Abstract
Background
Outcomes of severe hematochezia from ischemic colitis vs. other colonic diagnoses have not been well studied.
Objective
Our purposes were: 1) to compare demographics and outcomes of patients hospitalized with severe hematochezia from ischemic colitis vs. other colonic diagnoses, 2) to compare inpatient vs. outpatient start of bleeding from ischemic colitis, 3) to describe potential risk factors.
Design
Prospective cohort study.
Setting
Tertiary referral academic centers.
Patients
Patients referred for gastroenterology consultation about severe hematochezia.
Interventions
Colonoscopic therapy was provided as indicated.
Main outcome measurements
Rebleeding, surgery and length of hospital stay
Results
Of 550 patients in the last 12 years with severe colonic hematochezia, 65 were caused by ischemia. Major 30 day outcomes of ischemic colitis patients were significantly worse than patients with other colonic diagnoses. Patients with inpatient (vs. outpatient) ischemic colitis had significantly more and severe comorbidities at baseline and significantly higher rates of rebleeding, surgery & more hospital & intensive care unit days.
Limitations
Two-center study
Conclusions
Ischemic colitis was found more often in females and patients with anticoagulant usage, severe lung disease, higher creatinine, higher glucose, & more fresh frozen plasma transfused. Five patients with focal lesions had colonoscopic hemostasis. Major 30 day outcomes of ischemic colitis patients were significantly worse than patients with other colonic diagnoses. Comparing outpatient vs. inpatient start of ischemic colitis, inpatients had significantly worse outcomes.
Keywords: Ischemic Colitis, Severe Hematochezia, Hemostasis, Inpatient, Outpatient
Introduction
Ischemic colitis is the most common form of ischemic injury to the gastrointestinal (GI) tract, the second most common cause of inpatient hematochezia, and the third most common colonic cause of severe hematochezia.1-4 Ischemic colitis has a wide spectrum of injury and clinical severity ranging from mild reversible mucosal injury to severe, irreversible, transmural disease. The majority (75-85 percent) of patients have self-limited ischemic colitis and usually respond to medical therapy.5-6 If a focal ischemic ulcer with stigmata of hemorrhage is found, it can be treated endoscopically.4 Our purposes in this study were: (1) to compare demographics and outcomes of patients hospitalized for severe hematochezia from ischemic colitis vs. other colonic diagnoses, (2) to compare demographics and outcomes of patients whose hematochezia from ischemic colitis started as an outpatient vs. those who developed hematochezia from ischemic colitis as an inpatient, and (3) to describe potential risk factors of this disease vs. other colon diagnoses.
Methods
The study was approved by the University of California, Los Angeles Medical Center's and the Veterans Affairs Greater Los Angeles Medical Center's Institutional Review Boards. This study has been registered with ClinicalTrials.gov (ID 03-11-115-12). A total of 550 patients were prospectively evaluated after GI consultation after they were hospitalized in two academic medical centers in the last 12 years with severe hematochezia either that started as an outpatient or developed as an inpatient after admission for an unrelated reason. Inclusion criteria were: (1) bright red blood clots or burgundy colored stool documented by a health care worker, (2) clinical or laboratory evidence of significant blood loss, manifested by any one of the following: (a) more than three bloody bowel movements in eight hours (b) a decrease of two grams of hemoglobin (Hgb) from baseline, or (c) transfusion of more than three units of red blood cell (URBC). Exclusion criteria were: (1) age less than 18 years, (2) history of inflammatory bowel disease, (3) hypotension or shock refractory to resuscitation, (4) severe coagulopathy refractory to resuscitation (platelet count < 30,000; prothrombin time [PT]- more than 2 times normal; partial thromboplastin time [PTT]- more than 2 times normal), (5) acquired immune deficiency syndrome or neutropenia, (6) the inability to provide informed consent, and (7) documentation of anal disorders as a cause of bleeding such as internal hemorrhoids, anal fissures, and polyps or cancer of the anal canal.
Demographics and outcomes of all ischemic colitis patients were compared to all other colonic bleeding patients; outpatient ischemic colitis patients were compared to inpatient ischemic colitis patients. All patients were received six to ten liters of polyethelyne glycol based oral purgative (Golytely® or Colyte®) over four to six hours. Colonoscopy was performed within a mean of 12 hours of the GI consultation. The final diagnosis was made on colonoscopic findings, distribution, histopathology, and negative testing for other types of colitis. Patient outcomes were prospectively assessed during their hospitalization by a research study coordinator. Data were collected until patient discharge or 30 days, whichever came first. For rebleeding, we utilized the same criteria as the inclusion criteria to determine clinical or laboratory evidence of significant blood loss.
Data Management and Statistical Analysis
All data were de-identified and entered into data files by experienced data managers. SAS was used for data management and analyses. All analyses were performed in consultation with a biostatistician. Bivariate analysis was performed separately for each variable. P values were calculated by the Fisher's exact test for discrete variables and by the Student's t test or Wilcoxon rank-sum test for continuous variables. A p value of less than 0.05 was considered significant in these analyses.
Results
Patients, demographics and investigations
550 patients with colonic causes of severe hematochezia were identified. Sixty-five patients had ischemic colitis and 36 patients (55.4%) started bleeding as an outpatient whereas 29 patients (44.6%) developed bleeding for the first time while hospitalized for other reasons. Four hundred and eighty-five patients were diagnosed with other colonic ailments including diverticular bleeding, arteriovenous malformations (angioectasia), colon cancer, colonic polyps, delayed post- polypectomy bleeding, or non-ischemic colitis (Clostridium difficile, idiopathic, or inflammatory bowel diseases).
Results of the bivariate analyses comparing ischemic colitis with other colon causes are detailed in Table 1. For the 65 ischemic colitis group compared to the 485 with other colonic diagnoses, the mean ages, recent NSAIDs and/or aspirin usage, warfarin usage, shock or hypotension, baseline Hgb, baseline PT, baseline platelets, baseline white blood cell (WBC), URBC transfusion, units of platelets (UPlt) transfusion were not significantly different. However, female gender, any anticoagulant usage, moderate or severe lung disease; or serum creatinine, serum glucose, and units of fresh frozen plasma (UFFP) transfusion were all significantly higher in the ischemia group.
Table 1. Bivariate analyses between ischemic colitis and other colonic causes.
| Ischemic colitis (65) | other colonic causes (485) | p Value | |
|---|---|---|---|
| Age+ | 64.9±1.7 | 66.5±0.7 | 0.75 |
| Female | 32 (49.2%) | 172 (35.5%) | 0.0310 |
| NSAIDs and/or aspirin usage | 27 (41.5%) | 206 (42.5%) | 0.8860 |
| Warfarin usage | 10 (15.4%) | 43 (8.9%) | 0.0944 |
| Any anticoagulants usage* | 19 (29.2%) | 68 (14.0%) | 0.0016 |
| Shock or hypotension | 13 (20.0%) | 94 (19.4%) | 0.9058 |
| Moderate or severe lung disease | 27 (41.5%) | 130 (26.8%) | 0.0006 |
| Baseline Hgb+ | 9.7±0.4 | 9.4±0.1 | 0.74 |
| Baseline PT+ | 13.2±0.6 | 12.7±0.2 | 0.77 |
| Baseline platelets+ | 208,369±12,461 | 201,026±4,917 | 0.87 |
| Baseline WBC+ | 11.2±0.7 | 9.7±0.5 | 0.49 |
| Creatinine+ | 3.0±0.9 | 1.5±0.1 | 0 |
| Glucose+ | 151±10 | 130±3 | 0.01 |
| URBC before colonoscopy+ | 3.9±1.0 | 3.2±0.2 | 0.56 |
| UFFP before colonoscopy+ | 2.2±1.0 | 0.5±0.1 | 0 |
| UPlt before colonoscopy+ | 0.7±0.3 | 0.5±0.2 | 0.89 |
| Prognosis score+ | 2.5±0.16 | 2.4±0.1 | 0.93 |
expressed as mean±standard error (SE)
Warfarin, Heparin, Enoxaparin, and other anticoagulants
Treatment and outcomes
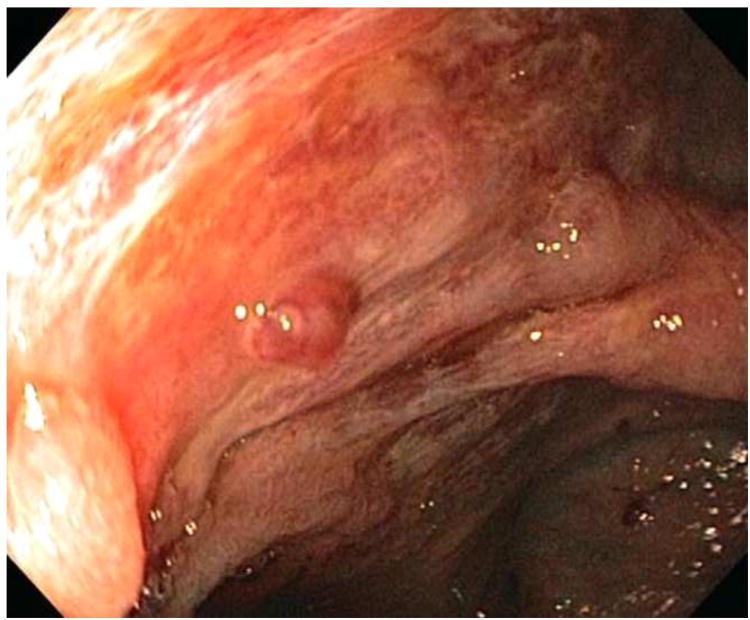
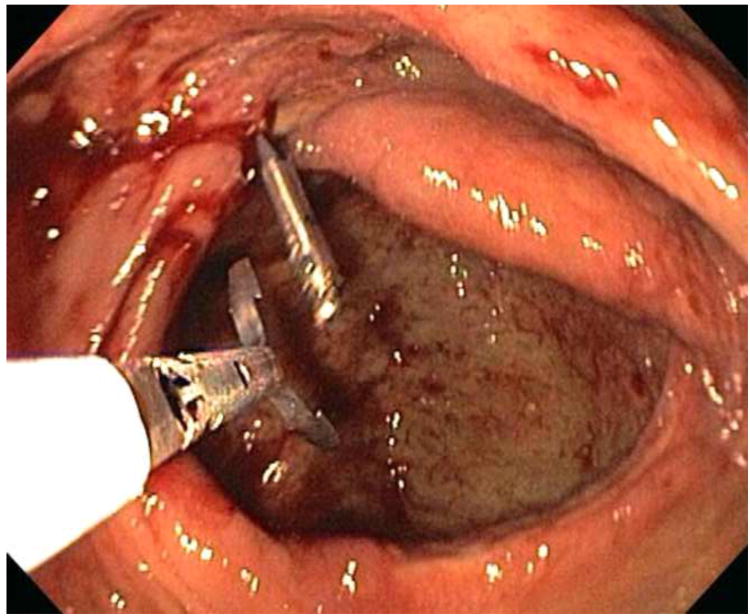
All ischemic colitis patients had more than one lesion on colonoscopy. These typically included ulcers, erosions, erythema, and loss of normal vascular pattern. In addition, a few patients also had a focal ulcer with major stigmata of recent hemorrhage. One patient had recurrent inpatient hematochezia while hospitalized and was found to have an ischemic ulcer with a non-bleeding visible vessel (NBVV) (Figure 1). This was successfully treated by combination therapy-epinephrine injection plus hemoclip placement (shown in Figure 2). On initial colonoscopy, four other patients had focal ulcers with major stigmata of hemorrhage, two with oozing bleeding, one with adherent clot, and one with NBVV. These were treated with either epinephrine injection (one patient) or hemoclipping (three patients). All other patients had diffuse or segmental lesions without stigmata, and none was amenable to endoscopic hemostasis. Fifty-six ischemia patients (86.2 %) responded to medical treatment (53 patients) alone (bowel rest, intravenous fluids, hemodynamic optimization, avoidance of vasoconstrictive drugs, antibiotic prophylaxis and treatment of comorbid conditions) or in combination with endoscopy therapy (three patients). Nine other ischemia patients (13.8%) were treated with surgery after initially failing medical treatment (seven patients) and/or endoscopic therapy (two patients) because they had recurrent severe bleeding requiring more transfusions. The overall mortality rate was 7.7% (5/65).
Figure 1. Focal ischemic ulcer with a non-bleeding visible vessel (NBVV).
Figure 2. Hemoclip placement on the NBVV of a focal ischemic ulcer.
Most outcomes at discharge or at 30 days of patients with ischemic colitis were significantly worse than patients with other colonic diagnoses (Refer to Table 2). The clinical course and outcomes of the ischemic colitis group and other colonic diagnoses group were significantly different for each of the following: (1) the rebleeding rate was significantly higher in the ischemic colitis group, (2) surgical intervention was significantly higher in ischemic colitis group, and (3) the mean number of hospital days was significantly higher in ischemic colitis group. Although clinically and arithmetically worse, the following were not significantly different: (1) death in both groups, and (2) the mean number of intensive care unit (ICU) days in both groups.
Table 2. Thirty day outcomes of 65 patients with ischemic colitis compared with 485 patients with other colonic diagnoses.
| Ischemic colitis | All other diagnoses | p value | |
|---|---|---|---|
| N | 65 | 485 | |
| Rebleeding | 18/65 (27.7%) | 61/485 (12.6%) | 0.0041 |
| Surgical intervention | 9/65 (13.9%) | 27/485 (5.6%) | 0.0113 |
| Mean ICU days* | 3.6 ± 1.0 | 2.2 ± 0.3 | 0.1 |
| Mean hospital days* | 11.8 ± 1.4 | 6.5 ± 0.4 | 0 |
| Death | 5/65 (7.7%) | 16/485 (3.3%) | 0.0826 |
Mean ± SE
Inpatient vs. outpatient ischemic colitis
Statistically significant differences were observed between patients with inpatient vs. outpatient start of hematochezia (See Table 3). NSAIDs usage was more frequently seen in the outpatient group compared to the inpatient group. In contrast, warfarin usage, any anticoagulant usage, anti-hypertensive usage, severe lung disease, severe heart disease, hematologic disorder, URBC transfusion before colonoscopy and prognosis score were all significantly higher in the inpatient group than outpatient group. Baseline Hgb and baseline PTT were also significantly different in the inpatient group compared to outpatient group. However, WBC, PT and creatinine were similar in both groups.
Table 3. Demographics by outpatient and inpatient ischemic colitis groups.
| Outpatient group (36 patients) | Inpatient group (29 patients) | p value | |
|---|---|---|---|
| Age* | 64.6±2.0 | 63.4±3.0 | 0.73 |
| NSAIDs usage | 14.0% | 0% | 0.0376 |
| NSAIDs and/or aspirin usage | 50% | 55.2% | 0.6781 |
| Warfarin usage | 5.6% | 27.6% | 0.0144 |
| Any anticoagulant usage | 8.3% | 55.2% | < 0.001 |
| Anti-hypertensives usage | 16.7% | 41.4% | 0.0269 |
| Hypotension or shock | 11.1% | 34.5% | 0.0227 |
| Severe lung disease | 2.8% | 62.1% | < 0.001 |
| Severe heart disease | 19.4% | 51.7% | 0.0122 |
| Severe renal disease | 8.3% | 58.6% | 0.0001 |
| Hematologic disorder+ | 52.8% | 85.7% | 0.0051 |
| URBC transfusion before colonoscopy* | 1.6±1.1 | 6.7±1.7 | 0.01 |
| Hospital days before urgent colonoscopy++ | 1.2±0.1 | 22.9±3.0 | 0 |
| Baseline Hgb* | 11.1±0.5 | 8.9±0.5 | 0 |
| Baseline PT* | 12.6±0.8 | 13.9±0.8 | 0.5 |
| Baseline PTT* | 27.5±0.7 | 32.5±1.7 | 0 |
| Baseline WBC* | 10.2±0.9 | 12.5±1.0 | 0.1 |
| Creatinine* | 1.7±0.3 | 4.5±1.9 | 0.27 |
| Prognosis score* | 1.8±0.1 | 3.3±0.2 | 0 |
Mean ± SE
Includes coagulation disorders or low platelet count
Mean hours between GI consultation and urgent colonoscopy was ≤ 12 hours
Inpatients developed severe hematochezia and had urgent colonoscopy a mean of 34.2±12.0 days after hospital admission for some other non-bleeding indication. In contrast, patients with outpatient ischemia and severe hematochezia had urgent colonoscopy a mean of 1.3±0.1 days after hospital admission.
No statistically significant differences were observed in the prevalence of the endoscopic stigmata of hemorrhage (active bleeding, NBVV, clot and clean lesion), nor the distribution of colonic lesions for inpatient vs. outpatient ischemic colitis. At baseline for resuscitation and between colonoscopic diagnosis and 30 days (or hospital discharge), the total URBC transfused were significantly higher in the inpatient group compared to the outpatient group (6.7±1.7 vs. 1.6±1.1). However, fresh frozen plasma and platelet transfusions were similar in both groups.
For 30 day outcomes, rebleeding rate, ICU days, hospital days, and surgical intervention were all significantly higher in the inpatient group. While the death rate was arithmetically higher in the inpatient group, this did not reach a statistically significant level (See Table 4). None of the 30 day outcomes were significantly different for the patients with outpatient development of severe hematochezia from ischemic colitis when compared to other colonic causes of hematochezia.
Table 4. Thirty day outcomes of inpatient vs. outpatient ischemic colitis.
| Outpatient group (36 patients) | Inpatient group (29 patients) | p value | |
|---|---|---|---|
| Rebleeding | 16.7% | 41.4% | 0.0269 |
| Total URBC* | 0.6±0.2 | 9.5±2.8 | 0 |
| Hospital days* | 4.8±1.0 | 20.8±1.8 | 0 |
| ICU days* | 0.6±0.5 | 7.6±2.1 | 0 |
| Surgical intervention | 5.6% | 24.1% | 0.0311 |
| Death | 2.8% | 13.8% | 0.098 |
Mean ± SE
Discussion
Because the presentation and colonoscopic findings of ischemic colitis often resemble other types of colitis, a high degree of suspicion is warranted. Although colonoscopy is a sensitive and specific method of evaluating the colon for patterns of ischemia, the final diagnosis is based upon histopathology and exclusion of other forms of colitis.7 For all patients in our study, the diagnosis of ischemic colitis was documented with biopsy specimens and negative testing for infectious causes of colitis. Ischemic colitis should be included in the differential diagnosis of patients who present either as outpatients or inpatients with severe hematochezia, particularly in older patients or those with multiple risk factors.
Conventional medical therapy is the mainstay of treatment for acute ischemic colitis and is associated with good clinical results even in elderly patients.8 However, early colonoscopy in suspected cases is vital for accurate diagnosis and risk stratification. Although the descending colon and splenic or hepatic flexures are the most susceptible areas to ischemic damage, complete colonoscopy is required to ensure that there is no ischemia in the proximal colon.
Our patient outcomes were more favorable than previous reports where approximately 20% of patients had clinical deterioration to peritonitis, colonic gangrene, or sepsis, necessitating surgical intervention with an associated 21 to 34 percent mortality rate.9-11 The potential reasons for the differences in complications, surgery, and mortality probably related the design of this study and types of patients included. First, we included all patients with severe hematochezia who were hospitalized and were evaluated by the GI Hemostasis services, regardless of what type of medical or surgical service they were on. Second, there may be differences in the diagnostic accuracy. In this study, the diagnosis was based upon colonoscopic pattern, histopathology, and exclusion of other forms of colitis. Third, the referral biases for colonoscopy vs. surgery or angiography are likely to be significantly different as are the principle indications for referral or hospitalization (GI bleeding vs. a surgical indication). We believe that the patients with ischemic colitis and severe hematochezia in this report are more typical of being patients referred to gastroenterologists in tertiary care medical centers rather than surgeons, as in some previous reports. 9-11
The pathophysiology for the ischemic injury to the colon in our patients most often had a pattern and clinical course of small vessel or mucosal ischemia (related to comorbidities) rather than thromboembolism (related to cardiac arrhythmias or large vessel stenosis or thromboembolism). There also is the possibility that early resuscitation, aggressive treatment of comorbidities, and prompt investigation (e.g. colonoscopy within 12 hours of consultation), expedited and improved the treatment, thereby leading to more favorable outcomes than previously reported.
When patients with ischemic colitis were compared to other patients with severe hematochezia and other colon diagnoses: 1) those caused by ischemic colitis had diffuse lesions including the five patients who also had a focal lesion with major stigmata of hemorrhage and were treated with colonoscopic hemostasis, and 2) major outcomes at 30 days were significantly worse.
Inpatient ischemic colitis cases were more severe than outpatient ischemic colitis cases and have significantly increased rates of severe lung disease or severe heart disease; poorer prognosis scores; significantly higher rates of rebleeding, and surgery; and more URBC transfused, ICU days, and total hospital days. The mortality rate of the inpatient ischemia group was arithmetically but not statistically higher. This may represent a Type II error and with more patients we anticipate that the mortality rate would be significantly different.
The substantial proportion of patients with inpatient ischemia (29/65 = 44.6%) made the overall 30 day outcomes of the ischemic colitis group worse compared with other colonic diagnoses. As further evidence for this conclusion, none of the outcomes for the outpatient ischemia group differed significantly from the patients with all other colon diagnoses.
In conclusion, compared to a large cohort of patients with severe hematochezia and non-ischemia colon diagnoses, those caused by ischemic colitis had: 1) significant risk factors including female gender; higher prevalence of any anticoagulant usage; significant lung disease; elevated creatinine or glucose; and more UFFP transfused, 2) diffuse rather than focal lesions for which medical therapy was indicated, except in five patients with focal ulcers and major stigmata of recent hemorrhage who required colonoscopic hemostasis, 3) significantly worse clinical outcomes up to 30 days after diagnosis, and 4) patients with inpatient ischemic colitis had significantly more risk factors and worse outcomes than patients with outpatient ischemic colitis or other colonic causes of severe hematochezia.
Capsule Summary.
What is already known on this topic
The majority of ischemic colitis patients have self-limited bleeding and usually respond to medical therapy.
What this study adds to our knowledge
Major outcomes of patients with ischemic colitis were significantly worse than patients with other colonic diagnoses of hematochezia.
Inpatient start of GI hemorrhage in patients with ischemic colitis had worse outcomes than those with outpatient start of bleeding.
If a focal ischemic ulcer with major stigmata of hemorrhage was found, endoscopic hemoclipping was feasible and safe.
Acknowledgments
The authors are grateful to Jeff Gornbein, DPH for biostatistical consultation; to Nan Sun, MS and Mary Ellen Jensen, MCS for data management; and to Martha Carrico, RN as the research coordinator.
This study was partially supported by research funds from NIH grants (K24 DK002650) and CURE Digestive Diseases Research Center Human Studies Core (P30 DK041301).
Acronyms
- GI
Gastrointestinal
- Hgb
Hemoglobin
- URBC
Units of red blood cell
- PT
Prothrombin time
- PTT
Partial thromboplastin time
- WBC
White blood cell
- UPlt
Units of platelets
- UFFP
Units of fresh frozen plasma
- NBVV
Non, bleeding visible vessel
- ICU
Intensive care unit
Footnotes
Author's contribution: Dennis M. Jensen, MD, Thomas O.G. Kovacs, MD, Rome Jutabha, MD, Gareth Dulai, MD, Gordon Ohning, MD and Gustavo A. Machicado, MD participated in the design of the study and performed the study. Dennis M. Jensen, MD and Disaya Chavalitdhamrong, MD participated in the study coordination and writing the manuscript. All authors read and approved the final manuscript
Disclosure: All authors disclosed no financial relationships relevant to this publication.
References
- 1.Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia - the role of urgent colonoscopy after purge. Gastroenterol. 1988;95:1569–74. doi: 10.1016/s0016-5085(88)80079-9. [DOI] [PubMed] [Google Scholar]
- 2.Jensen DM, Machicado GA. Colonoscopy for Diagnosis and Treatment of Severe Lower Gastrointestinal Bleeding: Routine Outcomes and Cost Analysis. Gastrointest Endos Clinics of North America. 1997;7:477–498. [PubMed] [Google Scholar]
- 3.Jensen DM, Machicado GA. Colonoscopy and Severe Hematochezia. In: Waye JD, Rex DK, Williams CB, editors. Colonoscopy – Principles and Practice. Blackwell Sciences; London: 2009. pp. 631–645. [Google Scholar]
- 4.Jensen DM, Machicado GA, Jutabha R, et al. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342:78–82. doi: 10.1056/NEJM200001133420202. [DOI] [PubMed] [Google Scholar]
- 5.Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J. 2005;98:217–22. doi: 10.1097/01.SMJ.0000145399.35851.10. [DOI] [PubMed] [Google Scholar]
- 6.Greenwald DA, Brandt LJ. Colonic ischemia. J Clin Gastroenterol. 1998;27:122–8. doi: 10.1097/00004836-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Sreenarasimhaiah J. Diagnosis and management of ischemic colitis. Curr Gastroenterol Rep. 2005;7:421–6. doi: 10.1007/s11894-005-0013-1. [DOI] [PubMed] [Google Scholar]
- 8.Anon R, Bosca MM, Sanchiz V, et al. Factors predicting poor prognosis in ischemic colitis. World J Gastroenterol. 2006;12:4875–8. doi: 10.3748/wjg.v12.i30.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scharff JR, Longo WE, Vartanian SM, et al. Ischemic colitis: spectrum of disease and outcome. Surgery. 2003;134:624–9. doi: 10.1016/s0039-6060(03)00308-8. [DOI] [PubMed] [Google Scholar]
- 10.Parish KL, Chapman WC, Williams LF. Ischemic colitis. An ever-changing spectrum? Am Surg. 1991;57:118–21. [PubMed] [Google Scholar]
- 11.Huguier M, Barrier A, Boelle PY, et al. Ischemic colitis. Am J Surg. 2006;192:679–84. doi: 10.1016/j.amjsurg.2005.09.018. [DOI] [PubMed] [Google Scholar]