Abstract
For several years, the literature has examined the association of depression and anxiety with inflammatory states such as atherosclerosis and cardiovascular disease, yet this association remains inconclusive. Several possible immune and endocrinological pathways have been postulated that associate depression and anxiety with inflammation and immune dysregulation. Anxiety and depression have usually been envisioned as two separate psychiatric conditions yet they share similar symptoms and are frequently encountered together among individuals. Individuals suffering from anxious-depression are more refractory to treatment and have been reported to have greater disability compared to individuals with anxiety or depression alone. With the current changes in the diagnostic manual for psychiatric disorders placing more emphasis on a dimensional approach for the diagnosis of psychiatric illnesses, the hypothesis presented is that anxious-depression should be considered as a chronic inflammatory phenomenon since it shares common physiopathological pathways and pharmacological treatments with inflammatory states. This hypothesis might help to investigate how different levels of inflammatory biomarkers could be correlated with symptoms of anxious-depression.
The Construct of Anxious-Depression
During the early 1990s the concept of mixed anxiety and depression was portrayed as an important condition, worthy of clinical attention, that was frequently found in primary care settings and did not meet full diagnostic criteria for major depression or an anxiety disorder; this diagnosis has been frequently referred as anxious-depression1. Early studies in community samples found increased suffering, disability and exacerbation of chronic medical conditions among patients with anxious-depression1. Similarly, subsyndromal anxiety and depressive states have been associated with loss of productivity and lack of adequate treatment2–4. Anxious-depression has been a condition of interest in the updated version of the Diagnostic and Statistical Manual for Mental disorders (DSM) due to the high co-occurrence of symptoms of anxiety and depression observed in clinical settings5. Consensus from experts is that more research is needed for anxious-depression to meet test-retest reliability as a well established diagnosis 6,7,8.
In behavioral health, diagnoses are usually conceptualized within a dimensional or categorical approach. The dimensional approach to study anxious-depression has been suggested as having some advantages over the traditional categorical approach 9. Traditional categorical approaches identify individuals suffering or not from a given behavioral health problem. The categorical approach has the disadvantage of not considering individuals below the established cutoff threshold, although some categorical classifications allow for a spectrum of continuity based on the severity of symptomatology, i.e. mild, moderate or severe. Individuals with subsyndromal classification of anxiety and depression states are usually not the focus of clinical attention2,4,10. Individuals with anxious-depression are frequently seen in primary care clinics and are better captured when a dimensional diagnostic approach is used with severity of symptoms within the spectrum5,11. A recent review of the literature found that the dimensional use of anxious-depression yields a more serious clinical presentation in which inflammatory markers could serve as direct measure of this commonly seeing condition in primary care settings12,13.
Our group has started to study the dimensional construct of anxious-depression. Following this dimensional approach, our group showed preliminary data on 16,000 individuals participating in a large epidemiological study in which states of anxiety and depression cluster together in an anxious-depression spectrum (mild, moderate, severe) 14. That spectrum-like disorder could be used as a single construct to study associations with chronic medical conditions resulting from pervasive inflammatory states such as diabetes, obesity, atherosclerosis and metabolic syndrome.
During the past two decades, there has been an increased interest in understanding the association between mood, anxiety symptoms and chronic medical conditions affected by inflammatory states such as atherosclerosis15,16. Since studies have shown that depression is an independent risk factor for cardiovascular disease17–19, in recent years, attention has shifted to anxiety disorders as a possible independent risk factor for inflammation and cardiovascular disease20. Because depression and anxiety coexist1, lessons learned from a number of studies looking at changes in depressive states have provided the ground work to study the association of anxious-depression states with atherosclerosis in which inflammation is considered to be the common biological pathway 21–24.
With the current need to integrate behavioral health in the primary care settings 25, it is reasonable to study anxious-depression and inflammation as a common chronic medical condition. Inflammation is considered to have a crucial role in the pathogenesis of affective states, atherosclerosis and metabolic disorders23,26,27. Therefore, the main hypothesis is to consider anxious-depression as a chronic inflammatory state considering the overlapping biological processes and shared treatments encountered in anxious-depression, immunological states and inflammation.
Anxious-Depression as an Inflammatory Phenomenon
Immunological Evidence
Biologically, anxiety and depressive symptoms have been associated with changes in the hypothalamic-pituitary-adrenocortical (HPA) axis, autonomic nervous system tone, immune response, endothelial integrity, coagulation and vascular reactivity; all of these systems are crucial in the generation of a systemic inflammatory response15,20,21,28–30. HPA axis dysregulation has been considered as the landmark biological connection between anxiety, depression, the central nervous system and systemic inflammatory response 31. Hyperactivation of the HPA axis is associated with hypersecretion of corticotropin-releasing factor (CRF), a peptide that mediates endocrine, autonomic and behavioral responses to states of anxiety and depression32. Hyperactivation of CRF has been considered a novel biomarker for depression and anxiety due to their close association with systemic depression, anxiety, stress and inflammation 32. Studies have reported that CRF may affect systemic inflammation via a peripheral paracrine effect similar to those observed in the HPA axis33,34. In fact peripheral CRF acts on receptors of inflamed tissue similar to the neuropeptide serotonin implicated in anxiety and depression as well as the novel Interleukin (IL)-1β, a potent pro-inflammatory cytokine 32,34,35.
IL-1β is present in peripheral immune cells as well as glia and neurons. This inflammatory cytokine has been shown to have a critical role in decreasing hypocampal neurons responsible for the production of serotonin in response to inflammatory states36. Furthermore, IL-1β has been shown to be a closely associated with states of depression and inflammation37. In summary, IL-1β is a novel systemic and neurologic biomarker that could explain how anxious-depression and inflammation are a similar phenomenon.
Although there are studies showing no association of mood states with inflammation38,39, there is evidence that anxious-depression states are associated with increased activation of T-helper lymphocytes and increased levels of pro-inflammatory cytokines such as IL-6, IL-1β, IL-2, IL-4, IL-1040 as well as acute phase reactants such as C-reactive protein and tumor necrosis factor-α (TNF-α)28,41. In a study performed in 38 medical students before and after an academic examination, investigators found that anxiety states are associated with increased levels of TNF-α, IL-6, IL-1 receptor antagonist (IL-1Ra), interferon gamma (IFN-gamma), IL-10 and IL-442. These findings provide further evidence that the production of the pro-inflammatory cytokines and thus inflammatory states clinically manifest as anxiety and depressive states. Furthermore, dysfunction in the tissue plasminogen activation factor has been implicated in the exacerbation of inflammatory states such as atherosclerosis in which anxiety and depressive states are the main clinical manifestation10,21,43–45.
The moderating effect of the proinflammatory cytokine indoleamine 2,3-dioxygenase (IDO) in reducing the availability of tryptophan for the synthesis of serotonin is another evidence of the commonality of anxiety and depression with inflammatory states. IDO is an inflammation-related enzyme, mainly expressed in antigen presenting cells that degrades tryptophan, a serotonin precursor, to kynurenine13,24. Furthermore, recent studies in rodents found that inflammatory states created by injection of bacterial endotoxines increased the activity of IDO as measured by increased kynurenine/tryptophan quotient which is directly observed by exacerbation of depression and anxiety-like behaviors46. Similarly, Maes and coworkers described increased activation of IDO during the acute inflammatory state of early puerperium observed in women. Maes et al. found increased symptoms of anxious-depression as the direct clinical manifestation of the inflammatory state of puerperium as measured by the direct activation of IDO47. This further demonstrates the link not only at a preclinical but clinical level of anxious-depression with inflammatory states where the immune system is the common pathway.
To further corroborate the above mentioned evidence, investigators have reported that inflammation reduces the availability of the enzyme cofactor tetrahydrobiopterin which is crucial in the activities of tryptophan hydroxylase and tyrosine hydroxylase, both rate-limiting enzymes essential for the synthesis of serotonin, dopamine and norepinhephrine. Deficit in these neuroamines is reflected in symptoms of anxious-depression48,49.
Genetic Evidence
In addition to the shared immune and cytokine activity seen in inflammation and anxious-depression, authors have reported that there is an up-regulation of genes associated with the expression of inflammatory cytokines among individuals endorsing high scores of depression and anxiety states50. Crucial genes expressed by leukocytes that have been associated with inflammation, cellular stress and mood states are STMN1, p16ink4a as well as FOS, DUSP1, TERT and the IL-6 gene. Among these pool of genes, FOS and DUSP1 have been reported good candidates to responses to stress, inflammation and anxiety states50,51. Teyssier and collaborators50 found a strong association of the leukocyte expression of the STMIN1 and p16ink4a with high scores of anxiety symptoms among patients diagnosed with depression. This study postulates that genes transcripts implicated in anxiety, depression and inflammation are highly correlated with each other which provides good evidence supporting the theory that anxious-depression is a chronic inflammatory state.
Treatments for the Anxious-Depression Inflammatory State
Common treatments for anxiety, depression and inflammation further supports the hypothesis that anxious-depression is an inflammatory state. Several studies have demonstrated that antidepressants, which are the pharmacological treatment of choice for anxiety and depression, have anti-inflammatory properties52,53. Several investigators demonstrated that infusing the commonly used selective serotonin reuptake inhibitor (SSRIs) antidepressants in whole blood produces a marked reduction in the pro-inflammatory cytokine interferon-gamma and increases the secretion of the anti-inflammatory cytokine IL-1053–55.
Chronic pain, a known chronic inflammatory condition, has traditionally been treated with different types of antidepressants such as tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), SSRIs, selective serotonin noradrenaline reuptake inhibitors (SNRIs) and norepinephrine reuptake inhibitors (NRIs)56. Furthermore, fibromyalgia which by definition is a systemic non-specific inflammatory state has shown to respond well to SSRIs and TCAs57.
Evidence shows that the administration of anti-TNF-α agents such as etanercept reduces anxious-depression behaviors in animals. Researchers found that TNF-α has well established role in inflammation but also a role in the modulation of anxious and depressive states providing a novel strategy to treat inflammatory conditions such as anxious-depression58. Similarly, infliximab a known monoclonal antibody that targets TNF-α has shown to reduce symptoms of anxious-depression among patients with high concentrations of the inflammatory marker C- reactive protein48.
Clinical Implications
The biological evidence presented on how anxious-depression and inflammation share the same physiopathological process has important clinical implications. Studies have extensively documented that metabolic syndrome and cardiovascular disease predicted increase in anxiety and depressive symptoms among patients59,60. Janszky et al. examined data from around 50,000 participants followed for an average of 37 years. The authors found that anxiety disorders were a significant predictor of chronic inflammation manifested as atherosclerosis and subsequent cardiovascular events20,61. On the same note, a meta-analysis by Roest and collaborators found that anxiety symptoms predicted cardiovascular events 11 years after the onset of anxiety symptoms20,62. To further validate the hypothesis that anxious-depression is an inflammatory state, a recent longitudinal study demonstrated over a 2 year period, that individuals with initial symptoms of anxiety and depression developed an increase in abdominal obesity and dyslipidemia 63. The common biological pathways between chronic inflammatory states such as obesity and affective disorders are considered critical areas of clinical research that have direct implication for the development of prevention and treatment programs 64.
Future Implications
For decades, the literature has emphasized the importance of addressing treatments for anxious-depression and chronic inflammation as one condition65. Anxious-depression is a proposed dimensional construct that could be used as a direct proxy measure of chronic inflammation. It is important to consider that the immune system has been described as the main regulator of inflammatory responses and mood states66. The immune system could be an important mediator for the proposed chronic inflammatory state of anxious-depression. For example, biological markers of immune dysregulation could be used to determine the level of inflammation among individuals experiencing symptoms of anxious-depression. These same biological markers could be used to study the association of chronic inflammatory conditions such as obesity and atherosclerosis with different dimensional states of anxious-depressive symptoms.
The new DSM-5 emphasizes the need to better operationalize the concept of anxious-depression5,6. The hypothesis postulated herein is that anxious-depression should not only be measured as a psychological/psychiatric construct but also as a state of chronic inflammation. Latent variables could be used to cluster individuals that have in common different levels of anxious-depression with different levels of common neuronal and peripheral inflammatory biomarkers. For instance, latent variable analyses could cluster different categories of anxious-depression with levels of IL-1β to evaluate the association of inflammation with anxious-depression. Latent variable models are becoming popular in behavioral medicine research because of their flexibility to measure different constructs (biological and psychological) and ease to be reproduced in other studies 67–69.
Conclusion
This manuscript proposes a hypothesis in which the dimensional construct of anxious-depression could serve as a direct measure chronic inflammation. Screening for negative emotional states such and anxiety and depression is considered essential in the initial work-up of patients with chronic inflammatory states such as metabolic syndrome and cardiovascular disease39,45,70,71, therefore, is viable to consider that anxious-depression is an inflammatory phenomenon that is contributing to the exacerbation of chronic medical conditions. As described in the multinational large case-control INTERHART study, around 33% of causes of myocardial infarction are associated with psychological symptoms, being anxiety and depressive symptoms the most common ones70,72.
The latent variable analytic approach will facilitate to study how clusters of anxious-depression are directly associated with inflammatory biomarkers. As several experts have cautioned in the past, it is important not to ignore the clustering of psychological factors that may act synergistically in the progression of a chronic illness73–75.
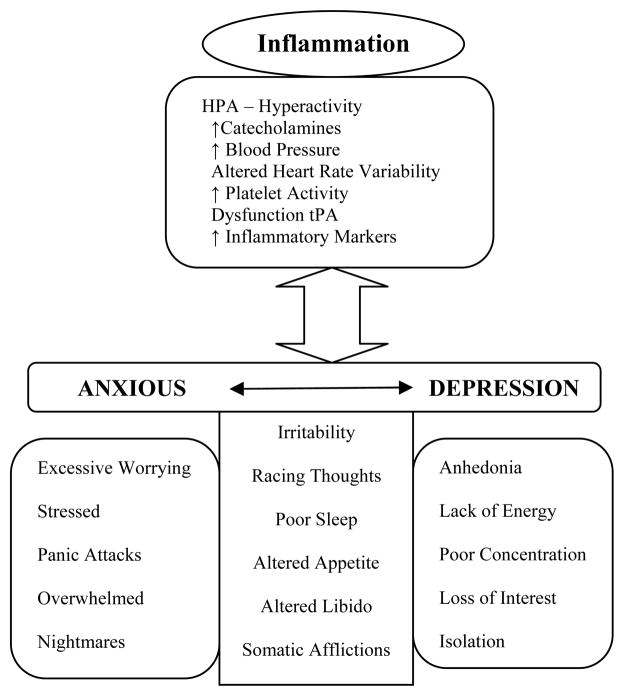
Figure 1. Theoretical Model of the Anxious-Depression as an Inflammatory State.
Inflammation is characterized by HPA hyperactivity, immune dysregulation, increased monoamine activity, increased pro-inflammatory cytokines, cardiovascular reactivity and altered coagulation factors. The different symptoms of anxious-depression share the same biological processes observed in inflammation. This theoretical model supports the hypothesis that anxious-depression is an inflammatory state.
Acknowledgments
The author has special gratitude for Joel E. Dimsdale, MD for his years of mentorship and support for this manuscript. Also special thanks to Mathew Allison, MD, MPH and Murray Stein, MD, MPH for their valuable feedback.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Katon W, Roy-Byrne PP. Mixed anxiety and depression. J Abnorm Psychol. 1991;100:337–45. doi: 10.1037//0021-843x.100.3.337. [DOI] [PubMed] [Google Scholar]
- 2.Judd LL, Schettler PJ, Akiskal HS. The prevalence, clinical relevance, and public health significance of subthreshold depressions. Psychiatr Clin North Am. 2002;25:685–98. doi: 10.1016/s0193-953x(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 3.Vahia IV, Meeks TW, Thompson WK, et al. Subthreshold depression and successful aging in older women. Am J Geriatr Psychiatry. 2010;18:212–20. doi: 10.1097/JGP.0b013e3181b7f10e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angst J, Merikangas KR, Preisig M. Subthreshold syndromes of depression and anxiety in the community. J Clin Psychiatry. 1997;58:6–10. [PubMed] [Google Scholar]
- 5.Goldberg DP, Simms LJ, Gater R, Krueger RF. Integration of Dimensional Spectra for Depression and Anxiety Into Categorical Diagnoses for General Medical Practice. In: Regier DA, Narrow WE, Kuhl EA, Kupfer DJ, editors. Evolution of DSM-V Conceptual Framework: Development, Dimensions, Disability, Spectra, and Gender/Culture. Arlington, VA: American Psychiatric Association; 2010. [Google Scholar]
- 6.APA Board of Trustees Aproves DSM. Psychiatric News. 2012 Dec Available at: http://alert.psychiatricnews.org/search?q=dsm+5.
- 7.Kraemer HC, Kupfer DJ, Clarke DE, Narrow WE, Regier DA. DSM-5: how reliable is reliable enough? Am J Psychiatry. 2012;169:13. doi: 10.1176/appi.ajp.2011.11010050. [DOI] [PubMed] [Google Scholar]
- 8.Wakefield J. DSM-5: Proposed Changes to Depressive Disorders. Curr Med Res Opin. 2011:1–25. doi: 10.1185/03007995.2011.653436. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Bjelland I, Lie SA, Dahl AA, Mykletun A, Stordal E, Kraemer HC. A dimensional versus a categorical approach to diagnosis: Anxiety and depression in the HUNT 2 study. International Journal of Methods in Psychiatric Research. 2009;18:128–37. doi: 10.1002/mpr.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjelland I, Dahl AA, Haung TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg DP, Prisciandaro JJ, Williams P. The primary health care version of ICD-11: the detection of common mental disorders in general medical settings. General Hospital Psychiatry. 2012;34:665–70. doi: 10.1016/j.genhosppsych.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Ionescu DF, Niciu MJ, Henter ID, Zarate C., Jr Defining anxious depression: a review of the literature. CNS Spectrums. 2013:1–9. doi: 10.1017/S1092852913000114. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almond M. Depression and Inflammation. Examining the link Current Psychiatry. 2013;12:25–32. [Google Scholar]
- 14.Camacho A, Gonzalez P, Buelna C, et al. Latent Profile Analyses of Anxious-Depression among Hispanics/Latinos: Results from the Hispanic Community Health Study/Study of Latinos. Poster presented at the International Society of CNS Clinical Trials and Methodology; Marina del Rey, CA. 2012. [Google Scholar]
- 15.Musselman D, Evans D, Nemeroff C. The Relationship of Depression to Cardiovascular Disease. Arch Gen Psychiatry. 1998;55:580–92. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 16.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and Risk of Stroke Morbidity and Mortality. JAMA: The Journal of the American Medical Association. 2011;306:1241–9. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grippo AJ, Johnson AK. Stress, Depression and Cardiovascular Dysregulation: A Review of Neurobiological Mechanisms and the Integration of Research from Preclinical Disease Models. Stress. 2009;12:1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan A, Lucas M, Sun Q, et al. Increased Mortality Risk in Women With Depression and Diabetes Mellitus. Arch Gen Psychiatry. 2011;68:42–50. doi: 10.1001/archgenpsychiatry.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith PJ, Blumenthal JA. Psychiatric and Behavioral Aspects of Cardiovascular Disease: Epidemiology, Mechanisms and Treatment. Rev Esp Cardiol. 2011;64:924–33. doi: 10.1016/j.recesp.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Dimsdale JE. What does Heart Disease have to do with Anxiety? J Am Coll Cardiol. 2010;56:47–8. doi: 10.1016/j.jacc.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Frasure-Smith N, Lesperance F. Depression and Anxiety as Predictors of 2-Year Cardiac Events in Patients with Stable Coronary Artery Disease. Arch Gen Psychiatry. 2008;65:62–71. doi: 10.1001/archgenpsychiatry.2007.4. [DOI] [PubMed] [Google Scholar]
- 22.Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic Attacks and Risk of Incident Cardiovascular Events Among Postmenopausal Women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64:1153–60. doi: 10.1001/archpsyc.64.10.1153. [DOI] [PubMed] [Google Scholar]
- 23.Capuron L, Su S, Miller AH, et al. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry. 2008;64:896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elovainio M, Hurme M, Jokela M, et al. Moderating effect of indoleamine 2,3-dioxygenase (IDO) activation in the association between depressive symptoms and carotid atherosclerosis: Evidence from the Young Finns study. Journal of Affective Disorders. 2011;133:611–4. doi: 10.1016/j.jad.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Grazier KL, Hegedus AM, Carli T, Neal D, Reynolds K. Ingegration of behavioral and physical health care for a Medicaid population through a public-public partnership. Psychiatr Serv. 2003;54:1508–12. doi: 10.1176/appi.ps.54.11.1508. [DOI] [PubMed] [Google Scholar]
- 26.Aldhahi W, Hamdy O. Adipokines, inflammation, and the endothelium in diabetes. Curr Diab Rep. 2003:34293–8. doi: 10.1007/s11892-003-0020-2. [DOI] [PubMed] [Google Scholar]
- 27.Cameron OG. Anxious-depressive comorbidity: effects on HPA axis and CNS noradrenergic functions. Essent Psychopharmacol. 2006;7:24–34. [PubMed] [Google Scholar]
- 28.Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.144. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Muldoon MF, Mackey RH, Sutton-Tyrrell K, Flory JD, Pollock BG, Manuck SB. Lower central serotonergic responsivity is associated with preclinical carotid artery atherosclerosis. Stroke. 2007;38:2228–33. doi: 10.1161/STROKEAHA.106.477638. [DOI] [PubMed] [Google Scholar]
- 30.Barrett JE, Barrett JA, Oxman TE, Gerber PD. The Prevalence of Psychiatric Disorders in a Primary Care Practice. Arch Gen Psychiatry. 1988;45:1100–6. doi: 10.1001/archpsyc.1988.01800360048007. [DOI] [PubMed] [Google Scholar]
- 31.Vreeburg SA, Zitman FG, van Pelt J, et al. Salivary Cortisol Levels in Persons With and Without Different Anxiety Disorders. Psychosomatic Medicine. 2010;72:340–7. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- 32.Arborelius L, Owens M, Plotsky P, Nemeroff C. The role of corticotropin-releasing factor in depression and anxiety disorders. Journal of Endocrinology. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 33.Schäfer M, Mousa SA, Stein C. Corticotropin-releasing factor in antinociception and inflammation. European Journal of Pharmacology. 1997;323:1–10. doi: 10.1016/s0014-2999(97)00057-5. [DOI] [PubMed] [Google Scholar]
- 34.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 35.Kopp S. The influence of neuropeptides, serotonin, and interleukin 1beta on temporomandibular joint pain and inflammation. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons. 1998;56:189–91. doi: 10.1016/s0278-2391(98)90867-9. [DOI] [PubMed] [Google Scholar]
- 36.Zhang K, Xu H, Cao L, Li K, Huang Q. Interleukin-1β inhibits the differentiation of hippocampal neural precursor cells into serotonergic neurons. Brain Res. 2013:1490. doi: 10.1016/j.brainres.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 37.van den Biggelaar AH, Gussekloo J, de Craen AJ, et al. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42:693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Vaccarino V, Johnson BD, Sheps DS, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50:2044–50. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 39.Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314–20. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brietzke E, Mansur RB, Grassi-Oliveira R, Soczynska JK, McIntyre RS. Inflammatory cytokines as an underlying mechanism of the comorbidity between bipolar disorder and migraine. Medical Hypotheses. 2012;78:601–5. doi: 10.1016/j.mehy.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 41.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 42.Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–8. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 43.Hou S-J, Yen F-C, Tsai S-J. Is dysfunction of the tissue plasminogen activator (tPA)-plasmin pathway a link between major depression and cardiovascular disease? Medical Hypotheses. 2009;72:166–8. doi: 10.1016/j.mehy.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Tsai S-J. The possible role of tissue-type plasminogen activator and the plasminogen system in the pathogenesis of major depression. Medical Hypotheses. 2006;66:319–22. doi: 10.1016/j.mehy.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Frasure-Smith N, Lesperance F, Talajic M. The Impact of negative emotions on prognosis following myocardial infaction: Is it more than Depression? Health Psychol. 1995;14:388–98. doi: 10.1037//0278-6133.14.5.388. [DOI] [PubMed] [Google Scholar]
- 46.Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O’Connor JC. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Hormones and Behavior. 2012;62:202–9. doi: 10.1016/j.yhbeh.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maes M, Verkerk R, Bonaccorso S, Ombelet W, Bosmans E, Scharpé S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. 2002;71:1837–48. doi: 10.1016/s0024-3205(02)01853-2. [DOI] [PubMed] [Google Scholar]
- 48.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–62. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teyssier J-R, Chauvet-Gelinier J-C, Ragot S, Bonin B. Up-Regulation of leucocytes Genes Implicated in Telomere Dysfunction and Cellular Senescence Correlates with Depression and Anxiety Severity Scores. PLoS ONE. 2012;7:e49677. doi: 10.1371/journal.pone.0049677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le-Niculescu H, Case NJ, Hulvershorn L, et al. Convergent functional genomic studies of omega-3 fatty acids in stress reactivity, bipolar disorder and alcoholism. Transl Psychiatry. 2011:1. doi: 10.1038/tp.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bot M, Carney RM, Freedland KE, et al. Inflammation and treatment response to sertraline in patients with coronary heart disease and comorbid major depression. J Psychosom Res. 2011;71:13–7. doi: 10.1016/j.jpsychores.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Littrell JL. Taking the Perspective that a Depressive State Reflects Inflammation: Implications for the Use of Antidepressants. Front Psychol. 2012;3:297. doi: 10.3389/fpsyg.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maes M, Song C, Lin AH, et al. Negative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology. 1999;20:370–79. doi: 10.1016/S0893-133X(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 55.Kubera M, Lin AH, Kenis G, Bosmans E, VanBockstaele D, Maes M. Anti-Inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol. 2001a;21:199–206. doi: 10.1097/00004714-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 56.Richards BL, Whittle SL, Buchbinder R. Antidepressants for pain management in rheumatoid arthritis. Cochrane Database Syst Rev. 2011;11:CD008920. doi: 10.1002/14651858.CD008920.pub2. [DOI] [PubMed] [Google Scholar]
- 57.Häuser W, Urrútia G, Tort S, Uçeyler N, Walitt B. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database Syst Rev. 2013;1:CD010292. doi: 10.1002/14651858.CD010292. [DOI] [PubMed] [Google Scholar]
- 58.Bayramgurler D, Karson AOC, Utkan T. Effects of long-term etanercept treatment on anxiety- and depression-like neurobehaviors in rats. Physiol Behav. 2013 Jun 12; doi: 10.1016/j.physbeh.2013.06.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 59.Rikknen K, Matthews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: Antecedent or consequence? Metabolism. 2002;51:1573–7. doi: 10.1053/meta.2002.36301. [DOI] [PubMed] [Google Scholar]
- 60.Skilton MR, Moulin P, Terra JL, Bonnet F. Associations between anxiety, depression, and the metabolic syndrome. Biol Psychiatry. 2007;62:1251–7. doi: 10.1016/j.biopsych.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 61.Janszky I, Ahnve S, Lundberg I, Hemmingsson T. Early-Onset Depression, Anxiety, and Risk of Subsequent Coronary Heart Disease: 37-Year Follow-Up of 49,321 Young Swedish Men. Journal of the American College of Cardiology. 2010;56:31–7. doi: 10.1016/j.jacc.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 62.Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. 2010;56:38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 63.van Reedt Dortland AK, Giltay EJ, van Veen T, Zitman FG, Penninx BW. Longitudinal relationship of depressive and anxiety symptoms with dyslipidemia and abdominal obesity. Psychosom Med. 2013;75:83–9. doi: 10.1097/PSY.0b013e318274d30f. [DOI] [PubMed] [Google Scholar]
- 64.Lopresti AL, Drummond PD. Obesity and psychiatric disorders: Commonalities in dysregulated biological pathways and their implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2013 doi: 10.1016/j.pnpbp.2013.05.005. [Epub-ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Simon GE, VonKorff M. Recognition, management, and outcomes of depression in primary care. Arch Fam Med. 1995;4:99–105. doi: 10.1001/archfami.4.2.99. [DOI] [PubMed] [Google Scholar]
- 66.Elenkov IJ, Chrousos GP. Stress Hormones, Proinflammatory and Antiinflammatory Cytokines, and Autoimmunity. Ann N Y Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 67.Llabre MM, Fitzpatrick SL. Revisiting measurement models in psychosomatic medicine research: a latent variable approach. Psychosom Med. 2012;74(2):169–77. doi: 10.1097/PSY.0b013e3182433a30. [DOI] [PubMed] [Google Scholar]
- 68.Roesch SC, Villodas M, Villodas F. Latent class/profile analysis in maltreatment research: A commentary on Nooner et al. Pears et al, and looking beyond Child Abuse & Neglect. 2010;34:155–60. doi: 10.1016/j.chiabu.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Roesch SC, Norman GJ, Villodas F, Sallis JF, Patrick K. Intervention-mediated effects for adult physical activity: A latent growth curve analysis. Soc Sci Med. 2010;71:494–501. doi: 10.1016/j.socscimed.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Känel R. Psychosocial stress and cardiovascular risk: current opinion. Swiss Med Wkly. 2012 Jan 20;142 doi: 10.4414/smw.2012.13502. [DOI] [PubMed] [Google Scholar]
- 71.Wassertheil-Smoller S, Shumaker S, Ockene J, et al. Depression and cardiovascular sequelae in postmenopausal women. The Women’s Health Initiative (WHI) Arch Intern Med. 2004;164:289–98. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]
- 72.Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–62. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 73.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 74.Kaplan GA. Where do shared pathways lead? Some reflections on a research agenda. Psychosom Med. 1995;57:208–12. doi: 10.1097/00006842-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications. Circulation. 1999;99:2192–217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]