Abstract
Notch signaling regulates many fundamental events including lateral inhibition and boundary formation to generate very reproducible patterns in developing tissues. Its targets include genes of the bHLH hairy and Enhancer of split [E(spl)] family, which contribute to many of these developmental decisions. One member of this family in Drosophila, deadpan (dpn), was originally found to have functions independent of Notch in promoting neural development. Employing genome-wide chromatin-immunoprecipitation we have identified several Notch responsive enhancers in dpn, demonstrating its direct regulation by Notch in a range of contexts including the Drosophila wing and eye. dpn expression largely overlaps that of several E(spl) genes and the combined knock-down leads to more severe phenotypes than either alone. In addition, Dpn contributes to the establishment of Cut expression at the wing dorsal-ventral (D/V) boundary; in its absence Cut expression is delayed. Furthermore, over-expression of Dpn inhibits expression from E(spl) gene enhancers, but not vice versa, suggesting that dpn contributes to a feed-back mechanism that limits E(spl) gene expression following Notch activation. Thus the combined actions of dpn and E(spl) appear to provide a mechanism that confers an initial rapid output from Notch activity which becomes self-limited via feedback between the targets.
Introduction
Notch signaling is a well-conserved pathway across metazoans involved in the regulation of many fundamental events including lateral inhibition and boundary formation. For example, in the Drosophila wing, activity of Notch is essential for restricting the width of the veins, the strut-like structures that confer rigidity, and for generating and maintaining the stable boundary between the dorsal and ventral cells, which acts as an organizer for patterning and growth of the whole wing. There the functions of Notch are implemented in part through the activities of wingless (wg) and cut (ct), which are expressed in a stripe at the D/V boundary in response to Notch activity.
Simple at first glance, Notch signaling is initiated by the activation of the Notch single-pass transmembrane receptor by the ligand Delta (Dl) or Serrate (Ser), which leads to its proteolysis and the release of the intracellular domain (NICD). NICD then enters the nucleus and affects transcriptional regulation by directly interacting with DNA-binding proteins of the CSL family [mammalian CBF1, Drosophila Suppressor of Hairless (Su(H)) and C.elagans LAG-1] [1], [2]. The downstream targets involved in implementing these actions include genes of the bHLH Hairy and Enhancer of split (HES) gene family, which in Drosophila are located within the 60 kb E(spl) complex. However, activity of the known targets cannot account for all of the functions of Notch, prompting us to perform genome wide chromatin immunoprecipitation (ChIP with anti-Su(H) antibody) to identify other Notch regulated genes [3], [4]. One gene which emerged from these analyses was deadpan (dpn), itself a member of the Hairy/Enhancer of Split (HES) subclass of repressor basic Helix-Loop-Helix (bHLH) proteins, but one that had been primarily linked to the specification of neural cells. More recently it has become evident that dpn is Notch regulated in some contexts although the extent to which it is a direct target and its relevance to Notch functions have remained enigmatic [5].
Until recently, studies of dpn have predominantly focused on its roles during Drosophila neurogenesis [6], where its ability to repress transcription of specific genes in the precursor cell is important in promoting neurogenesis [7], [8]. Its expression persists in the neural stem cells, so-called neuroblasts, where its functions are partially redundant with those of the conventional Drosophila E(spl) genes [5], [9]. In this context, it remains unclear whether or not dpn is a Notch-regulated target because its expression is unaffected in conditions of compromised Notch signaling although it responds to high levels of Notch activity in the mature intermediate neural precursor cells (INPs) of type II neuroblast (NB) lineage [5], [9], [10]. Furthermore, the regulation and functional relevance of dpn in other developmental processes is only just starting to emerge. Fundamental questions that remain are whether dpn is a direct target of Notch in other contexts, outside the nervous system, and how its function relates to that of the other E(spl) genes.
In this study, we show that there are multiple Notch responsive enhancers (NREs) in the dpn genomic region, which direct Notch dependent expression in a variety of tissues. Expression of dpn therefore is a widespread response to Notch activation. In addition, we demonstrate that it has a role in regulating the establishment of stable expression of cut at the D/V boundary in the wing discs and that it functions in a unidirectional feedback loop with the E(spl) genes, helping to self limit the Notch response.
Results
Multiple Notch Responsive Enhancers Associated with dpn
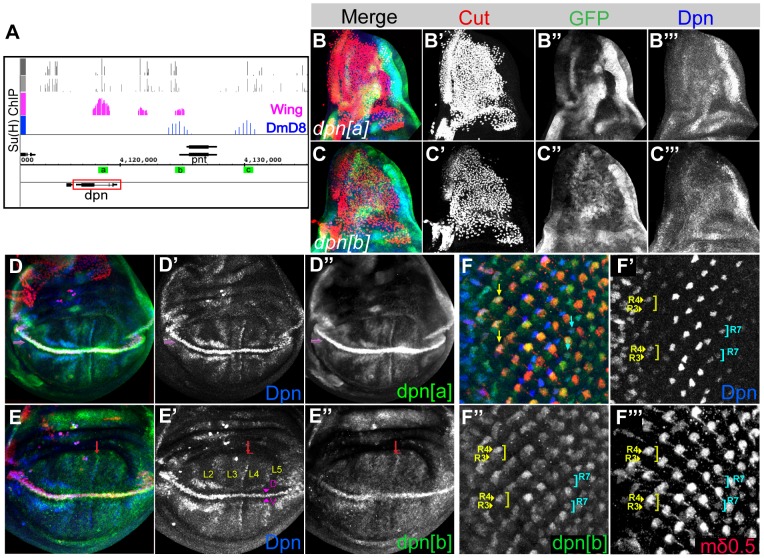
dpn was originally reported as a pan-neural gene [7] with little evidence to suggest that it could be regulated by Notch despite the fact that it is a member of the HES family. More recently it has emerged that there is direct input of Notch into a neuroblast enhancer, although the relevance of this regulation remains unclear [5]. dpn expression is also detected in a wide range of other tissues including the wing, eye and leg imaginal discs, where it overlaps with well-characterised Notch target genes ([7], [11], [12] and Fig. 1 ). Whether or not Dpn is directly regulated by Notch in these tissues has remained unclear, as the characterized neuroblast enhancer does not direct expression in the imaginal discs. However, our genome-wide analysis of Notch targets has indicated that dpn is likely to be directly regulated by Notch in other tissues besides the central nervous system (CNS) since we detect both Su(H) binding (by chromatin immunoprecipitation; ChIP) and changes in mRNA expression in several cells and tissues including wing imaginal discs (4.6 fold upregulated after Notch activation) and DmD8 cells (muscle progenitor related; 4.8-fold upregulated after Notch activation) ( Fig. 1A ).
Figure 1. Several Notch-regulated enhancers associated with dpn gene.
(A) Su(H) bound genomic regions obtained by chromatin immunoprecipitation (ChIP) using wing (pink) and DmD8 (blue) cells show strong overlap with Su(H) binding motifs (all motifs, light grey; conserved motifs, dark grey). Green a, b and c boxes represent peaks that are cloned into a reporter construct expressing GFP. (B–C) Thorax region of wing discs showing expression from dpn[a]GFP (B, green; B″ single channel) and dpn[b]GFP (C, green; C″ single channel) in relation to the adult muscle precursors (AMPs; red nuclei in B,C) which have similar characteristics to DmD8 cells. dpn[b]GFP (C′′) and endogenous Dpn (blue, single channel B′′′, C′′′) expression is detected in some of the AMPs. (D–E) Third instar wing discs immunostained with anti-Dpn (Blue, D, E; single channels D′, E′), anti-GFP (green D,E; single channel, D′′, E′′) and anti-Cut (red, D,E) antibodies. Both the dpn reporters [a]/[b] overlap with Dpn and Cut expression at the D/V boundary (purple arrows); dpn[a] also fully recapitulates Dpn expression in the interveins D-D″), whereas dpn[b] directs weak expression in those regions (e.g. red arrow; E-E″). (F) Expression of endogeneous Dpn (F′), and the dpn[b] reporter (F′′) overlap with E(spl)mδ0.5LacZ (F′′′), a Notch responsive enhancer, in third instar eye discs. Yellow and blue arrows mark the R4 and R7 cells, respectively.
Depending on the cellular origin, different locations for Su(H) binding at the dpn locus were detected, suggesting the existence of multiple Notch responsive enhancers. The major bound region in wing imaginal discs (dpn[a]) was located within the second intron of dpn, overlapping a pair of conserved Su(H) binding motifs. Of the two other more minor Su(H) bound regions detected in wing discs, one (dpn[b]) coincided with a region that was also occupied in DmD8 cells and that overlapped with the neuroblast enhancer identified previously [5]. A further prominent Su(H) bound region (dpn[c]) was detected in DmD8 cells but flanked the adjacent peanut gene, so its relationship to dpn was less clear. All the bound regions contained sequences that have good match to Su(H) binding motifs ( Fig. 1A ; grey bar graphs).
To assess whether these Su(H) occupied regions identify Notch regulated enhancers we inserted genomic fragments encompassing the bound regions upstream of reporter genes and assessed whether they directed dpn-like expression patterns. First, consistent with the results from the ChIP, dpn[b]GFP, but not dpn[a]GFP ( Fig. 1B′′ and C′′ ), recapitulated the endogenous Dpn ( Fig. 1B ′′′ and C′′′ ) expression in the adult muscle precursors (AMPs), which have similar characteristics to DmD8 cells. Second, dpn[a]GFP and dpn[b]GFP recapitulated key aspects of dpn expression in other tissues. dpn[a], the intronic enhancer bound by Su(H) in chromatin from wing discs, directed strong expression at the D/V margin and intervein regions, similar to endogenous dpn expression ( Fig. 1D ). Expression was also detected in the optic lobes of the brain, the leg joints and cone/support cells in the eye discs but not in neuroblasts or photoreceptors (Fig. S1). In contrast, dpn[b] gave weak but detectable expression at the D/V boundary and interveins in the wing, but it exhibited much stronger activity in the eye discs where it was detected in R3/R4 and R7 photoreceptors like endogenous Dpn, and was also strongly expressed in brain and leg discs ( Fig. 1E′′, F′′ and Fig. S1). dpn[c] did not show any significant expression in any of the tissues analyzed so its function remains unclear (Data not shown). These experiments demonstrate therefore that two of the Su(H) bound regions faithfully recapitulate endogenous dpn expression in specific tissues. Furthermore, their expression patterns resemble those of several E(spl) genes, which highlight known sites of Notch pathway activity, such as the D/V boundary and interveins in the wing as well as R4 and R7 photoreceptors in the eye [13], [14]. However, there are also some notable differences. For example, neither endogenous dpn nor the dpn enhancers exhibit strong expression in proneural territories unlike E(spl)m7 and E(spl)m8 [13]. In this respect the dpn expression appears most similar to E(spl)mβ, which is detected at low levels broadly through the thoracic proneural regions.
To address whether dpn[a] and dpn[b] respond to Notch activity, we tested the consequences of manipulating Notch activity in wing and eye imaginal discs. First, expression of NICD in the wing (driven by patched-Gal4 (ptc-Gal4)) was sufficient to elicit ectopic expression of dpn[a] and dpn[b], resembling the response of the endogenous dpn gene ( Fig. 2A and data not shown). Conversely, when Notch was down-regulated by RNAi the expression of dpn[a] and dpn[b] were ablated along with that of dpn itself ( Fig. 2B , and data not shown). Similar results were obtained with dpn[b] enhancer in the developing eye. NICD driven by sevenless-Gal4 (sev-Gal4, driving expression at R1, R3, R4, R6, and R7) resulted in ectopic dpn[b] expression so that firstly similar levels were detected in R3 and R4 (orange arrows, Fig. 2C″ ; compare to wild-type, blue arrows, Fig. 2D ) and secondly other photoreceptor cells started to express the reporter (orange circle, Fig. 2C″ ; compare to wild-type, blue circle, Fig. 2D ) correlating with the ectopic Dpn that was also detected (orange circle, Fig. 2C ′ ). Together these data demonstrate that dpn[a] and dpn[b] can mediate the Notch regulation of dpn in the wing, while only dpn[b] responds in photoreceptors. We note that the dpn[b] fragment is larger than the previous neuroblast enhancer that was identified in this region, containing additional Su(H) sites and flanking sequences that confer additional aspects of Notch regulated expression.
Figure 2. dpn[a] and dpn[b] respond to Notch.
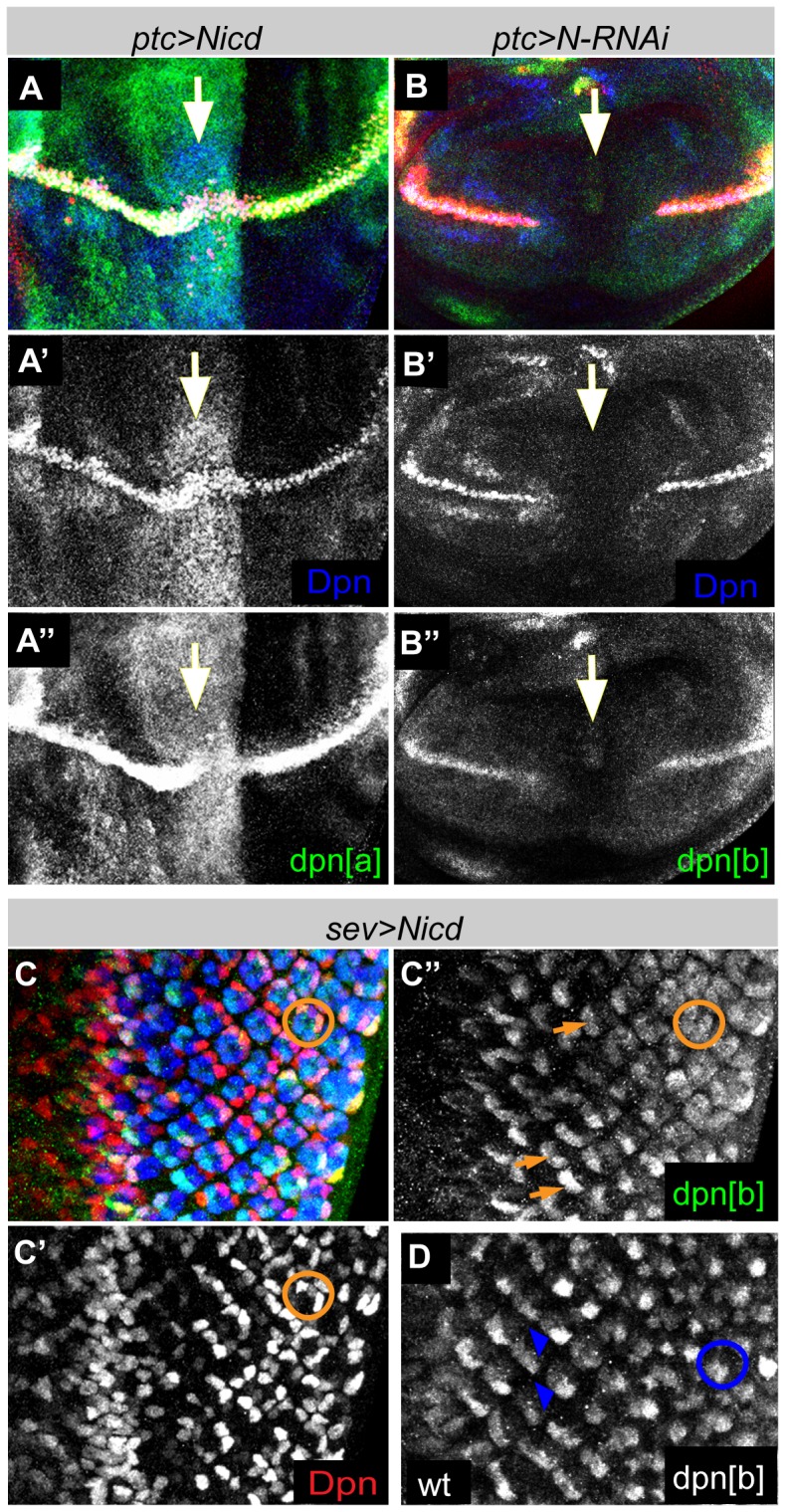
(A–B) Expression driven by dpn[a] or dpn[b] in third instar wing discs detected by immunostaining with anti-GFP (green) and anti-Dpn (blue). (A-A′′) Ectopic expression is detected when Notch intracellular domain (NICD) is expressed in the wing using patched-Gal4 (ptc>UAS-NICD) (position of ptc-expressing region indicated by white arrow). (B-B′′) Downregulation of Notch via RNAi using the same driver results in ablation of both endogenous (blue) and reporter (green) expressions at the D/V boundary (white arrow). (C-C′′) Ectopic expression of the NICD in R1, R3, R4, R6, and R7 with sev-Gal4 results in ectopic expression of endogenous Dpn (C′-red. Orange circle) and the GFP reporter (C′′-green. Orange circle and arrows) compared to wild type (D, blue circles and arrowheads).
Relationship between dpn and E(spl) gene functions
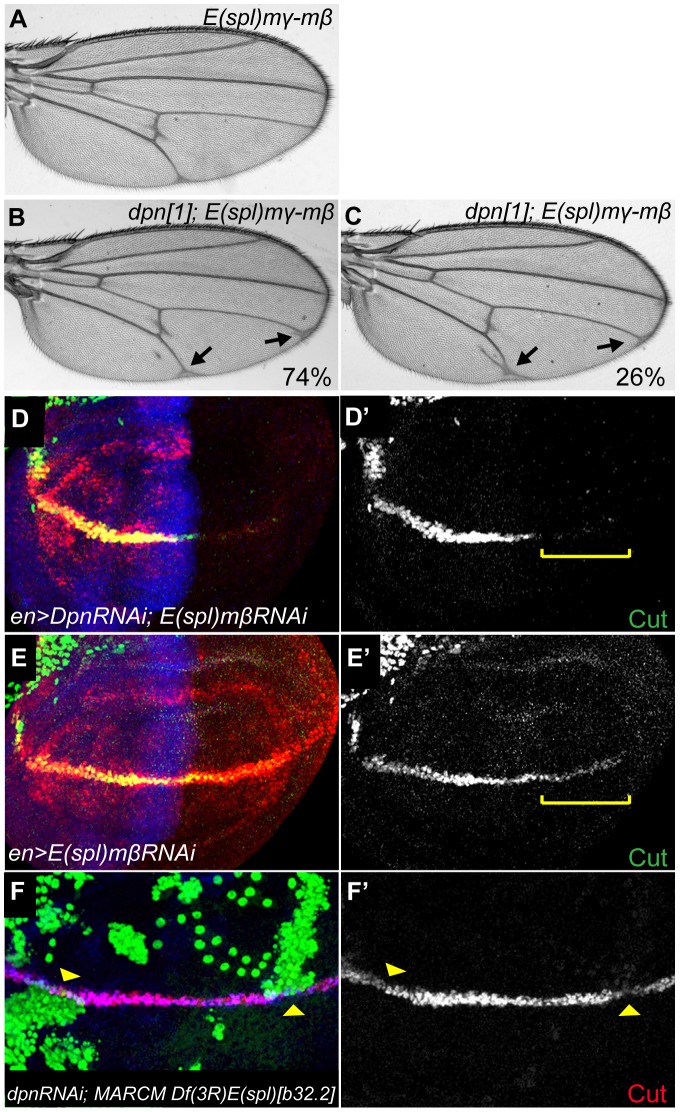
The expression of dpn in response to Notch activity, and the evidence showing direct binding of Su(H) in several contexts, supports the recent results indicating that dpn has a role in implementing Notch function in regulating proliferation [12]. This is exemplified by the fact that knock-down of dpn enhances the wing phenotype seen in Notch heterozygotes ( Fig 3A–F , Table S1 and see [12]). Twenty percent of the wings from N[55e11]/+ female flies had a single nick in the margin at the distal tip ( Fig. 3D ). In combination with dpn alleles both the extent and the frequency of wing nicks were enhanced. Thus, 97% of N[55e11]/+; dpn[6]/+ wings contained wing nicks and these frequently extended into the posterior of the wing margin ( Fig. 3F ). Slightly lower frequency was detected in combinations with dpn[1] where 78% of wings contained nicks, but again this was significantly enhanced compared to the N[55e11]/+ alone ( Fig. 3E comparing to 3D). In contrast, reductions in E(spl) function did not enhance the wing notching phenotype of Notch heterozygotes under these conditions (Table S1). These genetic interactions therefore support the hypothesis that dpn specifically contributes to Notch function at the D/V boundary in the wing.
Figure 3. dpn modifies Notch phenotypes and directly alters the expression of the Notch target cut.
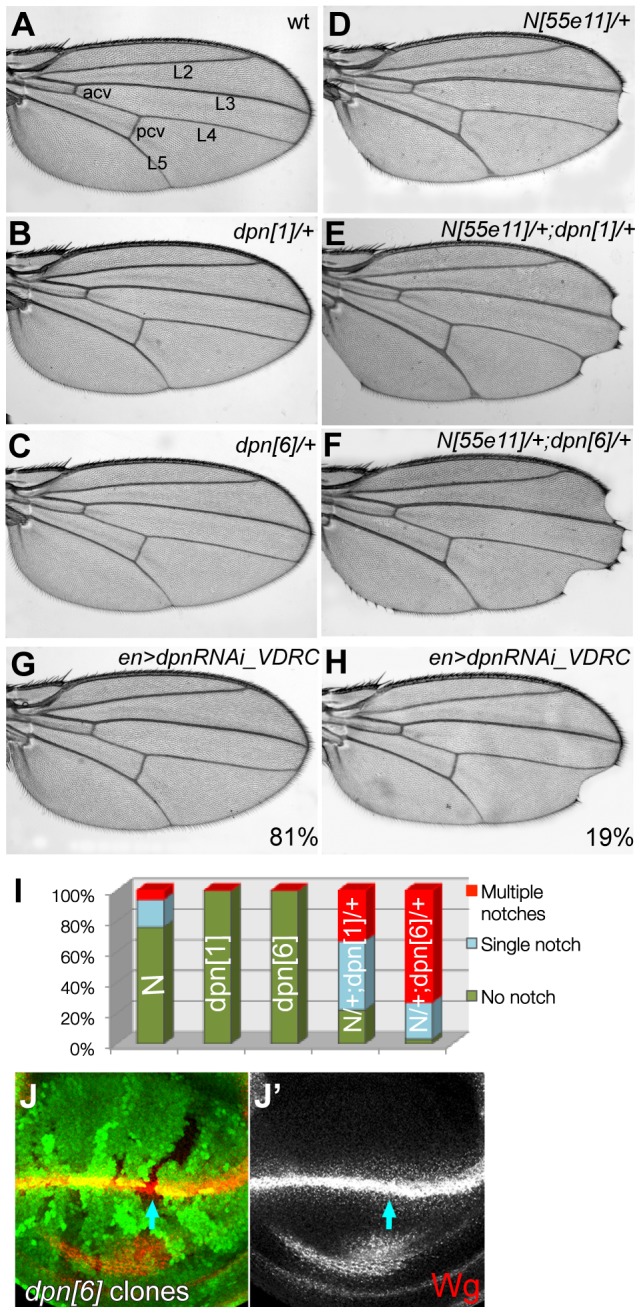
(A) Wild type wing showing the wing margin and the veins: anterior cross vein (acv), posterior cross vein (pcv), L2, L3, L4 and L5. (B–F) Notch (N[55e11]/+) heterozygous phenotype of mild distal wing notches (D) is genetically modified in flies also heterozygous for dpn alleles, dpn[1] (B,E) and dpn[6] (C, F). (G–H) dpn-RNAi driven by engrailed-Gal4 TubGal80 (en>) caused single nicks in 19% of the wings (H). See Table S1. (I) Quantification of nick occurrences in each phenotype, dpn alleles significantly enhance the wing notching of Notch heterozygotes (p<<0.01). (J-J′) dpn[6] homozygous mutant clones do not alter Wingless (Wg) expression at the D/V boundary.
Despite the genetic interactions with Notch, remarkably few defects were detected in dpn mutant clones or in tissue expressing RNAi targeting dpn (e.g. Fig 3J and Fig 4 ). For example, the majority of adult flies showed no defects under conditions where dpn-RNAi was expressed throughout the posterior of the wing using engrailed-Gal4. At best there was a low penetrance of wing nicks (19%) with one RNAi line, consistent with dpn having a subtle role in this process ( Fig. 3H ). Similar lack of phenotype was seen when E(spl) function was eliminated using a deficiency that spans the entire locus [15]. One possibility, given their shared Notch regulation, is that these HES genes have related functions as recently suggested [9], [12], so that the combined activity is required for the robust establishment of Notch function. Elimination of all the E(spl) genes causes embryonic lethality [15]. However, a newly generated deficiency, E(spl)mγ-mβ [DK33], that removes only two E(spl)bHLH genes (E(spl)mγ and E(spl)mβ) has a mild wing vein phenotype ( Fig. 4A ). We therefore assessed whether this phenotype could be modified by a reduction in the levels of dpn, by making combinations with heterozygous dpn alleles ( Fig. 4A–C ,Table S2). Wings from homozygous E(spl)mγ-mβ [DK33] flies had mild thickening in the posterior cross-vein (pcv) and tip of L5 ( Fig. 4A ). This mild phenotype was dominantly enhanced by removing a single copy of dpn, giving rise to prominent vein thickening at the tips of L4 and L5 ( Fig. 4B ) as well as to ectopic veins flanking L5 in 26% of wings ( Fig. 4C ). This genetic interaction suggests that dpn and E(spl) function together during wing vein development.
Figure 4. dpn and E(spl) genes may act redundantly.
(A–C) The mild wing vein phenotype of E(spl)mγ-mβ (A) is dominantly modified by dpn[1] (B and C). (B) Arrows point to vein thickening at the tips of L4 and L5, observed in 74% of dpn[1]/+ E(spl)mγ-mβ wings (C) 26% of dpn[1]/+ E(spl)mγ-mβ wings also showed ectopic veins surrounding L5 (arrow). See Table S2. (D–E) E(spl)mβ (E-E′) or both dpn and E(spl)mβ (D-D′) levels were knocked down by RNAi at early third instar wing discs. Cut expression (green) was dramatically reduced in the double RNAi (D′) compared to E(spl)mβ-RNAi alone (E′). (F-F′) E(spl)-C loss of function MARCM clones produce reduction in Cut expression (F′) when combined with dpn RNAi.
As there are few specific Notch targets known in the developing veins, we extended our analysis to Notch function at the D/V boundary in the wing, where there are several known targets of Notch activity including ct. In larvae, combined knock-down of E(spl)mβ and dpn expression in the posterior compartment of the third instar wing disc led to significant reduction of Cut level in comparison to E(spl)mβ alone, although we note that the latter caused a subtle decrease (compare Fig. 4D-D ′ to 4E-E′ ). To further assess whether dpn made a contribution to the regulation of ct expression, dpn function was eliminated in clones of cells that completely lacked all E(spl) functions using a deletion of the complex, Df(3R)E(spl)[b32.2], which has been combined with a gro+ transgene to rescue any effects on the neighbouring gro gene [15]. Such dpnRNAi; Df(3R)E(spl)[b32.2] double mutant clones had lost Cut staining, although knock down of either alone failed to significantly compromise ct expression under these conditions ( Fig 4F ;data not shown). Similar analysis in the eye disc also suggested that the genes have related functions. Knock down of dpn alone produced few if any defects (some rotational defects but no fate changes observed) while knock down of dpn and E(spl)mδ resulted in rare examples of symmetrical ommatidia (it was not possible to test the combinations with Df(3R)E(spl)[b32.2] as this leads to early defects in patterning; data not shown). Thus these data suggest that the dpn and E(spl) genes have overlapping functions in eye and wing development.
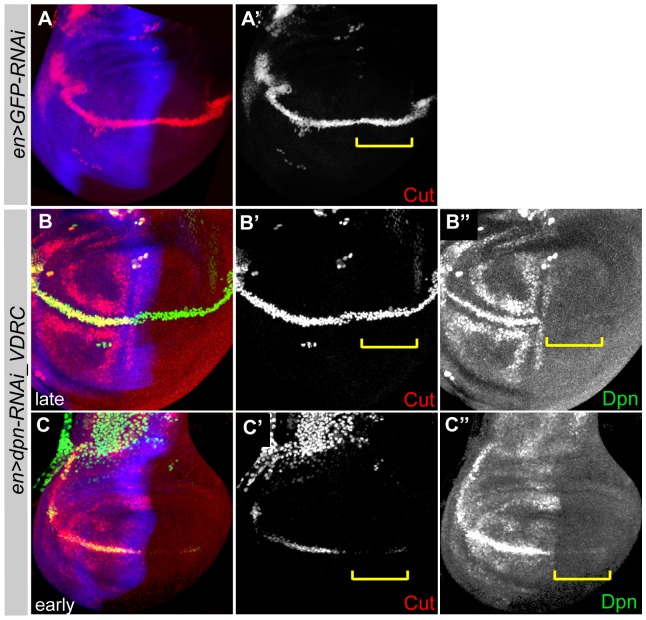
One surprising result emerged when we analyzed the consequences of ablating dpn in early wing discs ( Fig. 5 ). We found that the RNAi mediated knock down of dpn in the posterior compartment of the wing resulted in down-regulation of Cut at the D/V margin in mid third instar discs but not in older discs ( Fig. 5C ). Similar results were seen using independent RNAi lines, all produced defects at early but not at later stages (Fig. S2). Together the results suggest that dpn may make a contribution to Notch function at the D/V boundary, but that its absence can be compensated by another mechanism, to explain the recovery of cut expression at later stages, and the lack of phenotype in the adult wings. It is also striking that the phenotype is one of reduced Cut expression, because dpn and the E(spl)bHLH genes have characteristics of transcriptional repressors. This suggests that there may be an intermediary factor that is repressed by Dpn to enable the expression of Cut (see discussion). We therefore drew up a list of candidates, that are expressed in the relevant domain of the wing disc, and tested whether they were inhibited by Dpn overexpression or up-regulated in conditions of dpn-RNAi. None of those tested (e.g. msh, dve, others) showed any discernable change in response to changes in dpn (Fig. S3).
Figure 5. dpn regulates Cut expression.
dpn downregulation, mediated by en>RNAi, in the posterior compartment of late (B-B′′) and early (C-C′′) third instar wing discs. GFP-RNAi was used as a control (A-A′). Discs were stained with Cut (red) and Dpn (green). Yellow brackets (B′-B′′ and C′-C′′) represent the Cut expression at the posterior D/V boundary.
Regulation of E(spl)bHLH genes by dpn
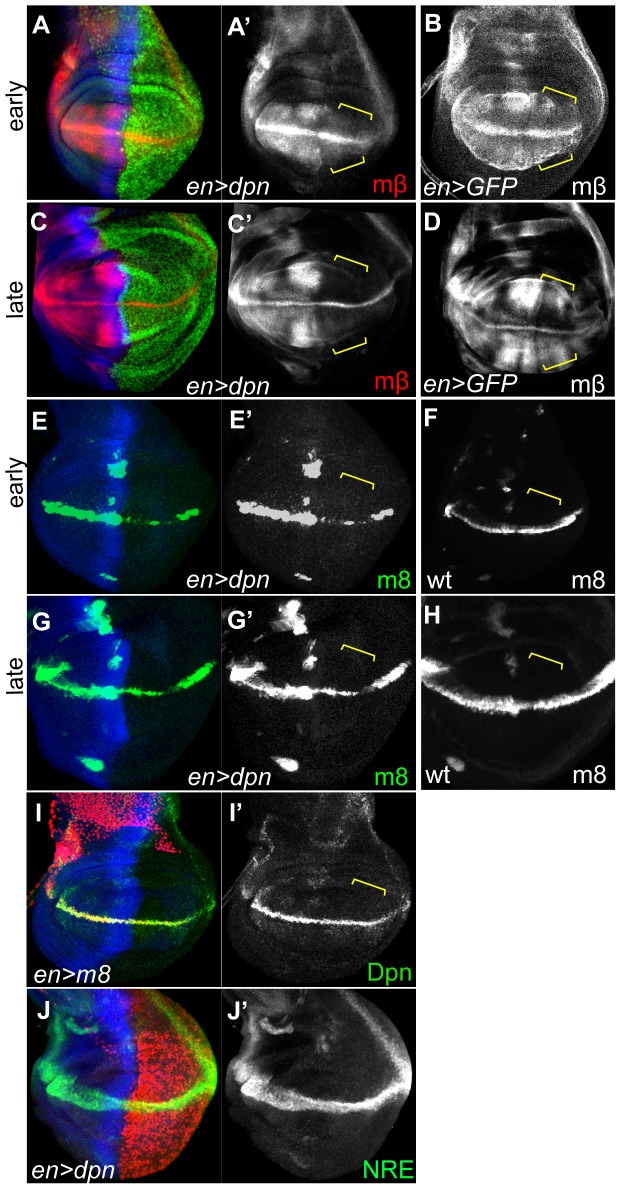
dpn and E(spl)bHLH genes are all regulated by Notch and have broadly similar expression patterns. This raises the possibility that there could be cross-regulatory interactions between these different HES genes, similar to what has been observed in somitogenesis [16]. We therefore tested whether ectopic expression of dpn or of E(spl)bHLH genes was able to modify the other's expression. First we found that overexpression of dpn (using enGal4 tubGal80ts), but not a control GFP, was sufficient to suppress the expression of two E(spl) reporters, E(spl)mβ1.5-CD2 and E(spl)m8-lacZ ( Figure 6A–H ). This reduction was even detected in late stage wing discs ( Fig 6C and 6G ). As there was no effect on a generic Notch reporter, NRE-GFP [17], under equivalent conditions ( Fig 6J-J ′ ), it is likely that dpn regulates the E(spl) enhancers directly rather than acting indirectly through Notch. In contrast, over-expression of E(spl)m8, E(spl)mβ, E(spl)m7 or E(spl)m5 ( Fig. 6I , Fig S4) all failed to modify the dpn expression at the D/V boundary. The data suggest therefore that there is a unidirectional feedback loop between Notch targeted HES genes, with dpn regulating E(spl)bHLH and not vice versa.
Figure 6. dpn regulates E(spl) genes.
Early (A, B, E and F) and late (C, D, G and H) third instar wing discs with en> driving dpn expression. Yellow brackets highlight the posterior compartment in which engrailed is expressed. E(spl)mβ (A′, C′) and E(spl)m8-lacZ (E′, G′) expression were reduced in both early and late instar discs, in comparison to GFP overexpression (B and D) and wild type E(spl)m8-lacZ (F and H) controls. (I) Misexpression of E(spl)m8 fails to alter Dpn expression. (J) Misexpression of dpn has no effect on NRE-GFP.
Discussion
HES genes are well-known targets of Notch activity. However, in Drosophila only the bHLH genes within the E(spl) Complex were originally thought to be directly downstream of Notch. The expression of another HES genes, dpn, appeared independent of Notch and indeed was associated with cells where Notch activity is considered to be down-regulated (embryonic neuroblasts). More recently it has emerged that dpn expression is under Notch regulation in some contexts [5], [9]. Our results extend these findings by demonstrating that dpn is directly bound by Su(H) in vivo. As the Su(H) occupied regions differ according to the tissue-type, it appears that dpn contains several Notch responsive enhancers and our results demonstrate that these direct Notch-dependent expression in different subsets of tissues. Nevertheless, it is striking that a single dpn enhancer, dpn[b] exhibits Notch related expression in both the eye and the wing discs yet these patterns are characteristic of distinct genes/enhancers from the E(spl) Complex [13].
Despite the clear regulation by Notch, there is however relatively few phenotypes resulting from loss of dpn in many tissues. For example, both the wing and eye disc exhibit robust expression of dpn but neither exhibit phenotypes when dpn function was ablated. However, genetic interactions demonstrate that dpn function is related to Notch and both our evidence, and that from recent studies [9], [12], indicate that it has partially redundant functions with the E(spl) genes. This is exemplified by the fact that absence of dpn or of E(spl) Complex alone has little effect on the D/V boundary, but the combined knock down leads to loss of key gene expression.
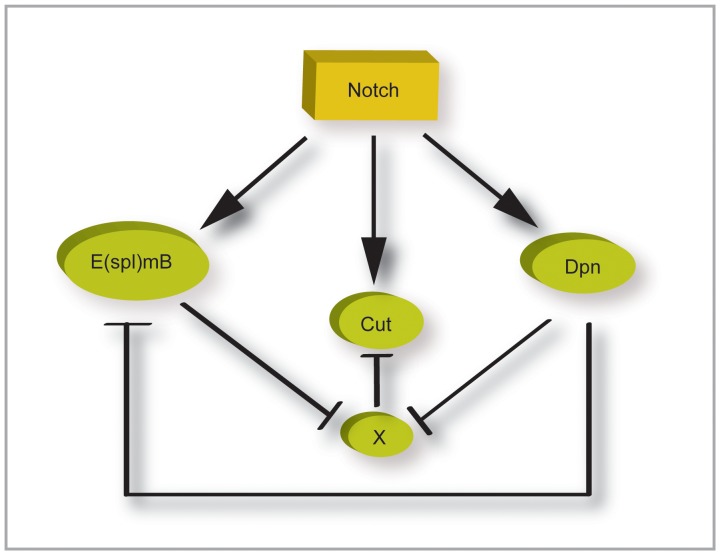
What then is the relevance of dpn in these contexts, especially given that there are 7 E(spl)bHLH genes that also appear to have largely redundant functions? We propose two components to dpn function to explain its importance in the Notch response. Clues for the first come from the fact that we could detect subtle phenotypes from reductions in dpn when we analyzed early developmental stages. Thus the absence of dpn led to a delay in the ability of Notch to up-regulate cut. Earlier studies also demonstrated a subtle decrease in cut expression in cells lacking E(spl) genes [18]. These results could be explained if both E(spl) and dpn make a contribution to cut regulation. We suggest that this must be indirect, via the inhibition of a repressor, since both dpn and E(spl)bHLH are thought to be dedicated repressors ( Fig. 7 ). So far, we have not identified another repressor that could act as an intermediary.
Figure 7. dpn contributes to the robustness of the Notch response.
Diagram summarizing regulatory interactions between the genes indicated. Involvement of additional gene, X, is inferred due to the fact that HES genes appear to function as dedicated repressors.
The second component of dpn function is suggested by the observation that Dpn can repress the enhancers derived from E(spl)bHLH genes but not vice versa. Futhermore, we observed that cells with high levels of Dpn often had lower levels of E(spl)bHLH on a cell by cell level. We therefore propose that there is a direct regulatory relationship between dpn and E(spl)bHLH, whereby dpn represses E(spl)bHLH expression ( Fig. 7 ). This could set a maximum threshold for E(spl) gene expression since, in previous studies, we have found that dpn shows a less rapid up-regulation following Notch activation than the E(spl) genes [19]. This is reminiscent of the differences seen between HES gene responses in the oscillatory clock involved in somitogenesis [20] and suggests that similar HES gene cross-regulatory network may underpin other Notch dependent processes.
Materials and Methods
Drosophila Genetics and Stocks
All alleles and stocks are described in FlyBase (www.flybase.org) unless indicated otherwise. The following mutant lines were used: dpn [1], dpn [6], Notch[55e11], E(spl)[b32.2] gro + FRT82B, E(spl)Δmγ-mβ [DK33-10.1] (see below). The following reporters were used: dpn[a]GFP, dpn[b]GFP, E(spl)mδ0.5-LacZ [21], E(spl)m8-LacZ [22] E(spl)mβ1.5-CD2 [23], NRE-GFP [17].
To assess the interactions between dpn and Notch, the following crosses were performed:
Notch[55e11]/FM7 females were mated to dpn [1] /CyO and dpn [6] /CyO males in independent crosses. Notch[55e11]/+; dpn [1] /+ and Notch[55e11]/+; dpn [6] /+ female adult wings were dissected out and analysed in terms of the wing nicks occurrence. As controls, Notch[55e11]/FM7, dpn [1] /CyO and dpn [6] /CyO females were individually crossed to wild type males in independent crosses. Notch[55e11]/+, dpn [1] /+ and dpn [6] /+ adult wings were analysed individually.
In overexpression and RNAi experiments, Gal4 driver stocks (engrailed:Gal4 Tub:Gal80ts, patched:Gal4 Tub:Gal80ts, sevenless-Gal4) were combined with UAS lines and larvae were shifted to 30°C 48 hours after egg laying. The following RNAi and overexpression lines were used: UAS-Notch-intra[79.2], UAS-dpn (from H.Vaessin), UAS-E(spl)m8 [24], UAS-dpn-RNAi (VDRC- v106181), UAS-Notch-RNAi (BL-7078), UAS-E(spl)mβ-RNAi (BL26202), UAS-GFP-RNAi (BL-9330). UAS-E(spl)m5 [18], UAS-m5RNAi (BL-26201), UAS-E(spl)m7, UAS-dpnRNAi (BL-26320), UAS-dpnRNAi (DGRC-8704R-4).
Mitotic clones were generated by FLP-mediated mitotic recombination [25]. Clones lacking dpn were obtained by crossing FRT42B dpn [6] /CyO males to hsp-FLP122; FRT42B/CyO virgin females. Control clones were generated using the wild type FRT42B chromosome. The progeny of these crosses were heat shocked at 37°C for 1 h between 48 and 72 h after egg-laying. Discs were dissected and analyzed 3 days after the induction of the clones.
To generate MARCM clones [26], E(spl)[b32.2] gro + FRT82B/TM6B or UAS-dpn-RNAi; E(spl)[b32.2] gro + FRT82B/TM6B were crossed to hs-FLP tubGal4 UAS-GFP; FRT82B tubGal80. Progeny were heat shocked at 37°C for 1 hr after 72 hours egg-laying then kept at 30°C until dissection.
Generation of E(spl)Δmγ-mβ [DK33-10.1]:
Small deficiencies in the E(spl)-Complex were generated in a cross to induce P-element mediated male recombination [27] using the P-element: P{lacW}K33 [28], where the transposon is inserted 61 bp upstream of the transcriptional start site of E(spl)mγ, oriented with the 3′ terminal repeats close to E(spl)mγ. One of the progeny had a deletion of approximately 6.2 Kb, starting inside the P-element (retaining the last 2361 bp of the 5′-end of the P{lacW}) deleting all the E(spl)mγ gene and most of E(spl)mβ coding region, terminating after the first 31amino acids.
For genetic interactions experiments, Notch[55e11]/FM7 females were mated to both E(spl)[b32.2] gro+FRT82B/TM6B and homozygous viable E(spl)Δmγ-mβ [DK33-10.1] males in independent crosses. Notch[55e11]/+; E(spl)[b32.2] gro+FRT82B/+ and Notch[55e11]/+; E(spl)Δmγ-mβ [DK33-10.1]/+ female adult wings were selected and analysed in terms of wing nick occurrences. The Chi-squared test was used to evaluate statistical significance.
Molecular Cloning
Putative NREs in dpn region:
ChIP-enriched regions (putative NREs in dpn) were amplified from Drosophila genomic DNA using primers containing specific restriction enzyme sequences and cloned into pGreenRabbit/pRedRabbit vectors [17] for in vivo reporter assays. Sequences of primers and restriction enzymes used for cloning dpn reporters are as follows:
dpn[a] chr2R- KpnI and XbaI
Left ACGTTTCGTGCCTCATATGTC
Right TTAAGGCACAAGTGTCCGAAG
dpn[b] chr2R-NotI and KpnI
Left AAAACAGGAGTCGCTTTGGA
Right GCAGTGTGACCCTGGAAAAT
dpn[c] chr2R-XbaI and KpnI
Left AGTGTGTGCGTGCGTAAAAG
Right CACAACAAAAGCGAACGAAA
Immunostaining
Antibody staining of wing and eye imaginal discs was performed as described previously [21] with the following antibodies: guinea pig anti-Dpn (1∶1000; gift of J. Skeath, Washington University in St Louis, MO, USA), rat anti-Ci (1∶20; Developmental Studies Hybridoma Bank [DSHB]), mouse anti-Cut (1∶20; DSHB), mouse anti-GFP (1∶500, Invitrogen), mouse anti β-gal (1∶20; DSHB), rabbit anti-Dve [29], rabbit anti-Msh [30]. Images were acquired with a scanning confocal microscope (Leica TCS SP2) and processed using Photoshop (Adobe).
Supporting Information
Reporter [a] expression was detected in the leg joints (A), the optic lobes of the brain (C), and cone/support cells in the eye discs (E) but not in neuroblasts or photoreceptors, while reporter [b] expression was detected in leg discs (B), in brain neuroblast lineages (D) and in R3–R4 and R7 photoreceptors (F).
(TIF)
dpn downregulation mediated by two different RNAi lines using the enGal4 system at the posterior compartment of early (A–C) and late (B–D) third instar wing discs. Discs were stained with Dpn (red), Cut (green) and Ci (blue).
(TIF)
(A-A′) Msh expression does not change at third instar wing discs after dpn misexpression at the posterior compartment (marked by the absence of blue) compared to wild type (B). (C-C′) Similarly Dve expression does not respond to dpn misexpression at the posterior compartment (marked by the absence of red) at third instar larval stages compared to wild type (D).
(TIF)
Neither E(spl)m5 misexpression (A-A′) nor E(spl)m5-RNAi driven by en-Gal4 changes Dpn expression (green in A and red in B) in the wing disc. Similarly dpn expression stays the same after misexpression (C-C′) or RNAi (D-D′) of E(spl)mβ. (E-E′) E(spl)m7 misexpression does not cause any difference in dpn expression.
(TIF)
Genetic interaction between Notch and dpn.
(DOC)
Genetic interaction between dpn and E(spl).
(DOC)
Acknowledgments
We are very grateful to J. Skeath (Dpn antibody), H. Vaessin (UAS-Dpn line), Fumio Matsuzaki (Dve antibody), Chris Q. Doe (Msh antibody), The Bloomington Stock Center (BL), Vienna Drosophila RNAi Center (VDRC), The Kyoto Stock Center (DGRC) and The Developmental Studies Hybridoma Bank (DSHB) for providing Drosophila strains and antibodies. We thank members of the Bray lab for valuable discussions. We also thank Kat Millen for generating recombinant fly strains and for excellent technical assistance.
Funding Statement
Research supported by an MRC programme grant G0800034 to SJB (http://www.mrc.ac.uk/index.htm). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689. [DOI] [PubMed] [Google Scholar]
- 2. Kopan R, Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137: 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krejci A, Bernard F, Housden BE, Collins S, Bray SJ (2009) Direct response to Notch activation: signaling crosstalk and incoherent logic. Sci Signal 2: ra1. [DOI] [PubMed] [Google Scholar]
- 4. Djiane A, Krejci A, Bernard F, Fexova S, Millen K, et al. (2013) Dissecting the mechanisms of Notch induced hyperplasia. EMBO J 32: 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. San-Juan BP, Baonza A (2011) The bHLH factor deadpan is a direct target of Notch signaling and regulates neuroblast self-renewal in Drosophila. Dev Biol 352: 70–82. [DOI] [PubMed] [Google Scholar]
- 6. Rushlow CA, Hogan A, Pinchin SM, Howe KM, Lardelli M, et al. (1989) The Drosophila hairy protein acts in both segmentation and bristle patterning and shows homology to N-myc. EMBO J 8: 3095–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bier E, Vaessin H, Younger-Shepherd S, Jan LY, Jan YN (1992) deadpan, an essential pan-neural gene in Drosophila, encodes a helix-loop-helix protein similar to the hairy gene product. Genes Dev 6: 2137–2151. [DOI] [PubMed] [Google Scholar]
- 8. Roark M, Sturtevant MA, Emery J, Vaessin H, Grell E, et al. (1995) scratch, a pan-neural gene encoding a zinc finger protein related to snail, promotes neuronal development. Genes Dev 9: 2384–2398. [DOI] [PubMed] [Google Scholar]
- 9. Zacharioudaki E, Magadi SS, Delidakis C (2012) bHLH-O proteins are crucial for Drosophila neuroblast self-renewal and mediate Notch-induced overproliferation. Development 139: 1258–1269. [DOI] [PubMed] [Google Scholar]
- 10. Zhu S, Wildonger J, Barshow S, Younger S, Huang Y, et al. (2012) The bHLH repressor Deadpan regulates the self-renewal and specification of Drosophila larval neural stem cells independently of Notch. PLoS One 7: e46724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Canon J, Banerjee U (2003) In vivo analysis of a developmental circuit for direct transcriptional activation and repression in the same cell by a Runx protein. Genes Dev 17: 838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. San Juan BP, Andrade-Zapata I, Baonza A (2012) The bHLH factors Dpn and members of the E(spl) complex mediate the function of Notch signalling regulating cell proliferation during wing disc development. Biol Open 1: 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Celis JF, de Celis J, Ligoxygakis P, Preiss A, Delidakis C, et al. (1996) Functional relationships between Notch, Su(H) and the bHLH genes of the E(spl) complex: the E(spl) genes mediate only a subset of Notch activities during imaginal development. Development 122: 2719–2728. [DOI] [PubMed] [Google Scholar]
- 14. Jennings B, Decelis J, Delidakis C, Preiss A, Bray S (1995) Role of Notch and Achaete-Scute Complex in the Expression of Enhancer of Split Bhlh Proteins. Development 121: 3745–3752. [Google Scholar]
- 15. Heitzler P, Bourouis M, Ruel L, Carteret C, Simpson P (1996) Genes of the Enhancer of split and achaete-scute complexes are required for a regulatory loop between Notch and Delta during lateral signalling in Drosophila. Development 122: 161–171. [DOI] [PubMed] [Google Scholar]
- 16. Shankaran SS, Sieger D, Schroter C, Czepe C, Pauly MC, et al. (2007) Completing the set of h/E(spl) cyclic genes in zebrafish: her12 and her15 reveal novel modes of expression and contribute to the segmentation clock. Dev Biol 304: 615–632. [DOI] [PubMed] [Google Scholar]
- 17. Housden BE, Millen K, Bray SJ (2012) Drosophila Reporter Vectors Compatible with PhiC31 Integrase Transgenesis Techniques and Their Use to Generate New Notch Reporter Fly Lines. G3 (Bethesda) 2: 79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ligoxygakis P, Bray SJ, Apidianakis Y, Delidakis C (1999) Ectopic expression of individual E(spl) genes has differential effects on different cell fate decisions and underscores the biphasic requirement for notch activity in wing margin establishment in Drosophila. Development 126: 2205–2214. [DOI] [PubMed] [Google Scholar]
- 19. Housden BE, Fu AQ, Krejci A, Bernard F, Fischer B, et al. (2013) Transcriptional dynamics elicited by a short pulse of notch activation involves feed-forward regulation by E(spl)/Hes genes. PLoS Genet 9: e1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schroter C, Ares S, Morelli LG, Isakova A, Hens K, et al. (2012) Topology and dynamics of the zebrafish segmentation clock core circuit. PLoS Biol 10: e1001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cooper MT, Bray SJ (1999) Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature 397: 526–530. [DOI] [PubMed] [Google Scholar]
- 22. Kramatschek B, Campos-Ortega JA (1994) Neuroectodermal transcription of the Drosophila neurogenic genes E(spl) and HLH-m5 is regulated by proneural genes. Development 120: 815–826. [DOI] [PubMed] [Google Scholar]
- 23. de Celis JF, Tyler DM, de Celis J, Bray SJ (1998) Notch signalling mediates segmentation of the Drosophila leg. Development 125: 4617–4626. [DOI] [PubMed] [Google Scholar]
- 24. Tata F, Hartley DA (1995) Inhibition of cell fate in Drosophila by Enhancer of split genes. Mech Dev 51: 305–315. [DOI] [PubMed] [Google Scholar]
- 25. Xu T, Rubin GM (1993) Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117: 1223–1237. [DOI] [PubMed] [Google Scholar]
- 26. Lee T, Luo L (2001) Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci 24: 251–254. [DOI] [PubMed] [Google Scholar]
- 27. Preston CR, Sved JA, Engels WR (1996) Flanking duplications and deletions associated with P-induced male recombination in Drosophila. Genetics 144: 1623–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. The I, Hannigan GE, Cowley GS, Reginald S, Zhong Y, et al. (1997) Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science 276: 791–794. [DOI] [PubMed] [Google Scholar]
- 29. Nakagoshi H, Hoshi M, Nabeshima Y, Matsuzaki F (1998) A novel homeobox gene mediates the Dpp signal to establish functional specificity within target cells. Genes Dev 12: 2724–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McDonald JA, Holbrook S, Isshiki T, Weiss J, Doe CQ, et al. (1998) Dorsoventral patterning in the Drosophila central nervous system: the vnd homeobox gene specifies ventral column identity. Genes Dev 12: 3603–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reporter [a] expression was detected in the leg joints (A), the optic lobes of the brain (C), and cone/support cells in the eye discs (E) but not in neuroblasts or photoreceptors, while reporter [b] expression was detected in leg discs (B), in brain neuroblast lineages (D) and in R3–R4 and R7 photoreceptors (F).
(TIF)
dpn downregulation mediated by two different RNAi lines using the enGal4 system at the posterior compartment of early (A–C) and late (B–D) third instar wing discs. Discs were stained with Dpn (red), Cut (green) and Ci (blue).
(TIF)
(A-A′) Msh expression does not change at third instar wing discs after dpn misexpression at the posterior compartment (marked by the absence of blue) compared to wild type (B). (C-C′) Similarly Dve expression does not respond to dpn misexpression at the posterior compartment (marked by the absence of red) at third instar larval stages compared to wild type (D).
(TIF)
Neither E(spl)m5 misexpression (A-A′) nor E(spl)m5-RNAi driven by en-Gal4 changes Dpn expression (green in A and red in B) in the wing disc. Similarly dpn expression stays the same after misexpression (C-C′) or RNAi (D-D′) of E(spl)mβ. (E-E′) E(spl)m7 misexpression does not cause any difference in dpn expression.
(TIF)
Genetic interaction between Notch and dpn.
(DOC)
Genetic interaction between dpn and E(spl).
(DOC)