Abstract
Generating an anti-tumor immune response is a multi-step process that is executed by effector T cells that can recognize and kill tumor targets. However, tumors employ multiple strategies to attenuate the effectiveness of T cell-mediated attack. This is achieved by interfering with nearly every step required for effective immunity, from deregulation of antigen-presenting cells, to establishment of a physical barrier at the vasculature that prevents homing of effector tumor-rejecting cells, and through the suppression of effector lymphocytes through the recruitment and activation of immunosuppressive cells like myeloid-derived suppressor cells (MDSCs), tolerogenic monocytes and T regulatory cells (Tregs). Here, we review the ways in which tumors exert immune suppression and highlight the new therapies that seek to reverse this phenomenon and promote anti-tumor immunity. Understanding anti-tumor immunity, and how it becomes disabled by tumors, will ultimately lead to improved immune therapies and prolonged survival of patients.
Introduction
The immune response against tumors is mounted by a multitude of immune cells. However, T cells remain potent mediators of anti-tumor immunity, and tumor infiltration by T cells is a good prognostic marker in a number of tumor types including ovarian, colon, breast renal, prostate, and cervical cancers (Galon et al., 2006; Hwang et al., 2012; Ma et al., 2012; Naito et al., 1998; Piersma et al., 2007; Zhang et al., 2003). The steps leading to an antitumor immune response are depicted in Figure 1. In some patients, these responses are activated spontaneously, but chemotherapy is also thought to promote antitumor immune responses.
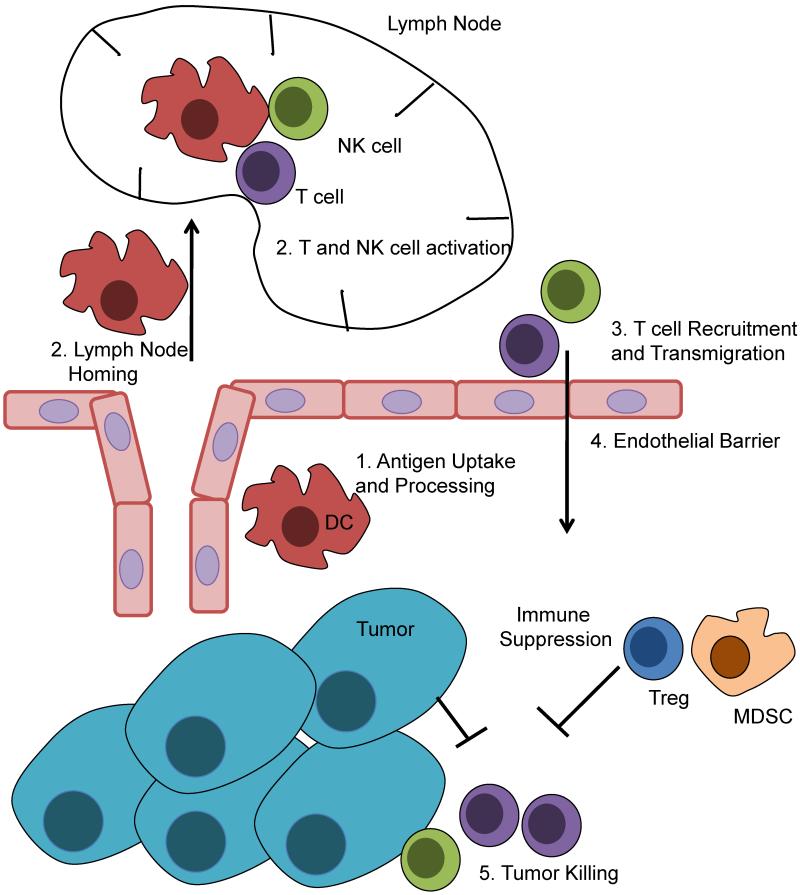
Figure 1.
Generation of an anti-tumor T cell response. Dendritic cells acquire tumor antigens from necrotic or apoptotic tumor cells, and then home to regional lymph nodes. Within the lymph nodes, DCs activate T cells (and NK cells) and they then traffic to the tumor site. Activated lymphocytes cross the tumor endothelial barrier, recognize tumor targets, and secrete cytokines and directly kill tumor targets. This process is under considerable suppression from the tumors, as they mount challenges to each step that prevents optimal T cell activation. Within the tumor site, suppressive cells like Tregs and MDSC are recruited by tumors and actively suppress lymphocytes from killing tumor targets.
Typically, the immune response begins at the tumor site, where professional antigen presenting cells (APCs) take up tumor antigens to be processed. These antigens may be some of the many mutational neo-antigens (Robbins et al., 2013), non-mutated genes that are overexpressed by cancer cells, or differentiation antigens related to the cancer’s tissue of origin (Segal et al., 2008). Although T cell priming is traditionally thought to occur exclusively in tumor-draining lymph nodes, spontaneously organized tertiary lymphoid organ features can be also encountered in tumors (de Chaisemartin et al., 2011), suggesting that T cell education can occur within the tumor stroma. Dendritic cells from tumors may present antigens in a tolerizing manner, stimulating T regulatory (Treg) cells (Steinman et al., 2000), which would oppose an antitumor response. In order to promote immunity rather than tolerance, it is believed that APCs require a robust maturation signal. Toll-like receptor (TLR) signaling from necrotic tumors cells may induce partial maturation (Cavassani et al., 2008), but chemotherapy drugs that induce immunogenic cell death can also stimulate an immune response (Zitvogel and Kroemer, 2009). Activated dendritic cells can also drive B and NK cell (Mellman and Steinman, 2001) responses that can play important roles in antitumor immunity. The exact type of T cell response required for optimal anti-tumor immunity is not entirely clear, however a potent CD8+ effector T cell response is certainly necessary. Additionally, either a CD4+ T helper 1 (Th1) or Th17 directed response appear to promote CD8+ effector T cell responses (Martin-Orozco et al., 2009; Mellman and Steinman, 2001; Steinman et al., 2000). Given that TILs are such an important prognostic marker for tumor progression across multiple tumor types, understanding the processes involved in their suppression is essential to developing new therapeutic strategies. In this review, we will detail the ways in which tumors suppress each step in the generation of an effective anti-tumor immune response, from generation of tumor-specific T cells, to their homing, engraftment and effective recognition of tumors. We also discuss recent and potential future therapeutic interventions to circumvent tumor-mediated immunosuppression.
Generation of tumor-reactive T cells
Dendritic cells (DCs) are extremely important for the coordination of an anti-tumor immune response. As professional APCs, they present tumor antigens to both B cells and T cells, generating an antigen-specific antitumor response. Tumors have a profound effect on the functions of dendritic cells (Gabrilovich, 2004). Defective dendritic cell function is often combined with deregulation of DC maturation, and in humans as well as in the mouse, tumor-infiltrating cells expressing DC markers also express markers of macrophages and immature monocytes, indicating recruitment of myeloid precursors with incomplete differentiation (Conejo-Garcia et al., 2004). Dendritic cells can have significant heterogeneity both in vitro and in vivo (Hashimoto et al., 2011), and include resident and bone-marrow derived myeloid dendritic cells and plasmacytoid dendritic cells. These cells have different functional properties, and they may contribute differently to tumor tolerance or rejection (Kim et al., 2007). For example, although DCs are important APCs, depletion of CD11c+ cells (primarily DCs) can actually inhibit tumor growth (Huarte et al., 2008), an effect that reflects the role of tumor-coopted tolerogenic dendritic cells in establishing tumor tolerance and dissemination (Labidi-Galy et al., 2011; Sawant et al., 2012).
Most tumor myeloid DCs present a phenotype of partially mature DCs expressing intermediate amounts of MHC class I and II and costimulatory molecules, and high amounts of coinhibitory molecules and immunosuppressive cytokines. In the mouse, such cells are unable to elicit antigen-specific effector T cells (Conejo-Garcia et al., 2004). Human DCs isolated from breast, neck/head, and lung cancer patients were also functionally impaired in a mixed leukocyte reaction, and this functional impairment corresponded to a more severe cancer diagnosis (higher stage) (Almand et al., 2000). Immature, or incompletely matured DCs may mediate tumor tolerance, inducing anergy of effector T cells and/or expansion of Treg cells in the lymph nodes or at tumor sites (Lutz and Schuler, 2002; Mahnke et al., 2002).
Gabrilovich and colleagues were the first to identify VEGF as a tumor factor capable of impairing both dendritic cell function and maturation from CD34+ hematopoietic precursors (Gabrilovich et al., 1996). Similar observations of defective DCs in cancer patients, with a dependence on or association with VEGF, have since been made (Della Porta et al., 2005; Takahashi et al., 2004). VEGF is an important regulator of hematopoiesis, and its artificial overexpression has led to widespread changes in the differentiation of multiple hematopoietic lineages. In patients, treatment with the VEGF blocking antibody bevacizumab has been shown to reverse maturation defects of in DCs (Almand et al., 2000; Fricke et al., 2007; Osada et al., 2008). Defective DC maturation that is reversible with VEGF blockade was also found in mouse models (Gabrilovich et al., 1999; Nair et al., 2003; Roland et al., 2009; Ishida et al., 1998). VEGF likely exerts effects on dendritic cells beyond its role in the suppression of normal hematopoiesis. Programmed death ligand 1 (PD-L1) is a major negative regulatory ligand of the B7 family that engages the cognate programmed death-1 (PD-1) receptor expressed on activated T cells, which transduces a signal that inhibits T-cell proliferation, cytokine production, and cytolytic function (Riley, 2009). PD-L1 is expressed on tumor cells, but it is also highly expressed on tumor-associated myeloid DCs in ovarian cancer patients (Curiel et al., 2003). Incubation of blood myeloid DCs with VEGF induced robust expression of PD-L1 on the cell surface offering a potential mechanism by which VEGF affects DC function (Curiel et al., 2003).
A number of other tumor-derived soluble mediators can also disrupt DC function and play critical role in defining the semi-mature, tolerogenic phenotype of tumor DCs, including transforming growth factor β (TGFβ) (Geissmann et al., 1999), interleukin 10 (IL-10) (Steinbrink et al., 1999), as well as macrophage colony-stimulating factor (M-CSF) and IL-6 (Menetrier-Caux et al., 1998). IL-10 also induces PD-L1 expression on DCs (Curiel et al., 2003). Additional mechanisms can contribute to DCs with a tolerogenic phenotype. Physiological stimuli found in the tumor microenvironment such as hypoxia (Elia et al., 2008) and lactic acid (Gottfried et al., 2006) can also influence DC phenotype and function. In vitro, DCs differentiated under these exposures tend to have a less mature phenotype, express immunosuppressive molecules like indoleamine 2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2), and fail to stimulate T cells efficiently (Elia et al., 2008; Gabrilovich et al., 2012; Gottfried et al., 2006). In particular, PGE2 signaling on DCs can induce the expression of immunosuppressive molecules such as IL-10 (Kalinski et al., 1997) and IDO (Braun et al., 2005), can suppress IL-12 production (Watchmaker et al., 2010), and inhibit chemokine expression (Muthuswamy et al., 2010).
Thus, it is not surprising that in many patients there are no detectable tumor-reactive T cells. For example, in ovarian cancer, tumor-reactive T cells were detected in the peripheral blood of only half the patients tested (Schlienger et al., 2003). The defective phenotype of DCs may contribute in more ways to deregulate T cell tumor attack, as properly mature DCs that express costimulatory ligands may be required in the periphery at the inflammatory site, to maintain an effective effector CD8+ T cell response (Dolfi et al., 2011), and these are typically absent in the tumor microenvironment. Finally, defective DCs fail to secrete appropriate chemokines that play a critical role in recruiting effector cells to tumors (Muthuswamy et al., 2012). As described above, disruption of normal DC function is an essential component of tumor-mediated immune suppression leading to tumor immune tolerance, and strategies aimed at relieving this immune suppression, or generating potent DC-vaccines ex vivo, is an active area of research that has already enjoyed some early successes.
Reaching the tumor site
Tumors have developed a number of unique ways to suppress the recruitment of T cells to the tumor site. The exact mechanisms have not been fully elucidated, but the disruption of normal chemokine elaboration is believed to be a contributing factor. Chemokines play a critical role in orchestrating T cell trafficking and the specialization of immune responses, and their alteration is probably tissue-specific. Of particular interest is the observation that tumors with high numbers of T cells express high amounts of established T cell-attracting chemokines including chemokine (C-C motif) ligand 2 (CCL2), CCL3, CCL4, CCL5, chemokine (C-X-C motif) ligand 9 (CXCL9), and CXCL10 (Harlin et al., 2009). It is unclear whether this is the result of an initial T cell activation, unleashing a chemokine response furthering T cell recruitment. However, the pattern of T cell infiltration in mouse models suggests that a few T cells infiltrate tumors initially, followed by a large influx of both specific and nonspecific T cells (Boissonnas et al., 2007). In skin tumors, aberrant epidermal growth factor receptor (EGFR)-Ras signaling has been shown to suppress the production of CCL27, a chemokine constitutively expressed by normal keratinocytes, and disruption of CCL27 expression in vivo prevented T cell homing to skin tumors and accelerated tumor outgrowth in a mouse model (Pivarcsi et al., 2007).
Tumor cells are well-known for aberrant post-translational modifications, and changes in cleavage, glycosylation, or deamination could result in dramatically altered activities of expressed chemokines (Loos et al., 2009); (Proost et al., 2007). For example, CCL2, an important chemokine for the recruitment of cytotoxic lymphocytes (CTLs) to the tumor site (Brown et al., 2007), undergoes nitrosylation induced by reactive nitrogen species, which abrogates its ability to attract tumor-specific CTLs, although it can still attract myeloid cells (MDSCs) to the tumor (Molon et al., 2011), highlighting a potent mechanisms of immune suppression. Furthermore, altered proteolytic processing of CXCL11, an important chemokine recruiting CXCR3+ effector T cells, can impair binding and signaling of the chemokine, ultimately reducing lymphocyte migration (Boissonnas et al., 2007; Harlin et al., 2009; Proost et al., 2007). Thus, deregulation of chemokine circuitries appears to be an important mechanism preventing proper orchestration of immune tumor rejection mechanisms.
Crossing the tumor vasculature
Although T cells can accumulate in the tumor stroma, they often fail to penetrate deeply into tumors in the epithelial compartment (Zhang et al., 2003; Boon et al., 2006; Dudley et al., 2002; Lurquin et al., 2005). The vascular endothelium plays a key role in leukocyte trafficking and mounting evidence indicates that the tumor vasculature establishes an active barrier that tumor-reactive T cells must cross in order to recognize and eliminate their tumor targets (Figure 2) (Boon et al., 2006; Dudley et al., 2002; Lurquin et al., 2005). Successful transmigration through the tumor endothelial barrier is required for optimal tumor regression. The prohibitive nature of the tumor endothelium can be mediated by the type and quantity of adhesion molecules expressed (Zitvogel et al., 2006) and is presumably maintained by local soluble tumor factors (Zitvogel et al., 2006), but precisely how the tumor vasculature establishes immune privilege is not well known.
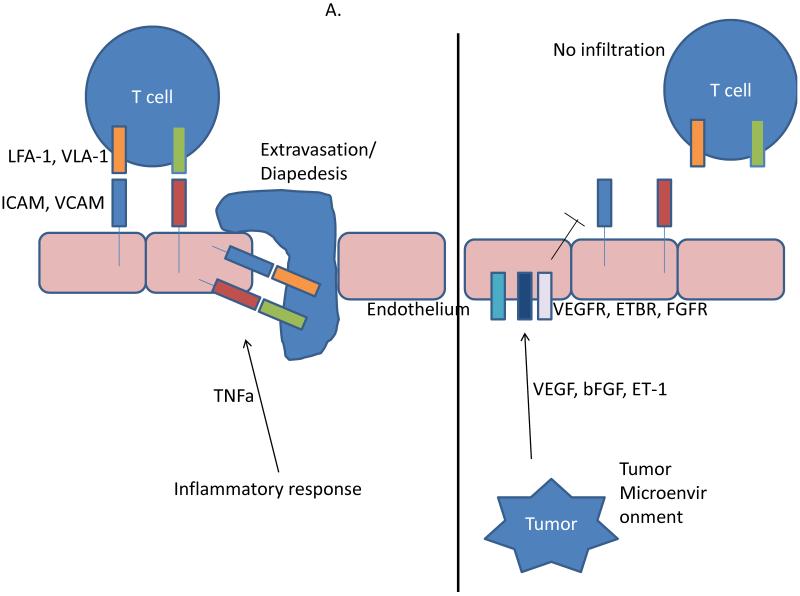
Figure 2.
Tumor endothelium in immune suppression. A, The endothelium is a physical barrier to T cell trafficking. During the normal inflammatory response, TNFα upregulates adhesion molecules ICAM and VCAM. While rolling, T cells bind to the adhesion molecules through LFA-1 and VLA-1, followed by extravasation through the endothelium and home to the site of inflammation. In the tumor microenvironment, tumor-derived angiogenic growth factors such as VEGF and ET-1 signal through their cognate receptors on the endothelium and block the expression of adhesion molecules, preventing T cell infiltration. B, The endothelium is a direct modulator of immune suppression. Under the influence of tumor- and stromal-derived factors (e.g., VEGF), the tumor endothelium expresses a number of immunosuppressive molecules such as TIM-3, IDO, PDL1/2, and PGE2. The expression of these molecules limit effector T cell activation. Further, the endothelium can also express a number of genes (e.g., TRAIL) that can directly kill effector T cells as they attempt to transverse the endothelial barrier.
T cells extravasate through the endothelium to the tumor in multi-step process that includes rolling and adhesion to endothelial cells, followed by diapedesis. Although the precise mechanisms are not entirely known, the consensus view is that in the tumor microenvironment angiogenic molecules, including VEGF, inhibit adhesion molecule expression on endothelial cells (Bouzin et al., 2007; Detmar et al., 1998; Dirkx et al., 2003; Griffioen et al., 1996a; Griffioen et al., 1996b; Min et al., 2005). Tumor necrosis factor-alpha (TNFα) is often expressed within the tumor microenvironment and is expressed, albeit in low amounts, by many malignant cells (Balkwill, 2009). Although TNFα is an activator of endothelial cells and T cell adhesion, in the presence of angiogenic growth factors like basic fibroblast growth factor (bFGF) or VEGF (Bouzin et al., 2007; Griffioen et al., 1996b) TNFα-induced T cell adhesion is minimal. In the presence of VEGF, TNFα stimulation is unable to induce expression of molecules intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule (VCAM-1) on endothelial cells (Bouzin et al., 2007; Griffioen et al., 1996b).
Endothelins and their receptors are upregulated in a number of cancers including ovarian, breast, renal, colon, and prostate cancer (Bagnato and Rosano, 2008; Nelson et al., 2003). Endothelin (ET) peptide ligands, ET-1, 2, 3, and 4 (Saida et al., 1989; Yanagisawa and Masaki, 1989) are potent regulators of endothelial cell biology and are involved in autocrine/paracrine loops that promote proliferation, protection from apoptosis, angiogenesis, vasculogenesis, and invasion and metastatic dissemination of tumors (Bagnato and Rosano, 2008; Nelson et al., 2003). Endothelins signal through two G protein-coupled receptors (GPCR), the endothelin-A receptor (ETAR) and ETBR, which exert opposite effects on the vasculature (Frommer and Muller-Ladner, 2008; Meidan and Levy, 2007). The use of specific endothelin receptor antagonists has been demonstrated to slow tumor growth in patients, or prevent tumor growth in mouse models (Bagnato and Rosano, 2008; Nelson et al., 2003). Endothelins interact with VEGF to regulate multiple aspects of angiogenesis including endothelial cell proliferation, migration, invasion, vessel formation, and neovascularization (Nelson et al., 2003). ET-1 can directly promote the expression of VEGF in cancer cell lines in vitro (Rosano et al., 2003; Salani et al., 2000; Spinella et al., 2007; Spinella et al., 2002), likely through increased HIF-1α expression (Salani et al., 2000). Furthermore, there is a correlation between ETBR and VEGF expression in a number of different tumor specimens (Kato et al., 2001). In addition to regulating vascular tension and angiogenesis, ETBR was recently demonstrated to suppress endothelial adhesion and T cell infiltration in tumors (Buckanovich et al., 2008). Endothelial ETBR was upregulated in ovarian tumors lacking intraepithelial tumor-infiltrating lymphocytes (TILs) (Buckanovich et al., 2008) and, similar to the absence of intraepithelial TILs (Zhang et al., 2003), ETBR overexpression was associated with poor survival. Endothelin 1 (ET1) signaling through ETBR was found to block T cell adhesion to the endothelium through suppression of ICAM-1 clustering on endothelial cell membranes (Buckanovich et al., 2008). ET1 is overexpressed in ovarian cancer (Bagnato et al., 1999), establishing a tumor cell–endothelium ET1–ETBR paracrine axis, which is upregulated specifically in the tumor microenvironment and suppresses T cell adhesion to endothelium, even in the presence of increased TNFα.
The imunosuppressive effects of VEGF as well as ETBR on tumor endothelium appear to be commonly mediated by nitric oxide (NO), as specific NO antagonists can abrogate the deregulation of CAMs induced by either molecule and restores T cell adhesion (Buckanovich et al., 2008; Bouzin et al., 2007). NO is a gaseous molecule and a highly reactive free radical. NO is highly important in the control of vascular functions regulating angiogenesis, vascular permeability, blood flow, and leukocyte-endothelial interactions (Fukumura et al., 2006). In tumors, inhibition of the NO producing enzyme, nitric oxide synthase (NOS), enhanced both rolling and adhesion of leukocytes on tumor vasculature (Fukumura et al., 1997). Although this effect may be due in part to direct effects of NO on T cells (Lukacs-Kornek et al., 2011; Bronte et al., 2005), NO also has a direct effect on endothelial cells that may limit anti-tumor immunity. NO reduces leukocyte-endothelial interactions by reducing the expression of important adhesion molecules P-selectin, ICAM-1, and VCAM-1, and conversely, NOS inhibitors increase expression of these adhesion molecules and leukocyte binding in normal blood vessels (De Caterina et al., 1995; Davenpeck et al., 1994; Kubes et al., 1991). Further, mice genetically deficient in the enzymes responsible for NO synthesis displayed enhanced leukocyte-endothelial interactions driven by P-selectin (Lefer et al., 1999).
It is emerging that the tumor vasculature also can shape the nature of T cell infiltration in tumors thorough mechanisms independent of adhesive interactions. Endothelial cells can for example express a number of mediators that suppress or kill effector lymphocytes such as Fas ligand (FasL) (Sata and Walsh, 1998), TNF-related apoptosis-inducing ligand (TRAIL) (Secchiero and Zauli, 2008), and possibly even CD31, a classical endothelial cell marker (Ma et al., 2010). Tumor endothelial cells can also express a robust repertoire of immunosuppressive molecules, both soluble and surface expressed including PD-L1 and PD-L2 (Frebel et al., 2012; Mazanet and Hughes, 2002; Rodig et al., 2003), B7-H3 (Zang et al., 2010), TIM-3 (Huang et al., 2010), and IL-10, TGFβ, and PGE2 (Hernandez et al., 2001; Pirtskhalaishvili and Nelson, 2000). Metabolic disruption of T cells through IDO expression (Batista et al., 2009; Blaschitz et al., 2011; Riesenberg et al., 2007) and arginase I (Fu et al., 2011) by endothelial cells has also been suggested, but whether these mechanisms are active in tumors are unknown. Thus, the function of tumor endothelial cells is largely immunosuppressive, maintained by tumor cells through paracrine mechanisms (Mulligan et al., 2009; Mulligan and Young, 2009).
Negotiating the tumor stroma space and suppressive leukocytes
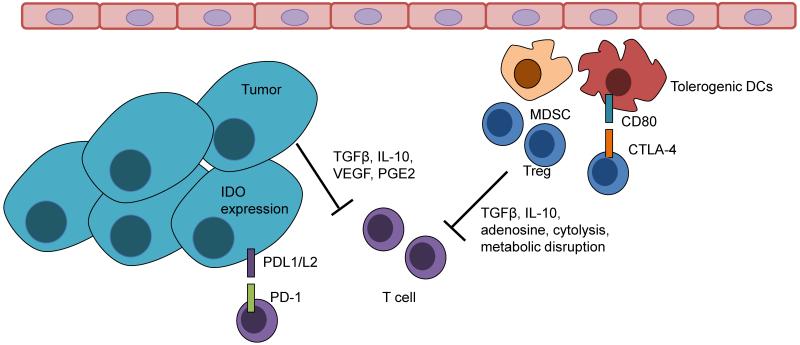
The effector T cells that manage to cross the endothelial barrier must navigate the tumor stroma until they encounter tumor cell targets. Many opportunities for encountering immunosuppressive signals exist within this space. Although resident stromal and immune cells can certainly contribute to immune suppression within the tumor microenvironment (Aggarwal and Pittenger, 2005; Dranoff and Fearon, 2013; Engels et al., 2012; Kammertoens et al., 2005; Motz and Coukos, 2011), immunosuppressive leukocyte populations such as Treg cells (Facciabene et al., 2012) and myeloid-derived suppressor cells (MDSCs) (Gabrilovich and Nagaraj, 2009) are also actively recruited and undergo local expansion to effectively suppress T cell effector functions. Infiltration of these cells types is frequently associated with a poor prognosis.
CD4+CD25+FoxP3+ Treg cells can be divided into natural Tregs (nTreg), which are derived from the thymus and maintained peripherally by TGFβ, and inducible Tregs (iTreg), which are induced from naïve CD4+ T cell precursors and exert suppressive functions similar to those observed for nTregs, controlled in both Treg subtypes by the forkhead box P3 (FoxP3) transcription factor (Curotto de Lafaille and Lafaille, 2009). Several studies have determined that Tregs that accumulate within tumors are generally clonally restricted (Sainz-Perez et al., 2012), and are specific for tumor antigens (Bonertz et al., 2009), expressing different T cell receptor (TCR) sequences than local effector T cells (Sainz-Perez et al., 2012). Treg are thus activated in an antigen-specific manner, but can suppress T cell function non-specifically through several mechanisms (Facciabene et al., 2012; Vignali et al., 2008). Tregs secrete soluble mediators such as TGFβ, IL-10, and IL-35, which can suppress effector T cell expansion and cytokine secretion (IFNγ, TNFα). Specifically, TGFβ and IL-10 have been shown experimentally to make significant contributions to tumor progression by attenuating antitumor immunity (Loser et al., 2007; Strauss et al., 2007). Although IL-2 signaling on Tregs is not required for their suppressive function, Tregs require IL-2 support to maintain metabolic homeostasis and their competitive fitness in vivo, (Fontenot et al., 2005). Thus, Treg cells depend on paracrine support from T effector cells, which secrete IL-2, for expanding and maintaining local tolerance. It is also believed that because of higher expression of the IL-2 receptor alpha (IL-2Rα, also known as CD25), Tregs can act as an IL-2 sink, “starving” effector T cells of IL-2 particularly within the tumor microenvironment, thus limiting effector T cell expansion and function (Pandiyan et al., 2007). In addition, Treg cells can suppress CTLs through the generation of adenosine (Deaglio et al., 2007). Tregs can also directly kill T cells via cytolysis mediated by TRAIL (Ren et al., 2007) or granzyme B (Gondek et al., 2005). Finally, Tregs can engage in crosstalk with DCs, inducing the expression of IDO, IL-10, and TGFβ expression from DCs through direct interactions (Facciabene et al., 2012).
Treg cells are actively recruited by tumors, and their accumulation has been associated with poor prognosis in some studies (Curiel et al., 2004). Chemokine ligand CCL22 is a potent Treg chemoattractant expressed in high amounts by both tumor cells and tumor macrophages recruiting Treg through C-C chemokine receptor 4 (CCR4) in ovarian cancer (Curiel et al., 2004) and Hodgkin’s lymphoma (Ishida et al., 2006). Recently, a link between Treg recruitment and hypoxia was discovered in ovarian cancer. Hypoxic ovarian cancer cells were shown to upregulate preferentially CCL28 among all chemokines (through hypoxia-inducible factor), which recruited CD4+CD25+FoxP3+ Treg cells through the cognate receptor CCR10 expressed preferentially on Tregs among T cell subsets (Facciabene et al., 2011). Forced overexpression of CCL28 in mouse ovarian cancer cells led to enhanced growth of intraperitoneal tumors, characterized by increased Treg infiltration and accelerated tumor growth (Facciabene et al., 2011). Of importance, depletion of CD25+ or CCR10+ cells eliminated Tregs from CCL28-overexpressing tumors and abrogated the tumor growth advantage conferred by CCL28 overexpression. It was found that besides immune suppression, CCL28-recruited Treg cells reprogrammed the tumor microenvironment towards angiogenesis (Facciabene et al., 2011). Recruitment of Tregs to areas of hypoxia could enhance their immunosuppressive capacity, as hypoxia-exposed Tregs are more effective at suppressing the proliferation of effector T cells (Ben-Shoshan et al., 2008).
In addition to recruiting nTregs, the tumor microenvironment promotes the continued expansion of nTregs (Valzasina et al., 2006) as well as the generation of iTregs (Liu et al., 2007). It is believed that IL-10 (Seo et al., 2001), TGFβ (Chen et al., 2003), and adenosine (Zarek et al., 2008) derived from either tumor cells or tumor-resident immunosuppressive DCs (Ghiringhelli et al., 2005) and Tie-2+ monocytes (TEMs) (Coffelt et al., 2011; Liu et al., 2007) may be responsible for this. Furthermore, TEMs can expand Tregs in vitro in a CD86-dependent manner, and in breast cancer patients TEMs expressing high amounts of CD86 are associated with high numbers of Tregs (Ibberson et al., 2013). CD86 expression in TEMs is dependent on TIE-2 and VEGFR signaling in vitro (Ibberson et al., 2013), highlighting the contribution of angiogenesis to this phenomenon.
There are a number of myeloid lineage cells that accumulate within tumors, but perhaps the best studied are MDSC. These cells are often found in large numbers within tumor sites as well as in the peripheral blood of cancer patients, and are potent inhibitors of effector T cell functions (Gallina et al., 2006). These cells can contribute to the suppression of T cells through production of arginase I and reactive oxygen species (ROS), IL-10 and TGFβ [reviewed by (Gabrilovich and Nagaraj, 2009)]. Additionally, MDSCs may expand Tregs within the tumor site (Huang et al., 2006). MDSCs may be recruited from the bone marrow and expanded in the peripheral blood through interactions with BV8 and endocrine-gland-derived VEGF (EG-VEGF) (LeCouter et al., 2004; Lin et al., 2002; Shojaei et al., 2007). Once in the blood, MDSCs can be recruited to tumors by a number of chemokines such as CCL2, CXCL5, CXCL12 and stem cell factor (SCF) (Murdoch et al., 2008). Expression of both BV8 and CXCL12 are increased by hypoxia (Du et al., 2008), so in a similar manner to Tregs, MDSCs may be recruited to sites of tumor hypoxia where they may directly influence angiogenesis. Once in the tumor, MDSCs exert potent effects on T cell effector function, but they also control other key events in tumors. Injection of MDSCs into tumors significantly enhanced blood vessel development (Yang et al., 2004), and tumor angiogenesis was reduced in tumor-bearing mice treated with a neutralizing anti-BV8 antibody, that reduced the numbers of infiltrating MDSC (Shojaei et al., 2007). However, MDSCs have been shown to have phenotypic plasticity, and they can acquire the characteristic of tumor-rejecting monocytes and even APCs given the right conditions are met. For example, cytokines like IL-12 and IFNγ have been shown to convert MDSCs into an APC-like cell that activates and enhances the functions of T cells either in vitro (Bronte et al., 2000) or in vivo (Kerkar et al., 2011).
The encounter with tumor cells
After T cells finally make their way through the barriers of the tumor vasculature and stroma, they are faced with a number of immunosuppressive factors that prevent effective recognition and/or attack of tumor cells (Figure 3). This topic has been reviewed extensively elsewhere (Whiteside, 2006; Zou, 2005). T cells depend largely on recognition of targets through major histocompatibility complex (MHC)-TCR interactions. It has become quite obvious that tumors express unique protein products that can be recognized by the immune system as ‘non-self’, ranging from overexpressed self proteins including cancer testis and other immune privileged site antigens, to novel mutational epitopes resulting from non-synonymous somatic mutations. Although most tested tumors harbor tens to hundreds of such mutations (Vogelstein et al., 2013), many of these epitopes are candidates for presentation (Segal et al., 2008), and T cells recognizing such tumor rejection epitopes have been recently identified in melanoma (Robbins et al., 2013), it is not known whether these mutations are in fact transcribed, translated in mutated protein products, or finally included in peptides presented on surface MHC molecules in all tumors. Many tumors presumably manage to eliminate presentation of immunogenic peptides through loss of expression or downregulation of the antigen presenting machinery, occurring in both MHC Ia (classical) (Marincola et al., 2000) and MHC Ib (non-classical) presentation (Carosella et al., 2003); (Rodgers and Cook, 2005). There are a multitude of mechanisms responsible for this aberrant expression, possibly stemming from selective pressure ranging from mutation, genetic loss, and epigenetic silencing of expression (Chang et al., 2005). This reduction is associated with histopathological markers of poor prognosis of disease and poor clinical outcomes (Marincola et al., 2000). However, the demonstration that CD8+ T cells recognizing mutational epitopes are already present in tumors and can in fact reject them under the optimized conditions of adoptive transfer, indicates that at the steady state tumors manage to attenuate recognition and attack by these T cells (Carosella et al., 2003; Chang et al., 2005; Marincola et al., 2000). Tumors express surface molecules that can directly kill T cells particularly of the TNF family of genes that include FasL and TRAIL (Whiteside, 2002). They can also express surface proteins such as PD-L1 and PD-L2 (Hamanishi et al., 2007) and B7-H4 (Kryczek et al., 2006), which can suppress T cell functions and arrest tumor rejection (Hirano et al., 2005; Munn and Mellor, 2004; Whiteside, 2002). In addition, the microenvironment close to tumor cells may be quite toxic for optimal CTL function. Tumors secrete a number of soluble mediators that can directly inhibit CTLs such as TGFβ, IL-10, PGE2, histamine, hydrogen peroxide, and adenosine (Whiteside, 2002). Deprivation of metabolic substrates due to competitive consumption by tumor cells and/or active depletion by enzymes such as IDO and arginase (Munn and Mellor, 2004) can further attenuate T cell effector function. Finally, the hypoxic conditions (Palazon et al., 2012) and the relatively lower extracellular pH characterizing the tumor interstitium can negatively affect CTL function (Aggarwal and Pittenger, 2005; Mendler et al., 2012)
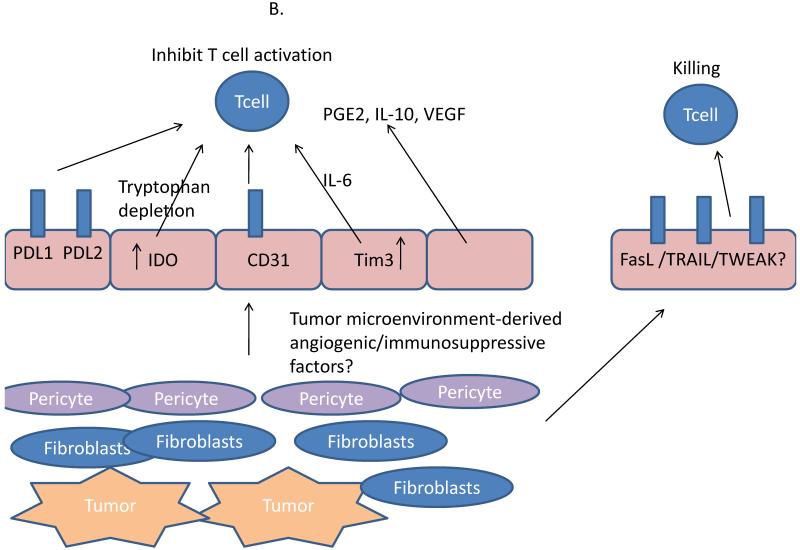
Figure 3.
After arrival within the tumor microenvironment, effector T cell functions are suppressed by a number of mechanisms including interactions with both soluble and cell surface-expressed mediators.
Therapies to restore antitumor immunity
The generation of competent tumor-specific CTLs and their recognition and rejection of tumor cells seems like an impossible task. Yet, recent successes indicate that this can be achieved in a proportion of patients with existing therapeutic means. It is obvious that most - if not all - tumors are potentially immunogenic, but the endogenous immune response is either entirely paralyzed or ineffective. Thus, major efforts should focus on attenuating tumor-associated immune suppression. Presently, there are multiple opportunities to target immunosuppressive pathways and mobilize antitumor immunity in vivo or ex vivo. Effective antitumor T cells can be expanded ex vivo from natural TILs, from endogenous high-avidity T cell clones recognizing tumor-associated antigens, or from T cells transduced with exogenous cloned TCRs or chimeric antigen receptors (CARs), while the host can be conditioned by lymhodepletion to optimize their engraftment; endogenous T cells can also be effectively activated by pharmacologic checkpoint inhibitors; the vasculature can be disrupted by low-dose metronomic chemotherapy or normalized by drugs targeting angiogenesis; soluble immunosuppressive factors such as IDO, IL-6, IL-10, TGFβ, and PGE2 can be individually blocked by specific pharmacologic inhibitors; suppressor cells can be depleted by specific chemotherapy drugs such cyclophosphamide (e.g. for Treg cells) or gemcitabine (e.g. for MDSCs); endogenous tumor-specific T cell immunity can be effectively boosted by exogenous vaccines; and tumor-associated antigen-presenting cells can be activated by specific drugs, including Toll-like receptor agonists, while tumor antigens can be released in situ by immunogenic cell death induced by specific chemotherapy drugs or radiation. The complexity of the immunosuppressive mechanisms at the tumor microenvironment and the availability of multiple therapeutic opportunities render the clinical advancement of cancer immunotherapy strategies a daunting task. The rational design of therapeutic strategies could be aided by classifying tumors based on the presence of pre-existing immunity. Robust biomarkers of pre-existing tumor immunity have not been validated yet, but TILs demonstrated by immunostaining could be a reasonable biomarker to classify tumors in those that already have pre-existing TILs, and those that do not. There is to date no data available on whether the TIL biomarker can predict responses to checkpoint blockade or other therapies activating T cells (such as bispecific antibodies) due to the lack of pre-treatment biopsies in most clinical trials. However, this classification could help designing immunomodulatory therapy, as immune escape mechanisms are likely to be quite different between these two main tumor immunophenotypes, at least with respect to T cell homing and engraftment. Validation of TILs is urgently needed to assess whether their presence in human tumors is a robust indication of endogenous antitumor immune response. Below, we discuss a number of immune therapies that have shown clinical efficacy or are under clinical development.
Tumors with pre-existing TILs
In tumors with pre-existing TILs, one can assume that the tumor microenvironment does not offer major barriers to T cell homing. Furthermore, the detection of tumor-reactive TILs would provide a strong indication that the tumor microenvironment does not entirely paralyze antigen-presenting mechanisms or the engraftment of tumor-reactive T cells. In melanoma, the success of adoptive TIL therapy is a testament to this hypothesis; all patients who receive TILs are preselected based on the availability of expandable tumor-reactive TILs, and a significant proportion of them experience objective responses. Thus, these TILs can reject tumors when appropriately activated and expanded, indicating that proper T cell activation is a major issue in these tumors, and that mechanisms limiting the function of CTLs must be operating in these tumors at the steady state. Attenuation of these immunosuppressive mechanisms could lead to clinical responses in many of these tumors. The first convincing evidence that activation of CTLs could produce objective clinical benefit in a significant number of patients was provided by Ipilimumab, a monoclonal antibody directed against CTLA-4 (cytotoxic T lymphocyte activation marker 4). CTLA-4 is a potent negative regulator of T cell activation that binds to members of the B7 family of co-stimulatory molecules (Chambers et al., 2001). Binding of CTLA-4 inhibits activation of T cells, thus attenuating excessive T cell activation. The significance of CTLA-4 as a negative regulator of the immune response comes from CTLA-4 knockout mice, which die at a very early age of autoimmune toxicity (Waterhouse et al., 1995). Ipiliumab has shown an overall survival benefit in two randomized, phase III trials in patients with advanced melanoma (Hodi et al., 2010; Robert et al., 2011). Although the response rates were modest, between 10% and 15%, a significant survival advantage was observed in the treated populations. The mechanisms of action of CTLA-4 blockade are still debated, including disinhibition of CTLA-4+ tumor-reactive TILs, but also depletion of CTLA-4high activated Treg cells (Egen et al., 2002; Kwon et al., 1999; Selby et al., 2013; Sutmuller et al., 2001; van Elsas et al., 2001).
PD-1 is an additional coinhibitory receptor expressed on activated T cells. Its function is important in peripheral tissues, where T cells can encounter the PD-1 ligands PD-L1 (B7-H1) and PD-L2 (B7-DC), which are expressed by tumor cells, stromal cells, or both (Dong et al., 2002). Inhibition of PD-1 signaling can enhance T-cell responses both in vitro and in vivo in preclinical tumor models (Iwai et al., 2002). In complementary pilot clinical studies using either anti-PD1 or anti-PD-L1 antibody, a large number of patients have demonstrated objective clinical responses (Brahmer et al., 2012; Topalian et al., 2012). Patients who responded the greatest appeared to be those whose tumors expressed PD-L1. Besides melanoma and renal cell cancer, patients with lung cancer and other solid tumors have shown objective responses, validating that tumor immunogenicity is a universal tumor property and it is not restricted to few tumor types traditionally considered immunogenic. A variety of additional coinhibitory (LAG-3, TIM-3, BTLA, 2B4, KLRG-1, CD160, etc) and costimulatory receptors (CD28, 4-1BB, OX-40, GITR etc.) have been identified. Their significance as therapeutic targets, whether complementary or redundant, will require careful preclinical characterization and clinical testing. Despite encouraging preclinical results with dual blockade of PD-1 and CTLA-4 (Duraiswamy et al., 2013), clinical data is still being accumulated (trial number NCT01024231). However, the fact that many patients still do not respond to CTLA-4 and/or PD-1 blockade may suggest that additional signals are required to effectively rescue TIL function.
An additional approach to activating TILs is the use of bispecific antibodies. This approach relies on the use of recombinant antibody structures with dual specificity that simultaneously engage both the TIL and an antigen on the tumor cell surface (Kontermann, 2012). For example, a recent approach using a bispecific antibody that recognizes both CD3ε (T cell activating domain) and CD19 (tumor-binding domain) achieved impressive clinical success (Topp et al., 2011). This approach could be used in a number of additional tumor targets to promote activation of TILs within the tumor microenvironment. This method activates TILs in a non-specific manner and therefore does not rely on the presence of endogenous tumor-specific T cells. However, tumors with pre-existing TILs may be more prone to respond, given that TILs are already available at the tumor microenvironment, and homing of additional T cells is not prevented by obvious local barriers.
Tumors lacking pre-existing TILs
Whereas tumors with pre-existing TILs appear intrinsically more immunogenic and easier to approach therapeutically, tumors lacking TILs may be more complex. Experiments in the mouse indicate that effective checkpoint blockade may not be sufficient to reenact tumor rejection mechanisms, and additional therapeutic interventions may be required to mobilize antitumor immunity (Kwon et al., 1999; Sutmuller et al., 2001; van Elsas et al., 2001). The molecular basis of immune escape in these tumors may be downregulation of the antigen presenting machinery in tumor cells, which may be due to epigenetic deregulation, and can be reversed with histone deacetylase inhibitors (Magner et al., 2000) or the use of low-dose IFNγ (Propper et al., 2003). Alternatively, a prohibitive tumor endothelial barrier may be the reason why T cells may not be able to home to tumors (Kandalaft et al., 2011). Proangiogenic signals can deregulate adhesive properties of the tumor endothelium and thus antiangiogenesis therapy could be contributory to activate antitumor immune response (Motz and Coukos, 2011). VEGF blockade in a transgenic mouse model of Her2/neu-driven breast cancer induced a de novo TIL infiltrate, which was responsible for enabling the therapeutic efficacy of VEGF blockade, as CD8+ T cell depletion abrogated the therapeutic efficacy of the anti-VEGFR-2 antibody DC101 (Manning et al., 2007). Given that VEGF inhibition has not proven beneficial in many human solid tumor types (Ebos and Kerbel, 2011), alternate or combinatorial approaches to block angiogenesis may be required. Lastly, given the likely paucity of pre-existing T cells in these tumors, maneuvers that expand the pool of tumor-specific T cells could prove beneficial. Molecularly defined monovalent vaccines have failed to produce substantial responses to date, but whole tumor antigen vaccines have performed better (Neller et al., 2008) and can greatly enhance checkpoint blockade to produce effective rejection of non-immunogenic tumors (Duraiswamy et al., 2013). This is possibly because they comprise all the potential mutational epitopes in addition to any other immunodominant epitopes. Future vaccine development based on mutational epitopes will test this hypothesis (Castle et al., 2012). Alternatively, in situ vaccination with exposure of tumor antigen through the use of immunogenic chemotherapy or radiation, in combination with tumor APC activation using appropriate Toll-like receptor agonists, could lead to the generation of tumor-reactive T cells and a switch of the tumor chemokine microenvironment by activated innate immune cells.
Specific targeting of immunosuppressive cells
In addition to promoting the activity of anti-tumor T cells, a number of approaches are aimed at inhibiting immune suppression within the tumor microenvironment. Specifically, a number of therapies have been developed to specifically deplete major immunosuppressive cells types. Reviewed extensively by Facciabene and colleagues (Facciabene et al., 2012), a number of direct and indirect methods exist to deplete Tregs within the tumor microenvironment. These include specific depletion with monoclonal antibodies (anti-CD25) and depletion with the use of chemotherapeutics such as cyclophosphamide (Facciabene et al., 2012). Further, additional immune cell targets like MDSCs have shown pre-clinical successes (Shojaei et al., 2007). This remains an area of active research.
Facing and deconvoluting the molecular complexity of tumor immune suppression
After decades of disappointment in immunotherapy, cancer immunotherapy using adoptive transfer of T cells or therapeutic antibodies that neutralize key checkpoint mechanisms (e.g. CTLA-4 or PD-1) has finally met the long-standing expectation to result in significant and reproducible clinical benefits. Immunotherapy is thus a feasible path forward to obtaining durable, long-lasting responses in cancer patients. Yet, many patients fail to respond, and among those who respond, many do not draw long-lasting benefits. Whereas adoptive therapy requires sophisticated infrastructures, checkpoint antibody therapy can be more easily distributed. Unfortunately, the mechanisms underlying therapeutic resistance to checkpoint blockade agents remain poorly understood, and the field faces a major gap in knowledge between our present understanding of mechanisms of tumor immune evasion at the steady state, and tumor resistance to immunomodulatory therapy. It is likely that checkpoint blockade fails because constitutive or adaptive tolerance mechanisms suppressing antigen-presentation and/or T cell homing and function are so powerful in many tumors that cannot be overcome solely by checkpoint blockade. A major challenge in addressing these mechanisms relates to the complexity of tumor microenvironment immunoregulatory systems, where tumor cells and an ever-growing list of tumor-infiltrating host leukocytes and stromal cells (including blood and lymphatic endothelial cells, and fibroblasts) can block T cell homing, engraftment or effector function, using diverse but often overlapping mechanisms. To translate this knowledge towards the development of rational (and personalized) therapies, one must develop knowledge and tools that allow integrating these pathways and deciphering their functional order and hierarchies (if any exist). For example, at the steady state, it is important to understand whether escape mechanisms differ among tumors, and if so, whether there are hierarchically dominant mechanisms, which could be targeted therapeutically first. From the therapeutic standpoint, it is unclear which of these mechanisms contribute to lack of response to checkpoint blockade, and how they may interact – when coexisting - to produce therapeutic resistance. Reversing the argument, it is unclear which of these escape mechanisms can be overcome by activating lymphocytes through checkpoint blockade alone, and which continue to act as a barrier to attenuate or abolish the effect of checkpoint blockade. It is likely that such mechanisms may not have a well-defined hierarchy, but their logic may be organized in networks. Although many of these resistance mechanisms are pre-existing, others may emerge in response to tumor adaptation to effective immune attack. Understanding this complex system requires new methods, including the development of high throughput experimental models that capture the heterogeneity of tumors among patients, and which allow systems pharmacology approaches producing large orthogonal datasets, and systems biology analytical approaches (Network, 2012).
Recognition of the complexity of biological networks has on one hand made it more difficult to identify appropriate therapeutic choices, but on the other hand, insights from systems biology suggest a new way of thinking about treatment resistance that may directly lead to new designs for trials. The tumor microenvironment coopts robust mechanisms of tissue repair, developed to promote homeostasis following injury, which summon overlapping cellular and molecular mechanisms promoting immune tolerance, angiogenesis, extracellular remodeling, and tissue repair. Successful immunotherapy must offer combinatorial strategies that make it impossible to block T cell responses, either by eliminating an essential, non-redundant central component, or alternatively, by simultaneously targeting multiple components that are able to compensate for each other’s activity. Such knowledge can only be developed effectively with experimental and analytical systems approaches and innovative adaptive designs in clinical trials.
Acknowledgments
This project was supported by NIH Transformative R01 CA156695 (GC), a grant by the Ovarian Cancer Research Fund (GTM), and the Ludwig Institute for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- Bagnato A, Rosano L. The endothelin axis in cancer. Int J Biochem Cell Biol. 2008;40:1443–1451. doi: 10.1016/j.biocel.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Bagnato A, Salani D, Di Castro V, Wu-Wong JR, Tecce R, Nicotra MR, Venuti A, Natali PG. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res. 1999;59:720–727. [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- Batista CE, Juhasz C, Muzik O, Kupsky WJ, Barger G, Chugani HT, Mittal S, Sood S, Chakraborty PK, Chugani DC. Imaging correlates of differential expression of indoleamine 2,3-dioxygenase in human brain tumors. Mol Imaging Biol. 2009;11:460–466. doi: 10.1007/s11307-009-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur J Immunol. 2008;38:2412–2418. doi: 10.1002/eji.200838318. [DOI] [PubMed] [Google Scholar]
- Blaschitz A, Gauster M, Fuchs D, Lang I, Maschke P, Ulrich D, Karpf E, Takikawa O, Schimek MG, Dohr G, et al. Vascular endothelial expression of indoleamine 2,3-dioxygenase 1 forms a positive gradient towards the feto-maternal interface. PLoS One. 2011;6:e21774. doi: 10.1371/journal.pone.0021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, Juenger S, Vlodavsky I, Khazaie K, Jaeger D, et al. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009;119:3311–3321. doi: 10.1172/JCI39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- Bouzin C, Brouet A, De Vriese J, Dewever J, Feron O. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J Immunol. 2007;178:1505–1511. doi: 10.4049/jimmunol.178.3.1505. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106:2375–2381. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, Battistini L, Iafrate M, Prayer-Galetti T, Pagano F, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Vishwanath RP, Aguilar B, Starr R, Najbauer J, Aboody KS, Jensen MC. Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. J Immunol. 2007;179:3332–3341. doi: 10.4049/jimmunol.179.5.3332. [DOI] [PubMed] [Google Scholar]
- Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O’Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- Carosella ED, Moreau P, Le Maoult J, Le Discorde M, Dausset J, Rouas-Freiss N. HLA-G molecules: from maternal-fetal tolerance to tissue acceptance. Adv Immunol. 2003;81:199–252. doi: 10.1016/s0065-2776(03)81006-4. [DOI] [PubMed] [Google Scholar]
- Castle JC, Kreiter S, Diekmann J, Lower M, van de Roemer N, de Graaf J, Selmi A, Diken M, Boegel S, Paret C, et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72:1081–1091. doi: 10.1158/0008-5472.CAN-11-3722. [DOI] [PubMed] [Google Scholar]
- Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- Chang CC, Campoli M, Ferrone S. Classical and nonclassical HLA class I antigen and NK Cell-activating ligand changes in malignant cells: current challenges and future directions. Adv Cancer Res. 2005;93:189–234. doi: 10.1016/S0065-230X(05)93006-6. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt SB, Chen YY, Muthana M, Welford AF, Tal AO, Scholz A, Plate KH, Reiss Y, Murdoch C, De Palma M, et al. Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. J Immunol. 2011;186:4183–4190. doi: 10.4049/jimmunol.1002802. [DOI] [PubMed] [Google Scholar]
- Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Davenpeck KL, Gauthier TW, Lefer AM. Inhibition of endothelial-derived nitric oxide promotes P-selectin expression and actions in the rat microcirculation. Gastroenterology. 1994;107:1050–1058. doi: 10.1016/0016-5085(94)90229-1. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr., Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71:6391–6399. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Porta M, Danova M, Rigolin GM, Brugnatelli S, Rovati B, Tronconi C, Fraulini C, Russo Rossi A, Riccardi A, Castoldi G. Dendritic cells and vascular endothelial growth factor in colorectal cancer: correlations with clinicobiological findings. Oncology. 2005;68:276–284. doi: 10.1159/000086784. [DOI] [PubMed] [Google Scholar]
- Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111:1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- Dirkx AE, Oude Egbrink MG, Kuijpers MJ, van der Niet ST, Heijnen VV, Bouma-ter Steege JC, Wagstaff J, Griffioen AW. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 2003;63:2322–2329. [PubMed] [Google Scholar]
- Dolfi DV, Duttagupta PA, Boesteanu AC, Mueller YM, Oliai CH, Borowski AB, Katsikis PD. Dendritic cells and CD28 costimulation are required to sustain virus-specific CD8+ T cell responses during the effector phase in vivo. J Immunol. 2011;186:4599–4608. doi: 10.4049/jimmunol.1001972. [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Dranoff G, Fearon D. Tumour immunology. Curr Opin Immunol. 2013;25:189–191. doi: 10.1016/j.coi.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual Blockade of PD-1 and CTLA-4 Combined with Tumor Vaccine Effectively Restores T Cell Rejection Function in Tumors. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-4100. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:316. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- Elia AR, Cappello P, Puppo M, Fraone T, Vanni C, Eva A, Musso T, Novelli F, Varesio L, Giovarelli M. Human dendritic cells differentiated in hypoxia down-modulate antigen uptake and change their chemokine expression profile. J Leukoc Biol. 2008;84:1472–1482. doi: 10.1189/jlb.0208082. [DOI] [PubMed] [Google Scholar]
- Engels B, Rowley DA, Schreiber H. Targeting stroma to treat cancers. Semin Cancer Biol. 2012;22:41–49. doi: 10.1016/j.semcancer.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Francavilla C, Maddaluno L, Cavallaro U. The functional role of cell adhesion molecules in tumor angiogenesis. Semin Cancer Biol. 2009;19:298–309. doi: 10.1016/j.semcancer.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Frebel H, Nindl V, Schuepbach RA, Braunschweiler T, Richter K, Vogel J, Wagner CA, Loffing-Cueni D, Kurrer M, Ludewig B, et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med. 2012;209:2485–2499. doi: 10.1084/jem.20121015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Lee JH, Sosman JA, Gabrilovich DI. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. 2007;13:4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- Frommer KW, Muller-Ladner U. Expression and function of ETA and ETB receptors in SSc. Rheumatology (Oxford) 2008;47(Suppl 5):v27–28. doi: 10.1093/rheumatology/ken274. [DOI] [PubMed] [Google Scholar]
- Fu H, Khan A, Coe D, Zaher S, Chai JG, Kropf P, Muller I, Larkin DF, George AJ. Arginine depletion as a mechanism for the immune privilege of corneal allografts. Eur J Immunol. 2011;41:2997–3005. doi: 10.1002/eji.201141683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Yuan F, Endo M, Jain RK. Role of nitric oxide in tumor microcirculation. Blood flow, vascular permeability, and leukocyte-endothelial interactions. Am J Pathol. 1997;150:713–725. [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res. 1999;5:2963–2970. [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, Amigorena S, Hermine O, Durandy A. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567–4575. [PubMed] [Google Scholar]
- Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- Griffioen AW, Damen CA, Blijham GH, Groenewegen G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood. 1996a;88:667–673. [PubMed] [Google Scholar]
- Griffioen AW, Damen CA, Martinotti S, Blijham GH, Groenewegen G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res. 1996b;56:1111–1117. [PubMed] [Google Scholar]
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez GL, Volpert OV, Iniguez MA, Lorenzo E, Martinez-Martinez S, Grau R, Fresno M, Redondo JM. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med. 2001;193:607–620. doi: 10.1084/jem.193.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- Huang X, Bai X, Cao Y, Wu J, Huang M, Tang D, Tao S, Zhu T, Liu Y, Yang Y, et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J Exp Med. 2010 doi: 10.1084/jem.20090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Buckanovich RJ, Benencia F, Stan RV, Keler T, Sarobe P, et al. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68:7684–7691. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124:192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibberson M, Bron S, Guex N, Faes-Van’t Hull E, Henry L, Ifticene-Treboux A, Lehr HA, Delaloye JF, Coukos G, Xenarios I, et al. TIE-2 and VEGFR kinase activities drive TIE-2-expressing monocytes immunosuppressive function in human breast tumors. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-3181. [DOI] [PubMed] [Google Scholar]
- Ishida T, Ishii T, Inagaki A, Yano H, Komatsu H, Iida S, Inagaki H, Ueda R. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res. 2006;66:5716–5722. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- Ishida T, Oyama T, Carbone DP, Gabrilovich DI. Defective function of Langerhans cells in tumor-bearing animals is the result of defective maturation from hemopoietic progenitors. J Immunol. 1998;161:4842–4851. [PubMed] [Google Scholar]
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- Kammertoens T, Schuler T, Blankenstein T. Immunotherapy: target the stroma to hit the tumor. Trends Mol Med. 2005;11:225–231. doi: 10.1016/j.molmed.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Kandalaft LE, Motz GT, Duraiswamy J, Coukos G. Tumor immune surveillance and ovarian cancer: lessons on immune mediated tumor rejection or tolerance. Cancer Metastasis Rev. 2011;30:141–151. doi: 10.1007/s10555-011-9289-9. [DOI] [PubMed] [Google Scholar]
- Kato T, Kameoka S, Kimura T, Soga N, Abe Y, Nishikawa T, Kobayashi M. Angiogenesis as a predictor of long-term survival for 377 Japanese patients with breast cancer. Breast Cancer Res Treat. 2001;70:65–74. doi: 10.1023/a:1012534724488. [DOI] [PubMed] [Google Scholar]
- Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K, Arihiro K. Potential functional role of plasmacytoid dendritic cells in cancer immunity. Immunology. 2007;121:149–157. doi: 10.1111/j.1365-2567.2007.02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontermann R. MAbs. 2012. Dual targeting strategies with bispecific antibodies; p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon ED, Foster BA, Hurwitz AA, Madias C, Allison JP, Greenberg NM, Burg MB. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc Natl Acad Sci U S A. 1999;96:15074–15079. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labidi-Galy SI, Sisirak V, Meeus P, Gobert M, Treilleux I, Bajard A, Combes JD, Faget J, Mithieux F, Cassignol A, et al. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res. 2011;71:5423–5434. doi: 10.1158/0008-5472.CAN-11-0367. [DOI] [PubMed] [Google Scholar]
- LeCouter J, Zlot C, Tejada M, Peale F, Ferrara N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc Natl Acad Sci U S A. 2004;101:16813–16818. doi: 10.1073/pnas.0407697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer DJ, Jones SP, Girod WG, Baines A, Grisham MB, Cockrell AS, Huang PL, Scalia R. Leukocyte-endothelial cell interactions in nitric oxide synthase-deficient mice. Am J Physiol. 1999;276:H1943–1950. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- Lin R, LeCouter J, Kowalski J, Ferrara N. Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. J Biol Chem. 2002;277:8724–8729. doi: 10.1074/jbc.M110594200. [DOI] [PubMed] [Google Scholar]
- Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25− T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- Loos T, Mortier A, Proost P. Chapter 1. Isolation, identification, and production of posttranslationally modified chemokines. Methods Enzymol. 2009;461:3–29. doi: 10.1016/S0076-6879(09)05401-9. [DOI] [PubMed] [Google Scholar]
- Loser K, Apelt J, Voskort M, Mohaupt M, Balkow S, Schwarz T, Grabbe S, Beissert S. IL-10 controls ultraviolet-induced carcinogenesis in mice. J Immunol. 2007;179:365–371. doi: 10.4049/jimmunol.179.1.365. [DOI] [PubMed] [Google Scholar]
- Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, Collier AR, Turley SJ. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol. 2011;12:1096–1104. doi: 10.1038/ni.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurquin C, Lethe B, De Plaen E, Corbiere V, Theate I, van Baren N, Coulie PG, Boon T. Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J Exp Med. 2005;201:249–257. doi: 10.1084/jem.20041378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- Ma C, Zhang Q, Ye J, Wang F, Zhang Y, Wevers E, Schwartz T, Hunborg P, Varvares MA, Hoft DF, et al. Tumor-infiltrating gammadelta T lymphocytes predict clinical outcome in human breast cancer. J Immunol. 2012;189:5029–5036. doi: 10.4049/jimmunol.1201892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Mauro C, Cornish GH, Chai JG, Coe D, Fu H, Patton D, Okkenhaug K, Franzoso G, Dyson J, et al. Ig gene-like molecule CD31 plays a nonredundant role in the regulation of T-cell immunity and tolerance. Proc Natl Acad Sci U S A. 2010;107:19461–19466. doi: 10.1073/pnas.1011748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magner WJ, Kazim AL, Stewart C, Romano MA, Catalano G, Grande C, Keiser N, Santaniello F, Tomasi TB. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J Immunol. 2000;165:7017–7024. doi: 10.4049/jimmunol.165.12.7017. [DOI] [PubMed] [Google Scholar]
- Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477–483. doi: 10.1046/j.1440-1711.2002.01115.x. [DOI] [PubMed] [Google Scholar]
- Manning EA, Ullman JG, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, Hicklin DJ, Jaffee EM, Emens LA. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13:3951–3959. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169:3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- Meidan R, Levy N. The ovarian endothelin network: an evolving story. Trends Endocrinol Metab. 2007;18:379–385. doi: 10.1016/j.tem.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int J Cancer. 2012;131:633–640. doi: 10.1002/ijc.26410. [DOI] [PubMed] [Google Scholar]
- Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]
- Min JK, Lee YM, Kim JH, Kim YM, Kim SW, Lee SY, Gho YS, Oh GT, Kwon YG. Hepatocyte growth factor suppresses vascular endothelial growth factor-induced expression of endothelial ICAM-1 and VCAM-1 by inhibiting the nuclear factor-kappaB pathway. Circ Res. 2005;96:300–307. doi: 10.1161/01.RES.0000155330.07887.EE. [DOI] [PubMed] [Google Scholar]
- Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11:702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- Mulligan JK, Day TA, Gillespie MB, Rosenzweig SA, Young MR. Secretion of vascular endothelial growth factor by oral squamous cell carcinoma cells skews endothelial cells to suppress T-cell functions. Hum Immunol. 2009;70:375–382. doi: 10.1016/j.humimm.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan JK, Young MR. Tumors induce the formation of suppressor endothelial cells in vivo. Cancer Immunol Immunother. 2009 doi: 10.1007/s00262-009-0747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Muthuswamy R, Berk E, Junecko BF, Zeh HJ, Zureikat AH, Normolle D, Luong TM, Reinhart TA, Bartlett DL, Kalinski P. NF-kappaB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cells. Cancer Res. 2012;72:3735–3743. doi: 10.1158/0008-5472.CAN-11-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy R, Mueller-Berghaus J, Haberkorn U, Reinhart TA, Schadendorf D, Kalinski P. PGE(2) transiently enhances DC expression of CCR7 but inhibits the ability of DCs to produce CCL19 and attract naive T cells. Blood. 2010;116:1454–1459. doi: 10.1182/blood-2009-12-258038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Boczkowski D, Moeller B, Dewhirst M, Vieweg J, Gilboa E. Synergy between tumor immunotherapy and antiangiogenic therapy. Blood. 2003;102:964–971. doi: 10.1182/blood-2002-12-3738. [DOI] [PubMed] [Google Scholar]
- Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- Neller MA, Lopez JA, Schmidt CW. Antigens for cancer immunotherapy. Semin Immunol. 2008;20:286–295. doi: 10.1016/j.smim.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3:110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- Network CGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115–1124. doi: 10.1007/s00262-007-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazon A, Aragones J, Morales-Kastresana A, de Landazuri MO, Melero I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res. 2012;18:1207–1213. doi: 10.1158/1078-0432.CCR-11-1591. [DOI] [PubMed] [Google Scholar]
- Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, Melief CJ, Kenter GG, Fleuren GJ, Offringa R, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- Pirtskhalaishvili G, Nelson JB. Endothelium-derived factors as paracrine mediators of prostate cancer progression. Prostate. 2000;44:77–87. doi: 10.1002/1097-0045(20000615)44:1<77::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Pivarcsi A, Muller A, Hippe A, Rieker J, van Lierop A, Steinhoff M, Seeliger S, Kubitza R, Pippirs U, Meller S, et al. Tumor immune escape by the loss of homeostatic chemokine expression. Proc Natl Acad Sci U S A. 2007;104:19055–19060. doi: 10.1073/pnas.0705673104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proost P, Mortier A, Loos T, Vandercappellen J, Gouwy M, Ronsse I, Schutyser E, Put W, Parmentier M, Struyf S, et al. Proteolytic processing of CXCL11 by CD13/aminopeptidase N impairs CXCR3 and CXCR7 binding and signaling and reduces lymphocyte and endothelial cell migration. Blood. 2007;110:37–44. doi: 10.1182/blood-2006-10-049072. [DOI] [PubMed] [Google Scholar]
- Propper DJ, Chao D, Braybrooke JP, Bahl P, Thavasu P, Balkwill F, Turley H, Dobbs N, Gatter K, Talbot DC, et al. Low-dose IFN-gamma induces tumor MHC expression in metastatic malignant melanoma. Clin Cancer Res. 2003;9:84–92. [PubMed] [Google Scholar]
- Ren X, Ye F, Jiang Z, Chu Y, Xiong S, Wang Y. Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4(+)CD25(+) regulatory T cells. Cell Death Differ. 2007;14:2076–2084. doi: 10.1038/sj.cdd.4402220. [DOI] [PubMed] [Google Scholar]
- Riesenberg R, Weiler C, Spring O, Eder M, Buchner A, Popp T, Castro M, Kammerer R, Takikawa O, Hatz RA, et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin Cancer Res. 2007;13:6993–7002. doi: 10.1158/1078-0432.CCR-07-0942. [DOI] [PubMed] [Google Scholar]
- Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013 doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, O’Day S, M DJ, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- Roland CL, Lynn KD, Toombs JE, Dineen SP, Udugamasooriya DG, Brekken RA. Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS One. 2009;4:e7669. doi: 10.1371/journal.pone.0007669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano L, Spinella F, Salani D, Di Castro V, Venuti A, Nicotra MR, Natali PG, Bagnato A. Therapeutic targeting of the endothelin a receptor in human ovarian carcinoma. Cancer Res. 2003;63:2447–2453. [PubMed] [Google Scholar]
- Saida K, Mitsui Y, Ishida N. A novel peptide, vasoactive intestinal contractor, of a new (endothelin) peptide family. Molecular cloning, expression, and biological activity. J Biol Chem. 1989;264:14613–14616. [PubMed] [Google Scholar]
- Sainz-Perez A, Lim A, Lemercier B, Leclerc C. The T-cell receptor repertoire of tumor-infiltrating regulatory T lymphocytes is skewed toward public sequences. Cancer Res. 2012;72:3557–3569. doi: 10.1158/0008-5472.CAN-12-0277. [DOI] [PubMed] [Google Scholar]
- Salani D, Taraboletti G, Rosano L, Di Castro V, Borsotti P, Giavazzi R, Bagnato A. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol. 2000;157:1703–1711. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sata M, Walsh K. TNFalpha regulation of Fas ligand expression on the vascular endothelium modulates leukocyte extravasation. Nat Med. 1998;4:415–420. doi: 10.1038/nm0498-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, Ponnazhagan S. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol. 2012;189:4258–4265. doi: 10.4049/jimmunol.1101855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlienger K, Chu CS, Woo EY, Rivers PM, Toll AJ, Hudson B, Maus MV, Riley JL, Choi Y, Coukos G, et al. TRANCE- and CD40 ligand-matured dendritic cells reveal MHC class I-restricted T cells specific for autologous tumor in late-stage ovarian cancer patients. Clin Cancer Res. 2003;9:1517–1527. [PubMed] [Google Scholar]
- Secchiero P, Zauli G. The puzzling role of TRAIL in endothelial cell biology. Arterioscler Thromb Vasc Biol. 2008;28:e4. doi: 10.1161/ATVBAHA.107.158451. author reply e5-6. [DOI] [PubMed] [Google Scholar]
- Segal NH, Parsons DW, Peggs KS, Velculescu V, Kinzler KW, Vogelstein B, Allison JP. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–892. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Anti-CTLA-4 Antibodies of IgG2a Isotype Enhance Antitumor Activity through Reduction of Intratumoral Regulatory T Cells. Cancer Immunology Research. 2013 doi: 10.1158/2326-6066.CIR-13-0013. Epub. [DOI] [PubMed] [Google Scholar]
- Seo N, Hayakawa S, Takigawa M, Tokura Y. Interleukin-10 expressed at early tumour sites induces subsequent generation of CD4(+) T-regulatory cells and systemic collapse of antitumour immunity. Immunology. 2001;103:449–457. doi: 10.1046/j.1365-2567.2001.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- Spinella F, Rosano L, Di Castro V, Decandia S, Nicotra MR, Natali PG, Bagnato A. Endothelin-1 and endothelin-3 promote invasive behavior via hypoxia-inducible factor-1alpha in human melanoma cells. Cancer Res. 2007;67:1725–1734. doi: 10.1158/0008-5472.CAN-06-2606. [DOI] [PubMed] [Google Scholar]
- Spinella F, Rosano L, Di Castro V, Natali PG, Bagnato A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1alpha in ovarian carcinoma cells. J Biol Chem. 2002;277:27850–27855. doi: 10.1074/jbc.M202421200. [DOI] [PubMed] [Google Scholar]
- Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–1642. [PubMed] [Google Scholar]
- Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Kono K, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines. Cancer Immunol Immunother. 2004;53:543–550. doi: 10.1007/s00262-003-0466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25− lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- van Elsas A, Sutmuller RP, Hurwitz AA, Ziskin J, Villasenor J, Medema JP, Overwijk WW, Restifo NP, Melief CJ, Offringa R, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr., Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchmaker PB, Berk E, Muthuswamy R, Mailliard RB, Urban JA, Kirkwood JM, Kalinski P. Independent regulation of chemokine responsiveness and cytolytic function versus CD8+ T cell expansion by dendritic cells. J Immunol. 2010;184:591–597. doi: 10.4049/jimmunol.0902062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. Tumor-induced death of immune cells: its mechanisms and consequences. Semin Cancer Biol. 2002;12:43–50. doi: 10.1006/scbi.2001.0402. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Masaki T. Molecular biology and biochemistry of the endothelins. Trends Pharmacol Sci. 1989;10:374–378. doi: 10.1016/0165-6147(89)90011-4. [DOI] [PubMed] [Google Scholar]
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Zang X, Sullivan PS, Soslow RA, Waitz R, Reuter VE, Wilton A, Thaler HT, Arul M, Slovin SF, Wei J, et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol. 2010;23:1104–1112. doi: 10.1038/modpathol.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Kroemer G. Anticancer immunochemotherapy using adjuvants with direct cytotoxic effects. J Clin Invest. 2009;119:2127–2130. doi: 10.1172/JCI39991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]