Abstract
While it is known that mice lacking melanocortin 4 receptor (MC4R) expression develop hyperphagia resulting in early-onset obesity, the specific neural circuits that mediate this process remain unclear. Here, we report that selective restoration of MC4R expression within dopamine-1 receptor-expressing neurons [MC4R/dopamine 1 receptor (D1R) mice] partially blunts the severe obesity seen in MC4R-nullmice by decreasing meal size, but not meal frequency, in the dark cycle. We also report that both acute cocaine-induced anorexia and the development of locomotor sensitization to repeated administration of cocaine are blunted in MC4R-null mice and normalized in MC4R/D1R mice. Neuronal retrograde tracing identifies the lateral hypothalamic area as the primary target ofMC4R-expressing neurons in the nucleus accumbens. Biochemical studies in the ventral striatum show that phosphorylation of DARPP-32Thr-34 and GluR1Ser-845 is diminished in MC4R-null mice after chronic cocaine administration but rescued in MC4R/D1R mice. These findings highlight a physiological role of MC4R-mediated signaling within D1R neurons in the long-term regulation of energy balance and behavioral responses to cocaine.
Keywords: Cocaine, dopamine-1 receptor, food intake, locomotor sensitization, melanocortin 4 receptor, obesity
The central melanocortin system is crucial for maintaining body energy homeostasis through melanocortin 3 receptor and melanocortin 4 receptor (MC4R) signaling (Cone 2005; Xu et al. 2011). The MC4R gene mutations are the most common monogenic cause of obesity in humans (Tao 2009) and mice lacking MC4Rs are markedly hyperphagic and obese (Balthasar et al. 2005; Huszar et al. 1997). One important question that remains to be answered is if MC4R-null mice are more sensitive to the effects of highly palatable foods such as high-fat diet (HFD). Several studies have reported that obesity in MC4R-null mice can be severely exacerbated by HFD feeding in both purified and chow-based diets (Albarado et al. 2004; Butler et al. 2001; Srisai et al. 2011), suggesting that the MC4R pathway might differentially affect consumption of palatable diets. In contrast, two recent articles show that MC4R-null mice display a paradoxical reduction in consumption of HFD when presented with either a two-choice diet test (Panaro & Cone 2013) or when required to perform effortful responding for HFD pellets (Cui et al. 2012a).
While several studies have implicated the hypothalamus and brainstem as important sites of MC4R action in regulat-ingmetabolic function (Xu et al. 2011), MC4Rs are expressed in other forebrain limbic regions including prefrontal cortex, hippocampus, amygdala and striatum (Liu et al. 2003). This broad anatomical distribution of MC4Rs supports recent findings that either pharmacologic or genetic manipulation of brainMC4R signaling affects measures of mood, anxiety and reward behaviors in rodents (Boghossian et al. 2010; Chaki & Okuyama 2005; Chuang et al. 2010). The nucleus accum-bens (NAc) within the ventral striatum is a well-established brain region known to regulate reward behaviors not only for drugs of abuse but also for natural rewards such as sucrose and HFD (Kelley 2004; Kelley et al. 2005). Although there is significant expression of MC4Rs in the NAc (Kishi et al. 2003), only a few studies have investigated the potential function of MC4R signaling in this brain region. Direct infusion of the MC3/4R antagonist SHU9119 into the NAc or global knockout of MC4R blocks behavioral responses to cocaine including locomotor sensitization, suggesting that MC4R signaling in the NAc is required for certain responses to drugs of abuse (Hsu et al. 2005). Recently, a separate study utilizing a shRNA-mediated knockdown approach has shown that MC4R signaling in the NAc is also required for stress-induced anhedonia, anorexia and body weight loss (Lim et al. 2012). While these pharmacological and viral-mediated gene-knockdown approaches shed light on previously unrecognized physiological functions of MC4Rs in the striatum, it remains to be elucidated whether MC4R signaling specifically in brain dopamine 1 receptor (D1R)-expressing neurons has an effect on the long-term regulation of body energy balance and is sufficient to mediate behavioral responses to drugs of abuse such as cocaine.
Using an in vivo loxP/Cre genetic approach, we have previously shown that MC4R expression in D1R neurons mediates procedural memory learning (Cui et al. 2012a). Using this mouse model combined with neuroanatomical and biochemical approaches, in this study, we further investigate the physiologic functions of MC4R expression in D1R neurons in terms of body energy homeostasis and behavioral responses to cocaine.
Materials and methods
Animals
Mice with a transcriptional blocking cassette flanked by loxP sites placed in front of the MC4R gene (MC4R-TB) and mice expressing Cre-recombinase under control of the dopamine 1 receptor promoter (D1R-Cre) were obtained as previously reported (Balthasar et al. 2005; Cui et al. 2012a). Mice were back-crossed six generations onto the C57BL/6 line. All pairings were conducted with male MC4R-TB with the D1R-Cre transgene mated to female mice heterozygous for MC4R-TB to generate the following groups: wild-type (WT), WT/D1R=Cre, MC4R-TB and MC4R-TB/D1R-Cre. We have previously reported that the D1R-Cre transgene restores expression of MC4R within the ventral striatum, lateral olfactory tract and in a subset of neurons in the paraventricular nucleus of the hypothalamus (PVH) (Cui et al. 2012a). Mice in which green fluorescent protein (GFP) expression is under the control of the MC4R gene promoter (MC4R-GFP mice) were generated and characterized as reported (Cui et al. 2012b; Liu et al. 2003). Mice were housed in the University of Texas Southwestern Medical Center (UTSWMC) vivarium in a temperature-controlled environment (lights on: 0600–1800) with ad lib access to water and standard chow (SC, 4% fat diet #7001, Harlan-Teklad, Madison, WI, USA) or HFD (42% kcal from anhydrous milk fat, TD.88137, Harlan-Teklad). All animal procedures were performed in accordance with UTSW Institutional Animal Care and Use Committee guidelines.
Assessment of body weight, food intake, energy expenditure and body composition
All pups were weaned and genotyped at 4 weeks of age. Body weight was measured weekly from 5 weeks of age either on SC [WT (n = 17), D1Cre (n = 20), MC4R-TB (n = 18) and MC4R-TB/D1Cre (n = 18) for male; WT (n = 18), D1Cre (n = 20), MC4R-TB (n = 18) and MC4R-TB/D1Cre (n = 17) for female] or on HFD [WT (n = 10), D1Cre (n = 10), MC4R-TB (n = 10) and MC4R-TB/D1Cre (n = 9) for male; WT (n = 10), D1Cre (n = 12),MC4R-TB (n = 14) and MC4R-TB/D1Cre (n = 14) for female]. For the food intake study, as divergence of body weight between MC4R-TB and MC4R/D1R groups was observed at 6–7 weeks of age, we generated another cohort of singly housed mice and measured food intake from 6 to 7 weeks of age either on SC [control (n = 18), MC4R-TB (n = 12) and MC4R-TB/D1Cre (n = 10) for male; control (n = 18), MC4R-TB (n = 8) and MC4R-TB/D1Cre (n = 8) for female] or on HFD [control (n = 11), MC4R-TB (n = 8) and MC4R-TB/D1Cre (n = 7) for male; control (n = 10), MC4R-TB (n = 13) and MC4R-TB/D1Cre (n = 10) for female]. For meal pattern and energy expenditure measurements, another cohort of male mice was generated. At 5–6 weeks of age (prior to significant divergence of body weight) mice were transferred to the UTSWMC metabolic phenotyping core for 4 days of acclimation and then placed in metabolic cages (TSE International Group, Chesterfield, MO, USA) for 4 days with free access to food and water [control (n = 10), MC4R-TB (n = 6) and MC4R-TB/D1Cre (n = 6)]. The following parameters were assessed: body weight, food intake, oxygen consumption, carbon dioxide production, heat production, locomotor activity and respiratory exchange ratio. Meal analysis was assessed using Lab Master Software (http://www.tse-systems.com/labmaster/drinkingfeeding.htm). Body composition was determined by nuclear magnetic resonance (NMR) analysis using a mq10 series Bruker Minispec (Bruker Optics, The Woodlands, TX, USA) in a separate cohort of 8- to 9-week-old male mice fed SC [control (n = 10),MC4R-TB (n = 7) and MC4R-TB/D1Cre (n = 7)].
Serum hormone assays
A small volume of blood sample was drawn from the tails of 8- to 9-week-old male mice [control (n = 12), MC4R-TB (n = 9) and MC4R-TB/D1Cre (n = 8)] fasted overnight (food removed at ZT11 and blood sample collected at ZT3). Plasma leptin (Cat# 90030, Crystal Chem Inc, Downers Grove, IL, USA), insulin (Cat# 90080, Crystal Chem Inc) and acylated ghrelin (Cat# A05117, SPI-Bio Bertin Pharma, Montignyle-Bretonneux, France) levels were determined by enzyme-linked immunosorbent assay according to instructions.
Retrograde tracing and fluorescent double immunohistochemistry
Stereotaxic surgery procedures were performed as previously described (Chamberlin et al. 1998). Briefly, 8- to 10-week-old male MC4R-GFP mice (n = 10) were anesthetized with ketamine HCl/xylazine HCl (80:12 mg/kg, i.p.) and restrained in a Kopf (Tujunga, CA, USA) stereotaxic apparatus. A small hole was drilled into the skull under aseptic conditions and a glass micropipette connected to an air pressure injector system or iontophoresis machine was positioned via the stereotaxic manipulator. Approximately 50 nl of 4% retrograde tracer, fluorogold (FG) (Fluorochrome, Denver, CO, USA), was slowly administered into the lateral hypothalamic area (−1.50mm from bregma, 1.10mm lateral and −4.90mm from the surface of the cortex). After injection, the glass micropipette was removed and the incisionwas closed with surgical staples. Mice were allowed to recover for 5–7 days post-surgery and then transcardially perfused with 4% paraformaldehyde and cryopreserved in 20% sucrose. Brains were then sectioned into 30-µm coronal sections (collecting 1:5 sections) and stored in cryoprotectant at −20°C until use. Brain sections were blocked and permeabilized with 3% normal donkey serum (NDS, Jackson Immuno Research, West Grove, PA, USA), 0.3% Triton X-100 in phosphate-buffered saline (PBS) for 30min at room temperature, rinsed with PBS and incubated with primary antibodies (diluted in 3% NDS and 0.3% Tween-20 in PBS) at 4°C overnight. Rabbit polyclonal antiserum against FG (1:2000, Chemicon, Billerica, MA, USA) and chicken anti-GFP polyclonal antiserum (1:1000, Aves, Tigard, OR, USA) were used. Immunostaining signals were then detected by Cy3-conjugated donkey anti-rabbit IgG secondary antibody (1:400, Jackson Immuno Research) and Cy2-conjugated donkey anti-chicken IgY secondary antibody (1:400, Jackson Immuno Research), respectively. Images were first taken by fluorescent microscopy (Nikon Eclipse, 80, Nikon Instruments Inc., Melville, NY, USA) and colocalization across all sections was determined by confocal microscope scanning (LSM510-META, Zeiss, Thornwood, NY, USA).
Repeated cocaine-induced locomotor sensitization and acute cocaine- and melanotan II (MTII)-induced anorexia
For locomotor sensitization to repeated cocaine administration, 5-to 6-week-old, non-obese male control (n = 8), MC4R-TB (n = 6) and MC4R/D1Cre (n = 6) littermates were used. To minimize the effect of neophobia on locomotion, mice were singly housed with reduced bedding 1 week before testing and locomotor activity was measured in the home cage throughout the experimental period. In the event of necessary exchange of dirty cages, new cages were provided after behavioral testing to allowmice to spend at least one overnight period in their new cages before the next trial. On each test day mice were placed in black locomotor testing boxes with open tops in a room with dim lighting without food and water. After 30min of habituation, either saline or cocaine (15mg/kg) (C5776, Sigma-Aldrich, St. Louis, MO, USA) was intraperitoneally (IP) injected and locomotor activity was monitored for 1 h with 5-min intervals by beam break sensors (San Diego Instruments, San Diego, CA, USA). Following the initial 3 days of saline administration, mice received IP cocaine (15mg/kg) for eight consecutive days, followed by a 6-day withdrawal period. Mice were then sequentially challenged with an intermediate dose (15mg/kg), a half dose (7.5mg/kg) and a double dose (30 mg/kg) of cocaine and saline with 6-, 3- and 3-day withdrawal intervals, respectively.
For acute cocaine- and MTII-induced suppression of food intake, control (n = 8), MC4R-TB (n = 11) and MC4R/D1R (n = 8) adult male mice were individually housed, handled and injected with saline daily for 5 days for acclimation. On day 6, mice received an IP injection of saline 30min before the dark cycle, and 4- and 24-h food intake was measured. On the following day, mice were given an IP injection of MTII (2 mg/kg) (H-3902, BACHEM, Torrance, CA, USA), and 4-and 24-h food intake was measured. After 3 days of continued handling and IP saline administration to eliminate any MTII effect, mice were given an IP injection of cocaine (15mg/kg), and 4- and 24-h food intake was measured. Food intake was normalized to the saline-injected condition and compared across genotypes.
Western blot analysis
After the last trial of saline on day 26 of the cocaine locomotor sensitization protocol, mice (n = 8 for control, n = 6 for MC4R-TB and n = 6 for -TB/D1Cre) received an additional IP injection of cocaine (15mg/kg) followed by dissection of the ventral striatum 30 min later. Fresh tissue was immediately frozen in liquid nitrogen and stored at −80°C until use. Frozen tissue was homogenized with ice-cold RIPA lysis buffer containing protease inhibitor and protein phosphatase inhibitor cocktails (sc-24948, Santa Cruz Biotech, Santa Cruz, CA, USA). Equal amounts of each sample (15 µg) were separated by 4–20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gradient gels (BioRad, Hercules, CA, USA) and transferred to a polyvinylidene fluoride membrane by electroblotting. Primary antibodies were diluted as follows: anti-phospho-DARPP-32Thr-34 antibody (1:1000, Cell Signaling Technology, Danvers, MA, USA), anti-phospho-GluR1Thr845 antibody (1:5000, Millipore, Billerica, MA, USA) and anti-beta-actin antibody (1:2000, Cell Signaling Technology). Membranes were then incubated with HRP-conjugated secondary antibody (Jackson Immuno Research) and signals were detected by chemiluminescence exposed to film. Signal intensity was measured and analyzed by ImageJ software (NIH, Bethesda, MD, USA). For each experiment, signal intensity of targeted proteins was normalized to beta-actin signal intensity as an internal control.
Statistics
Data are presented as mean±SEM. GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA, USA) was used to perform all statistical analyses. After finding no difference in food intake or body weight between WT groups with and without D1R-Cre expression, both groups were collectively classified as the ‘control’ group in future analysis to reduce animal usage and increase statistical power. Comparisons between groups were made by oneway analysis of variance (anova) with Tukey’s post hoc analysis or two-way anova with Bonferroni post hoc analysis as noted.P < 0.05 was considered to be statistically significant.
Results
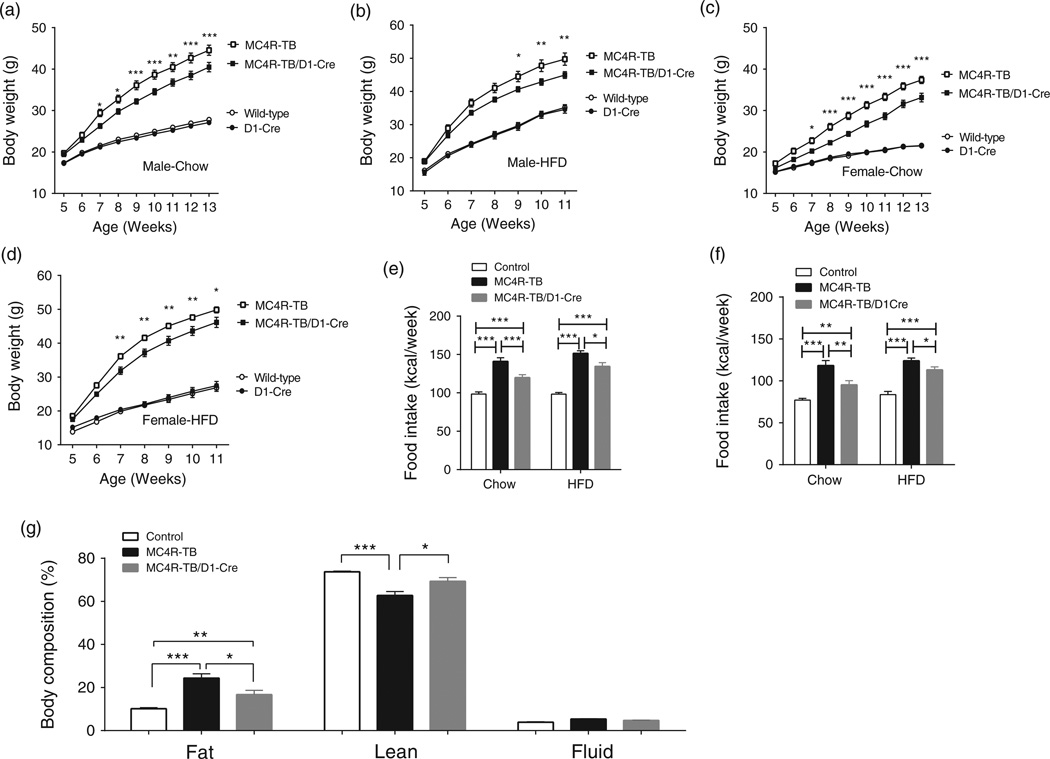
One week after weaning, mice received ad lib access to either SC or HFD and body weight was measured weekly for 8 weeks. Two-way anova of body weight showed a significant group×time interaction for both male (SC-fed condition, F 24,552 = 61.21, P < 0.0001, Fig. 1a; HFD-fed condition, F 18,210 = 22.89, P < 0.0001, Fig. 1b) and female (SC-fed condition, F 24,552 = 89.54, P < 0.0001, Fig. 1c; HFD-fed condition, F18,276 = 56.71, P <0.0001, Fig. 1d). Bonferroni post hoc analysis showed that there is no significant difference in body weight between WT and D1R-Cre genotypes in either male or female mice, indicating that the D1R-Cre transgene itself has no effect on body weight regulation (Fig. 1 a–d). Both male and female MC4R-TB mice on either SC or HFD were markedly obese comparedwithWTmice, which is consistent with previous reports (Balthasar et al. 2005). Bonferroni post hoc analysis showed that restoration of MC4Rs exclusively in D1R-expressing neurons significantly reduces body weight gain for both male and female mice on both SC and HFD (Fig 1 a–d). As divergence of body weight between MC4R-TB and MC4R/D1R mice occurred between 6 and 7weeks of age, we measured food intake during this time period to determine if changes in food intake contributed to the observed body weight differences. Consistent with the observed reduction in body weight, restoration of MC4Rs in D1R neurons significantly decreased food intake for both male (SC-fed condition, one-way ANOVA with Tukey’s post hoc analysis, F 2,37 = 37.3, P <0.0001; .HFD-fed condition, F 2,21 = 86.12, P <0.0001, Fig. 1e) and female mice (SC-fed condition, one-way ANOVA with Tukey’s post hoc analysis, F 2,25 = 34.29, P < 0.0001; HFD-fed condition, F2,30 = 34.57, P < 0.0001, Fig. 1f) on both SC and HFD. One-way ANOVA with Tukey’s post hoc analysis for relative body composition (%) showed that fat mass was significantly reduced in male MC4R/D1R mice receiving SC compared with MC4R-TBmice at 8–9 weeks of age (Fig. 1g).
Figure 1. MC4R signaling in D1R neurons regulates body weight and food intake.
(a–d) Restoration of MC4Rs specifically in D1R neurons results in a significant reduction in body weight on chow in male (a) and female (c) mice and on high-fat diet in male (b) and female (d) mice. *P < 0.05, **P< 0.01 and ***P< 0.001 compared with MC4R–TB group by two-way ANOVA with Bonferroni’s post hoc analysis.(e and f) Food intake for genotypes above was measured in separate cohorts of mice at 6–7 weeks of age. Restoration of MC4Rs in D1R neurons decreases intake of both chow and high-fat diet in male (e) and female (f) mice compared with MC4R-TB mice. (g) NMR body composition analysis in 8- to 9-week-old male mice. *P< 0.05, **P< 0.01 and ***P< 0.001 compared between the groups by one-way ANOVA with Tukey’s post hoc analysis. Data are presented as mean±SEM.
To understand the effect of MC4R expression in D1R neurons on metabolic regulation, fasting blood glucose and metabolic hormone levels were measured in young male mice that were body weight-matched between MC4R-TB and MC4R-TB/D1R groups. As expected, loss of MC4R signaling increased blood glucose, leptin and insulin levels and decreased acyl-ghrelin levels compared with WT mice. However, restoration of MC4Rs in D1R neurons did not affect any of these levels (data not shown).
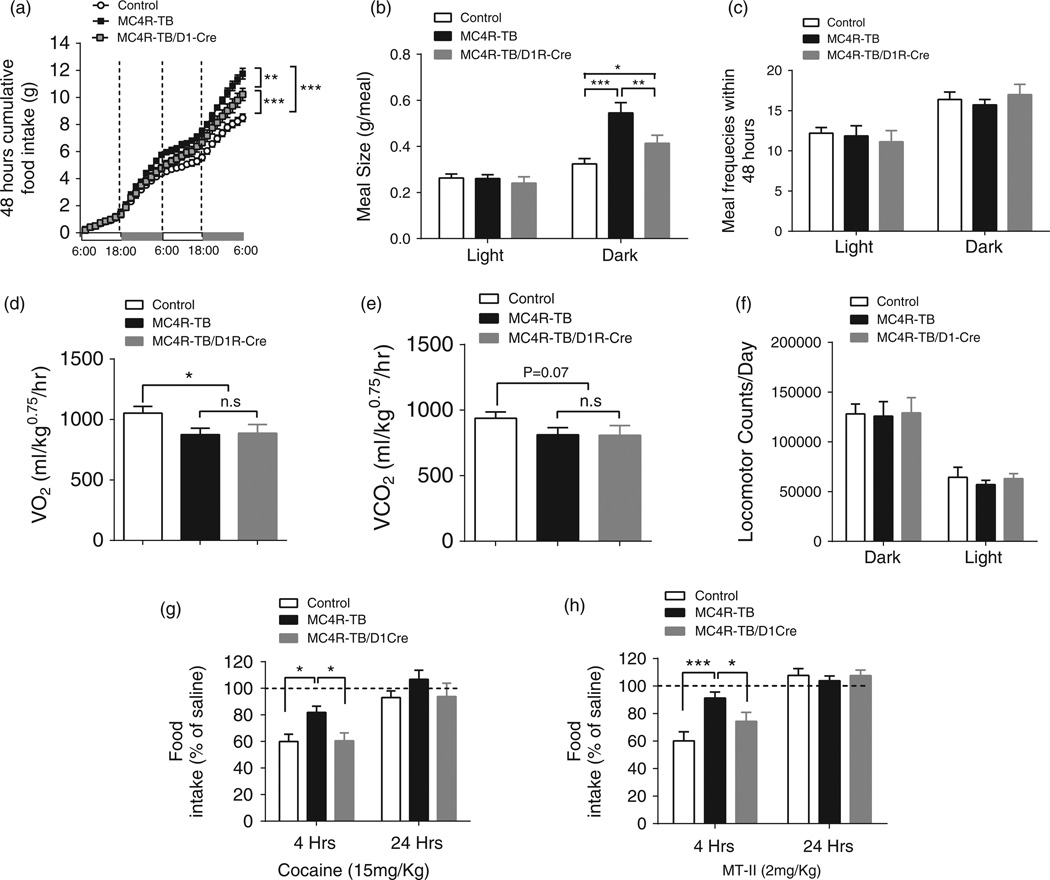
To further analyze the relative contribution of food intake and energy expenditure to the observed body weight phenotype, a cohort of mice was transferred to our metabolic phenotyping core before significant divergence of body weight occurred between MC4R–TB and MC4R/D1R mice (age of 5–6weeks). Two-way ANOVA of cumulative food intake shows a significant group×time interaction (F 62,496 = 15.28, P < 0.0001, Fig. 2a). Bonferroni post hoc analysis showed that, at the end of experiment, cumulative food intake was significantly lower in MC4R/D1R mice compared with MC4R-TB mice during this metabolic cage study (Fig. 2a), confirming our previous food intake results for male mice receiving SC under standard housing condition (Fig. 1e). Two-way ANOVA for meal pattern analysis showed significant group×time interaction for meal size (F2,20 = 7.965, P = 0.0029, Fig. 2b), but not for meal frequency (F2,42 = 0.4912, P = 0.615, Fig. 2c). Bonferroni post hoc analysis showed that significantly increased meal size in MC4R-TB mice in the dark cycle was reduced in MC4R-TB/D1R mice (Fig. 2b). In contrast to food intake, restoration of MC4R expression in D1R neurons did not affect oxygen consumption (Fig. 2d) or carbon dioxide production (Fig. 2e), indicating that MC4R signaling in D1R neurons does not affect energy expenditure. Neither nocturnal nor diurnal locomotor activity was significantly different between the groups during the test days (one-way ANOVA, F 2,21 = 1.219, P = 0.316, Fig. 2f).
Figure 2. MC4R signaling in D1R neurons regulates meal size and acute cocaine-induced anorexia.
(a–f) Metabolic cage analysis confirmed reduction of food intake in MC4R/D1R mice (a) and further showed that restoration of MC4Rs in D1R neurons reduces meal size (b), but not meal frequency (c), in the dark cycle. Oxygen consumption (d), carbon dioxide production (e) and daily locomotor activity (f) were not affected in MC4R–TB/D1R mice. (g and h) Acute (g) cocaine- and (h) MTII-induced suppression of 4-h food intake was significantly blocked in MC4R–TB mice but normalized in MC4R/D1R mice. *P< 0.05, **P< 0.01 and ***P < 0.001 compared between the groups by one-way ANOVA with Tukey’s post hoc analysis. Data are presented as mean±SEM.
While MC4R signaling in D1R neurons reduces food intake and body weight under standard housing conditions, the effect was modest. Because recent studies have shown that MC4R signaling in the NAc is required for certain behavioral responses to cocaine- (Hsu et al. 2005) and stress-induced anhedonia, hypophagia and body weight loss (Lim et al. 2012), we speculated that MC4R signaling in D1R neurons might suppress food intake under specific conditions. To test this hypothesis, we determined the effect of cocaine, which potently suppresses appetite (Clifford et al. 2007; Wellman et al. 2002), on food intake in our mouse models. An acute IP injection of cocaine (15mg/kg) induced a significant suppression of food intake in the control and MC4R/D1R groups after 4 h (one-way ANOVA followed by Tukey’s post hoc analysis, F 2,23 = 5.925, P = 0.0084, Fig. 2g), indicating that restoration of MC4R signaling in D1R neurons is sufficient to rescue the acute anorectic effect of cocaine. Additionally, we tested the effect of the melanocortin agonist MTII on food intake in our genetic model. Similar to cocaine, IP injection of MTII (2mg/kg) significantly suppressed 4-h food intake in WT and MC4R/D1R groups but not in MC4R–TB mice (one-way ANOVA followed by Tukey’s post hoc analysis, F 2,24 = 7.8145, P = 0.0024, Fig. 2h). No differences in 24-h food intake were observed after either cocaine (one-way ANOVA, followed by Tukey’s post hoc analysis, F 2,24 = 1.155, P = 0.332, Fig. 2g) or MT-II (one-way ANOVA, followed by Tukey’s post hoc analysis, F2,24 = 0.309, P = 0.737, Fig. 2h), confirming that the effects are reversible and not the result of developmental differences.
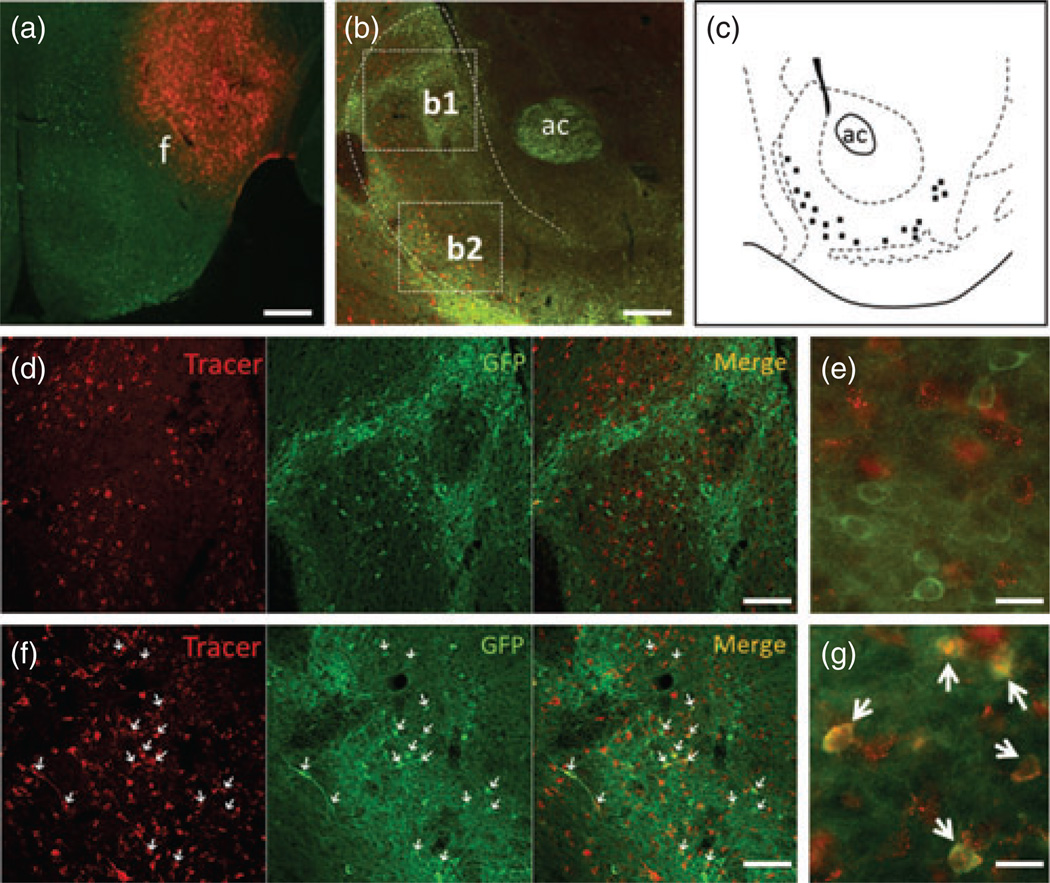
In addition to drugs of abuse, neurons in the NAc shell have also been implicated in inducing feeding behaviors through projections to the LHA, a historically recognized ‘feeding center’ in the brain (Stratford & Kelley 1997, 1999; Zheng et al. 2007). Altered food intake in MC4R-TB/D1Cre mice led us to hypothesize that MC4R-positive neurons in the NAc could directly project to the LHA to regulate food intake. To test this hypothesis, we performed neuronal retrograde tracing in mice expressing tau-conjugated GFP under control of the MC4R promoter (MC4R-GFP) (Cui et al. 2012b; Liu et al. 2003).Stereotaxic infusion of a small volume (∼50 nl) of the retrograde tracer FG into the LHA intensely labeled neurons in the NAc shell as expected (Fig. 3a,b,d,f). Dual immunostaining of FG and MC4R-GFP showed that retrograde tracer signals were colocalized in MC4R-GFP-labeled neurons predominantly in the ventrolateral portion of the NAc shell with no expression in the dorsomedial aspect of the NAc shell (Fig. 3b,d–g), indicating that MC4R-expressing neurons in the NAc shell do project to the LHA. This finding was confirmed in four successfully injected cases.
Figure 3. MC4R–positive neurons in the NAc shell project to the LHA.
Representative injection case showing that injection was exactly made into the LHA (a). Double immunohistochemistry for FG and MC4R-GFP showed that retrograde tracer-positive neurons in the ventrolateral part of the NAc shell were colocalized with MC4R-GFP (b). Schematic drawing showing the detailed distribution of FG/GFP double-positive neurons in the NAc shell (c). (d and f) Multichanneled zoom-in pictures for boxes b1 and b2 of (b), respectively. Pictures in (e) and (g) are high magnification from (d) and (f). f, fornix; ac, anterior commissure. Scale bars: 320 µm for (a and b); 80 µm for (d and f) and 20 µm for (e and g).
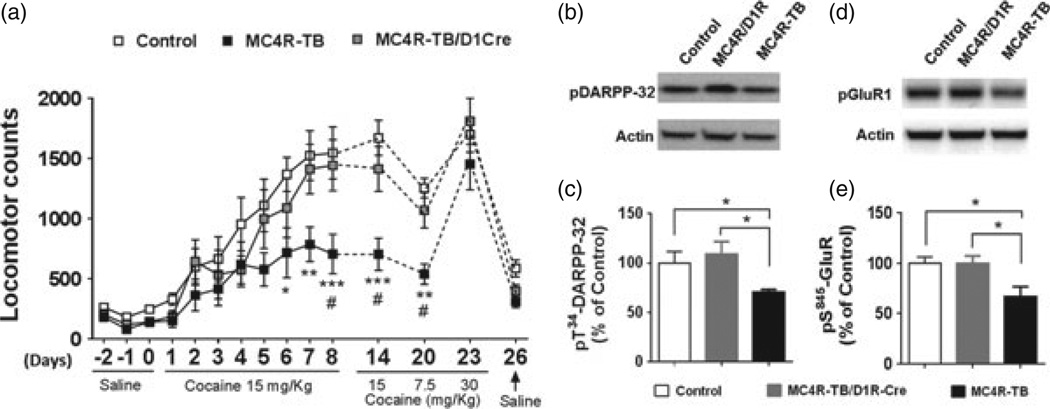
Previous pharmacological studies have shown that MC4R signaling in D1R neurons is required for certain behavioral responses to cocaine, including psychomotor sensitization (Alvaro et al. 2003; Hsu et al. 2005). Therefore, in this study, we decided to test cocaine-induced locomotor sensitization in our genetic mouse model. To minimize the potential effect of body weight on locomotor activity, we analyzed young, non-obese male mice at 5 weeks of age. Following 3 days of saline injection, mice received cocaine (15mg/kg, IP) for eight consecutive days. Two-way ANOVA of locomotor activity showed a significant group×time interaction (F28,238 = 1906, P = 0.0054, Fig. 4a). Bonferroni post hoc analysis showed that while WT mice developed locomotor sensitization after repeated cocaine administration as expected, this effect was significantly reduced in MC4R-TB mice consistent with previous reports (Fig. 4a) (Hsu et al. 2005). Restoring MC4Rs exclusively in D1R neurons was sufficient to completely normalize cocaine-induced locomotor sensitization (Fig. 4a). The development of locomotor sensitization upon repeated cocaine administration is accompanied by long-lasting changes in synaptic plasticity of striatal neurons (Russo et al. 2010). Therefore, mice were challenged with a full dose (15 mg/kg), a half dose (7.5mg/kg) and a double dose (30mg/kg) of cocaine. The MC4R/D1R mice showed elevated locomotor activity comparedwithMC4R-TBmice after 6 days of withdrawal when challenged with either a full or half dose of cocaine (Fig. 4a). However, the 30 mg/kg dose significantly elevated locomotor activity in MC4R-TB mice to a level comparable to WT and MC4R/D1R groups (Fig. 4a). This indicates that MC4R-TB mice are capable of mounting a full locomotor response to cocaine at higher dosages.
Figure 4. MC4R signaling in D1R neurons affects locomotor sensitization to cocaine and intracellular signaling in the ventral striatum.
(a) Development of locomotor sensitization to repeated cocaine administration was significantly blocked in MC4R-TB mice compared with control mice, which was fully normalized in MC4R/D1R mice. *P < 0.05, **P< 0.01 and ***P< 0.001 are for MC4R-TB vs. control groups; #P <0.01 for MC4R-TB vs. MC4R/D1R groups (two-way ANOVA with Bonferroni’s post hoc analysis). (b) Representative images of the blots of pT34-DARPP-32 and beta-actin. (c) Quantitative analysis showed that pT34-DARPP-32 levels after cocaine exposure were significantly decreased in MC4R-TB mice but normalized in MC4R/D1R mice. (d) Representative images for the blots of pS845-GluR1 and beta-actin. (e) Quantitative analysis showed that pS845-GluR1 levels after cocaine exposure were significantly decreased in MC4R-TB mice but normalized in MC4R/D1Rmice. *P< 0.05 by one-way ANOVA with Tukey’s post hoc analysis. Data are presented as mean±SEM.
Both D1R and MC4R are known to be Gs-coupled and activate the intracellular cAMP-PKA pathway (Damm et al. 2012; Nishi et al. 2011), and phosphorylation of dopamineand cAMP-regulated neuronal phosphoprotein-32 (DARPP-32) and the AMPA receptor subunit GluR1 are important targets downstream of D1R signaling (Nishi et al.2011). To understand the biochemical mechanism of MC4R action in D1R neurons, tissue punches of the ventral striatum were collected from mice after cocaine-induced locomotor sensitization. Western blot analysis showed that cocaineinduced phosphorylation ofDARPP-32 at Thr34 (pT34-DARPP-32; Fig. 4b) (one-way ANOVA followed by Tukey’s post hoc analysis, F 2,15 = 3.984, P = 0.041, Fig. 4c) and GluR1 at Ser845 (pS845-GluR1; Fig. 4d) (one-way ANOVA followed by Tukey’s post hoc analysis, F 2,15 = 4.664, P = 0.026, Fig. 4e) was significantly reduced in MC4R-null mice compared with the control group but normalized in MC4R/D1R mice, suggesting that modulation of DARPP-32 and GluR1 signaling may be an important intracellular target of MC4R signaling in D1R neurons in the ventral striatum.
Discussion
Using a genetic approach, we report that MC4R signaling within D1R-expressing neurons does affect food intake in MC4R-null mice. Our data suggest that under normal laboratory housing conditions, MC4R signaling in D1R neurons partially, but significantly suppresses food intake and body weight gain (∼10%), but when the system is stressed by MTII and cocaine, MC4R expression in D1R neurons alone is sufficient to mediate the full anorectic effects of both MTII and cocaine. This observation suggests that the primary physiological role of this circuit may be to induce anorexia in response to specific stressful stimuli such as exposure of drugs of abuse. This theory highlights an important interaction of dopamine and melanocortin signaling in regulation of stress responses and is consistent with literature reporting that D1R agonists and melanocortin agonists suppress food intake primarily through reduction in meal size, rather than meal frequency (Cooper et al. 2006; Rusk & Cooper 1989; Williams et al. 2002). Hence, it is likely that D1R/MC4R neurons identify a common neural substrate that mediates the anorectic effect of both D1R agonists and MTII.
One point that remains to be clarified is the relative contribution of the ventral striatum vs. the PVH in these effects. A recent article using shRNA to knockdown MC4R expression found that MC4R in the NAc is required for anorexia induced by restraint stress (Lim et al. 2012), suggesting that D1R neurons projecting from the ventral striatum can regulate food intake. However, a previous genetic study identified MC4R expression in Sim1-expressing neurons of the PVH and/or amygdala as a primary site of action on food intake as restoration of MC4R expression in these neurons reduces food intake and body weight by approximately 75% (Balthasar et al. 2005). We have previously shown that the MC4R-TB X D1R-Cre mouse line restoresMC4R expression in the ventral striatum, including the olfactory tubercle, as well as some cells of the PVH (Cui et al. 2012a). Given the known role of the PVH in regulation of food intake, we cannot completely rule out a role for this site in the regulation of food intake. While we do not know if PVH Sim1 neurons are also D1R-positive, it is unlikely that there is a significant overlap as Sim1-Cre reactivation produces a significantly larger reduction in food intake and body weight than D1R-Cre reactivation (Balthasar et al. 2005).
These data provide further support that MC4R signaling in D1R neurons does not differentially affect consumption of HFD. Prior studies suggested that MC4R mice are highly sensitive to a palatable HFD diet as they are markedly hyperphagic and rapidly gain body weight on HFD compared with chow-fed mice (Albarado et al. 2004; Butler et al. 2001; Srisai et al. 2011), possibly via both a deficit in satiety and enhanced reward value of HFD (Srisai et al. 2011). We showed previously that both MC4R-TB and MC4R-TB/D1R-Cre mice exhibit reduced operant responding for HFD pellets (Cui et al. 2012a). Given the known role of dopaminergic signaling in the ventral striatum in both drug and natural reward, we anticipated that restoration of MC4Rs in D1R neurons might preferentially reduce intake of palatable HFD. However, our results indicate that it is unlikely that loss of MC4R signaling in D1R neurons mediates the increased sensitivity to HFD observed in MC4R-null mice. Future studies are warranted to determine the neural substrates through which MC4Rs affect consumption of HFD, such as the LHA, which expresses MC4Rs and is an additional site of reward processing (Cui et al. 2012b).
Previous pharmacological studies have shown that intra-NAc infusion of the MC3/4R antagonist SHU9119 significantly blocks most behavioral effects of cocaine including locomotor sensitization (Hsu et al. 2005). Using a complementary genetic approach, in this study, we confirmed the requirement of MC4Rs in cocaine-induced locomotor sensitization and further showed that restoration of MC4Rs specifically in D1R neurons is sufficient to fully mediate this effect. In contrast to a previous study that utilized obese adult MC4R-null mice, in this study, we were able to show the effects in young, non-obese MC4R-null mice, indicating that MC4R action in the behavioral response to cocaine is not a non-specific effect secondary to obesity.
Finally, we sought to determine if there is a possible convergence of two Gs-coupled receptors, MC4R and D1R, within intracellular signaling cascades. After exposure to cocaine, phosphorylation at Thr34 of DARPP-32 and Ser845 of GluR1, two downstream targets of D1R signaling in the striatum (Nishi et al. 2011; Snyder et. al. 2000), was significantly decreased in MC4R-null mice but normalized when MC4Rs were specifically restored in D1R neurons. This finding suggests that expression of MC4Rs in the striatum is required for some chronic effects of cocaine exposure. Interestingly, Lim et al. have reported that striatal MC4Rs modulate synaptic strength by increasing endocytosis of GluR2-containing (also known as GluA2) AMPA receptors in D1R neurons (Lim et al. 2012). Because phosphorylation at S845-GluR1 increases both surface expression and the probability of opening of AMPA channels (Banke et al. 2000; Oh et al. 2006), these results collectively raise a possibility that MC4R signaling in D1R neurons specifically promotes the activity of GluR1 over GluR2 containing AMPA receptors within striatal neurons. More work is needed to test this hypothesis by examining the synaptic and electrophysiological properties of GluR1 and GluR2 containing APMA receptors.
In conclusion, here, we have described cocaine-induced anorexia and locomotor sensitization as two physiological functions of MC4Rs in D1R-expressing neurons. A greater understanding of this pathway may yield insights into disorders such as cocaine addiction and overeating.
Acknowledgments
This work was funded by the following grants: NIH Roadmap Grants for Mouse Metabolic Phenotyping Core at UT Southwestern Medical Center (DK081185-01, DK081182-01) and MH084058-01A1 (M.L.) and NARSAD Young Investigator Award (M.L.). We would like to thank Joel Elmquist, Brad Lowell and Gensat for use of mouse lines.
References
- Albarado DC, McClaine J, Stephens JM, Mynatt RL, Ye J, Bannon AW, Richards WG, Butler AA. Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology. 2004;145:243–252. doi: 10.1210/en.2003-0452. [DOI] [PubMed] [Google Scholar]
- Alvaro JD, Taylor JR, Duman RS. Molecular and behavioral interactions between central melanocortins and cocaine. J Pharmacol Exp Ther. 2003;304:391–399. doi: 10.1124/jpet.102.040311. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boghossian S, Park M, York DA. Melanocortin activity in the amygdala controls appetite for dietary fat. Am J Physiol Regul Integr Comp Physiol. 2010;298:R385–R393. doi: 10.1152/ajpregu.00591.2009. [DOI] [PubMed] [Google Scholar]
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4:605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- Chaki S, Okuyama S. Involvement of melanocortin-4 receptor in anxiety and depression. Peptides. 2005;26:1952–1964. doi: 10.1016/j.peptides.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Du B, de Lacalle S, Saper CB. Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain Res. 1998;793:169–175. doi: 10.1016/s0006-8993(98)00169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JC, Krishnan V, Yu HG, Mason B, Cui H, Wilkinson MB, Zigman JM, Elmquist JK, Nestler EJ, Lutter M. A beta3-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biol Psychiatry. 2010;67:1075–1082. doi: 10.1016/j.biopsych.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS, Davis KW, Elliott AE, Wellman PJ. Effects of ICV administration of the alpha1A-adrenoceptor antagonist 5-methylurapidil on concurrent measures of eating and locomotion after cocaine in the rat. Life Sci. 2007;81:1059–1065. doi: 10.1016/j.lfs.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Al-Naser HA, Clifton PG. The anorectic effect of the selective dopamine D1-receptor agonist A-77636 determined by meal pattern analysis in free-feeding rats. Eur J Pharmacol. 2006;532:253–257. doi: 10.1016/j.ejphar.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Cui H, Mason BL, Lee C, Nishi A, Elmquist JK, Lutter M. Melanocortin 4 receptor signaling in dopamine 1 receptor neurons is required for procedural memory learning. Physiol Behav. 2012a;106:201–210. doi: 10.1016/j.physbeh.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Sohn JW, Gautron L, Funahashi H, Williams KW, Elmquist JK, Lutter M. Neuroanatomy of melanocortin-4 receptor pathway in the lateral hypothalamic area. J Comp Neurol. 2012b;520:4168–4183. doi: 10.1002/cne.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm E, Buech TR, Gudermann T, Breit A. Melanocortin-induced PKA activation inhibits AMPK activity via ERK-1/2 and LKB-1 in hypothalamic GT1-7 cells. Mol Endocrinol. 2012;26:643–654. doi: 10.1210/me.2011-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu R, Taylor JR, Newton SS, Alvaro JD, Haile C, Han G, Hruby VJ, Nestler EJ, Duman RS. Blockade of melanocortin transmission inhibits cocaine reward. Eur J Neurosci. 2005;21:2233–2242. doi: 10.1111/j.1460-9568.2005.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatalhypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenicmice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Kuroiwa M, Shuto T. Mechanisms for the modulation of dopamine d(1) receptor signaling in striatal neurons. Front Neuroanat. 2011;5:43. doi: 10.3389/fnana.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Panaro BL, Cone RD. Melanocortin-4 receptor mutations paradoxically reduce preference for palatable foods. Proc Natl Acad Sci U S A. 2013;110:7050–7055. doi: 10.1073/pnas.1304707110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusk IN, Cooper SJ. The selective dopamine D1 receptor agonist SK&F 38393: its effects on palatability- and deprivation-induced feeding, and operant responding for food. Pharmacol Biochem Behav. 1989;34:17–22. doi: 10.1016/0091-3057(89)90346-8. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisai D, Gillum MP, Panaro BL, Zhang XM, Kotchabhakdi N, Shulman GI, Ellacott KL, Cone RD. Characterization of the hyperphagic response to dietary fat in the MC4R knockout mouse. Endocrinology. 2011;152:890–902. doi: 10.1210/en.2010-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX. Mutations in melanocortin-4 receptor and human obesity. Prog Mol Biol Transl Sci. 2009;88:173–204. doi: 10.1016/S1877-1173(09)88006-X. [DOI] [PubMed] [Google Scholar]
- Wellman P, Ho D, Cepeda-Benito A, Bellinger L, Nation J. Cocaine-induced hypophagia and hyperlocomotion in rats are attenuated by prazosin. Eur J Pharmacol. 2002;455:117–126. doi: 10.1016/s0014-2999(02)02616-x. [DOI] [PubMed] [Google Scholar]
- Williams DL, Grill HJ, Weiss SM, Baird JP, Kaplan JM. Behavioral processes underlying the intake suppressive effects of melanocortin 3/4 receptor activation in the rat. Psychopharmacology (Berl) 2002;161:47–53. doi: 10.1007/s00213-002-1022-5. [DOI] [PubMed] [Google Scholar]
- Xu Y, Elmquist JK, Fukuda M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann N Y Acad Sci. 2011;1243:1–14. doi: 10.1111/j.1749-6632.2011.06248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]