Abstract
Posttraumatic stress disorder (PTSD) is associated with functional abnormalities within aneurocircuitry that includes the hippocampus, amygdala, and medial prefrontal cortex. Evidence of structural abnormalities within these regions, and their association with PTSD severity and symptom burden is, however, sparse. The present study evaluated the relation between indices of gray matter volume and PTSD symptom severity using voxel-based morphometry. Fifteen individuals meeting DSM-IV criteria for PTSDcompleted the Clinician Administered PTSD Scale and underwent structural magnetic resonance imaging. Greater PTSD severity and avoidance/numbing were correlated withincreasedgray matter volume of the right amygdala-hippocampal complex. Greater hyper-arousal was associated with reducedgray matter volume in the left superior medial frontal gyrus. Findings are consistent with current neurocircuitry models of PTSD, which posit that the disorder is associated with structural and functional variance within this distributed network.
Keywords: magnetic resonance imaging, chronic post-traumatic stress disorder, prefrontal cortex, hippocampus, amygdala
1. Introduction
Individuals with posttraumatic stress disorder (PTSD) often show neurobiological differencesin the brain at the functional, structural, and neurochemical levels when compared to healthy controls (Shin & Liberzon, 2010). Neurocircuitry models of PTSD have focused on differences within several key brain regions including the amygdala, hippocampus, and medial prefrontal cortex (PFC) (Yehuda & LeDoux, 2007). This cortico-limbic neurocircuitry is posited to involve top-down emotional regulation of limbic and paralimbic structures by the medial prefrontal cortex. Dysfunction of the medial prefrontal cortex is hypothesized to lead to exaggerated amygdala responses, which are associated with enhanced fear acquisition and exaggerated expression of fear responses (Rauch, Shin, & Phelps, 2006). Functional magnetic resonance imaging (fMRI) data have generally found exaggerated responsiveness of the amygdala to emotionally arousing, but not necessarily trauma-related stimuli among individuals with PTSD (Etkin & Wager, 2007; Liberzon & Sripada, 2008; Rauch et al., 2000; Shin, Rauch, & Pitman, 2006), and greater severity of PTSD symptoms is correlated with increased amygdala responsiveness (Shin et al., 2006). Furthermore, exaggerated activation of the amygdala does not occur in isolation, but is generally associated with reduced activation within the ventromedial prefrontal cortex (VMPFC) (Etkin & Wager, 2007; Liberzon & Sripada, 2008; Shin et al., 2006), supporting the functional connectivity between these two regions. Interestingly, areas of the dorsomedialprefrontal cortex, such as the dorsal anterior cingulate cortex (ACC), which is involved in fear conditioning, show hyperresponsivenessin individuals with PTSD when engaged in recall of prior fear extinction learning (Milad et al., 2009). Evidence of hippocampal dysfunction also has been found in PTSD, although less consistently than altered functioning of the amygdala and VMPFC. The literature to date suggests that PTSD is associated with enhanced hippocampal activation at rest and during emotional tasks, but also hypoactivation of this structure during cognitive tasks (Liberzon & Sripada, 2008; Shin et al., 2006).
Volumetric studies of the brain have also revealeddifferences within frontal-limbic neurocircuitry among individuals with PTSD vs. Non-PTSD comparison subjects. Early studies showed reduced volume within the pregenual and subcallosal cortex, which also includes regions of the medial prefrontal and orbitofrontal cortex (Chen, Li, Xu, & Liu, 2009; Felmingham et al., 2009; Geuze et al., 2008; Sui et al., 2010; Tavanti et al., 2012; Thomaes et al., 2010), among women with PTSD compared to trauma-exposed women without PTSD (Rauch et al., 2003). This finding has been replicated across a number of studies (Chen et al., 2006; Eckart et al., 2011; Felmingham et al., 2009; Ferrari, Busatto, McGuire, & Crippa, 2008; Thomaes et al., 2010; Yamasue et al., 2003). Notably, reduced gray matter volume within these regions was not found in monozygotic twins discordant for the development of PTSD, suggesting that it reflects a consequence rather than a predisposing risk factor for the disorder (Kasai et al., 2008). Hippocampal gray matter reduction in PTSD vs. Non-PTSD subjects is among the mostreplicated finding in this literature (Bonne et al., 2008; Bremner et al., 1997; Chen et al., 2006; Emdad et al., 2006; Felmingham et al., 2009; Gurvits et al., 1996; Lindauer et al., 2004; Thomaes et al., 2010; Villarreal et al., 2002; Vythilingam et al., 2005; Wang et al., 2010; Winter & Irle, 2004; Yehuda et al., 2007), although some have failed to find such differences (Bonne et al., 2001; Fennema-Notestine, Stein, Kennedy, Archibald, &Jernigan, 2002; Golier et al., 2005; Pederson et al., 2004). Some evidence suggests that small hippocampal volume may confer a biological vulnerability to developing PTSD if exposed to a traumatic event (Gilbertson et al., 2002). Finally, amygdala volume has not been extensively studied in patients with PTSD. Although limited evidence suggests that PTSD may be associated with smaller volumes of this structure (Bremner et al., 1997), other studies have found no differences in amygdala volume compared to healthy controls (De Bellis, Hall, Boring, Frustaci, & Moritz, 2001; Fennema-Notestine, Stein, Kennedy, Archibald, & Jernigan, 2002; Lindauer et al., 2004; Wignall et al., 2004).
While PTSD appears to be associated with differences in brain morphology, there is limited research on the relation between gray matter volume and specific domains of PTSD symptomatology (i.e., re-experiencing, avoidance/numbing, and hyper-arousal symptoms). On the whole, greater severity of PTSD symptoms has been associated with reduced gray matter volume, particularly within the hippocampus (Gurvits et al., 1996; Lindauer et al., 2004; Villarreal et al., 2002; Vythilingam et al., 2005; Winter & Irle, 2004; Zhang et al., 2011), and less consistently for bilateral calcarine cortex (Zhang et al., 2011), left ACC (Yamasue et al., 2003), and regions of the cerebellum (Baldacara et al., 2011). With regard to specific symptom clusters, the severity of re-experiencing symptomshas been associated with reduced gray matter in the left middle temporal gyrus, left operculum/insula, right inferior occipital and precentral gyrus (Kroes, Whalley, Rugg, & Brewin, 2011b), left frontal cortex (Tavanti et al., 2012), left cerebellum and cerebellar vermis (Baldacara et al., 2011). Similarly, greater avoidance/numbing symptoms have been associated with reduced gray matter in the left ACC (Araki et al., 2005), left cerebellum and cerebellar vermis (Baldacara et al., 2011). Finally, hyper-arousal symptoms were inversely related to gray matter density of the dorsal ACC (Thomaes et al., 2010), left cerebellum and cerebellar vermisin one study (Baldacara et al., 2011). Few studies, however, have specifically examined the relation between PTSD symptoms and amygdala volume (Matsuoka, Yamawaki, Inagaki, Akechi, & Uchitomi, 2003; Rogers et al., 2009). Matsuoka et al. (Matsuoka et al., 2003) found smaller left amygdala volume in Non-PTSD cancer survivorsendorsing clinically significant intrusive recollectionscompared to cancer survivors without a history of intrusive memories. Volumetric amygdala differences did not emerge when the sample was dichotomized with regard to avoidance/ numbing or hyper-arousal. Rogers et al. (Rogers et al., 2009) reported smaller bilateral amygdala volumes in patients with a lifetimediagnosis of PTSD compared to trauma-exposed non-PTSD subjects. Furthermore, in the PTSD-positive group, greater avoidance involved smaller amygdala volume in the left hemisphere. Given the sparse literature, we, therefore, conducted a voxel-based morphometry (VBM) study to investigate whether the severity of symptom presentation among the three clusters (i.e., re-experiencing, avoidance, and hyper-arousal) was related to gray matter volume, particularly within the amygdala, given its prominence in neurocircuitry models of PTSD. Based on functional and morphometric findings in PTSD, we hypothesized that the clinical severity of PTSD symptoms would be inversely related to the gray matter volume of hippocampus and medial PFC. Given the scarcity and inconsistency in the literature on amygdala volume in PTSD, we did not form a directional hypothesisabout the relationship of PTSD symptomatology with amygdala volume.
2. Materials and method
2.1 Participants
As part of a larger neuroimaging study comparing three anxiety disorders (14 individuals with Panic Disorder, 15 individuals with Specific Animal Phobia, 22 healthy controls), we studied fifteen right-handed adults (mean age = 35.6 years, SD = 12.7, range: 22–58; 10 females, 5 males) meeting criteria for PTSD based on a clinical interview. Potential volunteers were excluded for known medical or neurological conditions including any history of seizures, significant head trauma, or known structural abnormalities. Volunteers with contraindications for MRI scanning, including pregnancy, were excluded. Participants were recruited via flyers posted in community centers, physician referrals, and local newspaper and internet advertisements within the Boston metropolitan area. Participants were paid $150for their time, and all provided written informed consent prior to enrollment. The McLean Hospital Institutional Review Board reviewed and approved all study procedures in accordance with the most recent Declaration of Helsinki.
2.2. Materials and Procedure
Each volunteer underwent a psychiatric interview using the Structured Clinical Interview for DSM-IV Disorders Patient Edition (SCID-I/P) (First, Spitzer, Gibbon, & Williams, 2002), administered by a trained research staff memberto ensure that the DSM-IV diagnosis of PTSD and exclusion criteria were met. To assess symptom severity, the Clinician Administered PTSD Scale (CAPS) (Blake et al., 1995) was administered to each PTSD participantbefore scanning. Current PTSD symptom severity ranged from mildto extreme (40.0% mild– CAPS total 20–39; 33.3% moderate– CAPS total 40–59; 13.3% severe– CAPS total 60–79; 13.4% extreme– CAPS total ≥80) (Weathers, Keane, & Davidson, 2001).Table A.1 presents descriptive statistics for the CAPS. The clinical sample comprised a variety of non-combat traumatic experiences, includingrape/sexual assault (n=5), robbery (n=3), childhood sexual abuse (n=2), physical assault (n=3), traffic accident (n=1), and sudden death of a close friend (n=1). The mean time since trauma was 13 years (SD 13.5; range: 0.4 – 43.0 years). In addition to the PTSD diagnosis, 40% of patients also met DSM-IV criteria for up to two comorbid disorders:current specific phobia (n=2);lifetimepanic disorder without agoraphobia (n=1);lifetime major depressive disorder (n=2); lifetime alcohol substance abuse (n=2). No participants were on daily psychiatric medication, with only one reporting use of Ambien up to two times a week.
Table A.1.
Mean, standard deviation (SD) and range of CAPS scores in PTSD subjects (n =15)
| CAPS measure | Mean±SD | (range) |
|---|---|---|
| Total Sum score | 50.0±20.25 | (23–93) |
| Re-experiencing | 15.1±5.41 | (5–27) |
| Avoidance/ Numbing | 20.0±12.18 | (4–50) |
| Hyper-arousal | 14.93±7.59 | (5–30) |
Note on PTSD Severity. CAPS total 20–39 = mild; CAPS total 40–59 - moderate; CAPS total 60–79 = severe; CAPS total ≥80 = extreme)
2.3 MRI parameters
MRI was conducted at 3.0 Tesla (SIEMENS Tim Trio) using a quadrature or 12-channelheadcoil. A T1-weighted 3DMPRAGE sequence (TR/ TE/ flip angle = 2.53s/3.39ms/7°) was used to obtain 128 sagittal slices (256×256 matrix) with a slice thickness 1.33mm and a voxel size of 1.3mmx1mm x1.3mm.
2.4 Voxel-based morphometry (VBM)
VBM analysis was performed with the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html; version r435) in SPM8 (Wellcome Department of Imaging Neuroscience Group, London, UK; http://www.fil.ion.ucl.ac.uk/spm; update revision number 4667). Preprocessing usedthe VBM8 default settings for modulated VBM (i.e., total brain volume served as a covariate). In brief, T1-weighted structural images were DARTEL-normalized to Montreal Neurological Institute (MNI) space and then segmented into graymatter, white matter and cerebrospinal fluid. Normalized gray matter images were then smoothed with a 10mm full-width at half-maximum (FWHM) Gaussian kernel.
2.5 Statistical Analysis
Descriptive statistics and bivariate pearson correlations between the non-normally distributed CAPS subscale scores were calculated using IBM SPSS Statistics 20.0 for Macintosh. In SPM8, normalized smoothed gray matter images were entered into a series ofrandom effectsmultiple regression whole-brain analyses (absolute threshold: 0.2; p<.001, uncorrected, cluster threshold k≥30) in SPM8. The primary model assessed gray matter correlates of overall PTSD severity and thus included the CAPS Total sum score as the non-centered regressor. Three additional modelsassessed gray matter correlates of each of the three PTSD symptom clusters, i.e., re-experiencing, avoidance/numbing, and hyper-arousal, and thusincluded these subscale scores as GMV non-centered regressors. All models included age and gender as non-centered nuisance covariates.
3. Results
Re-experiencing did not correlate with avoidance/numbing (r =.11, p=.71) or hyper-arousal (r =.10, p=.72). Avoidance/numbing was positively associated with hyper-arousal (r =.83, p<.001).
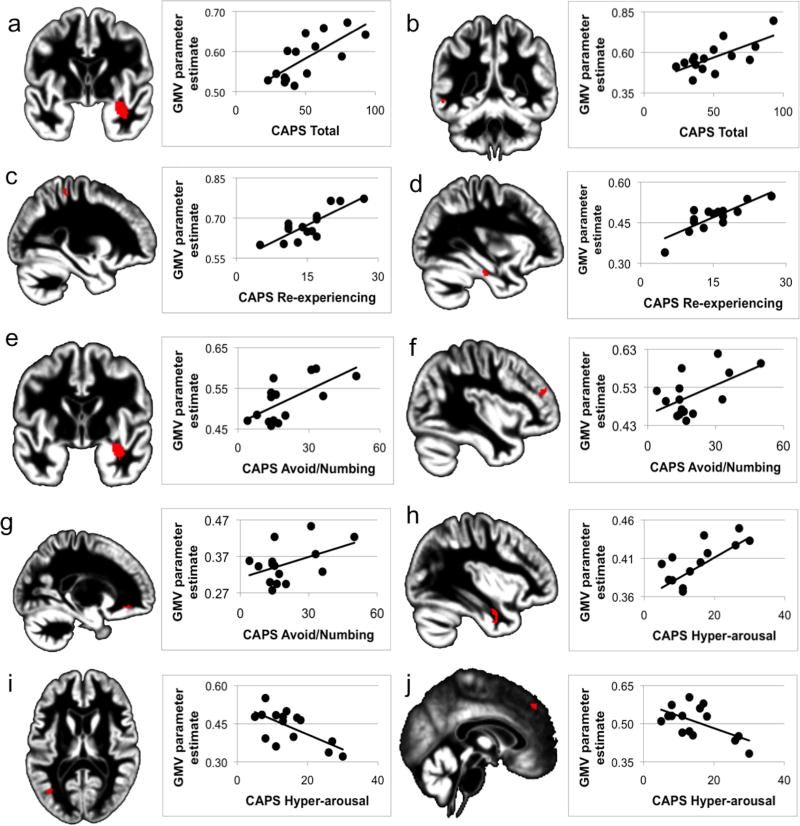
Severity of PTSD as assessed by CAPS Total and symptom cluster scores predicted gray matter volume (GMV) of both subcortical and cortical structures (Table A.2). Specifically, PTSD severity correlated positivelywithGMV within the right amygdala-hippocampal complex and left middle temporal gyrus (Figure A.1a and A.1b).
Table A.2.
Gray matter correlates of PTSD severity and symptomatology
| CAPS measure | Brain region | Volume | Peak-level coordinates | T | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Positive correlations | ||||||
| Sum score | R. AHC | 468 | 34 | −1 | −26 | 6.82 |
| L. Temporal Middle Gyrus | 34 | −56 | −46 | −9 | 4.50 | |
| Re-experiencing | R. Postcentral Gyrus | 58 | 27 | −37 | 58 | 5.50 |
| L. Fusiform Gyrus | 30 | −32 | −22 | 26 | 4.64 | |
| Avoidance | R. AHC | 350 | 36 | −3 | −24 | 6.64 |
| R. Cerebellum | 141 | 8 | −43 | −56 | 5.57 | |
| L. Cerebellum | 83 | −21 | −46 | −62 | 4.79 | |
| R. Frontal Middle Gyrus | 61 | 40 | 44 | 19 | 5.01 | |
| L. Frontal inferior orbital gyrus | 50 | −20 | 36 | −11 | 4.76 | |
| Hyper-arousal | R. Fusiform Gyrus/ Temporal Inferior Gyrus | 87 | 38 | −1 | −35 | 7.09 |
| Negative correlations | ||||||
| Hyper-arousal | L. Temporal Middle Gyrus | 37 | −36 | −67 | 12 | 5.26 |
| R. Frontal Superior Medial Gyrus | 57 | 2 | 39 | 40 | 4.82 | |
Notes. L = left hemisphere, R = right hemisphere. Atlas coordinates are listed in the standard space of the Montreal Neurological Institute (MNI). Volumes are in voxels (1.5mm × 1.5mm × 1.5mm). All clusters are significant at p < .001 (uncorrected), k ≥ 30. CAPS – Clinician Administered PTSD scale, AHC – Amygdala-Hippocampus Complex.
Figure A.1.
Brain regions with significant correlations between gray matter volume (GMV) and PTSD severity and symptomatology superimposed on normalized meanstudy-specific structural image and corresponding scatterplots.
At the symptom cluster level, re-experiencing correlated positively with GMV of right postcentral gyrus and left fusiform gyrus (Figure A.1c and A.1d). Greater endorsement of avoidance/numbing symptoms was associated with greater GMV within the rightamygdala-hippocampal complex, bilateral cerebellum, the orbital region of the left inferior frontal gyrus and the middle frontalgyrus (Figure A.1e to A.1g; cerebellum not shown). Greater hyper-arousal was correlated with greater GMV within the right fusiform gyrus and temporal inferior gyrus (Figure A.1h). Also, greater hyper-arousal was inversely correlated with GMV within the left temporal middle gyrus and right superior medial frontal gyrus (Figure A.1i and A.1j).
4. Discussion
In the present study of participants meeting criteria for PTSD, severity of posttraumatic stress symptoms was associated with gray matter volume in several brain regions, including those involved in the hypothesized fear neurocircuitry. Specifically, greater PTSD severity, as defined by the CAPS Total sum score (Blake et al., 1995), as well as greater endorsement of symptoms consistent with effortful avoidance and emotional numbing, was associated with larger gray matter volume within the right amygdala and right anterior hippocampus. Unlike findings for the amygdala, greater hyper-arousal was correlated with smaller gray matter volume in the right medial prefrontal cortex. The present findings support the hypothesized contribution of these regionsto PTSD symptomatology (Sierra-Mercado, Padilla-Coreano, & Quirk, 2011), suggesting that the severity of PTSD symptoms is associated with increased volume within limbic structures involved in immediate threat processing, attribution of affective salience, and contextual memory functions (Etkin, 2010) and reduced gray matter volume within emotional appraisal and regulation regions of the prefrontal cortex.
Our data also appear to reflect the close link between memory and emotion in PTSD, as overall symptom severity, was associated with larger volume of the amygdala and hippocampus. Functional neuroimaging studies have reliably shown that the amygdala is hyper-responsive among individuals with PTSD (Etkin & Wager, 2007; Shin & Liberzon, 2010). In addition to its role in detecting and orienting attention towardpotential threat (Khoury-Malhame et al., 2011), the amygdala also plays a critical role in modulating hippocampal memory encoding (Packard & Teather, 1998), and evidence suggests that the independent memory systems of the amygdala and hippocampus interact to encode the emotional significance of events (Phelps, 2004). Animal research has shown thatchronic stress induces significant increases indendritic length, the number of dendritic branching points, and synaptic interconnectivity in the amygdala (Mitra, Jadhav, McEwen, Vyas, & Chattarji, 2005; Vyas, Mitra, Shankaranarayana Rao, & Chattarji, 2002). It is not inconceivable that present findings of a relation between gray matter volume in these regions may parallel this stress-induced morphologic remodelingseen in animal studies and may even reflect the outcome of similar stress-induced remodeling processes. Testing this proposition, however, would require longitudinal research combining measures of functional and structural brain integrity.
PTSD is a heterogeneous disorder with regard to the symptoms that may be exhibited (Zoellner & Rothbaum, 2011), and this heterogeneity was also reflected in the voxel-based morphometric neurocorrelates. Specifically, there was minimal overlap in the brain regions that were predicted by the three symptom clusters re-experiencing, avoidance/ numbing, and hyper-arousal. This is in line with the clinical observation that symptoms may occur independent of each other (McMillen, North, & Smith, 2000) and suggests that different symptom clusters may not only be present as clinical entities, but also with differential neurocorrelates. Interestingly, of the three symptom clusters, only avoidance/ numbing predicted gray matter volume of the right amygdala-hippocampus complex. Avoidance/ numbing is central to the PTSD diagnosis (McMillen et al., 2000), and our data suggest that it may be particularly linked to the central components of the fear neurocircuitry, particularly the amygdala and hippocampus. This finding needs to be confirmed in a larger independent sample.
Our findings also support the hypothesized role of the medial PFC in modulating amygdala responses and regulating the experience of anxiety symptoms such as hyper-arousal, as we found gray matter volume of the right medial PFC to be inversely related to symptoms of hyper-arousal in the present sample of individuals with PTSD. This is consistent with neurocircuitry models that propose the exaggerated responsiveness of the amygdala in individuals with PTSD might perhaps be secondary to a dysfunction in top-down regulation by the medial prefrontal cortex (Etkin, 2010; Liberzon & Sripada, 2008; Rauch et al., 2006). A meta-analytic review of the neuroimaging literature demonstrated that PTSD was consistently associated with reduced functional activation within medial prefrontal cortex, a feature that appeared to discriminate that condition from other forms of anxiety disorders (Etkin & Wager, 2007). Notably, animal research has shown that repeated stress yields significant reductions in the number and length of dendrites as well as their spine density in the medial PFC (Radley et al., 2006), and human studies suggest that reduced volume of the medial PFC emerges as a function of exposure to traumatic stress rather than as a pre-disposing vulnerability to the effects of stress (Kasai et al., 2008). Interestingly, in contrast to the significant correlations for avoidance/ numbing, hyper-arousal symptoms did not predict gray matter volume of the right amygdala and hippocampus. We found this somewhatsurprising, particularly as there was a positive correlation between avoidance/numbing and hyper-arousal. However, this lack of association is in line with recent animal research that showed hyper-arousal to be independent of mesial temporal functions such as contextual fear memory (Siegmund & Wotjak, 2007). This finding, however, needs independent replication.
In addition to the apriori hypothesized correlations between PTSD symptom severity and specific structures of the fear neurocircuitry, we also found correlations between PTSD symptomatology and a variety of clusters in non-hypothesized regions, including temporal and frontal regions. Due to their post-hoc nature, we do not interpret these findings, but merely report them for completeness. These findings are consistent with the anatomical location, but not necessarily the direction, of previously reported associations between PTSD trauma load andgray matter volume of the postcentral gyrus, middle frontal gyrus, fusiform, gyrus and frontal inferior gyrus (Felmingham et al., 2009; Kroes, Rugg, Whalley, & Brewin, 2011a; Nardo et al., 2010; Sui et al., 2010; Tavanti et al., 2012). We also found structural volume withinthe cerebellum to be positively associated with greater endorsement of PTSD symptomatology. The cerebellum has long been implicated in emotion regulation (Schutter & van Honk, 2005), though little is known about the exact nature of its contribution. Similar to limbic regions, evidence forcerebellar structural abnormalities in PTSD is mixed with some findings pointing toward left-hemispheric volumetric reductions and negative associations with PTSD symptoms (Baldacara et al., 2011; Nardo et al., 2010), while others reportedneither volumereductions nor associations with PTSD symptomatology (Levitt et al., 2006). Our results indicate that PTSD severity is related to structural remodeling and increased gray matter within bilateral cerebellum. In contrast, no other regions showed reduced gray matter volume with increasing symptom severity. Overall, our data suggest that cortical and subcortical regions beyond the amygdala, hippocampus and medial PFC are likely be involved in PTSD, although these findings should be interpreted with caution, as they were not hypothesized a priori.
The positive correlation between the right amygdala-hippocampal complex and PTSD severity and avoidance/ numbing respectively may be interpreted two ways. It may relate to increased gray matter volume in either the hippocampus or the amygdala. Alternatively, it may reflect increased gray matter volume at the interface of the amygdala and hippocampus. Due to the methodological limitations of VBM and the influence of structural image acquisition, as well as the VBM pre-processing parameters such as original voxel size, 10mm smoothing kernel and reslicing, a structure-specific interpretation remains imprecise.
While the present findings suggest that PTSD symptoms are associated with variance in structural volumes within the primary nodes of the hypothesized cortico-limbic neurocircuitry, several caveats should be considered. First, while the correlations observed between symptom severity and gray matter volumes within the cortico-limbic PTSD neurocircuitry are generally congruent with the direction of activation and deactivation observed among functional neuroimaging studies (Rauch et al., 2000; 2006; Shin et al., 2006), some of the findings are not consistent with several of the existing volumetric studies on PTSD. Past research has mostly, but not exclusively shown an inverse relationship between PTSD symptomatology and GMV of the hippocampus (Bonne et al., 2008; 2001; Bremner et al., 1997; Chen et al., 2006; Emdad et al., 2006; Felmingham et al., 2009; Fennema-Notestine et al., 2002; Golier et al., 2005; Gurvits et al., 1996; Lindauer et al., 2004; Pederson et al., 2004; Thomaes et al., 2010; Villarreal et al., 2002; Vythilingam et al., 2005; Wang et al., 2010; Winter & Irle, 2004; Yehuda et al., 2007). This negative association, however, was primarily reported for the left hippocampus, whereas our findings were specifically observed in the right mesial temporal structures. Furthermore, the relationship between symptom severity and the right amygdala-hippocampal volumes seen in our study is consistent with findings from a recent meta analysis of brain morphology studies of PTSD, which showed that these structures tended to be larger on the right than the left among this population (Woon & Hedges, 2009). Also, our sample was comparatively modest in size and comprised a range of PTSD severities, mostly mild to moderate. This variation in PTSD severity allowed the correlational analyses to be conducted; however, a larger sample size would have been desirable. Likewise, the range of PTSD severities included in the current investigation restricts generalizability of our findings beyond a comparable sample composition. Furthermore, the relatively small sample comprised more females than males. This imbalance and limited statistical power prevented us from investigating whether gender-specific differences in PTSD symptom severity might be reflected at the brain-morphological level. Despite potentially higher prevalence of traumatic events in men, prior work suggests that women seem more vulnerable to develop PTSD following a traumatic event (Gavranidou & Rosner, 2003), though there is no consistent evidence for gender-specific symptom presentation in PTSD (Freedman et al., 2002). Future studies should address this important issue, as sex differences in these associations may emerge with larger samples. Comorbid psychiatric disorders such as Major Depressive Disorder (MDD) that are known to be associated with brain morphological changes (Amico et al., 2011) are a frequent characteristic of clinical samples such as ours, and therefore complicate interpretation. Although we cannot exclude the possibility that these comorbidities may have influenced our data, the majority of our participants had no additional history of other psychiatric disorders as determined by clinical interview, so the influences are likely to be modest. Lastly, readers should bear the correlational nature of the current investigation in mind. Because the data were collected cross-sectionally, the causal direction of the associations found cannot be deduced. The gray matter variations we observed mayhave preceded the trauma and led to a greater emotional response to the events, or conversely, may have emerged a consequence of trauma. The current literature suggests that the former may be true for the hippocampal region (Gilbertson et al., 2002), but the latter may be the case for the medial regions of the PFC, with research in both animals and humans pointing towards brain-morphological changes in the anterior cingulate cortex occurring as a function of exposure to traumatic stress (Kasai et al., 2008; Radley et al., 2006). A longitudinal study using a large, balance sampleand at least two assessment waves might be able to successfully address this important issue.
5. Conclusions
Overall, the present data confirm that PTSD severity is associated with variability in gray matter volume and suggest the potential for brain morphological remodeling, but also suggest that this remodeling is not necessarily unidirectional towards gray matter atrophy with greater PTSD symptomatology. Rather our data suggest that the three different symptom clusters may be associated with differential structural remodeling processes in associated, but not necessarily overlapping brain regions. More specifically, gray matter appears to be reduced within the medial PFC and increasedwithin the amygdala-hippocampal complexin association with greater severity of PTSD symptoms. These findings are consistent with current neurocircuitry models of the disorder that emphasize dysfunction of cortico-limbic affect processing systems.
Acknowledgments
Funding: This research was supported by an NIMH grant (R01 MH70730-01A2).
Footnotes
Conflicts of interests: None declared.
Contributor Information
Mareen Weber, Email: mweber@mclean.harvard.edu.
William D.S. Killgore, Email: killgore@mclean.harvard.edu.
References
- Amico F, Meisenzahl E, Koutsouleris N, Reiser M, Moller HJ, Frodl T. Structural MRI correlates for vulnerability and resilience to major depressive disorder. J Psychiatry Neurosci. 2011;36(1):15–22. doi: 10.1503/jpn.090186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Kasai K, Yamasue H, Kato N, Kudo N, Ohtani T, et al. Association between lower P300 amplitude and smaller anterior cingulate cortex volume in patients with posttraumatic stress disorder: a study of victims of Tokyo subway sarin attack. Neuroimage. 2005;25(1):43–50. doi: 10.1016/j.neuroimage.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Baldacara L, Jackowski AP, Schoedl A, Pupo M, Andreoli SB, Mello MF, et al. Reduced cerebellar left hemisphere and vermal volume in adults with PTSD from a community sample. J Psychiatr Res. 2011;45(12):1627–1633. doi: 10.1016/j.jpsychires.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158(8):1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne O, Vythilingam M, Inagaki M, Wood S, Neumeister A, Nugent AC, et al. Reduced posterior hippocampal volume in posttraumatic stress disorder. J Clin Psychiatry. 2008;69(7):1087–1091. doi: 10.4088/jcp.v69n0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse–a preliminary report. Biological Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Li L, Xu B, Liu J. Insular cortex involvement in declarative memory deficits in patients with post-traumatic stress disorder. BMC Psychiatry. 2009;9:39. doi: 10.1186/1471-244X-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xia W, Li L, Liu J, He Z, Zhang Z, et al. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Res. 2006;146(1):65–72. doi: 10.1016/j.pscychresns.2005.09.006. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological Psychiatry. 2001;50(4):305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- Eckart C, Stoppel C, Kaufmann J, Tempelmann C, Hinrichs H, Elbert T, et al. Structural alterations in lateral prefrontal, parietal and posterior midline regions of men with chronic posttraumatic stress disorder. J Psychiatry Neurosci. 2011;36(3):176–186. doi: 10.1503/jpn.100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdad R, Bonekamp D, Sondergaard HP, Bjorklund T, Agartz I, Ingvar M, Theorell T. Morphometric and psychometric comparisons between non-substance-abusing patients with posttraumatic stress disorder and normal controls. Psychother Psychosom. 2006;75(2):122–132. doi: 10.1159/000090897. [DOI] [PubMed] [Google Scholar]
- Etkin A. Functional neuroanatomy of anxiety: a neural circuit perspective. Current Topics in Behavioral Neurosciences. 2010;2:251–277. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Whitford TJ, Falconer E, Kemp AH, Peduto A, Bryant RA. Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport. 2009;20(16):1402–1406. doi: 10.1097/WNR.0b013e3283300fbc. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biological Psychiatry. 2002;52(11):1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- Ferrari MC, Busatto GF, McGuire PK, Crippa JA. Structural magnetic resonance imaging in anxiety disorders: an update of research findings. Rev Bras Psiquiatr. 2008;30(3):251–264. doi: 10.1590/s1516-44462008000300013. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Freedman SA, Gluck N, Tuval-Mashiach R, Brandes D, Peri T, Shalev AY. Gender differences in responses to traumatic events: A prospective study. Journal of Traumatic Stress. 2002;15(5):407–413. doi: 10.1023/A:1020189425935. [DOI] [PubMed] [Google Scholar]
- Gavranidou M, Rosner R. The weaker sex? Gender and post-traumatic stress disorder. Depress Anxiety. 2003;17(3):130–139. doi: 10.1002/da.10103. [DOI] [PubMed] [Google Scholar]
- Geuze E, Westenberg HG, Heinecke A, de Kloet CS, Goebel R, Vermetten E. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008;41(3):675–681. doi: 10.1016/j.neuroimage.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golier JA, Yehuda R, De Santi S, Segal S, Dolan S, de Leon MJ. Absence of hippocampal volume differences in survivors of the Nazi Holocaust with and without posttraumatic stress disorder. Psychiatry Res. 2005;139(1):53–64. doi: 10.1016/j.pscychresns.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biological Psychiatry. 1996;40(11):1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological Psychiatry. 2008;63(6):550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury-Malhame El M, Reynaud E, Soriano A, Michael K, Salgado-Pineda P, Zendjidjian X, et al. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia. 2011;49(7):1969–1973. doi: 10.1016/j.neuropsychologia.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Kroes MC, Rugg MD, Whalley MG, Brewin CR. Structural brain abnormalities common to posttraumatic stress disorder and depression. J Psychiatry Neurosci. 2011a;36(4):256–265. doi: 10.1503/jpn.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes MC, Whalley MG, Rugg MD, Brewin CR. Association between flashbacks and structural brain abnormalities in posttraumatic stress disorder. Eur Psychiatry. 2011b;26(8):525–531. doi: 10.1016/j.eurpsy.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, Chen QC, May FS, Gilbertson MW, Shenton ME, Pitman RK. Volume of cerebellar vermis in monozygotic twins discordant for combat exposure: lack of relationship to post-traumatic stress disorder. Psychiatry Res. 2006;148:2–3. 143–149. doi: 10.1016/j.pscychresns.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, et al. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biological Psychiatry. 2004;56(5):356–363. doi: 10.1016/j.biopsych.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Yamawaki S, Inagaki M, Akechi T, Uchitomi Y. A volumetric study of amygdala in cancer survivors with intrusive recollections. Biological Psychiatry. 2003;54(7):736–743. doi: 10.1016/s0006-3223(02)01907-8. [DOI] [PubMed] [Google Scholar]
- McMillen JC, North CS, Smith EM. What parts of PTSD are normal: intrusion, avoidance, or arousal? Data from the Northridge, California, earthquake. J Trauma Stress. 2000;13(1):57–75. doi: 10.1023/A:1007768830246. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardo D, Hogberg G, Looi JC, Larsson S, Hallstrom T, Pagani M. Gray matter density in limbic and paralimbic cortices is associated with trauma load and EMDR outcome in PTSD patients. J Psychiatr Res. 2010;44(7):477–485. doi: 10.1016/j.jpsychires.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Amygdala modulation of multiple memory systems: hippocampus and caudate-putamen. Neurobiology of Learning and Memory. 1998;69(2):163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- Pederson CL, Maurer SH, Kaminski PL, Zander KA, Peters CM, Stokes-Crowe LA, Osborn RE. Hippocampal volume and memory performance in a community-based sample of women with posttraumatic stress disorder secondary to child abuse. J Trauma Stress. 2004;17(1):37–40. doi: 10.1023/B:JOTS.0000014674.84517.46. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14(2):198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biological Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, et al. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14(7):913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Yamasue H, Abe O, Yamada H, Ohtani T, Iwanami A, et al. Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Res. 2009;174(3):210–216. doi: 10.1016/j.pscychresns.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J. The cerebellum on the rise in human emotion. Cerebellum. 2005;4(4):290–294. doi: 10.1080/14734220500348584. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. Hyperarousal does not depend on trauma-related contextual memory in an animal model of Posttraumatic Stress Disorder. Physiol Behav. 2007;90(1):103–107. doi: 10.1016/j.physbeh.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2011;36(2):529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui SG, Wu MX, King ME, Zhang Y, Ling L, Xu JM, et al. Abnormal grey matter in vicitims of rape with PTSD in Mainland China: a voxel-based morphometry study. Acta Neuropsychiatrica. 2010;22:118–126. doi: 10.1111/j.1601-5215.2010.00459.x. [DOI] [PubMed] [Google Scholar]
- Tavanti M, Battaglini M, Borgogni F, Bossini L, Calossi S, Marino D, et al. Evidence of diffuse damage in frontal and occipital cortex in the brain of patients with post-traumatic stress disorder. Neurol Sci. 2012;33(1):59–68. doi: 10.1007/s10072-011-0659-4. [DOI] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, van Balkom AJ, Smit JH, Veltman DJ. Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. J Clin Psychiatry. 2010;71(12):1636–1644. doi: 10.4088/JCP.08m04754blu. [DOI] [PubMed] [Google Scholar]
- Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, et al. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biological Psychiatry. 2002;52(2):119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Luckenbaugh DA, Lam T, Morgan CA, 3, Lipschitz D, Charney DS, et al. Smaller head of the hippocampus in Gulf War-related posttraumatic stress disorder. Psychiatry Res. 2005;139(2):89–99. doi: 10.1016/j.pscychresns.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67(3):296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Wignall EL, Dickson JM, Vaughan P, Farrow TF, Wilkinson ID, Hunter MD, Woodruff PW. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biological Psychiatry. 2004;56(11):832–836. doi: 10.1016/j.biopsych.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Winter H, Irle E. Hippocampal volume in adult burn patients with and without posttraumatic stress disorder. Am J Psychiatry. 2004;161(12):2194–2200. doi: 10.1176/appi.ajp.161.12.2194. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Amygdala volume in adults with posttraumatic stress disorder: a meta-analysis. J Neuropsychiatry Clin Neurosci. 2009;21(1):5–12. doi: 10.1176/appi.neuropsych.21.1.5. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A. 2003;100(15):9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56(1):19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Tischler L, Harvey PD, Newmark R, Yang RK, Buchsbaum MS. Hippocampal volume in aging combat veterans with and without post-traumatic stress disorder: relation to risk and resilience factors. J Psychiatr Res. 2007;41(5):435–445. doi: 10.1016/j.jpsychires.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Zhang J, Tan Q, Yin H, Zhang X, Huan Y, Tang L, et al. Decreased gray matter volume in the left hippocampus and bilateral calcarine cortex in coal mine flood disaster survivors with recent onset PTSD. Psychiatry Res. 2011;192(2):84–90. doi: 10.1016/j.pscychresns.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Zoellner LA, Rothbaum BO. PTSD not an anxiety disorder? DSM committee proposal turns back the hands of time. Depression and Anxiety. 2011 doi: 10.1002/da.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]