
| T wave complexity | 0.34 ± 0.16 | 0.36 ± 0.18* |
| TaVR (μV) | −219 ± 96.9 | −201 ± 86.9* |
| TaVL (μV) | 94 ± 95.5 | 75 ± 80.3* |
| TV1 (μV) | −133 ± 145.6 | −12 ± 119.8* |
| SToV (μV) | 54 ± 27.9 | 36 ± 19.8* |
| ToV (μV) | 148 ± 56.7 | 104 ± 41.1* |
| TpV (μV) | 390 ± 141.3 | 315 ± 122.5* |
| VTo/VTp (μV) | 0.39 ± 0.09 | 0.36 ± 0.11* |
Θ(R|STT) and Θ(Rp|Tp) = spatial angle (Θ) between mean QRS and STT and between Rp and Tp vectors, respectively; Θ(Tinit|Tterm) = spatial angle between the initial T vectors from quintiles 1 –3 and the terminal T vectors from quintiles 4 and 5; Θ(Tp|Tref) = spatial angle between Tp vector and T reference (Tref) vector; LVH = left ventricular hypertrophy; MI = myocardial infarction; QToa, QTea, and QTpa = QT onset and QT end intervals, respectively, rate-adjusted with formulas listed in Table 1; RNDPV = QRS non-dipolar voltage from singular value decomposition (square root of pooled variance of components 4 to 8); RTepi and RTendo = epicardial and endocardial repolarization time, respectively (see “Methods” section); ToV/TpV = ratio of To and Tp spatial vector magnitudes; TpTe = interval from QTp to end of global T wave, representing global repolarization time gradient; TpTxd = interval from Tp to Txd (TpTxd represents left ventricular cross-mural repolarization time gradient); T wave complexity = ratio of the second to the first principal component from singular value decomposition of the T wave; Txd = inflection point at T wave downstroke; V in SToV, ToV, and TpV = spatial magnitudes of STo, To, and Tp vectors.
p <0.001.
p <0.05, z-test for proportions and t test for gender differences.
New-onset HF occurred in 695 men (11.9%) and 721 women (9.5%). Summary results for the ECG predictors of incident HF are presented in Table 3 for multivariate-adjusted single ECG variable models such that the risk of incident HF was evaluated separately for each of the 23 ECG variables. A significant gender interaction with incident HF was found for 13 ECG variables, which have been listed separately for men and women in Table 3. For most of these 13 variables, the HRs were slightly stronger for the men than the women, and for 4 of the variables, the HR was significant for men only. The highest increased risk of incident HF in men, 1.76-fold, was observed for the spatial angle Θ(Tinit|Tterm) and 1.71-fold increased risk for T-wave aVR amplitude. Of the remaining 10 ECG variables with no significant gender interaction, the HRs were significant for 5, with the highest level of increased risk of incident HF for 2 of the spatial angles, 1.63-fold for Θ(R|STT) and 1.59-fold for Θ(Tp|Tref).
Table 3. Hazard ratios (HRs) with 95% confidence intervals (CIs) for electrocardiographic (ECG) predictors of incident heart failure (HF) from multivariate-adjusted risk model in men and women.
| ECG Variable | Multivariate-Adjusted Single ECG Variable Model* | |||
|---|---|---|---|---|
|
| ||||
| Men | Women | p Value (Gender Interaction) | ||
| RNDPV (μV) | 1.34 (1.09–1.64) | 1.50 (1.26–1.78) | <0.001 | |
| QToa (ms) | 1.57 (1.33–1.86) | 1.32 (1.16–1.51) | 0.018 | |
| QTpa (ms) | 1.55 (1.31–1.83) | 1.35 (1.19–1.54) | 0.0027 | |
| TpTe (ms) | 1.40 (1.17–1.67) | 1.09 (0.95–1.25) | 0.037 | |
| RTepi (ms) | 1.65 (1.39–1.94) | 1.38 (1.21–1.57) | 0.006 | |
| RTendo (ms) | 1.30 (1.09–1.55) | 1.39 (1.22–1.57) | 0.016 | |
| Θ(Tp|Tref)(°) | 1.65 (1.40–1.95) | 1.07 (0.90–1.28) | <0.001 | |
| Θ(Tinit|Tterm) (°) | 1.76 (1.49–2.07) | 1.32 (1.12–1.57) | 0.024 | |
| T wave complexity | 1.57 (1.33–1.86) | 1.31 (1.16–1.48) | <0.001 | |
| STo Amp. aVR (μV) | 1.45 (1.23–1.71) | 1.27 (1.12–1.44) | 0.024 | |
| T Amp. aVR (μV) | 1.71 (1.45–2.01) | 1.51 (1.34–1.70) | 0.037 | |
| ToV (μV) | 1.31 (1.10–1.55) | 1.05 (0.92–1.20) | <0.001 | |
| TpV (μV) | 1.31 (1.10–1.55) | 1.21 (0.07–1.37) | 0.040 | |
|
| ||||
| Men and Women | ||||
|
| ||||
| Heart rate (beats/min) | 1.50 (1.33–1.69) | 0.508 | ||
| QRS duration (ms) | 1.12 (0.98–1.28) | 0.810 | ||
| TpTe interval (ms) | 1.09 (0.95–1.25) | 0.845 | ||
| QTea (ms) | 1.29 (1.14–1.46) | 0.134 | ||
| TpTxd (ms) | 1.05 (0.93–1.19) | 0.845 | ||
| Θ(R|STT) (°) | 1.63 (1.45–1.83) | 0.558 | ||
| Θ(Rp|Tp) | 1.59 (1.41–1.79) | 0.130 | ||
| Θ(Tterm|Tref) (°) | 1.24 (1.09–1.40) | — | ||
| STo Amp. V6 (μV) | 1.34 (1.19–1.52) | 0.062 | ||
| STo Amp. aVL (μV) | 1.19 (1.04–1.37) | 0.851 | ||
| T Amp. aVL (μV) | 1.32 (1.17–1.49) | 0.120 | ||
| T Amp. V1 (μV) | 1.24 (1.09–1.41) | 0.068 | ||
| ToV/TpV | 1.26 (1.10–1.44) | 0.445 | ||
| SToV (μV) | 1.01 (0.88–1.16) | 0.177 | ||
HRs evaluated for quintile 5 (quintile 1 for QToa and TpV) as the test group, with the remaining 4 quintiles as the reference group.
HRs for ECG variables with significant gender interaction for HF risk are listed separately for men and women, otherwise the gender groups were combined.
Θ(R|STT) = spatial angle between the mean QRS and STT vectors; Θ(Rp|Tp) = spatial angle between peak QRS and T vectors (Rp and Tp, respectively); Θ(Tinit|Tterm) = spatial angle between initial and terminal T vectors from T wave quintiles 1–3 and 4 and 5, respectively; Θ(Tp|Tref) = spatial angle between Tp vector and T reference (Tref) vector; Θ(Tterm|Tref) = spatial angle between mean terminal T vector from T wave quintiles 4 and 5 and Tref vector; QToa and QTpa and QTea = rate-adjusted QT onset, QT peak, and QT end intervals (formulas listed in Table 1); RNDPV = QRS nondipolar voltage from singular value decomposition (square root of pooled variance of components 4–8); RTepi and RTendo = epicardial and endocardial repolarization time, respectively (see Figure 1 and “Methods” section); STo Amp. = ST onset amplitude at end of QRS (the J-point); ToV/TpV = ratio of To and Tp vector magnitudes; TpTe = interval from the peak to the end of the global T wave, considered as global RT gradient; TpTxd = interval from Tp to inflection point at T wave downstroke (Txd) considered as cross-mural repolarization time gradient (XMRTgrad); T wave complexity = ratio of second to first principal component from singular value decomposition of T wave; V in SToV, ToV, and TpV = vector magnitude of STo, To, and Tp vectors, respectively.
Adjusted for age, race, education level, smoking status, alcohol status, asthma, cancer, diabetes, hypertension, family history of coronary heart disease and stroke, body mass index, systolic and diastolic blood pressure, high-density lipoprotein, low-density lipoprotein, triglycerides, white blood cells, glucose, creatinine, and uric acid.
Statistically significant.
Independent ECG predictors for incident HF were identified after considering collinearity among the ECG variables, as described in the “Methods” section. The correlation matrix in Table 4 lists the ECG variables with low correlation (r <0.5) chosen to evaluate independent predictors of incident HF. The risk of incident HF for these 8 variables was evaluated by entering them simultaneously into the multiple ECG variable models, and each variable was thus adjusted to the other ECG variables without any additional adjustment. Four ECG variables for men and three for women were significant independent predictors of incident HF (Table 5). Θ(Tp|Tref) was a significant independent predictor in men (HF risk increased 31%) and in women (HF risk increased 46%). The other independent predictors were the epicardial RT (62% increased risk) and Tp−T wave end interval (27% increased risk) in men and the QRS nondipolar voltage (46% increased risk) in women.
Table 4. Correlations among electrocardiographic (ECG) variables selected for evaluation of independent electrocardiographic (ECG) predictors of heart failure (HF).
| Θ(R|STT) | Θ(Tp|Tref) | RTepi | RNDPV | TpTe | ToV/TpV | T Wave Complexity | QRS Duration (ms) | |
|---|---|---|---|---|---|---|---|---|
| Θ(R|STT) (°) | 1 | |||||||
| Θ(Tp|Tref) (°) | 0.346 | 1 | ||||||
| RTepi (ms) | −0.07 | 0.29 | 1 | |||||
| RNDPV (μV) | 0.05 | 0.07 | −0.11 | 1 | ||||
| TpTe (ms) | 0.18 | 0.07 | −0.34 | 0.18 | 1 | |||
| ToV/TpV | 0.36 | 0.02 | 0.23 | 0.23 | 0.21 | 1 | ||
| T wave complexity | 0.20 | 0.09 | 0.09 | 0.02 | 0.01 | 0.28 | 1 | |
| QRS duration (ms) | 0.24 | 0.03 | −0.06 | 0.28 | 0.04 | 0.14 | 0.04 | 1 |
Abbreviations as in Table 3.
Table 5. Hazard ratios (HRs) and 95% confidence intervals (CIs) for independent predictors* of incident heart failure (HF) by gender and cardiovascular disease status at baseline.
| CVD-Free Men | CVD-Free Women | ||
|---|---|---|---|
|
|
|
||
| ECG Predictors With Test Group Threshold | HR (95% CI) | ECG Predictors With Test Group Threshold | HR (95% CI) |
| RTepi >329 ms | 1.62 (1.34–1.97)† | RNDPV >54 μV | 1.46 (1.24–1.73)† |
| TpTe >110 ms | 1.27 (1.06–1.54)‡ | Θ(R|STT) >64° | 1.54 (1.29–1.85)† |
| Θ(Tp|Tref) >28° | 1.31 (1.07–1.61)§ | Θ(Tp|Tref) >28° | 1.46 (1.21–1.76)† |
| T complexity | 1.32 (1.10–1.58)§ | ||
Θ(R|STT) = spatial angle between the mean QRS and STT vectors; Θ(Tp|Tref) = spatial angle between T peak vector and reference T vector (Tref) with x, y, z components (0.686, 0.528, −0.501) (direction of repolarization is diametrically opposite to Tref vector); CVD = cardiovascular disease; RNDPV = non-dipolar QRS voltage; RTepi = epicardial repolarization time; TpTe = T peak-T end interval (global RTgrad); T wave complexity = ratio of second to first principal component of T wave from singular value decomposition.
Independent predictors were obtained by entering the predictors significant on univariate single ECG parameter models simultaneously into Cox regression model and adjusting each variable to other significant ECG predictors. HRs were evaluated for quintile 5. with quintiles 1–4 as the reference group.
p <0.001.
p <0.05.
p <0.01 for HR.
Discussion
The most salient results from the present investigation can be summarized as follows. First, most of the ECG variables evaluated were predictors of hospitalized HF as significant as single ECG variables with adjustment for demographic and clinical variable. Second, after controlling for collinearity among the ECG variables, the significant independent predictors of hospitalized incident HF in women were Θ(R|STT), with 54% increased risk, Θ(Tp|Tref), with 46% increased risk, and QRS nondipolar voltage with 46% increased risk. Third, in men, the significant independent predictors were Θ(Tp|Tref), with 31% increased risk, RTepi, with 62% increased risk, Tp−T wave end interval, with 62% increased risk, and T wave complexity, with 32% increased risk.
It should be noted that this set of independent predictors of incident HF is not unique, because many ECG variables were highly correlated. In particular, if QTp or QTe had been chosen for evaluation of independent predictors, instead of RTepi, the HRs would have been close to those observed for RTepi. Similarly, some STT variables such as the T- wave aVR amplitude and ST onset amplitude in V6 would likely be significant predictors of incident HF if chosen for evaluation instead of Θ(Tp|Tref) or Θ(R|STT) because of the high level of correlation between them.
Concerning the validity of the repolarization model, electrophysiologic evidence,21–23 and electrocardiographic potential theory applied to the generation of T waves support the concept that with normal direction of the repolarization sequence, RTepi coincides with the time point of Tp. The timing of the endocardial RT was less clear. The repolarization model assumes that the inflexion point at global T wave downstroke occurs when the largest number of left ventricular myocytes leaves phase 3 of their action potential within the same increment of RT. It is conceivable that this occurs when most endocardial myocytes have reached the end of phase 3 of their repolarization. The repolarization model uses the TpTxd interval (where Txd is the time point of the minimum slope [inflexion point] at the T wave downstroke) and RTepi to derive a tentative estimate for a representative value for endocardial RT.
Θ(Tp|Tref) is a measure of deviation of the direction of the repolarization sequence from normal reference direction during initial repolarization phases dominated by regional cross-mural repolarization of the left ventricular lateral wall. Figure 2 shows a progressive decrease in T wave V5 amplitude with Θ(Tp|Tref) widening from its median value of 17° to 42° at the 95th percentile of the Θ(Tp|Tref) distribution. The T wave at lead V5 becomes flat and slightly negative at Θ(Tp|Tref), widening to 65°, the 98% normal limit. Although not shown, a parallel change occurs in T wave of lead aVR, which becomes progressively less negative and turns positive at abnormal widening of Θ(Tp|Tref). The trends in the men were closely similar to those in the women. Θ(Tp|Tref) and other spatial angles reflecting deviation of the repolarization sequence from normal reference direction might have utility in the evaluation of regional contributions to the prolongation of the overall QT interval and global RT, particularly in various abnormal clinical conditions and as an abnormal response to cardioactive agents.
Figure 2.
TV5 waveforms in women free of cardiovascular disease with widening of the spatial angle between Tp and normal T reference vectors, Θ(Tp|Tref). A progressive decrease occurred in TV5 amplitude with Θ(Tp|Tref) widening from the median 17° to 42° at the 95th percentile. TV5 becomes flat and slightly negative at Θ(Tp|Tref), widening to 65°, the 98% normal limit. As a parallel change (not shown), aVR amplitudes became progressively less negative and turned positive at abnormal widening of Θ(Tp|Tref). The trends in men were closely similar to those in women.
Coronary heart disease, valvular heart disease, and hypertensive heart disease are clinically known to be the primary determinants of HF. In our study population, the prevalence of ECG left ventricular hypertrophy was low (1.5% in men and 3.9% in women), just as was the prevalence of Q wave myocardial infarction (3.0% in men and 1.4% in women). Thus, in these cardiovascular disease-free men and women, the ECG predictors of incident HF might be early manifestations of otherwise undetected coronary heart disease or hypertensive heart disease.
In an earlier ARIC study with 9 years of follow-up, the QRS nondipolar voltage was an independent ECG predictor of risk of new-onset HF only in women, just as it was in the present ARIC study with 14 years of follow-up. In postmenopausal women in the Women's Health Initiative study, the independent predictors of incident HF were wide Θ(R|STT), ST-segment depression in V1, increased T wave V1 amplitude, QT prolongation, and increased heart rate variability.7 QRS nondipolar voltage was the only significant depolarization-related predictor in women in that study. Previous studies have postulated microvascular disease and fragmentation of ventricular conduction as a possible mechanism for QRS nondipolar voltage as a predictor of incident HF. However, in the Women's Ischemia Syndrome Study (WISE), a wide QRS/T angle, QRS duration, and prolonged QT interval were independent predictors of incident fatal and nonfatal coronary heart disease events, but the QRS nondipolar voltage was not.24 The question of the mechanism for increased risk of incident HF for QRS nondipolar voltage in women remains open.
Incident HF events in the study group were identified by hospitalization and evidence of HF as the discharge diagnosis, without, however, performing centralized adjudication. The study population consisted of men and women free of clinically manifest cardiovascular disease; however, no clinical data with evaluation of cardiac function were available for the present study.
Acknowledgments
The Atherosclerosis Risk in Communities Study was performed as a collaborative study. We thank the staff and participants of the ARIC study for their important contributions.
This work was supported by contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C from the National Heart, Lung, and Blood Institute (Bethesda, Maryland).
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Zhang Y, Post WS, Blasco-Colmenares E, Dalal D, Tomaselli GF, Guallar E. Electrocardiographic QT interval and mortality: a metaanalysis. Epidemiology. 2011;22:660–670. doi: 10.1097/EDE.0b013e318225768b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–2597. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- 3.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 4.Shah R, Hondeghem LM. Refining detection of drug-induced proarrhythmia: QT interval and TRIaD. Heart Rhythm. 2005;2:758–772. doi: 10.1016/j.hrthm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Huikuri HV, Castellanos A, Myerburg RJ. SCD due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 6.Rautaharju PM, Prineas RJ, Wood J, Zhang ZM, Crow R, Heiss G. Electrocardiographic predictors of new-onset heart failure in men and in women free of coronary heart disease (from the Atherosclerosis in Communities [ARIC] Study) Am J Cardiol. 2007;100:1437–1441. doi: 10.1016/j.amjcard.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 7.Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic predictors of incident congestive heart failure and all-cause mortality in postmenopausal women. The Women's Health Initiative Circulation. 2006;113:481–489. doi: 10.1161/CIRCULATIONAHA.105.537415. [DOI] [PubMed] [Google Scholar]
- 8.Rautaharju PM, Zhou SH, Gregg RE, Startt-Selvester RH. Electrocardiographic estimates of action potential durations and transmural repolarization time gradients in healthy subjects and in acute coronary syndrome patients—profound differences by sex and by presence vs absence of diagnostic ST elevation. J Electrocardiol. 2011;44:309–319. doi: 10.1016/j.jelectrocard.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Rautaharju PM, Zhou SH, Gregg RE, Startt-Selvester RH. Electrocardiographic estimates of regional action potential durations and repolarization time subintervals reveal ischemia-induced abnormalities in acute coronary syndrome not evident from global QT. J Electrocardiol. 2011;44:718–724. doi: 10.1016/j.jelectrocard.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Rautaharju PM, Zhou SH, Gregg RE, Startt-Selvester RH. Heart rate, gender differences, and presence versus absence of diagnostic ST elevation as determinants of spatial QRS|T angle widening in acute coronary syndrome. Am J Cardiol. 2011;107:744–750. doi: 10.1016/j.amjcard.2011.02.333. [DOI] [PubMed] [Google Scholar]
- 11.The ARIC Investigators. The Atherosclerosis in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 12.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Eillismd OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 13.Vitelli LL, Crow RS, Shahar E, Hutchinson RG, Rautaharju PM, Folsom AR. Electrocardiographic findings in a healthy biracial population: the ARIC Study. Am J Cardiol. 1998;81:453–459. doi: 10.1016/s0002-9149(97)00937-5. [DOI] [PubMed] [Google Scholar]
- 14.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 15.Rose GA, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular Survey Methods. Geneva, Switzerland: World Health Organization; 1982. [Google Scholar]
- 16.Blackburn H, Keys A, Simonson E, Rautaharju P, Punsar S. The electrocardiogram in population studies: a classification system. Circulation. 1960;21:1160–1175. doi: 10.1161/01.cir.21.6.1160. [DOI] [PubMed] [Google Scholar]
- 17.Wolf HK, MacInnis PJ, Stock S, Helppi RK, Rautaharju PM. The Dalhousie program: a comprehensive analysis program for rest and exercise electrocardiograms. In: Zywiets C, Schneider B, editors. Computer Application on ECG and VCG Analysis. Amsterdam: North Holland Publishing; 1973. pp. 231–240. [Google Scholar]
- 18.Rautaharju PM, MacInnis PJ, Warren JW, Wolf HK, Rykers PM, Calhoun HP. Methodology of ECG interpretation in the Dalhousie Program: NOVACODE ECG classification procedures for clinical trials and population health surveys. Methods Inf Med. 1990;29:362–374. [PubMed] [Google Scholar]
- 19.Horáĉek BM, Warren JW, Field DQ, Feldman CL. Statistical and deterministic approaches to designing transformations of electrocardiographic leads. J Electrocardiol. 2002;35(suppl):41–52. doi: 10.1054/jelc.2002.37154. [DOI] [PubMed] [Google Scholar]
- 20.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfied P. Improved sex-specific criteria for left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987;75:565–572. doi: 10.1161/01.cir.75.3.565. [DOI] [PubMed] [Google Scholar]
- 21.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–1936. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 22.Zhu TG, Patel C, Martin S, Quan X, Wu Y, Burke JF, Chernick M, Kowey PR, Yan GX. Ventricular transmural repolarization sequence: its relationship with ventricular relaxation and role in ventricular diastolic function. Eur Heart J. 2009;30:372–380. doi: 10.1093/eurheartj/ehn585. [DOI] [PubMed] [Google Scholar]
- 23.Xia Y, Liang Y, Kongstad O, Liao Q, Holm M, Olsson B, Yan S. In vivo validation of the coincidence of the peak and end of the T wave with full repolarization of the epicardium and endocardium. Heart Rhythm. 2005;2:162–169. doi: 10.1016/j.hrthm.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Triola B, Olson MB, Reis SE, Rautaharju P, Merz CN, Kelsey CF, Shaw LJ, Sharaf BL, Sopko G, Saba S. Electrocardiographic predictors of cardiovascular outcome in women: the NHLBI-sponsored WISE study. J Am Coll Cardiol. 2005;45:51–56. doi: 10.1016/j.jacc.2004.09.082. [DOI] [PubMed] [Google Scholar]