Abstract
Objectives
MDM2 SNP309 polymorphism (T>G) has been correlated with an increased risk of cancer in multiple tumor types. MDM2 overexpression in has shown to be weakly associated with distant tumor metastases, and downregulation of MDM2 via antisense oligonucleotides in vitro has resulted in the radiosensitization of prostate cancer cell lines. Based on these results, we decided to evaluate the role of MDM2 SNP309 in the context of histopathologic parameters and clinical outcomes in prostate cancer tumors.
Materials and Methods
The population consisted of 212 consecutive prostate cancer patients who underwent radical prostatectomy between 1997 and 1999 at Vanderbilt University Medical Center. Two hundred eight of the samples were successfully genotyped for the MDM2 SNP309 polymorphism. Correlations between the polymorphism, recurrence, and survival data were analyzed using univariate and multivariate genetic models.
Results
The only prognostic factor predictive of overall survival in our study was Gleason score (P < 0.005). Using chi square analysis, we determined that the MDM2 SNP309 polymorphism had no significant association with race (P = 0.7512), patient’s age at diagnosis (P = 0.6820), pre-prostatectomy PSA level (P = 0.8606), Gleason’s score (P = 0.4839), surgical margin status (P = 1.0000), extracapsular extension (P = 0.6175) and disease stage (P = 0.4945). In addition, there was no significant difference in 3 year recurrence-free survival (P = 0.218) or 8 year overall survival (P = 0.376).
Conclusions
Our study finds no evidence for association of the MDM2 SNP309 polymorphism with clinicopathologic variables, recurrence risk, and overall survival outcome in prostate cancer.
Keywords: promotor, polymorphism, mdm2, prostate cancer, prognosis
Introduction
Mouse double-minute 2 (MDM2) is an E3-ubiquitin ligase that binds, inhibits and promotes the degradation of the tumor suppressor protein, p53 1. A strong correlation between overexpression of MDM2, tumor proliferation, and an early onset of tumorigenesis exists 2–6. In fact, the MDM2 gene is amplified in approximately 30% of the osteosarcomas and soft tissue sarcomas 7–9.
Bond et al 10 demonstrated that a single nucleotide polymorphism in the promoter region of the MDM2 gene (−309 T/G; SNP309) could result in elevated MDM2 levels with increased p53 degradation. Interestingly, Bond and co-workers found that the effects of this polymorphism on enhanced tumorigenesis occurs in a gender-specific and hormone-dependent manner 11. Based on these findings, we performed genotypic analysis of MDM2 SNP309 in tumor tissues of 212 patients with prostate cancer status post prostatectomy and evaluated the association of this polymorphism with clinicopathologic and prognostic parameters. To our knowledge, the current investigation represents the largest report evaluating the clinical effects of MDM2 SNP 309 in prostate cancer outcome.
Materials and Methods
Study Population
The population consisted of 212 consecutive prostate cancer patients who underwent radical prostatectomy between 1997 and 1999 at Vanderbilt University Medical Center. Ninety-eight percent of the patients were Caucasian with the remainder being 4 African-American patients. The median follow-up for overall survival was 9.1 years (mean 8.3 ± 2.4 years), and for assessment of prostate cancer recurrence was 3.4 years (mean 4.4 ± 3.9 years). Recurrence post-prostatectomy was classified as biochemical, local or distant. All patients had histologically confirmed adenocarcinoma. Two hundred eight of the samples were successfully genotyped for the MDM2 SNP309 polymorphism. This study was approved by the Vanderbilt University Institutional Review Board (IRB No. 030986).
Specimen preparation and deoxyribonucleic acid extraction
Utilizing a standard microtome with disposable blades, we cut 5-μm thick sections of representative areas of normal prostate glands from paraffin-embedded blocks and stained these tissues with hematoxylin and eosin. We subsequently examined the slides under a microscope to verify the absence of prostate cancer. A 5-μm thick section (approximately 1 μg) from each patient was used for DNA extraction. The sections were deparaffinized twice with xylene at room temperature for 30 minutes, washed twice with 100% ethanol, and after complete ethanol evaporation, the tissue was completely lysed with proteinase K. The QIAamp DNA Mini Kit (QIAGEN Inc., Valencia, CA) was used to extract and purify DNA from the tissues according to the manufacturer’s protocol.
Genotyping
Genomic DNA samples were obtained from patient tumor specimens and processed as described previously 12,13. MDM2 SNP309 (rs2279744) genotyping was performed using Pyrosequencing™ technology (Biotage AB, Uppsala, Sweden).14 The primers were: 5′-GGGGTGGTTCGGAG GTCT-3′ (sense); and 5′-Biotin -GTGACCCGACAG GCACCT-3′ (antisense). A 113 bp fragment was amplified from 10 ng genomic DNA. Single stranded DNA was isolated from the PCR reactions using the Pyrosequencing Vacuum Prep Workstation and transferred into a 96-well plate. A sequencing primer, 5′ GGGCTGCGGGGCCGCT, was annealed to the single stranded DNA. The plate was then transferred to the Pyrosequencing PSQ96MA by dispensing the nucleotides in the following order [G/T]CGGCGCGGGAGGTCCGGATGATCGC. The genotype was determined using SNP Software (Biotage AB).
Statistical Analysis
Testing of adherence to Hardy-Weinberg equilibrium was performed using a Chi-square goodness of fit test. Overall survival was determined as the time between the date of surgery and the date of death or last follow-up. Recurrence-free survival was calculated from the date of surgery to the date of recurrence (biochemical, local or distant) or last follow-up, as described previously 12,13. The data were censored for live (or recurrence-free) patients as of their last follow-up visits. Biochemical recurrence was defined as a prostate-specific antigen (PSA) detection of > 0.1 ng/ml in at least two consecutive lab draws. Kaplan-Meier survival curves were calculated for each genotype and were compared using the log rank test. Association of the genotypes to various clinicopathologic characteristics was assessed using the Fisher’s exact test. Multivariate analysis was performed using the Cox proportional hazards model. MDM2SNP 309 had no evidence of association with our endpoints, but we used the model to validate known variables predictive of our studied outcomes.
Results
The MDM2 SNP309 genotype frequencies among the prostate cancer cohort were in Hardy-Weinberg equilibrium (P = 0.705). Two hundred and six of the 212 samples gave analyzable results. The frequencies of the SNP309 genotypes were: 16.5% (35 of 212) for G/G; 48.6% (103 of 212) for G/T; and 32.5% (69/212) for T/T. Log-rank univariate analyses of known prognostic factors, including pre-prostatectomy PSA levels (P < 0.045), Gleason score (P < 0.001), surgical margin status (P < 0.001), extracapsular extension status (P < 0.001) and T stage (P < 0.001) were predictive of relapse-free survival.
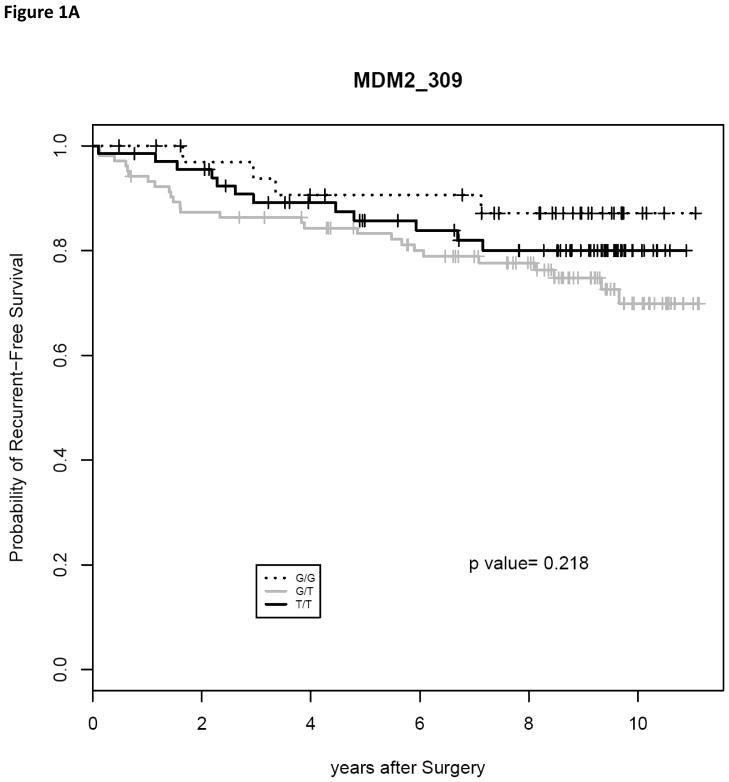
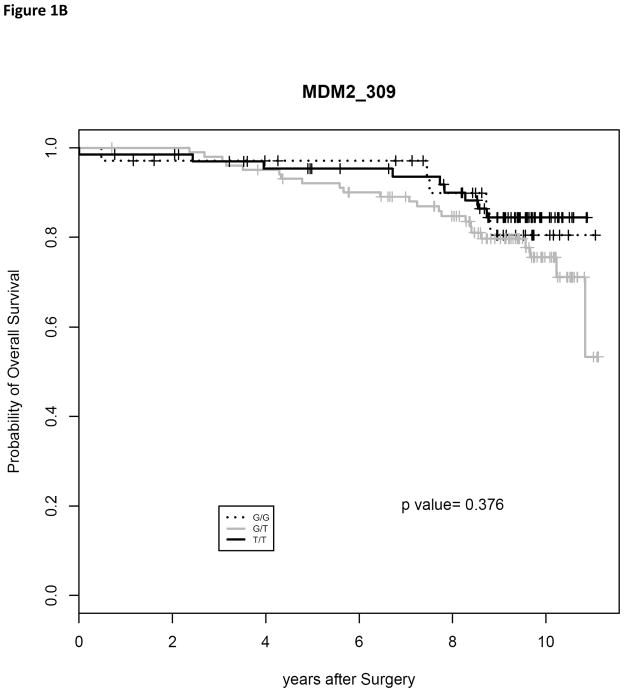
The only one of these prognostic factors predictive of overall survival in our study was Gleason score (P < 0.005). Using chi square analysis, we determined that the MDM2 SNP309 polymorphism had no significant association with race (P = 0.7512), patient’s age at diagnosis (P = 0.6820), pre-prostatectomy PSA level (P = 0.8606), Gleason’s score (P = 0.4839), surgical margin status (P = 1.0000), extracapsular extension (P = 0.6175) and disease stage (P = 0.4945) (Results in Table 1). We utilized the Kaplan-Meier method to determine the recurrence and survival rates, and the log-rank test was used to test the difference in these rates between the different genotypes. There was no significant difference in 3 year recurrence-free survival (Fig. 1A, P = 0.218) or 8 year overall survival (Fig. 1B, P = 0.376).
Table 1.
Association of MDM2 SNP309 polymorphism with clinicopathologic parameters in prostate cancer
| FACTORS | T/T (N=68) | T/G (N=103) | G/G (N=35) | Missing (N=6) | TOTAL (N=212) | P Value |
|---|---|---|---|---|---|---|
| Age at diagnosis (yrs), n(%) | 0.6820 | |||||
| ≤ 65 | 47 (69.12) | 73 (70.87) | 22 (62.86) | 6 (100.00) | 148 (69.81) | |
| > 65 | 21 (30.88) | 30 (29.13) | 13 (37.14) | 0 (0.00) | 64 (30.19) | |
| Race, n(%) | 0.7512 | |||||
| Black | 1 (1.47) | 1 (0.97) | 1 (2.86) | 1 (16.67) | 4 (1.89) | |
| White | 67 (98.53) | 102 (99.03) | 34 (97.14) | 5 (83.33) | 208 (98.11) | |
| PSA at diagnosis (ng/ml), n(%) | 0.8606 | |||||
| ≤ 4 | 8 (11.76) | 10 (9.71) | 4 (11.43) | 0 (0.00) | 22 (10.38) | |
| 4–10 | 30 (44.12) | 60 (58.25) | 21 (60.00) | 2 (33.33) | 113 (53.30) | |
| > 10 | 14 (20.59) | 20 (19.42) | 7 (20.00) | 2 (33.33) | 43 (20.28) | |
| Missing | 16 ( 23.53) | 13 ( 12.62) | 3 (8.57) | 2 (33.33) | 34 (16.04) | |
| Gleason score, n(%) | 0.4839 | |||||
| ≤ 6 | 44 (64.71) | 60 (58.25) | 21 (60.00) | 5 (83.33) | 130 (61.32) | |
| 7 | 22 (32.35) | 37 (35.92) | 10 (28.57) | 1 (16.67) | 70 (33.02) | |
| > 7 | 2 (2.94) | 6 (5.83) | 4 (11.43) | 0 (0.00) | 12 (5.66) | |
| Pathologic Stage*, n(%) | 0.4945 | |||||
| Not palpable (T1) | 5 (7.35) | 3 (2.91) | 0 (0.00) | 0 (0.00) | 8 (3.77) | |
| Confined to prostate (T2) | 42 (61.76) | 64 (62.14) | 23 (65.71) | 5 (83.33) | 134 (63.21) | |
| Locally advanced (T3) | 17 (25.00) | 28 (27.18) | 11 (31.43) | 1 (16.67) | 57 (26.89) | |
| Missing Data | 4 (5.88) | 8 (7.77) | 1 (2.86) | 0 (0.00) | 13 (6.13) | |
| Extracapsular extension, n(%) | 0.6175 | |||||
| Negative | 38 (55.88) | 64 (62.14) | 19 (54.29) | 4 (66.67) | 125 (58.96) | |
| Positive | 20 (29.41) | 32 (31.07) | 14 (40.00) | 1 (16.67) | 67 (31.60) | |
| Missing | 10 (14.71) | 7 (6.80) | 2 (5.71) | 1 (16.67) | 20 (9.43) | |
| Surgical Margin, n(%) | 1.0000 | |||||
| Negative | 44 (64.71) | 65 (63.11) | 44 (64.71) | 2 (33.33) | 133 (62.74) | |
| Positive | 24 (35.29) | 37 (35.92) | 24 (35.29) | 4 (66.67) | 78 (36.79) | |
| Missing | 0 (0.00) | 1 (0.97) | 0 (0.00) | 0 (0.00) | 1 (0.47) |
Based on the 2006 American Joint Committee on Cancer TNM Staging
Abbreviations: PSA = prostate-specific antigen.
Fig. 1.
Kaplan-Meier estimates of recurrence-free survival (RFS) and overall survival (OS) following radical prostatectomy as treatment for localized prostate cancer. (A) RFS curves were plotted for the MDM2 SNP309 genotypes. (B) OS curves were plotted for the MDM2 SNP309 genotypes.
To determine independent risk factors for recurrence, multivariate analyses using the Cox proportional hazards regression model were performed. In this model the recessive genotypes of SNP309 were not found to be independent predictors of recurrence (G/T: hazard ratio of 3.480 (1.031 – 11.746, 95% CI), P = 0.045; T/T: hazard ratio of 2.159 (0.581 – 8.030, 95% CI), P=0.251) or overall survival (G/T: hazard ratio of 2.478 (0.736 – 8.347, 95% CI), P = 0.143; T/T: hazard ratio of 1.564 (0.400 – 6.116, 95% CI), P = 0.521).
Discussion
MDM2 SNP309 polymorphism (T>G) is associated with an increased risk of cancer in multiple tumor types including renal cancer 15, non-small cell lung cancer 16, oral squamous cancer17, and gastric cancer 18. In B cell lymphoma a significant association between MDM2 SNP309 polymorphism and relapse-free and overall survival rates was observed 19. MDM2 overexpression in prostate cancers has also been shown to be weakly associated with distant metastases in a small trial 3. Furthermore downregulation of MDM2 via antisense oligonucleotides in vitro has resulted in the radiosensitization of prostate cancer cell lines 20. Based on these results we evaluated the role of MDM2 SNP309 in the context of histopathologic parameters and clinical outcomes in prostate cancer tumors and found no statistically significant association.
Recently there has been a report evaluating the risk of MDM2 SNP309 in prostate cancer in a case control design 21. The study included 145 affected men who underwent radical prostatectomy, as well as 124 patient controls. They concluded that there was no correlation between the MDM2 SNP 309 polymorphism and an increased risk of cancer. The authors also analyzed histological, pathological and recurrence parameters from which no significant association was found. The major weakness of their study was that only 65 of the 145 affected prostate patients were evaluable for prognostic parameters, and that over 10% of the pathologic data was missing. In contrast, the present study consists of a larger sample size, a greater median follow-up, and a more complete database for all patient endpoints than the only other published study of its kind.
Our study similarly revealed no association between the MDM2 SNP309 polymorphism and clinicopathologic outcomes in prostate cancer. Of note several limitations from this present study may obscure the understanding of MDM2 polymorphism in association with tumorigenesis. First the population studied was primarily non-Jewish Caucasian (98%), thus limiting the extrapolation to other races. However, the frequency of G allele of MDM2 SNP309 is much lower in African-Americans and intermediate in Caucasians 22. Notably our study patients had clinical low risk features, but more intermediate pathologic features. Although 53% of these patients had PSA values in the normal range (<4 ng/ml), ~27% of our patients had pathologic T3 disease. This degree of clinical understaging was in line with historical rates at VUMC (30% for open retropubic radical prostactectomies)23.
Unlike this study, previous studies have examined MDM2 SNP309 polymorphism in the context of p53 status. MDM2 SNP309 polymorphism has been shown to enhance tumorigenesis when co-existent with mutated p53, as in patients with Li-Fraumeni Syndrome 10. Not only does the T > G allelic change in MDM2 SNP309 lead to increased occurrence of tumors in a lifetime for these patients, but also leads to an earlier onset of cancer 24. As such several studies have pooled genotypes according to p53 mutation status, and have either shown an increased risk of tumorigenesis or poor prognosis with MDM2 SNP309 polymorphism 13,18. Although the present data did not take into consideration p53 mutation status, it is worth noting that p53 alterations represent a rare event in prostate cancer tumorigenesis in which p53 mutation occurs with only the most aggressive, metastatic prostate cancer transitioning from androgen-dependent to androgen-independent growth 25. Likewise, p53 alterations may be of less relevance in prostate carcinogenesis than in other cancers.
It has been recently suggested that MDM2 SNP309 polymorphism and subsequent changes in the affinity of the promoter to hormones such as estrogen could affect breast tumorigenesis 11,26,27, although other data have noted no such association 28,29. One study showed that the G-allele of MDM2 SNP309 accelerates colorectal formation in women and not in men and hypothesized the effects of female-specific hormones such as estrogen on the observed finding 11. In the same way, prostate tumors with MDM2 overexpression may be of value in tumorigenesis only when the tumor retains androgen-sensitivity. We have not attempted to evaluate this association in our study, as we have not seen a significant correlation of MDM2 SNP309 with any of our endpoints.
In conclusion there is no evidence in our study that MDM2 SNP309 polymorphism is significantly associated with clinicopathologic variables, recurrence risk, and overall survival endpoints in prostate cancer. It is possible that the study is underpowered, and additional patient numbers could detect a difference in our evaluated endpoints. Additionally it is possible that a statistical difference in local recurrence and/or overall survival rates may have been detectable with longer follow-up. However consistent with other recently reported studies MDM2 SNP309, we have found no association of this biomarker with prostate cancer risk or prognosis.
Acknowledgments
Grant Support: This work was supported in part by the DOD grant PC031161 (PI: Bo Lu) and R01 CA122756 (Qiuyin Cai).
References
- 1.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 387:296–9. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 2.Hu W, Feng Z, Ma L, Wagner J, Rice JJ, Stolovitzky G, Levine AJ. A single nucleotide polymorphism in the MDM2 gene disrupts the oscillation of p53 and MDM2 levels in cells. Cancer Res. 67:2757–65. doi: 10.1158/0008-5472.CAN-06-2656. [DOI] [PubMed] [Google Scholar]
- 3.Khor LY, Desilvio M, Al-Saleem T, Hammond ME, Grignon DJ, Sause W, Pilepich M, Okunieff P, Sandler H, Pollack A. MDM2 as a predictor of prostate carcinoma outcome: an analysis of Radiation Therapy Oncology Group Protocol 8610. Cancer. 104:962–7. doi: 10.1002/cncr.21261. [DOI] [PubMed] [Google Scholar]
- 4.Leite KR, Franco MF, Srougi M, Nesrallah LJ, Nesrallah A, Bevilacqua RG, Darini E, Carvalho CM, Meirelles MI, Santana I, et al. Abnormal expression of MDM2 in prostate carcinoma. Mod Pathol. 2001;14:428–36. doi: 10.1038/modpathol.3880330. [DOI] [PubMed] [Google Scholar]
- 5.Nayak MS, Yang JM, Hait WN. Effect of a single nucleotide polymorphism in the murine double minute 2 promoter (SNP309) on the sensitivity to topoisomerase II-targeting drugs. Cancer Res. 67:5831–9. doi: 10.1158/0008-5472.CAN-06-4533. [DOI] [PubMed] [Google Scholar]
- 6.Osman I, Drobnjak M, Fazzari M, Ferrara J, Scher HI, Cordon-Cardo C. Inactivation of the p53 pathway in prostate cancer: impact on tumor progression. Clin Cancer Res. 5:2082–8. [PubMed] [Google Scholar]
- 7.Cordon-Cardo C, Latres E, Drobnjak M, Oliva MR, Pollack D, Woodruff JM, Marechal V, Chen J, Brennan MF, Levine AJ. Molecular abnormalities of mdm2 and p53 genes in adult soft tissue sarcomas. Cancer Res. 54:794–9. [PubMed] [Google Scholar]
- 8.Leach FS, Tokino T, Meltzer P, Burrell M, Oliner JD, Smith S, Hill DE, Sidransky D, Kinzler KW, Vogelstein B. p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 53:2231–4. [PubMed] [Google Scholar]
- 9.Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 358:80–3. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- 10.Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 11.Bond GL, Menin C, Bertorelle R, Alhopuro P, Aaltonen LA, Levine AJ. MDM2 SNP309 accelerates colorectal tumour formation in women. J Med Genet. 43:950–2. doi: 10.1136/jmg.2006.043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browning REt, Li H, Shinohara ET, Cai Q, Chen H, Courtney R, Cao C, Zheng W, Lu B. ATM polymorphism IVS62+60G>A is not associated with disease aggressiveness in prostate cancer. Urology. 67:1320–3. doi: 10.1016/j.urology.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Lind H, Zienolddiny S, Ekstrom PO, Skaug V, Haugen A. Association of a functional polymorphism in the promoter of the MDM2 gene with risk of nonsmall cell lung cancer. Int J Cancer. 119:718–21. doi: 10.1002/ijc.21872. [DOI] [PubMed] [Google Scholar]
- 14.Fakhrai-Rad H, Pourmand N, Ronaghi M. Pyrosequencing: an accurate detection platform for single nucleotide polymorphisms. Hum Mutat. 19:479–85. doi: 10.1002/humu.10078. [DOI] [PubMed] [Google Scholar]
- 15.Hirata H, Hinoda Y, Kikuno N, Kawamoto K, Suehiro Y, Tanaka Y, Dahiya R. MDM2 SNP309 polymorphism as risk factor for susceptibility and poor prognosis in renal cell carcinoma. Clin Cancer Res. 13:4123–9. doi: 10.1158/1078-0432.CCR-07-0609. [DOI] [PubMed] [Google Scholar]
- 16.Han JY, Lee GK, Jang DH, Lee SY, Lee JS. Association of p53 codon 72 polymorphism and MDM2 SNP309 with clinical outcome of advanced nonsmall cell lung cancer. Cancer. 113:799–807. doi: 10.1002/cncr.23668. [DOI] [PubMed] [Google Scholar]
- 17.Tu HF, Chen HW, Kao SY, Lin SC, Liu CJ, Chang KW. MDM2 SNP 309 and p53 codon 72 polymorphisms are associated with the outcome of oral carcinoma patients receiving postoperative irradiation. Radiother Oncol. 87:243–52. doi: 10.1016/j.radonc.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Ohmiya N, Taguchi A, Mabuchi N, Itoh A, Hirooka Y, Niwa Y, Goto H. MDM2 promoter polymorphism is associated with both an increased susceptibility to gastric carcinoma and poor prognosis. J Clin Oncol. 24:4434–40. doi: 10.1200/JCO.2005.04.1459. [DOI] [PubMed] [Google Scholar]
- 19.Gryshchenko I, Hofbauer S, Stoecher M, Daniel PT, Steurer M, Gaiger A, Eigenberger K, Greil R, Tinhofer I. MDM2 SNP309 is associated with poor outcome in B-cell chronic lymphocytic leukemia. J Clin Oncol. 26:2252–7. doi: 10.1200/JCO.2007.11.5212. [DOI] [PubMed] [Google Scholar]
- 20.Mu Z, Hachem P, Agrawal S, Pollack A. Antisense MDM2 sensitizes prostate cancer cells to androgen deprivation, radiation, and the combination. Int J Radiat Oncol Biol Phys. 58:336–43. doi: 10.1016/j.ijrobp.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Stoehr R, Hitzenbichler F, Kneitz B, Hammerschmied CG, Burger M, Tannapfel A, Hartmann A. Mdm2-SNP309 polymorphism in prostate cancer: no evidence for association with increased risk or histopathological tumour characteristics. Br J Cancer. 99:78–82. doi: 10.1038/sj.bjc.6604441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris SL, Gil G, Robins H, Hu W, Hirshfield K, Bond E, Bond G, Levine AJ. Detection of functional single-nucleotide polymorphisms that affect apoptosis. Proc Natl Acad Sci U S A. 102:16297–302. doi: 10.1073/pnas.0508390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith JA, Jr, Chan RC, Chang SS, Herrell SD, Clark PE, Baumgartner R, Cookson MS. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007;178:2385–9. doi: 10.1016/j.juro.2007.08.008. discussion 2389–90. [DOI] [PubMed] [Google Scholar]
- 24.Bond GL, Hu W, Levine A. A single nucleotide polymorphism in the MDM2 gene: from a molecular and cellular explanation to clinical effect. Cancer Res. 65:5481–4. doi: 10.1158/0008-5472.CAN-05-0825. [DOI] [PubMed] [Google Scholar]
- 25.Dong JT. Prevalent mutations in prostate cancer. J Cell Biochem. 97:433–47. doi: 10.1002/jcb.20696. [DOI] [PubMed] [Google Scholar]
- 26.Bond GL, Hirshfield KM, Kirchhoff T, Alexe G, Bond EE, Robins H, Bartel F, Taubert H, Wuerl P, Hait W, et al. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res. 2006;66:5104–10. doi: 10.1158/0008-5472.CAN-06-0180. [DOI] [PubMed] [Google Scholar]
- 27.Saji S, Okumura N, Eguchi H, Nakashima S, Suzuki A, Toi M, Nozawa Y, Saji S, Hayashi S. MDM2 enhances the function of estrogen receptor alpha in human breast cancer cells. Biochem Biophys Res Commun. 281:259–65. doi: 10.1006/bbrc.2001.4339. [DOI] [PubMed] [Google Scholar]
- 28.Boersma BJ, Howe TM, Goodman JE, Yfantis HG, Lee DH, Chanock S, JAmbs S. Association of breast cancer outcome with status of p53 and MDM2 SNP309. J Natl Cancer Inst. 98:911–9. doi: 10.1093/jnci/djj245. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt MK, Reincke S, Broeks A, Braaf LM, Hogervorst FB, Tollenaar RA, Johnson N, Fletcher O, Peto J, Tommiska J, et al. Do MDM2 SNP309 and TP53 R72P interact in breast cancer susceptibility? A large pooled series from the breast cancer association consortium. Cancer Res. 2007;67:9584–90. doi: 10.1158/0008-5472.CAN-07-0738. [DOI] [PubMed] [Google Scholar]