Abstract
We tested the hypothesis that antagonism of progesterone receptor (PR) in newborn rats alters carotid body and respiratory responses to hypoxia and nicotinic receptor agonists. Rats were treated with the PR antagonist mifepristone (daily oral gavage 40 μg/g/d) or vehicle between post-natal days 3 and 15. In 11–14-day-old rats, we used in vitro carotid body/carotid sinus nerve preparation and whole body plethysmography to assess the carotid body and ventilatory responses to hypoxia (65 mmHg in vitro, 10% O2 in vivo) and to nicotinic receptor agonists (as an excitatory modulator of carotid body activity—nicotine 100 μM for in vitro studies, and epibatidine 5 μg/kg, i.p., which mainly acts on peripheral nicotinic receptors, for in vivo studies). The carotid body responses to hypoxia and nicotine were drastically reduced by mifepristone. Compared with vehicle, mifepristone-treated rats had a reduced body weight. The ventilatory response to epibatidine was attenuated; however, the hypoxic ventilatory response was similar between vehicle and mifepristone-treated pups. Immunohistochemical staining revealed that mifepristone treatment did not change carotid body morphology. We conclude that PR activity is a critical factor ensuring proper carotid body function in newborn rats.
Keywords: carotid sinus nerve, whole-body plethysmography, hypoxia, nicotine receptor agonist, progesterone receptor antagonist, newborn
Progesterone is a respiratory stimulant that acts both on the central nervous system (CNS) and on peripheral chemoreceptors to enhance resting minute ventilation (Bayliss et al., 1987), the hypoxic ventilatory response (Joseph et al., 2002), and the carotid sinus nerve response to hypoxia (Hannhart et al., 1990). These effects of progesterone are well described in adults and also occur in newborn rats in which chronic progesterone exposure enhances the hypoxic ventilatory response and reduces apnea frequency under normoxia or hypoxia (Lefter et al., 2007, 2008). This has potential far-reaching implications because progesterone-based hormone replacement therapy reduces the occurrence of sleep apneas in postmenopausal women (Shahar et al., 2003), and it has been suggested that progesterone might be used for treatment of apneas in preterm neonates (Finer et al., 2006).
Circulating progesterone levels are supposed to be low after birth in mammals. However, neural groups within the central and peripheral nervous systems synthesize steroid hormones (referred to as neurosteroids), including progesterone (Zwain and Yen, 1999), which play a key role in the reproductive behavior through its action in the brain (Micevych and Sinchak, 2008; Micevych et al., 2008). In sensory structures of the peripheral nervous system during mouse embryonic development, there is dense immunohistochemical staining for the P450 side-chain cleavage enzyme (P450scc, a key enzyme in neurosteroid synthesis) (Compagnone et al., 1995), and we have reported that P450scc and the progesterone receptor (a member of the nuclear hormone receptor superfamily of ligand-activated transcription factors), are both densely expressed in the carotid body of fetal, newborn, and adult rats (Joseph et al., 2006). Concomitant expression of these proteins suggests that progesterone acts as an autocrine factor in the carotid body. To test this hypothesis, we treated newborn rats with the progesterone receptor antagonist mifepristone for up to 12 consecutive days between postnatal days 3 and 15, and we used both in vitro and in vivo approaches to assess carotid body and ventilatory responses to hypoxia. Because acetylcholine is an important transmitter in the carotid body response to hypoxia (Conde and Monteiro, 2006; Shirahata et al., 2007) and shows increased function during postnatal development in rats (Niane et al., 2009), we also tested carotid body and ventilatory responses to a nicotine cholinergic receptor agonist. Our results are consistent with a critical role of progesterone receptor for adequate development of carotid body responses to hypoxia. In vivo, the ventilatory response to epibatidine was also reduced by mifepristone treatment, but hypoxic ventilatory response was not affected suggesting the development of compensatory responses.
EXPERIMENTAL PROCEDURES
Animals
The local committee on animal care and use reviewed and approved all experimental protocols. We applied strategies to reduce animal use and suffering as recommended by our local committee. Adult male and female rats were ordered from Charles-River (St.-Constant, Québec, Canada) and were used for mating. At birth, all litters were reduced to 12 pups to avoid specific effects on growth, and pups were randomly assigned to a vehicle (5% ethanol in propylene glycol) or a mifepristone (mifepristone −40 μg/g/d (Lonstein et al., 2001)) group (daily oral gavage (0.05 ml/10 g) from postnatal days 3 to 15). We used a total of 35 vehicle pups (from 18 litters) and 37 mifepristone pups (from 10 litters) throughout the study. All recordings were performed in males.
In vitro recordings of carotid sinus nerve activity
We used rats aged 11–14 days to perform carotid sinus nerve recording with a standard in vitro preparation (Peng et al., 2004) as previously described (Niane et al., 2009). Briefly, the carotid bifurcation was dissected en bloc with the carotid body and carotid sinus nerve left intact. Each carotid bifurcation was pinned in a small-volume tissue bath that was continuously superfused (2 ml/min) with a gassed (95% O2 and 5% CO2) bicarbonate-buffered saline solution. The carotid body and sinus nerve were cleaned, sectioned, and transferred to a heated (36 °C) recording chamber that was superfused (2 ml/min) with a gassed (21% O2/5% CO2) solution.
Extracellular recordings were made using a glass suction electrode (A-M Systems, Carlsborg, WA, USA) connected to a differential input amplifier (NL100AK, Digitimer, Hertfordshire, UK); the signal was preamplified, filtered (30 –1500 Hz), amplified using standard Neurolog modules (NL104A, AC Preamplifier; NL125/6, Filter; NL106, AC/DC Amplifier, Digitimer), and then fed to an A/D converter (Micro1401, Cambridge Electronic Design, Cambridge, UK) and data acquisition software (Spike 2 software, CED). A reference electrode was in contact with the carotid body surface, whereas a ground electrode was in the recording chamber. Chemoreceptor discharges were discriminated as activity that was 25% above baseline noise. Experiments began when the carotid sinus nerve discharge rate was stable under normoxic (PO2≈150 mmHg), normocapnic (PCO2≈40 mmHg/pH=7.38, measured from the reservoir bath) conditions. The in vitro preparation was superfused with a solution that was bubbled with 5% O2/5% CO2 in N2 (hypoxia—PO2=65 mmHg). Hypoxia was maintained for 5 min to achieve a steady-state response. The super-fusion line was then switched to the normoxic solution for 5–10 min before initiating superfusion with nicotine (100 ≈M) for 5 min.
In vivo ventilatory recordings using whole-body plethysmography
Respiratory recordings were performed in 10 –12-day-old rat pups using whole-body flow-through plethysmography (Emka technologies, Paris, France) as previously described (Lefter et al., 2007, 2008; Niane et al., 2009). Airflow through the chamber was set at ~100 ml/min, and the temperature inside the chamber at 30 °C using a temperature control loop. Oxygen and CO2 levels were analyzed for the calculation of O2 uptake and CO2 production. All signals were stored on a computer and used to calculate respiratory parameters minute ventilation (V̇E), respiratory frequency (fR), and tidal volume (VT), O2 uptake, and CO2 production. O2 and CO2 ventilatory equivalents were calculated as V̇E/V̇O2, and V̇E/V̇CO2 respectively.
Baseline recording
After a period of habituation in the plethysmograph chamber (10 –15 min), the chamber was opened, and rectal temperature was measured. The animal was then returned to the chamber and allowed an additional 10 min before the start of the experiment. The rectal temperature value was used to correct tidal volume using the standard equation (Drorbough and Fenn, 1955; Bartlett and Tenney, 1970) as previously described (Lefter et al., 2007, 2008; Niane et al., 2009). Different groups of animals were used to test the effects of mifepristone treatment on ventilatory responses to epibatidine or hypoxia stimuli.
Ventilatory response to cholinergic nicotine receptor agonist
Ventilatory responses to epibatidine was tested under normoxic conditions. Baseline recording was performed as described previously. The chamber was then opened, and the pup received a saline injection (1 μl/g body weight, i.p.) followed by the measurements of ventilatory variables for 30 min. The plethysmograph chamber was reopen, the rat then received an i.p. injection of epibatidine (i.p. 5 μg/kg; potent agonist of peripheral nicotinic acetylcholine receptors, nAChR), and recording was resumed for 30 min. This dose of epibatidine was chosen based on our previous studies (Niane et al., 2009).
Ventilatory response to hypoxia
After baseline recording for 10 –15 min under normoxia, the rat were exposed to 10% O2 for 30 min. At the end of the exposure, the chamber was opened, and the rectal temperature was immediately measured.
Carotid body morphology
Ten-day-old male rat pups were deeply anaesthetized, and the carotid bifurcation was removed, postfixed, and cryoprotected as previously described (Joseph et al., 2006). A standard immunohistochemistry protocol for tyrosine hydroxylase (TH) staining (TH is the rate-limiting enzyme for catecholamine synthesis and is used as a specific marker of chemosensitive cells) followed by Hematoxylin/Eosin counterstaining was performed on serial slices (10-μm thick) of the carotid bifurcation as previously described (Joseph et al., 2006). We then performed morphological analysis of the carotid body slices by measuring the area (μm2) of the carotid body in each slice and the area of the stained glomic tissue. Each specific area was summed to calculate the carotid body and glomic tissue volume (μm3).
Data analysis
All results are reported as mean±SEM. For carotid sinus nerve responses, second-by-second averages of in vitro values were obtained; 50 s of activity under baseline conditions and peak activity were averaged to calculate the mean value. For the ventilatory recordings in rats, 5 min of baseline ventilatory and metabolic variables were averaged. For responses to saline and epibatidine injections, all variables were averaged every 2 min. For responses to hypoxia, a minute-by-minute average was calculated for the first 10 min (early phase), and values between 25 and 30 min of hypoxia were then averaged (late phase).
All statistical analyses were performed using StatView software (v. 5.0). The effects of mifepristone treatment on baseline values were tested by ANOVA with treatment as the grouping variable. Respiratory or CSN responses to hypoxia or drugs were analyzed with ANOVA by using an analysis for repeated measures when necessary. P<0.05 was considered statistically significant.
RESULTS
Mifepristone reduces carotid sinus nerve responses to nicotine and hypoxia
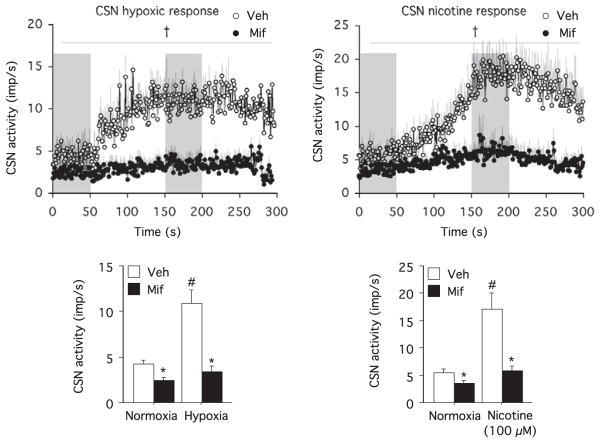
Representative recordings of carotid sinus nerve activity (impulses/seconds) under baseline conditions, in response to hypoxia, and in response to nicotine superfusion are shown in Fig. 1. As shown in the lower trace, the carotid sinus nerve responses to hypoxia and nicotine were almost abolished in the mifepristone-treated rats. Group results for hypoxic and nicotine responses are shown in Fig. 2. Carotid sinus nerve activity was reduced under normoxia in mifepristone vs. vehicle rats (normoxic carotid sinus nerve activity was 4.2±0.4 imp/s and 2.3±0.3 in vehicle-treated and mifepristone-treated rats, respectively: P=0.003), whereas mifepristone rats showed suppressed carotid sinus nerve responses to hypoxia and nicotine (P-value for group×treatment<0.0001).
Fig. 1.
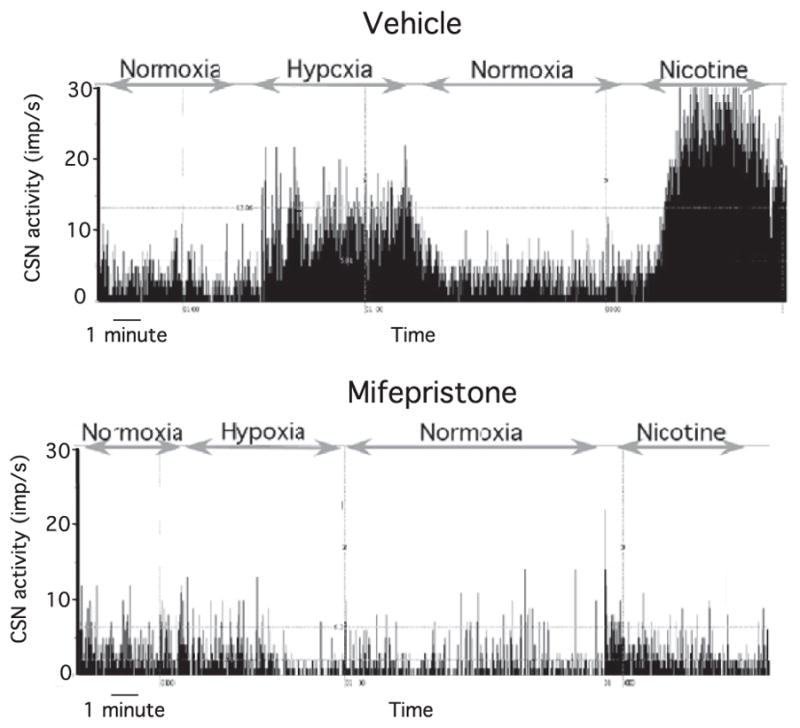
Representative recordings of carotid sinus nerve activity in vitro (impulses/second) under baseline conditions, in response to hypoxia, and during nicotine superfusion (100 μM) in a vehicle (upper trace) pup and a mifepristone (lower trace) pup.
Fig. 2.
Hypoxic and nicotinic carotid sinus nerve responses in vitro in vehicle (n=7 for hypoxia and n=6 for nicotine from three litters) and mifepristone (n=14 for hypoxia and n=11 for nicotine from three litters). Upper panels show the second-by-second hypoxic (left) and nicotinic responses (right), and the gray zones show the values used to construct the bar graph (lower panels). All values are mean±SEM. † P<0.05 for group×hypoxia or group×nicotine. * P<0.05 vs. Veh. # P<0.05 vs. baseline.
Mifepristone reduces ventilatory responses to epibatidine but not to hypoxia
Mifepristone-treated pups had a reduced body weight (23.0±0.9 vs. 27.3±0.7, P=0.0008) and higher rectal temperature (35.8±0.1 vs. 35.5±0.1, P=0.03) compared with vehicle.
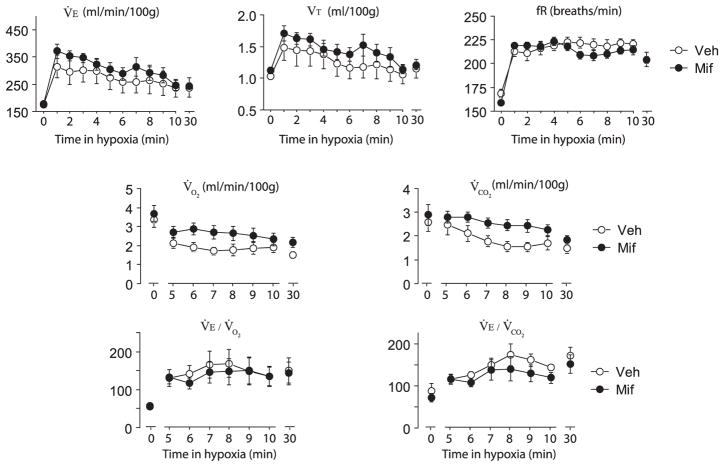
During hypoxia, V̇E, fR, and VT increased similarly in the vehicle and mifepristone pups (Fig. 3). The first 4 minutes of the metabolic rate measurements were discarded to allow equilibrium of gas composition between inflow and outflow lines. V̇O2 and V̇CO2 declined during the hypoxic exposure, with a tendency toward a treatment effect (P=0.06 for both variable), without treatment×group interaction. V̇E/V̇O2 and V̇E/V̇CO2 increased also similarly between groups. At the end of the hypoxic exposure, rectal temperature was higher in the mifepristone compared with the vehicle pups (mifepristone, 33.4±0.1 °C vs. vehicle, 33.0±0.2 °C, P=0.03).
Fig. 3.
Ventilatory and metabolic responses to hypoxia in vehicle (n=8, from four litters) and mifepristone (n=10, from two litters) pups. Minute ventilation (V̇E), tidal volume (VT in ml/100 g), respiratory frequency (fR in breaths/min), O2 uptake (V̇O2) CO2 production (V̇CO2) and O2 ans CO2 ventilatory equivalents (V̇E/V̇O2 and V̇E/V̇CO2) under normoxia or after 10 or 30 min of hypoxic exposure. Values are mean±SEM.
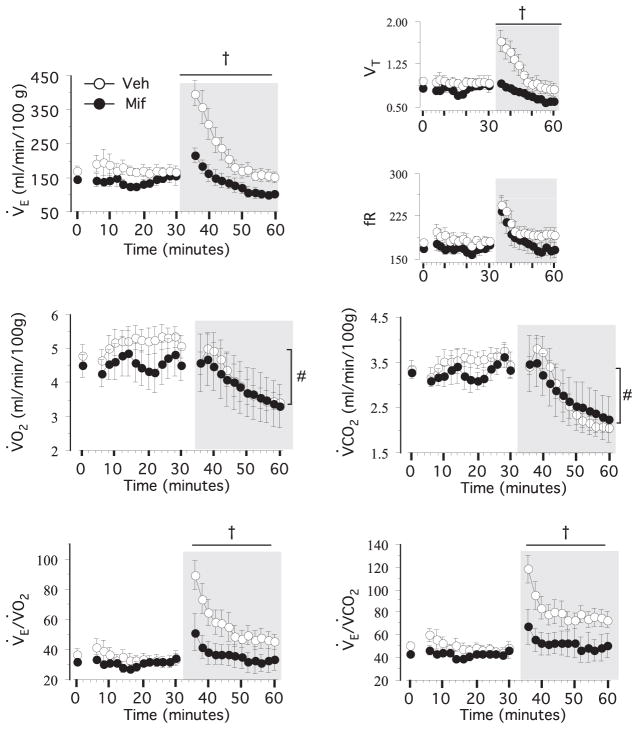
The respiratory responses to saline and epibatidine injections are presented in Fig. 4. The respiratory variables remained stable following saline injection and increased substantially for 10 –15 min following the epibatidine injection in vehicle rats. In mifepristone-treated rats, the tidal volume and minute ventilation responses were drastically reduced (P-value for group×treatment<0.0001). Because the chamber was opened before each injection, the first 4 minutes of the measurements were discarded to ensure that the animals were quiet and allow equilibrium in the gas composition in the outflow line to be achieved. The metabolic rate decreased slowly following the epibatidine injection in both the vehicle and mifepristone pups, and both V̇E/V̇O2 and V̇E/V̇CO2 were higher in the vehicle pups at 4 – 6 min following the injection (P for group×treatment=0.0003 and 0.02, respectively).
Fig. 4.
Ventilatory and metabolic response to epibatidine in vehicle (n=6 from three litters) and mifepristone (n=3 from one litter) pups. Minute-by-minute values at baseline (at t0), following saline injection up to 30 min, and following epibatidine injection (gray area) are shown. Minute ventilation (V̇E), tidal volume (VT in ml/100 g), fR in breaths/min, O2 uptake (V̇O2), CO2 production (V̇CO2), and O2 and CO2 ventilatory equivalents (V̇E/V̇O2 and V̇E/V̇CO2) are also shown. Values are mean±SEM. † P<0.05 for group or group×hypoxia. *P<0.05 vs. Vehicle at specific time points. # P<0.05 for epibatidine injection.
Mifepristone does not alter carotid body morphology
Mifepristone treatment did not alter carotid body size or the fraction of the glomic tissue in the carotid body (Fig. 5), indicating that this drug does not alter carotid body morphological growth.
Fig. 5.
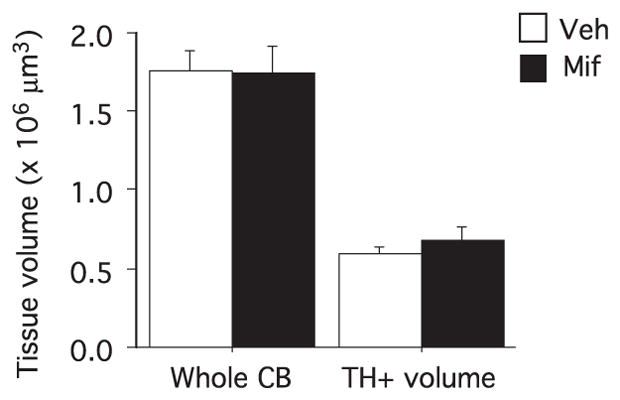
Carotid body morphology in Vehicle (n=7 from four litters) and mifepristone (n=9 from three litters) pups. Tissue volume (×106 μm3) shows the area of the carotid body and the area of the tyrosine hydroxylase-stained cells (TH+). Values are mean±SEM. No difference between groups.
DISCUSSION
This study shows that in newborn rats antagonism of the progesterone receptor drastically reduces carotid body responses to hypoxia and nicotine, this however does not affect the ventilatory response evoked by severe hypoxia (10% O2), but drastically reduces the ventilatory response to epibatidine. These findings are consistent with the wealth of data showing that progesterone is an important respiratory stimulant in adults (Bayliss et al., 1987; Hannhart et al., 1990; Joseph et al., 2002), and the data showing that in newborn rats, progesterone affects the hypoxic ventilatory response without affecting baseline ventilation (Lefter et al., 2007, 2008). Because progesterone promotes the expression of neural growth factors (Gibbs, 1999; Kaur et al., 2007) that are involved in the postnatal development of peripheral chemoreceptors (Brady et al., 1999; Wang and Bisgard, 2005), we also tested the hypothesis that mifepristone treatment impairs carotid body growth. However, carotid body and glomic tissue size were not affected in mifepristone-treated pups, and these findings do not support that hypothesis.
Mifepristone had also reduced body weight in rat pups. In adult dogs, progesterone induces growth hormone secretion, but this effect is mediated by the mammary glands rather than pituitary gland (Kooistra and Okkens, 2002), which discard this option in newborn male rats. However, it appears that mechanisms related to postnatal body growth are under the influence of either the progesterone or corticosteroid receptor and warrants further investigation to be better understood.
Methodological considerations
Mifepristone is a synthetic steroid compound that binds with very high affinity to the rat progesterone and gluco-corticoid receptors (Healy et al., 1983a,b; Jung-Testas and Baulieu, 1983; Schreiber et al., 1983; Moguilewsky and Philibert, 1984) forming an inactive complex. This is one of the few available antagonists for these receptors. Mifepristone also binds weakly to the androgen receptor (9% of testosterone binding activity), and it might also have weak anti-androgenic activity (Schreiber et al., 1983). Obviously these pharmacological properties should be kept in mind when further discussing these results. At the dose used (40 μg/g/d), however, mifepristone prevents the facilitation of sexual behavior induced by relevant stimuli in adult female rats (Auger et al., 1997), thus clearly indicating an anti-progesterone activity, and impairs sexual behavior in adult male rats treated between postnatal days 1 and 10 (Lonstein et al., 2001), indicating that it is also effective in newborn rats.
Respiratory and metabolic recordings in rats around 10 –12 days of age are performed at an ambient temperature of 30 °C based on data showing that in 8-day-old rats, this is the lower critical temperature below which metabolic rate increases (Mortola and Naso, 1998). Contrastingly, other evidence indicates that in 8-day-old rats, 30 °C is below the thermo-neutral zone (Blumberg and Sokoloff, 1998). These discrepancies might be due to subtle, uncontrolled conditions between laboratory settings and should be kept in mind for further interpretations of our results. In particular, the fact that mifepristone slightly increases rectal temperature, might either be due to a general thermogenic effect, higher thermogenic response specific to cold exposure (below the lower critical ambient temperature), or both.
Sources of steroid hormones in newborn rats
In pups, progesterone can be supplied either by maternal milk (Rodriguez-Palmero et al., 1999) or through local synthesis in neural networks (Compagnone et al., 1995; Zwain and Yen, 1999). Previous studies have reported immunochemical staining of P450scc, which is involved in progesterone synthesis, in the sensory nervous system in fetal mice (Compagnone et al., 1995), whereas we have reported dense P450scc staining in the carotid bodies of fetal, newborn, and adult male rats (Joseph et al., 2006). Taken together, these findings support the hypothesis of a local progesterone synthesis in the carotid body that would be able to bind and activate progesterone receptors in an autocrine loop. Interestingly, a similar autocrine function of progesterone has been reported in the hypothalamus in which local progesterone synthesis (induced by estradiol) is necessary to induce the surge of luteinizing hormone associated with proestrus (Micevych et al., 2003; Micevych and Sinchak, 2008). In addition to the presence of progesterone, progesterone-independent activation of the progesterone receptor through phosphorylation has also been described (Mani et al., 2009). In particular, dopamine might activate the progesterone receptor in the CNS by intracellular phosphorylation pathways (Mani et al., 2009), and because dopamine is a predominant neuromodulator in the carotid body (Gonzalez et al., 1994), it might also contribute to the activation of the progesterone receptor at this level.
Mifepristone reduces carotid body and ventilatory responses to a nicotinic cholinergic receptor agonist
Acetylcholine is considered an excitatory transmitter between carotid body type I cells and sensitive endings of the carotid sinus nerve (Shirahata et al., 2007). The current model proposes that acetylcholine released from glomic cells during hypoxic exposure binds to postsynaptic cholinergic receptors to initiate action potentials in the carotid sinus nerve. In addition, acetylcholine binds to presynaptic nAChR that contain the α4 subunit to further increase neurotransmitter release (Conde and Monteiro, 2006). Therefore, the dramatic mifepristone-mediated reduction in the responses to nAChR agonists, both in vivo and in vitro is presumably linked to decreased expression of nicotinic acetylcholine receptors. Furthermore, the expression of nicotinic cholinergic receptors in carotid bodies is developmentally regulated in cats (Bairam et al., 2007), and in rats, the respiratory response to epibatidine increases during postnatal development (Niane et al., 2009). Interestingly, progesterone enhances the mRNA expression of the nicotine acetylcholine receptor α5 subunit in mice (Gangitano et al., 2009). To our knowledge, additional data on the regulation of nAChR expression by progesterone are not available in the literature. These results suggest that the reduced response of the carotid body to hypoxia in rats following mifepristone treatment was mainly due to a decreased response to acetylcholine. Whether this effect implies pre- or postsynaptic changes in nAChR expression and/or function remains to be determined, and we could not exclude alterations of other neurotransmitters such as dopamine, whose inhibitory function on ventilation control is inhibited by progesterone in adult and newborn rats (Joseph et al., 2002; Lefter et al., 2007).
Contrasting effects of mifepristone on the carotid body and ventilatory responses to hypoxia
In response to reduced arterial O2 levels, carotid body type I cells are depolarized and release neurotransmitters that act upon the apposed sensitive nerve terminals to generate postsynaptic action potentials. The chemosensory inputs to the carotid sinus nerve are mainly sent to the nucleus tractus solitarius in the brainstem (Finley and Katz, 1992), where they are integrated to produce the final respiratory output. Most of these processes are capable of responding to stimuli deprivation with significant plasticity (Forster, 2003), contributing to the restoration of functional phenotypes. In particular, one intriguing aspect of this study is the contrast between the in vitro and in vivo effects that were observed in rats for the hypoxic response; following mifepristone treatment, the carotid body response to hypoxia was drastically reduced, but the ventilatory response to severe hypoxia (10% O2) was similar.
Differential effects of the hypoxic responses in vitro vs. in vivo have been previously reported, which illustrates the striking plasticity of the O2-sensing systems and the neurological integration that govern the adequate physiological response to hypoxia. For example, in dopamine receptor D2 knock-out mice, the carotid sinus nerve response to hypoxia is approximately 50% lower than in wild-type mice, but the respiratory frequency response to hypoxia is normal (Prieto-Lloret et al., 2007). In adult rats subjected to bilateral chemodenervation, the hypoxic ventilatory response gradually returns to normal levels (Roux et al., 2000b), but with a different respiratory pattern. Hence, upon chronic reduction or deprivation of carotid body chemosensory afferents, compensatory mechanisms are able to partially restore the ventilatory responses under hypoxia (10% O2), presumably through changes in the CNS, as reported in chemodenervated adult rats (Roux et al., 2000a,c). Our results suggest that similar mechanisms might have developed following mifepristone treatment in newborn rats. Because mifepristone is a mixed antagonist of the progesterone and glucocorticoid receptors, these results might also indicate a yet undefined role of gluco-corticoids on respiratory control development.
CONCLUSIONS
We conclude that signaling through the progesterone receptor is required for proper carotid body function and respiratory chemoreflex in newborn rats. Disorders linked to respiratory control immaturity such as apnea in preterm neonates, or dysfunction in sudden infant death syndrome, remain a major burden (Finer et al., 2006; Halloran and Alexander, 2006). Breastfeeding notably reduces the risk of sudden infant death syndrome (Ford et al., 1993; McVea et al., 2000; Vennemann et al., 2009) and sleep-disordered breathing in 4 –10-year-old infants (Montgomery-Downs et al., 2007). Our study suggests that one of the potential links between these outcomes might be perinatal exposure to progesterone contributing to enhance respiratory chemoreflex and protective responses to hypoxia.
Acknowledgments
The authors acknowledge Jorge Soliz for a critical review of the paper and the technical assistance of Van Diep Doan, Sylvie Viger, and Mélanie Pelletier. V J. is a research scholar from FRSQ. L Niane has a fellowship from RSR FRSQ and Fondation des Etoiles. This study was funded by a CIHR grant to V J. (MOP 102715).
Abbreviations
- CNS
central nervous system
- nAChR
nicotinic acetylcholine receptors
References
- Auger AP, Moffatt CA, Blaustein JD. Progesterone-independent activation of rat brain progestin receptors by reproductive stimuli. Endocrinology. 1997;138:511–514. doi: 10.1210/endo.138.1.4986. [DOI] [PubMed] [Google Scholar]
- Bairam A, Joseph V, Lajeunesse Y, Kinkead R. Developmental profile of cholinergic and purinergic traits and receptors in peripheral chemoreflex pathway in cats. Neuroscience. 2007;146:1841–1853. doi: 10.1016/j.neuroscience.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Bartlett D, Tenney SM. Control of breathing in experimental anemia. Respir Physiol. 1970;10:384–395. doi: 10.1016/0034-5687(70)90056-3. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Millhorn DE, Gallman EA, Cidlowski JA. Progesterone stimulates ventilation through a central nervous system steroid receptor-mediated mechanism in the cat. Proc Natl Acad Aci U S A. 1987;84:7788–7792. doi: 10.1073/pnas.84.21.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Sokoloff G. Thermoregulatory competence and behavioral expression in the young of altricial species—revisited. Dev Psychobiol. 1998;33:107–123. doi: 10.1002/(sici)1098-2302(199809)33:2<107::aid-dev2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Brady R, Zaidi SI, Mayer C, Katz DM. BDNF is a target-derived survival factor for arterial baroreceptor and chemoafferent primary sensory neurons. J Neurosci. 1999;19:2131–2142. doi: 10.1523/JNEUROSCI.19-06-02131.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Bulfone A, Rubenstein JL, Mellon SH. Expression of the steroidogenic enzyme P450scc in the central and peripheral nervous systems during rodent embryogenesis. Endocrinology. 1995;136:2689–2696. doi: 10.1210/endo.136.6.7750493. [DOI] [PubMed] [Google Scholar]
- Conde SV, Monteiro EC. Activation of nicotinic ACh receptors with alpha4 subunits induces adenosine release at the rat carotid body. Br J Pharmacol. 2006;147:783–789. doi: 10.1038/sj.bjp.0706676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbough JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81– 87. [PubMed] [Google Scholar]
- Finer NN, Higgins R, Kattwinkel J, Martin RJ. Summary proceedings from the apnea-of-prematurity group. Pediatrics. 2006;117:S47–S51. doi: 10.1542/peds.2005-0620H. [DOI] [PubMed] [Google Scholar]
- Finley J, Katz D. The central organization of carotid-body afferent projections to the brainstem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Ford RP, Taylor BJ, Mitchell EA, Enright SA, Stewart AW, Becroft DM, Scragg R, Hassall IB, Barry DM, Allen EM, et al. Breast-feeding and the risk of sudden infant death syndrome. Int J Epidemiol. 1993;22:885– 890. doi: 10.1093/ije/22.5.885. [DOI] [PubMed] [Google Scholar]
- Forster HV. Plasticity in the control of breathing following sensory denervation. J Appl Physiol. 2003;94:784–794. doi: 10.1152/japplphysiol.00602.2002. [DOI] [PubMed] [Google Scholar]
- Gangitano D, Salas R, Teng Y, Perez E, De Biasi M. Progesterone modulation of alpha5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav. 2009;8:398– 406. doi: 10.1111/j.1601-183X.2009.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemo-receptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829– 898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Halloran DR, Alexander GR. Preterm delivery and age of SIDS death. Ann Epidemiol. 2006;16:600– 606. doi: 10.1016/j.annepidem.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Hannhart B, Pickett CK, Moore LG. Effects of estrogen and progesterone on carotid body neural output responsiveness to hypoxia. J Appl Physiol. 1990;68:1909–1916. doi: 10.1152/jappl.1990.68.5.1909. [DOI] [PubMed] [Google Scholar]
- Healy DL, Baulieu EE, Hodgen GD. Induction of menstruation by an antiprogesterone steroid (RU 486) in primates: site of action, dose-response relationships, and hormonal effects. Fertil Steril. 1983a;40:253–257. doi: 10.1016/s0015-0282(16)47246-8. [DOI] [PubMed] [Google Scholar]
- Healy DL, Chrousos GP, Schulte HM, Williams RF, Gold PW, Baulieu EE, Hodgen GD. Pituitary and adrenal responses to the anti-progesterone and anti-glucocorticoid steroid RU 486 in primates. J Clin Endocrinol Metab. 1983b;57:863– 865. doi: 10.1210/jcem-57-4-863. [DOI] [PubMed] [Google Scholar]
- Joseph V, Doan VD, Morency CE, Lajeunesse Y, Bairam A. Expression of sex-steroid receptors and steroidogenic enzymes in the carotid body of adult and newborn male rats. Brain Res. 2006;1073–1074:71–82. doi: 10.1016/j.brainres.2005.12.075. [DOI] [PubMed] [Google Scholar]
- Joseph V, Soliz J, Soria R, Pequignot J, Favier R, Spielvogel H, Pequignot JM. Dopaminergic metabolism in carotid bodies and high altitude acclimatization in female rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R765–R773. doi: 10.1152/ajpregu.00398.2001. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Baulieu EE. Inhibition of glucocorticosteroid action in cultured L-929 mouse fibroblasts by RU 486, a new anti-glucocorticosteroid of high affinity for the glucocorticosteroid receptor. Exp Cell Res. 1983;147:177–182. doi: 10.1016/0014-4827(83)90282-3. [DOI] [PubMed] [Google Scholar]
- Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85:2441–2449. doi: 10.1002/jnr.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra HS, Okkens AC. Secretion of growth hormone and prolactin during progression of the luteal phase in healthy dogs: a review. Mol Cell Endocrinol. 2002;197:167–172. doi: 10.1016/s0303-7207(02)00271-x. [DOI] [PubMed] [Google Scholar]
- Lefter R, Doan VD, Joseph V. Contrasting effects of estradiol and progesterone on respiratory pattern and hypoxic ventilatory response in newborn male rats. Respir Physiol Neurobiol. 2008;164:312–318. doi: 10.1016/j.resp.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Lefter R, Morency CE, Joseph V. Progesterone increases hypoxic ventilatory response and reduces apneas in newborn rats. Respir Physiol Neurobiol. 2007;156:9–16. doi: 10.1016/j.resp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Quadros PS, Wagner CK. Effects of neonatal RU486 on adult sexual, parental, and fearful behaviors in rats. Behav Neurosci. 2001;115:58–70. doi: 10.1037/0735-7044.115.1.58. [DOI] [PubMed] [Google Scholar]
- Mani SK, Portillo W, Reyna A. Steroid hormone action in the brain: cross-talk between signalling pathways. J Neuroendocrinol. 2009;21:243–247. doi: 10.1111/j.1365-2826.2009.01844.x. [DOI] [PubMed] [Google Scholar]
- McVea KL, Turner PD, Peppler DK. The role of breastfeeding in sudden infant death syndrome. J Hum Lact. 2000;16:13–20. doi: 10.1177/089033440001600104. [DOI] [PubMed] [Google Scholar]
- Micevych P, Sinchak K. Synthesis and function of hypothalamic neuroprogesterone in reproduction. Endocrinology. 2008;149:2739–2742. doi: 10.1210/en.2008-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78:29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- Micevych P, Soma KK, Sinchak K. Neuroprogesterone: key to estrogen positive feedback? Brain Res Rev. 2008;57:470– 480. doi: 10.1016/j.brainresrev.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moguilewsky M, Philibert D. RU 38486: potent antiglucocorticoid activity correlated with strong binding to the cytosolic glucocorticoid receptor followed by an impaired activation. J Steroid Biochem. 1984;20:271–276. doi: 10.1016/0022-4731(84)90216-4. [DOI] [PubMed] [Google Scholar]
- Montgomery-Downs HE, Crabtree VM, Sans Capdevila O, Gozal D. Infant-feeding methods and childhood sleep-disordered breathing. Pediatrics. 2007;120:1030–1035. doi: 10.1542/peds.2007-0722. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Naso L. Thermogenesis in newborn rats after prenatal or postnatal hypoxia. J Appl Physiol. 1998;85:84–90. doi: 10.1152/jappl.1998.85.1.84. [DOI] [PubMed] [Google Scholar]
- Niane L, Joseph V, Bairam A. Role of cholinergic-nicotinic receptors on hypoxic chemoreflex during postnatal development in rats. Respir Physiol Neurobiol. 2009;169:323–322. doi: 10.1016/j.resp.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J Appl Physiol. 2004;97:2020–2025. doi: 10.1152/japplphysiol.00876.2003. [DOI] [PubMed] [Google Scholar]
- Prieto-Lloret J, Donnelly DF, Rico AJ, Moratalla R, González C, Rigual RJ. Hypoxia transduction by carotid body chemoreceptors in mice lacking dopamine D(2) receptors. J Appl Physiol. 2007;103:1269–1275. doi: 10.1152/japplphysiol.00391.2007. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Palmero M, Koletzko B, Kunz C, Jensen R. Nutritional and biochemical properties of human milk: II. Lipids, micronutrients, and bioactive factors. Clin Perinatol. 1999;26:335–359. [PubMed] [Google Scholar]
- Roux JC, Pequignot JM, Dumas S, Pascual O, Ghilini G, Pequignot J, Mallet J, Denavit-Saubie M. O2-sensing after carotid chemodenervation: hypoxic ventilatory responsiveness and upregulation of tyrosine hydroxylase mRNA in brainstem catecholaminergic cells. Eur J Neurosci. 2000a;12:3181–3190. doi: 10.1046/j.1460-9568.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- Roux JC, Peyronnet J, Pascual O, Dalmaz Y, Pequignot JM. Ventilatory and central neurochemical reorganisation of O2 chemoreflex after carotid sinus nerve transection in rat. J Physiol. 2000b;522 (Pt 3):493–501. doi: 10.1111/j.1469-7793.2000.t01-4-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux JC, Peyronnet J, Pascual O, Dalmaz Y, Pequignot JM. Ventilatory and central neurochemical reorganisation of O2 chemoreflex after carotid sinus nerve transection in rat. J Physiol (Lond) 2000c;522 (Pt 3):493–501. doi: 10.1111/j.1469-7793.2000.t01-4-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber JR, Hsueh AJ, Baulieu EE. Binding of the anti-progestin RU-486 to rat ovary steroid receptors. Contraception. 1983;28:77–85. doi: 10.1016/s0010-7824(83)80008-0. [DOI] [PubMed] [Google Scholar]
- Shahar E, Redline S, Young T, Boland LL, Baldwin CM, Nieto FJ, O’Connor GT, Rapoport DM, Robbins JA. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:1186–1192. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- Shirahata M, Balbir A, Otsubo T, Fitzgerald RS. Role of acetylcholine in neurotransmission of the carotid body. Respir Physiol Neurobiol. 2007;157:93–105. doi: 10.1016/j.resp.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Vennemann MM, Bajanowski T, Brinkmann B, Jorch G, Yücesan K, Sauerland C, Mitchell EA. Does breastfeeding reduce the risk of sudden infant death syndrome? Pediatrics. 2009;123:e406– e410. doi: 10.1542/peds.2008-2145. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Bisgard GE. Postnatal growth of the carotid body. Respir Physiol Neurobiol. 2005;149:181–190. doi: 10.1016/j.resp.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]