Abstract
Progesterone and corticosterone are key modulators of the respiratory control system. While progesterone is widely recognized as an important respiratory stimulant in adult and newborn animals, much remains to be described regarding the underlying mechanisms. We review the potential implication of nuclear and membrane progesterone receptors in adults and in newborns. This raises intriguing questions regarding the contribution of progesterone as a protective factor against some respiratory control disorders during early life. We then discuss our current understanding of the central integration of stressful stimuli and the responses they elicit. The fact that this system interacts with the respiratory control system, either because both share some common neural pathways in the brainstem and hypothalamus, or because corticosterone directly modulates the function of the respiratory control network, is a fascinating field of research that has emerged over the past few years. Finally, we review the short- and long-term consequences of disruption of stress circuitry during postnatal development on these systems.
Keywords: Sex, Hormones, Stress, Progesterone, Corticosterone
1. Introduction
Regulation of sexual function and behavior, as well as physiological stress responses mainly rely on integrated neuroendocrine systems that share some basic properties. Amongst these systems there is a key implication that steroid hormones are the chemical messengers carried by the blood from a site of synthesis, and released to the target tissues which are either peripheral organs or elements of the central and peripheral nervous system. A variety of effectors are necessary for the expression of the related processes allowing an efficient response. For example, corticosteroid release from the adrenal cortex following exposure to stress activates glycolysis from the liver and glucose uptake by muscles to sustain the high energy demand associated with the “fight or flight” response. At the same time, these hormones act on hippocampal neurons contributing to the cognitive processes that create and strengthen memory information related to the stressful events and the appropriate behavioral responses (Gore and Roberts, 2003; McEwen, 2008; Joels and Baram, 2009). Similarly, ovarian and placental steroid hormones promote growth of uterine tissue throughout gestation, suppress uterine contractions, and modulate catecholamine synthesis in uterine arteries to maintain a high blood flow. These hormones also act on the central nervous system to ensure timely expression of maternal behavior related to care and feeding of the offspring. The objective of this review is to discuss how these neuroendocrine systems act to modulate the peripheral and central structures that regulate breathing.
1.1. Historical perspective of the impact of hormones on breathing
In the early 20th century it was reported that adult women had lower alveolar PCO2 than men (Fitzgerald and Haldane, 1905). Interestingly, this difference was also apparent in young girls vs. boys (8–15 years). To visualize the full extent of this difference we took the raw data and group categories published by Fitzgerald and Haldane (1905) to construct a graph (Fig. 1) and run a statistical analysis (ANOVA). Corresponding to Tables I–IV in the original paper, adults are subjects above 17 years, whereas boys and girls are those ranging from 7.5 to 15.5 years. Using these criteria, we found that there are sex (p = 0.0003) and age (p = 0.015) effects on PACO2, but no sex × age interaction (p = 0.4).
Fig. 1.
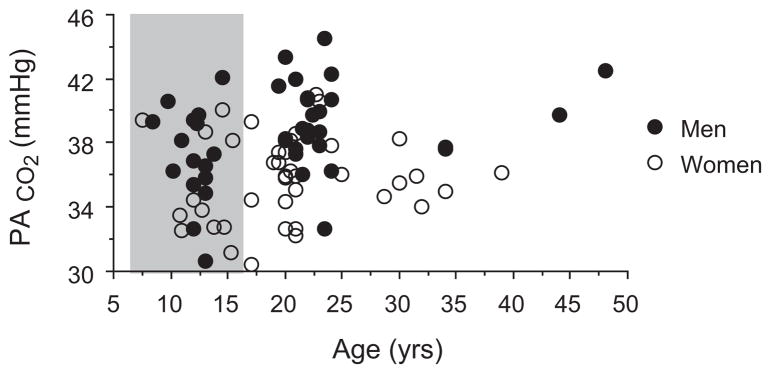
Illustration of sex-specific effect of ventilatory control in adult (white area) and pre-pubertal boys and girls (grey area). Alveolar PCO2 plotted as a function of age.
Data taken from Fitzgerald and Haldane (1905).
Following a study published a few years later by Hasselbach showing the hyperventilation associated with pregnancy in women (Hasselbalch and Gammeltoft, 1915), many subsequent studies focused on the function of ovarian hormones on respiratory control in adult subjects, providing remarkable advances in our understanding of the contribution of these hormones to the respiratory control system. In 1935, the factor necessary for the morphological transformation of the uterus to prepare for, and maintain gestation was isolated, crystallized, and named “progesterone” (Allen, 1935). It was later confirmed that fluctuating levels of plasma progesterone correlated with cyclical ventilatory stimulation (see Dempsey et al., 1986 for review). The respiratory stimulant effect of progesterone during gestation likely ensures adequate outflow of CO2 from the fetus to the maternal circulation, avoiding a potentially harmful fetal acidosis. Several reviews of the respiratory effects of progesterone have been published (Dempsey et al., 1986; Saaresranta and Polo, 2002; Soliz and Joseph, 2005; Behan and Wenninger, 2008) giving a substantial overview of the implication of progesterone on respiratory control and some underlying mechanisms. This part of the current review will mainly discuss the receptor subtypes that are involved in these effects and the relevance of progesterone in the newborn. While most of this section relates to progesterone, the reader should keep in mind that other sex hormones (estradiol and testosterone) are also involved in respiratory control (White et al., 1985; Lefter et al., 2008), and where relevant, some of their effects will be mentioned.
2. Impact of progesterone on respiratory regulation
2.1. In adults
In adults, exogenous administration of progesterone reduces CO2 retention in patients suffering from chronic obstructive pulmonary disease (COPD), decreases the occurrence of periodic breathing and episodic apneas in subjects sleeping at high altitude, and, in patients with chronic mountain sickness, improves arterial oxygen saturation and decreases the frequency of episodes of severe desaturation during sleep (see Dempsey et al., 1986 for review). The prevalence of sleep apnea is higher in adult men compared to women (Block et al., 1979), and because the risk factor for sleep apnea increases after menopause (Young et al., 2003), it was proposed that hormone replacement therapy in women could reduce apnea frequency. This hypothesis was firmly supported in a study that analyzed the frequency and severity of apneic events during sleep in a total of 2854 women aged 50 years and older, of whom 525 were taking estrogen alone and 382 were taking estrogen and progesterone. Overall, the odds ratio for an apnea index > 15 episodes/h was 0.55, and the odds ratio of arterial oxygen saturation < 90% for 10% or more of the recording time was 0.75 in women taking estradiol and 0.41 in women taking estradiol + progesterone as compared with women not taking any hormones (Shahar et al., 2003). The authors concluded that these data indicate that hormone replacement therapy could have a significant role in the prevention or treatment of sleep-disordered breathing in postmenopausal women. Despite this clear demonstration, the underlying mechanisms remain poorly understood.
2.2. In newborn
High levels of progesterone during pregnancy are also encountered in the fetal circulation. This is an important concern in preterm neonates because the circulating levels of progesterone and other placental hormones are much lower than in in utero fetuses (Trotter and Pohlandt, 2000). In parallel, preterm neonates have an extremely elevated frequency of apnea and unstable breathing patterns (Finer et al., 2006) due to the immaturity of the respiratory control system. In this population, tactile and mechanical respiratory support can be used to reduce apnea frequency, and when these approaches fail, the most common practice is to use methylxanthines (caffeine or theophylline) as respiratory stimulant drugs. While methylxanthines are thus far considered safe drugs, they are not always effective to relieve apneas, and it has been suggested that progesterone (or progestins in general) might be used as alternative or supplemental drugs (Finer et al., 2006). While this suggestion is based on a wealth of relevant data in adults, much less is known about the effects of progesterone as a respiratory stimulant in the newborn. By using 10 day old rats, fed by mothers implanted 1 day after delivery with osmotic mini-pumps delivering progesterone, estradiol, or estradiol + progesterone, we reported that progesterone increases the hypoxic ventilatory response and reduces apnea frequency in newborn rats (Lefter et al., 2007). Surprisingly, the frequency of sighs, a specific breathing pattern characterized by high inspiratory volume and a very rapid expiration, was increased by estradiol (by about 75%), while estradiol + progesterone increased sigh-frequency three-fold (Lefter et al., 2008). In control animals about 30% of these sighs were followed by an apnea, but remarkably, this effect was balanced by the progesterone treatment such that apnea frequency did not increase in proportion to sigh frequency (Lefter et al., 2008).
One particularly intriguing question in newborns is whether, without pharmacological intervention, progesterone that is present in breast milk at concentration equal to or lower than in maternal plasma (Grosvenor et al., 1993), or progesterone that is directly synthesized by neurons and glial cells (Zwain and Yen, 1999), could have a role in respiratory control during development. From a clinical perspective, it is striking to observe that preterm birth – which deprives the newborn of maternal progesterone secretion – is an important risk factor for Sudden Infant Death Syndrome (SIDS), while breastfeeding notably reduces the risk of SIDS (Vennemann et al., 2009). Along the same line of evidence, whereas preterm birth is a risk factor for development of sleep apnea during infancy (Hibbs et al., 2008), breastfeeding also decreases sleep-disordered breathing in 4–10-year-old infants (Montgomery-Downs et al., 2007). Hence, it is tempting to postulate that one of the potential links between these outcomes might be perinatal exposure to progesterone contributing to an enhanced respiratory chemoreflex, protective responses to hypoxia, and long-term modulation of the respiratory control system.
To address this hypothesis of an endogenous stimulatory effect of progesterone on the hypoxic chemoreflex in newborn rats, we recently performed a study with mifepristone, a progesterone receptor antagonist (Joseph et al., 2012). Unfortunately mifepristone is not specific, being a progesterone and a glucocorticoid receptor antagonist with weak anti-androgenic activity. With these limitations in mind, we used mifepristone to ask whether it would affect the function of peripheral chemoreceptors in newborn rats. After daily gavage between postnatal days 3–15, we used the ex vivo carotid body/carotid sinus nerve preparation and whole body plethysmography to determine whether this treatment would alter hypoxic sensitivity. Carotid body responses to both hypoxia and a cholinergic nicotinic agonist as measured in the ex vivo carotid body preparation were suppressed in rats treated with mifepristone, and the ventilatory response to the nicotinic agonist was also reduced in vivo (but not the hypoxic ventilatory response) (Joseph et al., 2012). While apparently conflicting, these results are in line with previous studies showing that chronic alteration of peripheral chemoreceptor activity may in some cases fail to result in a corresponding alteration of the hypoxic ventilatory response (see Discussion in Joseph et al., 2012; Bavis et al., 2011). These results are encouraging, and while not definitive, they clearly support the hypothesis that extra-ovarian sources of progesterone in newborn rats are important for the function of peripheral chemoreceptors. The fact that we previously localized the p450 side-chain-cleavage enzyme in the carotid body of fetal, newborn and adult rats (Joseph et al., 2006) strongly supports the hypothesis that the carotid bodies may be able to synthesize progesterone, which could then act as an autocrine factor supporting peripheral chemoreceptor function.
3. Progesterone receptors
3.1. Genomic (or nuclear) progesterone receptors
The most-widely known progesterone receptors are nuclear receptors belonging to the super-family of steroid receptors, which are transcription factors able to modulate the expression of target genes with a specific response element in their promoter (Brinton et al., 2008). Two major isoforms of the progesterone receptor (PR-A and PR-B) are synthesized by alternative mRNA splicing. Several other isoforms have also been identified (Brinton et al., 2008). These receptors are localized in both central and peripheral areas that are important for respiratory control. In the peripheral chemoreceptors of adults, newborn and fetal rats, we reported a dense immunostaining for progesterone receptors, apparently localized in clusters of chemosensitive cells (Joseph et al., 2006). Furthermore, by using Western-blot analysis of the carotid bodies and the superior cervical ganglion of adult rats, we showed that the PR-A isoform (at 80 kDa) was predominant, while the PR-C isoform was also present (at 60 kDa). PR mRNA was also present in the carotid bodies of adult male rats (Joseph et al., 2006). In the central nervous system, PR receptors are localized in brainstem areas involved in respiratory control such as the nucleus tractus solitarius (Haywood et al., 1999), locus coeruleus (Helena et al., 2009), and in hypothalamic nuclei including the preoptic area, the paraventricular, ventromedial, dorsomedial nuclei and the arcuate nucleus (Romeo et al., 2005; Brinton et al., 2008).
Experimental studies have implicated progesterone receptors in respiratory control. In rats, injection of progesterone (1 mg/kg i.p.) reduces the frequency of apneas recorded during sleep (identified by behavioral criteria) by 50% at 14 weeks of age, and by almost 80% at 26 weeks of age, whereas no effects were seen in younger animals (at 4 weeks). This effect was abolished when mifepristone (5 mg/kg, a progesterone receptor antagonist) was injected 1.5 h before progesterone injection (Yamazaki et al., 2005). In anesthetized cats, the stimulatory effect of progesterone administration (i.v.) on phrenic nerve activity is also suppressed by pre-treatment with mifepristone (Bayliss et al., 1987). Because the same authors reported that stimulation of phrenic nerve activity by progesterone is mediated by the hypothalamus, and also elicited by direct application of progesterone to the NTS (Bayliss et al., 1987, 1990), a functional role for these progesterone receptors in the central nervous system seems evident.
To further explore the role of receptors in respiratory control, we used mice in which the progesterone receptor was deleted (PRKO) (Lydon et al., 1995) to record (with whole body plethysmography) resting minute ventilation and metabolic rate under normoxic conditions, and in response to hypoxia (10% O2). These recordings were performed in adult and 10-day old mice of both sexes. To take into account the differences in body weight observed between P10 and adult mice, we plotted the log of each variable (V̇E, VT, V̇O2, V̇CO2) against the log of body weight for all the animals used (not shown), and used the slope of the correlation obtained as the allometric scaling factor. Accordingly, normalized minute ventilation is calculated as V̇E/bw0.855 (where V̇E is the absolute value of minute ventilation in ml/min, bw is body weight in g, and 0.855 is the slope of the linear regression between log(bw) and log(V̇E)). The allometric scaling factors for the other variables are reported in Fig. 2. In 10-day old mice there were no sex or genotype-specific effects under normoxia (not shown). In adults, for minute ventilation there was a prominent sex effect (p < 0.0001) and a genotype effect (p < 0.03), however the power for the genotype effect is low (0.6) and thus should be interpreted cautiously (Fig. 2). Other reported variables in normoxia showed specific sex effects, but no genotype (although for both VT and V̇CO2 there was a trend with p values at 0.06 and 0.07 respectively). In response to hypoxia, there were specific effects of sex (p = 0.002), genotype (p = 0.02), and a sex × hypoxia interaction (0.01), with females showing higher ventilation in hypoxia than males, and PRKO mice showing lower ventilation in hypoxia than wild-type mice (Fig. 2). We conclude from these results that PRKO adult mice have lower minute ventilation, with a tendency towards a reduced metabolic rate, but that PR expression does not explain the sex-specific differences observed. However, more extensive studies in 10–12 day-old PRKO mice have been conducted showing that PR expression is required to sustain high minute ventilation, and avoid an exaggerated ventilatory roll-off during exposure to moderate levels of hypoxia (14 and 12% O2), likely indicating an effect at the level of the central nervous system. We have yet to verify if a similar effect occur in adults. Nonetheless, because progesterone has been extensively reported as a respiratory stimulant, and because progesterone receptors have been identified as one of the mediators of this effect, it is surprising that gender specific effects of resting ventilation were not affected in adult PRKO mice. Accordingly, some sex-specific effects on breathing might be mediated by other classes of progesterone receptors, such as membrane receptors.
Fig. 2.
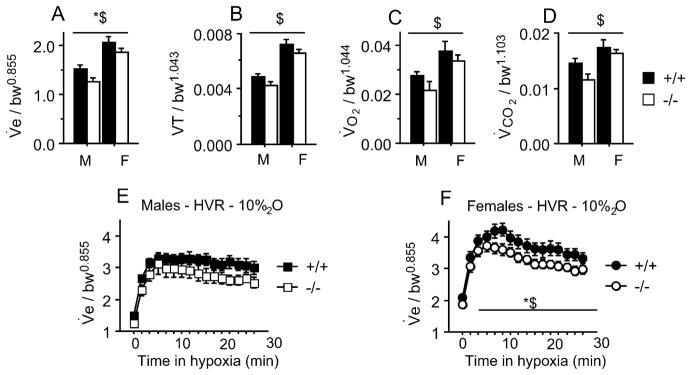
(A–D) Normoxic respiratory and metabolic parameters recorded in adult mice – male (M) and female (F), wild type (+/+) or KO (−/−) for the progesterone receptors. (A) Minute ventilation (ml/min/g0.855), (B) tidal volume (ml/g1.043), (C) O2 consumption rate (ml/min/g1.044 ), (D) CO2 production rate (ml/min/g1.103 ). (E and F) Hypoxic response for minute ventilation (10% O2 – 30 min) in males (E) and females (F). *p < 0.05 −/− vs. +/+ mice. $ p < 0.05 males vs. females. Number of animals for each group: males: (+/+ n = 13; −/− n = 6), females (+/+ n = 13; −/− n = 19).
3.2. Membrane progesterone receptors
While activation of the nuclear progesterone receptor is expected to induce measurable, physiological responses coinciding with de novo protein synthesis (by at least 30–45 min), some findings indicate rapid (within 2–3 min) effects of progesterone administration. One example is the effect of progesterone administration on neuronal responses to hypoxia recorded from NTS neurons in brainstem slices of adult rats. In this preparation, application of progesterone (1 μM) had no effect on basal neuronal activity, but it restored post-synaptic input resistance in neurons inhibited by hypoxia, reduced the excitatory effects of hypoxia on neurons responding to hypoxia by depolarization, and increased the rate of action potential firing (Pascual et al., 2002). Because of this dual action, the authors concluded that progesterone interacted with the molecular pathways involved in hypoxia signaling upstream of the activated channels, rather than by interaction with specific post-synaptic receptors. Whatever this interaction between progesterone and the hypoxia signaling pathway is, these finding are truly intriguing and may be related to the expression of membrane progesterone receptors. Indeed, a family of membrane progesterone receptors has been identified and recently characterized. These receptors have seven transmembrane domains (a pattern typical of G protein-coupled receptors), are expressed in steroid target tissues, selectively bind progesterone at physiologically relevant concentrations, and regulate intracellular signaling pathways likely via specific G proteins (see Labombarda et al., 2010; Intlekofer and Petersen, 2011 and references therein). Three subtypes of these receptors (also known as “progestin and adipoQ receptors”) have been characterized, namely mPRα, mPRβ, and mPRγ. Each subtype is encoded by a distinct gene and has been identified in the spinal cord of mice (Labombarda et al., 2010) as well as in the brain of rats (Intlekofer and Petersen, 2011). In addition, progesterone receptor membrane component 1 (Pgrmc1) and Pgrmc2 might also mediate some non-genomic effects of progesterone in the brain (Intlekofer and Petersen, 2011). Pgrmc1 has been identified in the NTS and pre-Bötzinger complex in adult rats (Tan et al., 2012).
4. Interim conclusion
As a conclusion to this part of the review, while progesterone is proven to be a potent and clinically relevant respiratory stimulant in adults, much remains to be understood regarding the diverse mechanisms by which it exerts its effects. In particular research efforts should be directed to discriminate the roles of various progesterone receptors in the respiratory stimulant effect. Furthermore, the notion that extra-ovarian progesterone synthesis might also play a crucial role in respiratory regulation is emerging as a new and appealing hypothesis. This is of particular importance as respiratory disorders in infancy and during early postnatal development are a worrying clinical issue.
5. Stress and respiratory regulation
5.1. What is stress?
The concept of stress began to emerge in 1929 when Walter Cannon described the “fight or flight” response. He was the first to demonstrate that an organism experiencing a life threatening injury or perceiving a dangerous situation quickly responds by releasing multiple hormones that help it to survive. Hans Selye then coined the concept of the “general adaptation syndrome” to explain how stress and its consequences affect the health of the organism and how stress can cause both defense and damage (Selye, 1950). An important aspect of this original concept was that, unlike its popular conception, stress encompasses both the stimulus and the biological response. Stress is now generally defined as “an actual or anticipated disruption of homeostasis or an anticipated threat to well-being” (Ulrich-Lai and Herman, 2009), and there is a strong tendency to distinguish the event from the response (Drolet et al., 2001; McEwen, 2008). As the definition suggests, stressful stimuli are commonly divided into systemic or neurogenic (discussed below) and we now know that the attributes of the stimulus (e.g. nature, duration) determine which neural circuits are activated by a particular stressor.
5.2. Associative vs. processive stress
The stress response is mediated by largely overlapping circuits located in the limbic forebrain, the hypothalamus, and the brainstem. The ensuing contributions from the sensory, motor, endocrine, and autonomic systems are activated in accordance with the stressor modality and aim to re-establish and maintain homeostasis and adapt to novel environments when appropriate (Drolet et al., 2001; Ulrich-Lai and Herman, 2009). Amongst these, the sympatho-adrenomedullary and hypothalamic-pituitary-adrenocortical (HPA) axes are the main neuroendocrine systems recruited during stress (Ulrich-Lai and Herman, 2009). Multiple limbic forebrain structures, including the amygdala, hippocampus, and the prefrontal cortex, involved in higher order sensory processing and memory, exert an important top down influence on the degree of HPA activation (Ulrich-Lai and Herman, 2009). Consequently, the involvement (or absence) of limbic pathways in triggering a stress response is an important distinction in the way stressful stimuli are handled (Drolet et al., 2001; Ulrich-Lai and Herman, 2009). Typically, stressors that activate the limbic pathways are referred to as “processive stress” because they require processing of multiple sensory modalities and mnemonic reference; these stimuli are mainly psychological and neurogenic stressors. In rodents for instance, restraint, immobilization, and exposure to predator odor are commonly used in the laboratory as psychogenic stressors. By contrast, limbic insensitive stressors are those that represent a direct threat to homeostasis (e.g. hemorrhage, hypoxia). As they tend to require immediate attention, systemic stressors usually bypass cognitive processing to avoid potential delays in the transmission of an excitatory signal to the paraventricular nucleus of the hypothalamus (PVN) where the initiation of a neuroendocrine response to the stressor takes place. Sensory information associated with systemic stress is conveyed directly to the PVN by visceral efferent pathways through brainstem autonomic nuclei such as the parabrachial nucleus, the NTS, and the ventrolateral medulla (Drolet et al., 2001; Ulrich-Lai and Herman, 2009; King et al., 2012). Although useful, these categories do not take into account situations in which stressors can activate different components of each pathway, either because of their mixed nature or the fact that an apparently benign situation becomes stressful with repetition. Currently, most research protocols used in the field of respiratory control – either to investigate acute (reflexive) responses or to elicit plasticity – use systemic stressors (hypoxia, intermittent hypoxia). Because it activates limbic structures at relatively low levels (FiCO2 ~0.05) in humans (Corfield et al., 1995; Harper et al., 2005), increasing CO2 in the inspired gas should be viewed as a mixed stressor. Of note, CO2 inhalation is a potent anxiogen and is commonly used to diagnose patients with panic disorders (Preter and Klein, 2008).
5.3. The paraventricular nucleus of the hypothalamus coordinates the stress response and exerts top down regulatory influence on the respiratory control system
The hypothalamus represents “a microcosm of homeostatic control mechanisms” that ensures synchronization between the environment and cognitive and physiological functions (Saper, 2004). To do so, the hypothalamus integrates afferent information originating from virtually every sensory and autonomic system that pertains to the internal and external milieu, and then responds rapidly by releasing its neurotransmitters and peptides to targets within the CNS and the pituitary gland (Gore and Roberts, 2003). Amongst the numerous groups of hypothalamic neurons that regulate the HPA axis and autonomic responses, the paraventricular nucleus (PVN) stands out as the main structure responsible for the orchestration of the neuroendocrine responses to stressful stimuli. The PVN is one of the most vascularized structures within the CNS (Ambach and Palkovits, 1974) and is capable of activating each of the major physiological responses available to the brain to mobilize bodily resources in response to environmental challenges.
The anatomical location, structural organization, and subdivisions of the PVN has been reviewed recently (Behan and Kinkead, 2011). For the purpose of the present review, it is sufficient to mention that the most common classification of PVN neurons distinguishes between three major cell groups: neurons that project to the posterior pituitary (neural lobe), neurons that project to the median eminence (anterior pituitary), and those that project outside the hypothalamus and are mainly associated with the autonomic nervous system (Swanson and Sawchenko, 1983). Considering its widespread projections throughout the CNS, the PVN can influence breathing via numerous indirect pathways. For instance, the PVN modulates the activity of several groups of monoaminergic neurons (raphé nuclei, locus coeruleus Holstege, 1987; Toth et al., 1999) which in turn exert important activity on respiratory neurons. In addition to these indirect effects, axons from the autonomic region of the PVN project directly onto (1) cranial and spinal motoneurons that produce the motor command, including inspiratory phrenic and hypoglossal motoneurons (Kc et al., 2002; Mack et al., 2007), (2) neurons of the pre-Bötzinger complex that contribute to respiratory rhythm generation (Kc et al., 2002), and (3) groups of neurons that regulate respiratory activity and breathing patterns. Specifically, the PVN has direct and bidirectional connexions with the parabrachial complex (Luiten et al., 1985) and the NTS (Luiten et al., 1985; Blevins et al., 2003; King et al., 2012). Thus, besides integrating afferent signals associated with somatic and cognitive functions to regulate the neuroendocrine response to stress, the PVN has the projections necessary to exert a top down influence on respiratory regulation on both an acute and long term time scale (see Kc et al., 2002; Behan and Kinkead, 2011 for a comprehensive review of this literature).
5.4. Stress hormones and peptides modulate breathing
The neurochemicals produced and released by the PVN onto neurons regulating breathing are both diverse and numerous, and their impact on respiratory activity is considerable. In addition to their acute stimulatory effects on breathing, many of these substances (e.g. corticotropin releasing hormone – CRH) also trigger important changes in stress-related neurochemicals and hormones in the blood stream. However, many of the key mediators of the stress response act on more distant target sites where their effects typically occur on a longer time scale (minutes to hours to months; Joels and Baram, 2009). Here, we will mainly discuss steroid hormones for which the ability to modulate the O2 chemoreflex has been demonstrated experimentally or seems highly likely.
5.4.1. Corticosteroids
Corticosteroids are synthesized from cholesterol within the adrenal cortex. Their synthetic pathways diverge to produce two major classes of hormones: gluco- and mineralococorticoids and each class binds to specific receptors. Corticosteroids are secreted from the adrenal glands in hourly pulses, with the largest amplitude at the start of the circadian period (Joels et al., 2008). They readily cross the blood–brain barrier and the rhythmicity of the secretion process helps coordinate and synchronize daily activities and sleep (Joels et al., 2008). During stress, the rapid rise in plasma adrenocorticotropic hormone (ACTH) can interrupt this rhythm and cause a premature burst of corticosteroid secretion. Glucocorticoids are the end product of the HPA axis; the main hormone is cortisol (humans) and corticosterone (rodents). Their role is related to the control of energy metabolism (from appetite to energy allocation and replenishment), and the different phases of the stress response from perception to physiological and behavioral adaptation (Joels et al., 2008). By contrast, aldosterone is the primary mineralocorticoid. It is the end product of the renin–angiotensin system; aldosterone secretion is also stimulated by ACTH. As the name indicates, the main function of the mineralocorticoids is associated with the regulation of electrolyte homeostasis (Joels et al., 2008) but as we discuss below, mineralocorticoids likely influence respiratory regulation.
5.4.2. Corticosteroid receptors: “classical” (genomic) and “rapid” (membrane) action
Rapid gluco- and mineralocorticosteroid signaling has received much attention in recent years and there is now substantial evidence that these effects are mediated by one or more membrane-associated corticosteroid receptors coupled to downstream G-protein-dependent signaling cascades. This mechanism has been studied extensively in the context of HPA axis regulation (Tasker et al., 2006); however, the presence of membrane-associated glucocorticoid receptors on neurons involved in respiratory control other than the PVN has not been evaluated.
More is known about the “delayed”, classical effects of corticosteroids. Within the central nervous system, the mineralo- and glucocorticoid receptors reside in the cytoplasm of the target cell in the unbound state. When attached to their ligand, they translocate into the nucleus where they bind to specific sites on the promoter of the responsive gene. As a result, both receptors act as transcription factors, as they can stimulate (or inhibit) gene transcription (Gore and Roberts, 2003).
Nuclear glucocorticoid receptors are widely distributed within the CNS, including the hypothalamus and, more specifically, the CRH-containing cells of the PVN (Gore and Roberts, 2003). During acute stress, these receptors are strategically located within the HPA axis to provide negative feedback. Dexamethasone is a synthetic glucocorticoid with a higher affinity for glucocorticoid receptors than its natural ligand, and with minimal affinity for mineralocorticoid receptors (Reul et al., 2000). Owing to these properties, dexamethasone activates the negative feedback system of the HPA axis and elicits a reduction of plasma cortisol levels (Basu et al., 2002). The fact that glucocorticoid receptor expression is highly dynamic, and that elevation of corticosteroid levels reduces receptor expression (Herman et al., 2008) explains why the basal level of HPA activity often becomes elevated during chronic stress.
Regions associated with respiratory regulation that express nuclear glucocorticoid receptors include the dorsal raphé nuclei, the locus coeruleus, the NTS and the parabrachial nucleus (lateral and medial divisions) (Morimoto et al., 1996). As we discuss below, chronic corticosterone elevation has a significant effect on the hypoxic ventilatory response.
5.4.3. Evidence supporting a role for aldosterone in respiratory regulation
Glucocorticoids normally circulate at levels 100–1000 times that of aldosterone, and their affinity for nuclear mineralocorticosteroid receptors is ~10 times greater than for the primary ligand, aldosterone (Gore and Roberts, 2003; Joels et al., 2008). To circumvent this situation and confer selectivity for aldosterone, target tissues express the enzyme 11-β-hydroxysteroid dehydrogenase type 2 (HSD2) which inactivates other adrenal steroids (Joel and Arthur, 2006; Gomez-Sanchez et al., 2010). Outside the brain, expression of HSD2 is restricted to specialized aldosterone-sensitive cells in a limited number of epithelial tissues such as the distal nephron of the kidney. Within the central nervous system, few areas contain neurons that may be sensitive to aldosterone. The enzymes required for aldosterone synthesis from cholesterol are expressed in rat and human brains, and expression of aldosterone synthase mRNA has been detected in rat brainstem (Gomez-Sanchez et al., 2010). HSD2-immunoreactive neurons have been located in the ventrolateral division of the ventromedial hypothalamic nucleus and a few scattered neurons in the medial vestibular nucleus, just rostral to the NTS.
In the present context, it is interesting to note that the caudal region of the NTS, which receives afferent projections from the carotid bodies, contains dense nuclear mineralocorticoid receptors, and a subpopulation of these neurons express HSD2 (Joel and Arthur, 2006). The ubiquitous expression of the transcription factor Phox2b by the HSD2 neurons suggests that they are developmentally related to other Phox2b-dependent neurons of the NTS (Geerling et al., 2008). While aldosterone is typically associated with regulation of sodium uptake, the sum of these observations and the fact that Phox2b is essential for the development of the autonomic nervous system raises the possibility that aldosterone also plays a role in respiratory regulation.
5.5. Early life experience, PVN function, and regulation of the O2 chemoreflex gain
For many species, having a stress response that is in tune with external resources and/or external pressures (e.g. predators) can be a determining factor in their ability to survive. It is not surprising therefore that newborns that develop in challenging conditions, would be more sensitive and more responsive to stress later in life. This biological principle has been the subject of extensive research over the past decade. We now know that in mammals the mother plays an important role in transmitting this information and contributes to early life programming of the stress response. The precise mechanism(s) by which the information regarding the degree of stress experienced by the mother is transmitted to her offspring is highly debated, yet we know that neonatal maternal separation (NMS) is a form stress that has persistent and sex-specific effects on PVN function. These effects on the regulation of the stress response have significant clinical implications because increased stress responsiveness contributes to a broad range of psychological and physiological disorders including hypertension, asthma, states of panic and anxiety, depression, and sleep disorders (Herman and Cullinan, 1997; Ritz and Kullowatz, 2005; Sparrenberger et al., 2008). Based on this knowledge and the strong influence of the PVN on respiratory related brain structures, we determined whether a non-systemic stress such as NMS could affect the development of respiratory control systems. The following section will mainly discuss data showing that neonatal stress interferes with respiratory control development in ways that alter the hypoxic ventilatory response and predispose to respiratory instabilities.
5.6. Neonatal stress and sex-specific effects on the developmental trajectory of the respiratory control system
The maternal separation protocol used in our studies was derived from that of Wigger and Neumann (1999) in which the entire litter was separated daily from the mother from postnatal days 3 to 12 (3 h/day; 09:00–12:00). Separated pups were placed in a temperature (35 °C) and humidity (45%) controlled incubator and isolated from each other by a partition. This temperature maintains rat pups within their thermoneutral range and thus requires no additional energy expenditure. For these studies, physiological and neuroanatomical measurements obtained from NMS rats were compared to data from animals maintained under standard rearing conditions in which the nest was not disturbed during the first two weeks post partum. These animals are considered the most desirable and appropriate control group for such studies (Lehmann and Feldon, 2000). Note that for each group, rats originated from at least two different litters to ensure that treatment-related differences were not due to a litter-specific effect. All experimental protocols have their limitations, and the fact that pups are separated from their mother for 3 h makes it possible that stress related to starvation and dehydration contributes to the effects of NMS, thus raising questions about the nature and specificity of this protocol. These issues have been discussed in detail recently (Gulemetova and Kinkead, 2011). Despite these potential limitations, this NMS protocol effectively disrupts PVN function and the HPA axis. Using in situ hybridization we demonstrated that c-fos mRNA expression levels within the PVN adult male (but not female) rats previously subjected to NMS are higher than that of undisturbed rats. This result is supported by measurements of plasma ACTH and corticosterone levels which both show that baseline levels of these hormones were greater in NMS rats than controls; again, these effects were also sex specific (Genest et al., 2004). Following neonatal stress, disruption of HPA regulation in male rats persists until adulthood. However, this effect is not permanent, as triad housing (a form of environmental enrichment) during the juvenile period reverses the effects of NMS on the HPA axis (Fournier et al., 2011). As we discuss below, the impact of early life stress on brain development also affects respiratory regulation at various stages throughout the life of an animal.
5.6.1. O2 chemoreflex and respiratory instability in newborn
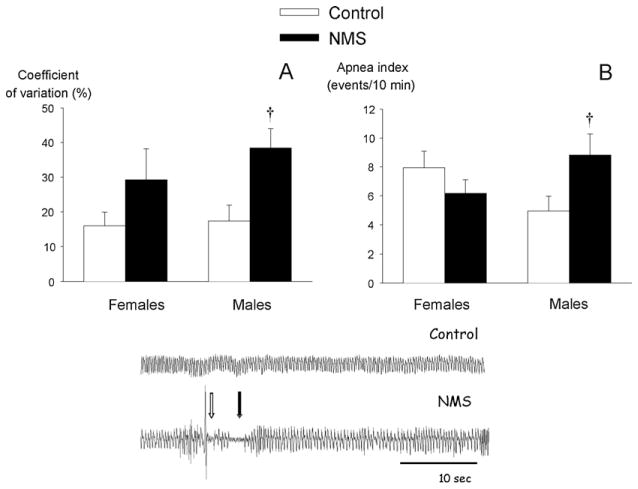
We recently assessed the consequences of NMS on respiratory control on the day that followed the last stress session (post natal day 13; Gulemetova and Kinkead, 2011). At this age, plasma corticosterone levels measured in stressed pups were higher than control, thus indicating that either the 24 h recovery period was not sufficient for hormone levels to return to baseline, or that disruption of HPA axis regulation is already noticeable (Gulemetova and Kinkead, 2011). These interpretations are not mutually exclusive. The fact that, unlike adults, NMS-related enhancement of baseline corticosterone was similar between sexes, suggests that hormonal changes that take place during development influence this process. Breathing measurements with whole body plethysmography showed that respiratory activity is more variable in NMS rats than in control animals. This result was accompanied by a sex-specific increase in apnea frequency (Fig. 3). Note that in these experiments, an apnea was defined according to the criteria commonly used in rodents and newborn lambs. Specifically, an apnea was defined as an interruption of airflow for at least two breathing cycles (at P12 for instance, an apneic pause > 0.75 s). The apneic index is the sum of two types of apneic pauses: spontaneous and post-sigh apneas. A spontaneous apnea was characterized by an interruption of flow that was not preceded by any significant change in tidal volume, whereas a post-sigh apnea was preceded by a breath with an amplitude twice the resting tidal volume. Note that in Fig. 3, the white and black arrows indicate a post-sigh and a spontaneous apnea, respectively.
Fig. 3.
Effects of neonatal maternal separation (NMS; black bars) on two indicators or respiratory instability: (A) coefficient of variation (CV) of the breathing cycle and (B) the number of apneic events (apnea index) per unit time. NMS data are compared to that of controls that were undisturbed during the same period. These measurements were obtained in male and female pups under normoxic conditions. The middle panel shows representative plethysmographic recordings from control and NMS pups (males). The white and black arrows indicate a post-sigh and a spontaneous apnea, respectively. Data are presented as means ± 1 SEM. † indicates a mean statistically different from corresponding control value at p < 0.05.
From Gulemetova and Kinkead (2011), with permission.
When PaO2 drops, an excessive or insufficient hypoxic ventilatory response (HVR) leads to transient hypocapnia which then contributes to respiratory instability and apneas. In rat pups the incidence of apneas correlates positively with the magnitude of the HVR (Julien et al., 2008). In pre-term infants, an augmented O2 chemoreflex, as indicated by the rapid change in breathing that occurs upon exposure to moderate hypoxia or hyperoxia, exacerbates periodic breathing and promotes apneas (Al-Matary et al., 2004). As the HVR of NMS and control pups was essentially similar, it would appear that other mechanisms are responsible for the respiratory instability and higher apnea frequency observed following stress. However, the use of a relatively severe level of hypoxia (FiO2 = 0.09) capable of inducing respiratory depression shortly after the induction of hypoxia, could obscure differences related to peripheral chemoreceptor function that usually occur at the early stage of the response. This in turn could explain why unlike adults, a chronic increase in corticosterone does not coincide with an increase in HVR. On the other hand, a reduced ventilatory response to CO2 contributes to respiratory disorders in the newborn such as apnea of prematurity (Katz-Salamon and Milerad, 1998). Considering that NMS has persistent and sex-specific consequences on CO2 chemoreflex development (Genest et al., 2007b), similar disruption in pups could contribute to respiratory instability; however, this hypothesis has yet to be investigated.
5.6.2. O2 chemoreflex and respiratory instability in adults
During hypoxia (FiO2 = 0.12; 20 min), the HVR of adult females exposed to NMS during early life is 30% lower than controls, and this effect reflects a reduced breathing frequency response during hypoxia (Genest et al., 2004). The physiological significance of this effect is uncertain, but in awake NMS females, this reduced responsiveness to hypoxia is accompanied by an excessive ventilatory response to CO2 (Genest et al., 2007b). Results from recent experiments led us to propose that these females share features of patients suffering from panic and anxiety-related respiratory disorders (Dumont et al., 2011).
In contrast, male rats exposed to NMS during early life are hypertensive and show a HVR greater than controls, owing mainly to a larger tidal volume response (Genest et al., 2004, 2007a). Potentiation of the inspiratory motor output response to hypoxia by NMS was confirmed with phrenic nerve recording in anesthetized rats (Kinkead et al., 2005a). By comparison with controls, mean enhancement of the HVR by NMS varies between 25 and 35%. This effect may seem trivial, but is comparable to the 30% enhancement of O2 chemoreflex reported in patients suffering from obstructive sleep apnea relative to healthy subjects (Narkiewicz et al., 1999). The clinical relevance of the effects of NMS on respiratory regulation was assessed in male rats instrumented to monitor sleep–wake states while recording ventilatory activity at rest, and in response to a brisk hypoxic challenge (FiO2 from 0.21 to 0.12 in 90 s). Results show that the variability in ventilatory activity (as indicated by the coefficient of variation) was higher in NMS rats than in controls across all sleep–wake states (Kinkead et al., 2009). During non-REM sleep the magnitude of the HVR was positively correlated with the coefficient of variation (Fig. 4). Of note, the values from NMS rats are clustered on the high end of the regression. Although apneic events were not quantified in that study, these results nonetheless indicate that the stress-related increase in HPA function augments the O2 chemoreflex gain. Considering the importance of “loop gain” in the pathophysiology of sleep disordered breathing, these results point to new and highly promising research avenues in the etiology of this prominent respiratory disorder.
Fig. 4.
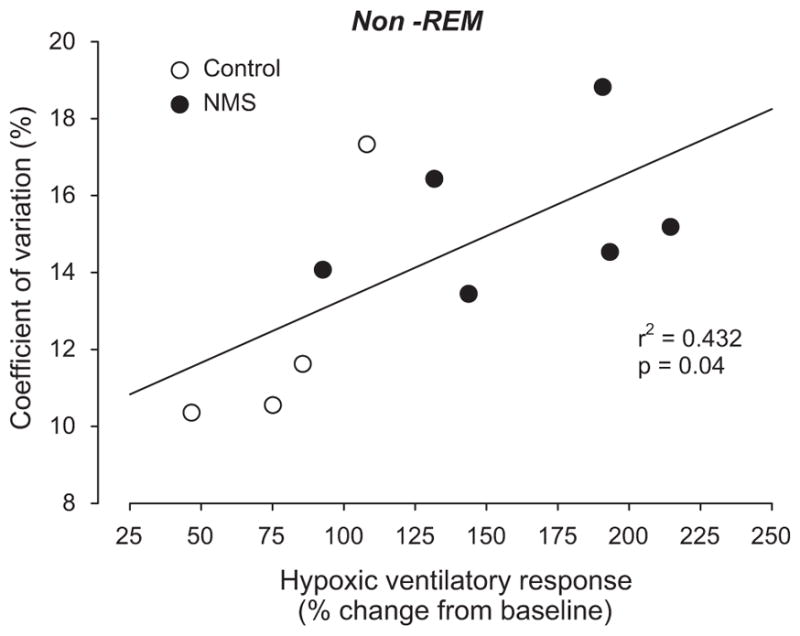
Correlation between the minute ventilation response to hypoxia (last 20 s of hypoxia expressed as a percentage change from baseline) and coefficient of variation for this variable during the 2.5 h of recording under normoxia. These measurements were obtained during non-REM sleep. Open circles: control rats (n = 5); black circles: rats previously subjected to neonatal maternal separation (NMS; n = 6).
From Kinkead et al. (2009), with permission.
5.6.3. Mechanisms
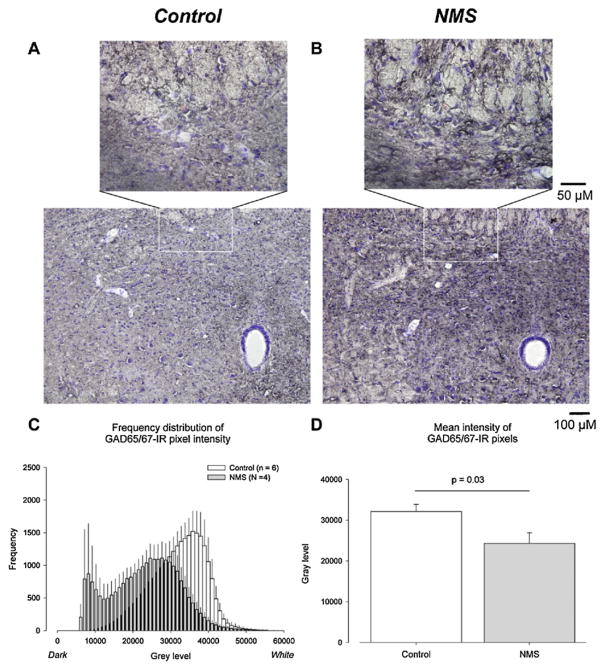
To address the neural mechanisms underlying the physiological phenotype of NMS rats, we used electrical stimulation of the carotid sinus nerve to activate the neural pathways involved in the hypoxic chemoreflex. Because hypoxia is not used in this approach, the carotid bodies (which may be affected by NMS) are not activated, and the potential confounding effects of brain hypoxia are eliminated. Although the frequency response to carotid sinus nerve stimulation was similar between groups, the phrenic burst amplitude increase was greater in NMS than in control rats. Therefore, enhancement of peripheral chemoreceptor O2 sensitivity likely accounts for the NMS-related enhancement of the frequency response (see below), and central integration of the chemoafferent signal is more efficient (higher gain) in rats subjected to NMS than controls (Kinkead et al., 2005a). To explain these results, we initially proposed that NMS decreases inhibitory (GABAergic) modulation in key integrative regions. However, the use of unilateral microinjections of GABA agonists/antagonists and GABAA receptor autoradiography in the NTS and paraventricular hypothalamus nucleus indicate that NMS rats have a greater number of GABAA receptors in these regions and a greater sensitivity to GABAergic agents. Moreover, comparison of glutamic acid decarboxylase (GAD; enzyme involved in GABA synthesis) immunostaining in the NTS showed that the signal intensity observed in NMS rats was greater than controls (Fig. 5). These data suggests that the capacity for inhibitory modulation is greater in NMS rats. From a functional perspective, however, this effect is obviously not sufficient to maintain the hypoxic ventilatory response at level close to that of controls. Other inhibitory neurotransmissions (e.g. endogenous opiates) could be involved, and as yet, these have not been investigated. Nevertheless, we now propose that NMS disrupts the balance between excitatory (glutamatergic) and inhibitory (GABAergic) modulation. Although this “imbalance” was shown to occur in CNS regions involved in the integration of relevant sensory afferents signals, it may occur at other levels (e.g. motoneurons) that have yet to be studied (Genest et al., 2007b; Kinkead et al., 2008).
Fig. 5.
Neonatal maternal separation (NMS) augments GAD65/67 immunostainning in the caudal NTS (bregma −14.3 to −14.6). Representative NTS photomicrographs comparing GAD65/67 immunoreactivity between (A) controls and (B) NMS rats. (C) Comparison of frequency distribution histograms for gray scale GAD65/67 immunoreactive pixel intensities between control (open bars) and NMS rats (gray bars). (D) Comparison of the mean gray scale intensity values between NTS sections from controls and NMS rats. For each section, the gray scale intensity of three circular areas of interests (diameter = 50 μm) positioned within the NTS were analyzed. Note that low gray level values correspond to darker pixels. Data in (D) is expressed as mean ± 1 SEM.
From Kinkead et al. (2008), with permission.
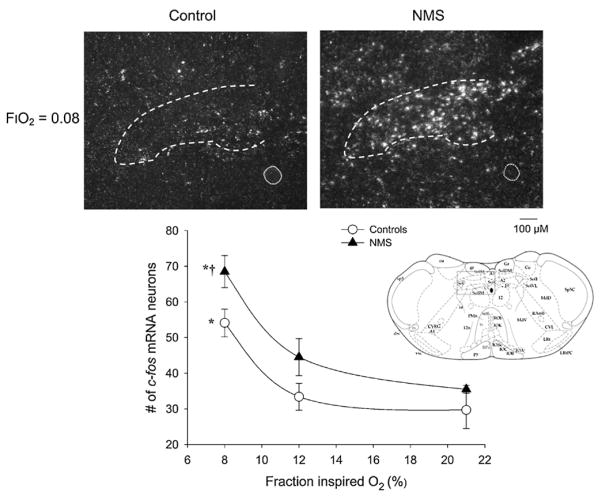
The use of the anesthetized rat preparation also allowed us to compare the temporal dynamics of the hypoxic ventilatory response and to demonstrate that, similar to awake animals (Genest et al., 2004, 2007a), the rapid increase in respiratory frequency measured in males at the onset of hypoxia is greater in NMS rats vs. controls. Since the rapidity and magnitude of this component of the hypoxic ventilatory response is hypothesized to reflect the responsiveness of the carotid bodies to a PaO2 decrease (Powell et al., 1998), our results strongly suggest that NMS enhances carotid body responsiveness in male rats. This interpretation is supported by the fact that carotid sinus nerve stimulation did not reproduce the stronger frequency response that typifies NMS rats (Kinkead et al., 2005a). Moreover, hypoxic exposure augments c-Fos mRNA expression within the caudal NTS in an intensity-dependent fashion; within this region, the response measured in NMS rats is greater than in controls (Kinkead et al., 2008) (Fig. 6). Note that since the NTS integrates and “redistributes” visceral sensory information to key CNS structures, it was not surprising to see that a similar augmentation of the c-Fos mRNA response to hypoxia was also measured in the locus coeruleus (Kinkead and Gulemetova, 2010).
Fig. 6.
Neonatal maternal separation (NMS) augments c-fos m-RNA expression levels in the caudal NTS of awake rats following exposure to one of three fraction of inspired O2 level (FiO2 ) for 20 min: normoxia (FiO2 = 0.21), moderate hypoxia (FiO2 = 0.12), or severe hypoxia (FiO2 = 0.21). Top panels: representative photomicrographs comparing c-fos m-RNA in situ hybridization signal after exposure to severe hypoxia between control rats (left) and rats subjected to neonatal maternal separation (NMS; right). The dotted circle represents the central canal. Lower panel: relationship between the number of c-fos mRNA positive neurons within the NTS, and inspired O2 level for controls (open circles) and NMS rats (closed triangles) in the caudal NTS. Data are expressed as means ± SEM. Each value represents the mean number of c-fos mRNA-containing perikarya were counted bilaterally from sections corresponding to the rostro-caudal coordinates −14.3 to −14.60 from bregma for each experimental condition. For each rat, a mean number of c-fos positive neurons was obtained by averaging perikarya counted for several sections (the number of sections ranged between 1 and 3). * indicates means that are statistically different from baseline value at p < 0.05. † indicates means that are statistically different from corresponding control value at p < 0.05.
Adapted with permission from Kinkead et al. (2008).
Based on these data, we then examined the potential impact of NMS on carotid body function by assessing two molecular indicators of dopaminergic neurotransmission within this structure: tyrosine hydroxylase and dopamine D2 receptor mRNA expression (Kinkead et al., 2005b). Densitometric analysis of the RT-PCR mRNA expression signals obtained from carotid bodies harvested from both groups of adult animals showed that NMS enhanced tyrosine hydroxylase mRNA expression in male, but not female rats. Neonatal maternal separation also increased dopamine D2 receptor mRNA expression; however, this effect was not sex-specific. While we are aware of the limitations inherent to such semi-quantitative measurements, these results are nonetheless consistent with the hypothesis that NMS disrupts carotid body function. However, further investigation with a more direct (electrophysiological) approach is necessary.
5.6.4. Chronic corticosterone elevation and enhancement of the hypoxic ventilatory response in adult rats
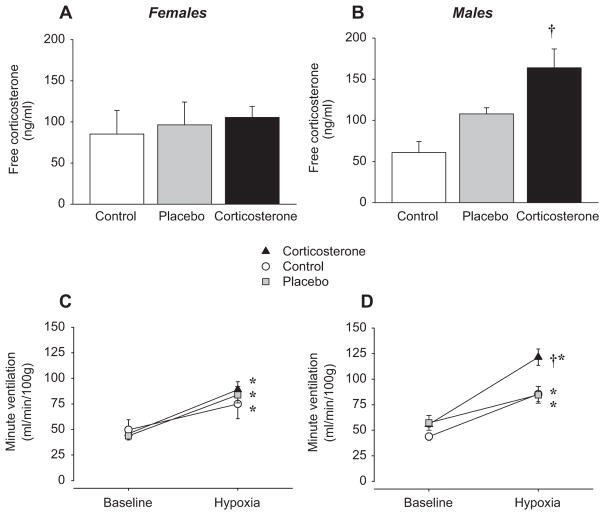
Acute administration of the synthetic glucocorticoid dexamethasone affects catecholamine synthesis within the carotid bodies (Joseph et al., 1998), and glucocorticoid over-exposure during early life also leads to cardio-respiratory disorders such as hypertension in rat (O’Regan et al., 2001). Chronic elevation of plasma corticosterone levels is an important feature of male NMS rats; based on these previous studies, corticosterone could play a prominent role in the respiratory phenotype observed in NMS rats. To address this issue, we tested the hypothesis that chronic corticosterone elevation is sufficient to augment the HVR. In these experiments, we chronically implanted either placebo or “slow release” corticosterone pellets in untreated (no-NMS) male and female rats to increase plasma corticosterone levels over 14 days (Fournier et al., 2007). At the end of treatment, plasma corticosterone levels of males were comparable to levels observed in NMS rats (Fig. 7B); however, this protocol failed to increase plasma corticosterone levels of females significantly (Fig. 7A). The reasons underlying this unexpected result are unknown. Subsequent plethysmographic measurements in the awake animal showed that male (but not female) rats subjected to chronic corticosterone elevation had a hypoxic ventilatory response 62% greater than placebo-treated rats (Fig. 7C and D); again, this effect was caused mainly be an increase in tidal volume response (Fournier et al., 2007). As a whole, these results are strikingly similar to those reported following NMS, and support our initial hypothesis that corticosterone alone is sufficient to produce this phenotype. Thus, the acute and long-term effects of this hormone must also be considered in the programming of the hypoxic ventilatory response also.
Fig. 7.
Comparison of ‘resting’ free plasma corticosterone levels in (A) female and (B) male rats following chronic subcutaneous implantation of a “slow release” corticosterone pellets (3 × 100 mg/pellet; active over 21 days). Data were also obtained from rats that received a sham (placebo) implant or no implant (control). For all three groups (corticosterone, placebo, and control) minute ventilation was measured under resting (normoxia) and hypoxic conditions (12% O2, 20 min) with whole body plethysmography 14 days after implants were placed subcutaneously. For each group, data were obtained in (C) female and (D) male rats. Note that to ensure that ventilatory measurements did not affect “resting” corticosterone levels, blood samples were taken 4 ± 1 days after ventilatory measurements were completed (i.e. 18 ± 1 day post surgery). Data are expressed as means ± 1 SEM. *Value different from corresponding baseline value at p < 0.05. *Value different from corresponding control value at p < 0.05.
Redrawn with permission from Fournier et al. (2007).
6. Concluding remarks and future directions
By their ability to modulate gene expression via nuclear receptors, steroid hormones can have a profound influence on the central and peripheral components of the respiratory control system. Keeping in mind that hypoxia is a systemic stress that can trigger the release of corticosteroids as well as sex hormones, results from recent research discussed in this brief review highlight how steroid hormones can “shape” the O2 chemoreflex gain and influence its developmental trajectory. It will be interesting to see how activation of membrane receptors influences this vital chemoreflex.
As in the case with a range of respiratory diseases, these manifestations of respiratory plasticity can differ substantially between males and females. As a recent editorial has pointed out, understanding the mechanisms underlying this sexual dimorphism is a highly promising (and necessary) research avenue (Cahill, 2006; McCarthy et al., 2012; Miller, 2012).
Footnotes
This paper is part of a special issue entitled “Development of the Carotid Body”, guest-edited by John L. Carroll, David F. Donnelly and Aida Bairam.
References
- Al-Matary A, Kutbi I, Qurashi M, Khalil M, Alvaro R, Kwiatkowski K, Cates D, Rigatto H. Increased peripheral chemoreceptor activity may be critical in destabilizing breathing in neonates. Seminars in Perinatology. 2004;28:264–272. doi: 10.1053/j.semperi.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Allen WM. The isolation of crystalline progestin. Science. 1935;82:89–93. doi: 10.1126/science.82.2118.89. [DOI] [PubMed] [Google Scholar]
- Ambach G, Palkovits M. Blood supply of the rat hypothalamus. II. Nucleus paraventricularis. Acta Morphologica Academiae Scientiarum Hungaricae. 1974;22:311–320. [PubMed] [Google Scholar]
- Basu M, Sawhney RC, Kumar S, Pal K, Prasad R, Selvamurthy W. Hypothalamic-pituitary-adrenal axis following glucocorticoid prophylaxis against acute mountain sickness. Hormone and Metabolic Research. 2002;34:318–324. doi: 10.1055/s-2002-33260. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Dmitrieff EF, Young KM, Piro SE. Hypoxic ventilatory response of adult rats and mice after developmental hyperoxia. Respiratory Physiology & Neurobiology. 2011;177:342–346. doi: 10.1016/j.resp.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Millhorn DE, Gallman EA, Cidlowski JA. Progesterone stimulates ventilation through a central nervous system steroid receptor-mediated mechanism in the cat. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:7788–7792. doi: 10.1073/pnas.84.21.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Cidlowski JA, Millhorn DE. The stimulation of respiration by progesterone in ovariectomized cat is mediated by an estrogen-dependent hypothalamic mechanism requiring gene expression. Endocrinology. 1990;126:519–527. doi: 10.1210/endo-126-1-519. [DOI] [PubMed] [Google Scholar]
- Behan M, Wenninger JM. Sex steroidal hormones and respiratory control. Respiratory Physiology & Neurobiology. 2008;164:213–221. doi: 10.1016/j.resp.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Kinkead R. Comprehensive Physiology. John Wiley & Sons, Inc; 2011. Neuronal control of breathing: sex and stress hormones. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Research. 2003;993:30–41. doi: 10.1016/j.brainres.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Block A, Boysen P, Wynne J, Hunt L. Sleep apnea, hypopnea and oxygen desaturation in normal subjects. A strong male predominance. New England Journal of Medicine. 1979;300:513–517. doi: 10.1056/NEJM197903083001001. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Frontiers in Neuroendocrinology. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Corfield DR, Fink GR, Ramsay SC, Murphy K, Harty HR, Watson JD, Adams L, Frackowiak RS, Guz A. Evidence for limbic system activation during CO2 -stimulated breathing in man. The Journal of Physiology. 1995;488:77–84. doi: 10.1113/jphysiol.1995.sp020947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Olson EB, Skatrud JB. Hormones and neurochemicals in the regulation of breathing. In: Soc AP, editor. Handbook of Physiology: The Respiratory System. pt 1. II. Control of Breathing; Bethesda, MD: 1986. pp. 181–221. sec 3. chapt 7. [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Dumont FS, Biancardi V, Kinkead R. Hypercapnic ventilatory response of anesthetized female rats subjected to neonatal maternal separation: insight into the origins of panic attacks? Respiratory Physiology & Neurobiology. 2011;175:288–295. doi: 10.1016/j.resp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Finer NN, Higgins R, Kattwinkel J, Martin RJ. Summary proceedings from the apnea-of-prematurity group. Pediatrics. 2006;117:S47–S51. doi: 10.1542/peds.2005-0620H. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MP, Haldane JS. The normal alveolar carbonic acid pressure in man. The Journal of Physiology. 1905;32:486–494. doi: 10.1113/jphysiol.1905.sp001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier S, Allard M, Gulemetova R, Joseph V, Kinkead R. Chronic corticosterone elevation and sex-specific augmentation of the hypoxic ventilatory response in awake rats. Journal of Physiology. 2007;584:951–962. doi: 10.1113/jphysiol.2007.141655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier S, Joseph V, Kinkead R. Influence of juvenile housing conditions on the ventilatory, thermoregulatory, and endocrine responses to hypoxia of adult male rats. Journal of Applied Physiology. 2011;111:516–523. doi: 10.1152/japplphysiol.00370.2011. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Chimenti PC, Loewy AD. Phox2b expression in the aldosterone-sensitive HSD2 neurons of the NTS. Brain Research. 2008;1226:82–88. doi: 10.1016/j.brainres.2008.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation and sex-specific plasticity of the hypoxic ventilatory response in awake rat. Journal of Physiology. 2004;554:543–557. doi: 10.1113/jphysiol.2003.052894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genest SE, Balon N, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation and enhancement of the hypoxic ventilatory response: the role of GABAergic neurotransmission within the paraventricular nucleus of the hypothalamus. Journal of Physiology. 2007a;554 (2):299–314. doi: 10.1113/jphysiol.2007.135160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation induces sex-specific augmentation of the hypercapnic ventilatory response in awake rat. Journal of Applied Physiology. 2007b;102:1416–1421. doi: 10.1152/japplphysiol.00454.2006. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Gomez-Sanchez CM, Plonczynski M, Gomez-Sanchez CE. Aldosterone synthesis in the brain contributes to Dahl salt-sensitive rat hypertension. Experimental Physiology. 2010;95:120–130. doi: 10.1113/expphysiol.2009.048900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Roberts JL. Neuroendocrine systems. In: Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ, editors. Fundamental Neuroscience. Academic Press; Amsterdam: 2003. pp. 1031–1065. [Google Scholar]
- Grosvenor CE, Picciano MF, Baumrucker CR. Hormones and growth factors in milk. Endocrine Reviews. 1993;14:710–728. doi: 10.1210/edrv-14-6-710. [DOI] [PubMed] [Google Scholar]
- Gulemetova R, Kinkead R. Neonatal stress increases respiratory instability in rat pups. Respiratory Physiology & Neurobiology. 2011;176:103–109. doi: 10.1016/j.resp.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Harper RM, Macey PM, Woo MA, Macey KE, Keens TG, Gozal D, Alger JR. Hypercapnic exposure in congenital central hypoventilation syndrome reveals CNS respiratory control mechanisms. Journal of Neurophysiology. 2005;93:1647–1658. doi: 10.1152/jn.00863.2004. [DOI] [PubMed] [Google Scholar]
- Hasselbalch K, Gammeltoft S. Die neuralitatisie gravideson des gravidesn organismus. Biochemische Zeitschrift. 1915;68:206–264. [Google Scholar]
- Haywood SA, Simonian SX, van der Beek EM, Bicknell RJ, Herbison AE. Fluctuating estrogen and progesterone receptor expression in brainstem norepinephrine neurons through the rat estrous cycle. Endocrinology. 1999;140:3255–3263. doi: 10.1210/endo.140.7.6869. [DOI] [PubMed] [Google Scholar]
- Helena C, Gustafsson JA, Korach K, Pfaff D, Anselmo-Franci JA, Ogawa S. Effects of estrogen receptor alpha and beta gene deletion on estrogenic induction of progesterone receptors in the locus coeruleus in female mice. Endocrine. 2009;36:169–177. doi: 10.1007/s12020-009-9207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Flak J, Jankord R, Inga DNaRL. Progress in Brain Research. Vol. 170. Elsevier; 2008. Chronic stress plasticity in the hypothalamic paraventricular nucleus; pp. 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs AM, Johnson NL, Rosen CL, Kirchner HL, Martin R, Storfer-Isser A, Redline S. Prenatal and neonatal risk factors for sleep disordered breathing in school-aged children born preterm. Journal of Pediatrics. 2008;153:176–182. doi: 10.1016/j.jpeds.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G. Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: an HRP and autoradiographic tracing study in the cat. The Journal of Comparative Neurology. 1987;260:98–126. doi: 10.1002/cne.902600109. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA, Petersen SL. Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain. Neuroscience. 2011;172:55–65. doi: 10.1016/j.neuroscience.2010.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel CG, Arthur DL. Aldosterone-sensitive neurons in the nucleus of the solitary tract: efferent projections. The Journal of Comparative Neurology. 2006;497:223–250. doi: 10.1002/cne.20993. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H, DeRijk R, de Kloet ER. The coming out of the brain mineralocorticoid receptor. Trends in Neurosciences. 2008;31:1–7. doi: 10.1016/j.tins.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Joels M, Baram TZ. The neuro-symphony of stress. Nature Reviews Neuroscience. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph V, Dalmaz Y, Cottet-Emard JM, Pequignot JM. Dexamethasone’s influence on tyrosine hydroxylase activity in the chemoreflex pathway and on the hypoxic ventilatory response. Pflugers Archiv. 1998;435:834–839. doi: 10.1007/s004240050591. [DOI] [PubMed] [Google Scholar]
- Joseph V, Doan VD, Morency CE, Lajeunesse Y, Bairam A. Expression of sex-steroid receptors and steroidogenic enzymes in the carotid body of adult and newborn male rats. Brain Research. 2006;1073–1074:71–82. doi: 10.1016/j.brainres.2005.12.075. [DOI] [PubMed] [Google Scholar]
- Joseph V, Niane LM, Bairam A. Antagonism of progesterone receptor suppresses carotid body responses to hypoxia and nicotine in rat pups. Neuroscience. 2012;207:103–109. doi: 10.1016/j.neuroscience.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Bairam A, Joseph V. Chronic intermittent hypoxia reduces ventilatory long-term facilitation and enhances apnea frequency in newborn rats. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2008;294:R1356–R1366. doi: 10.1152/ajpregu.00884.2007. [DOI] [PubMed] [Google Scholar]
- Katz-Salamon M, Milerad J. The divergent ventilatory and heart rate response to moderate hypercapnia in infants with apnoea of infancy. Archives of Disease in Childhood. 1998;79:231–236. doi: 10.1136/adc.79.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kc P, Haxhiu MA, Tolentino-Silva FP, Wu M, Trouth CO, Mack SO. Paraventricular vasopressin-containing neurons project to brain stem and spinal cord respiratory-related sites. Respiratory Physiology & Neurobiology. 2002;133:75–88. doi: 10.1016/s1569-9048(02)00131-3. [DOI] [PubMed] [Google Scholar]
- King TL, Heesch CM, Clark CG, Kline DD, Hasser EM. Hypoxia activates nucleus tractus solitarii neurons projecting to the paraventricular nucleus of the hypothalamus. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2012;302 (10):R1219–R1232. doi: 10.1152/ajpregu.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Gulemetova R, Bairam A. Neonatal maternal separation enhances phrenic responses to hypoxia and carotid sinus nerve stimulation in the adult anesthetised rat. Journal of Applied Physiology. 2005a;99:189–196. doi: 10.1152/japplphysiol.00070.2005. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Joseph V, Lajeunesse Y, Bairam A. Neonatal maternal separation enhances dopamine D2 receptor and tyrosine hydroxylase mRNA expression levels in carotid body of rats. Canadian Journal of Physiology and Pharmacology. 2005b;83:76–84. doi: 10.1139/y04-106. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Balon N, Genest SE, Gulemetova R, Laforest S, Drolet G. Neonatal maternal separation and enhancement of the inspiratory (phrenic) response to hypoxia in adult rats: disruption of GABAergic neurotransmission in the nucleus tractus solitarius. European Journal of Neuroscience. 2008;27:1174–1188. doi: 10.1111/j.1460-9568.2008.06082.x. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Montandon G, Bairam A, Lajeunesse Y, Horner RL. Neonatal maternal separation disrupts regulation of sleep and breathing in adult male rats. Sleep. 2009;32:1611–1620. doi: 10.1093/sleep/32.12.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Gulemetova R. Neonatal maternal separation and neuroendocrine programming of the respiratory control system in rats. Biological Psychology. 2010;84:26–38. doi: 10.1016/j.biopsycho.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Labombarda F, Meffre D, Delespierre B, Krivokapic-Blondiaux S, Chastre A, Thomas P, Pang Y, Lydon JP, Gonzalez SL, De Nicola AF, Schumacher M, Guennoun R. Membrane progesterone receptors localization in the mouse spinal cord. Neuroscience. 2010;166:94–106. doi: 10.1016/j.neuroscience.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Lefter R, Morency CE, Joseph V. Progesterone increases hypoxic ventilatory response and reduces apneas in newborn rats. Respiratory Physiology & Neurobiology. 2007;156:9–16. doi: 10.1016/j.resp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Lefter R, Doan VD, Joseph V. Contrasting effects of estradiol and progesterone on respiratory pattern and hypoxic ventilatory response in newborn male rats. Respiratory Physiology & Neurobiology. 2008;164:312–318. doi: 10.1016/j.resp.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Reviews in the Neurosciences. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Luiten PGM, ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Research. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes and Development. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Mack SO, Wu M, Kc P, Haxhiu MA. Stimulation of the hypothalamic paraventricular nucleus modulates cardiorespiratory responses via oxytocinergic innervation of neurons in pre-Botzinger complex. Journal of Applied Physiology. 2007;102:189–199. doi: 10.1152/japplphysiol.00522.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. The Journal of Neuroscience. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VM. In pursuit of scientific excellence: sex matters. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2012;302:R1023–R1024. doi: 10.1152/ajpregu.00105.2012. [DOI] [PubMed] [Google Scholar]
- Montgomery-Downs HE, Crabtree VM, Sans Capdevila O, Gozal D. Infant-feeding methods and childhood sleep-disordered breathing. Pediatrics. 2007;120:1030–1035. doi: 10.1542/peds.2007-0722. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neuroscience Research. 1996;26:235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJH, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–1189. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- O’Regan D, Welberg LL, Holmes MC, Seckl JR. Glucocorticoid programming of pituitary–adrenal function: mechanisms and physiological consequences. Seminars in Neonatology. 2001;6:319–329. doi: 10.1053/siny.2001.0067. [DOI] [PubMed] [Google Scholar]
- Pascual O, Morin-Surun MP, Barna B, Denavit-Saubie M, Pequignot JM, Champagnat J. Progesterone reverses the neuronal responses to hypoxia in rat nucleus tractus solitarius in vitro. The Journal of Physiology. 2002;544:511–520. doi: 10.1113/jphysiol.2002.023994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respiration Physiology. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Preter M, Klein DF. Panic, suffocation false alarms, separation anxiety and endogenous opioids. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32:603–612. doi: 10.1016/j.pnpbp.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JMHM, Gesing A, Droste S, Stec ISM, Weber A, Bachmann C, Bilang-Bleuel A, Holsboer F, Linthorst ACE. The brain mineralocorticoid receptor: greedy for ligand, mysterious in function. European Journal of Pharmacology. 2000;405:235–249. doi: 10.1016/s0014-2999(00)00677-4. [DOI] [PubMed] [Google Scholar]
- Ritz T, Kullowatz A. Effects of stress and emotion on lung function in health and asthma. Current Respiratory Medicine Reviews. 2005;1:208–219. [Google Scholar]
- Romeo RD, Bellani R, McEwen BS. Stress-induced progesterone secretion and progesterone receptor immunoreactivity in the paraventricular nucleus are modulated by pubertal development in male rats. Stress. 2005;8:265–271. doi: 10.1080/10253890500489320. [DOI] [PubMed] [Google Scholar]
- Saaresranta T, Polo O. Hormones and breathing. Chest. 2002;122:2165–2182. doi: 10.1378/chest.122.6.2165. [DOI] [PubMed] [Google Scholar]
- Saper CB. Central autonomic system. In: Paxinos G, editor. The Rat Nervous System. Elsevier Academic Press; San Diego: 2004. pp. 761–796. [Google Scholar]
- Selye H. Stress and the general adaptation syndrome. British Medical Journal. 1950;1:1383–1392. doi: 10.1136/bmj.1.4667.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahar E, Redline S, Young T, Boland LL, Baldwin CM, Nieto FJ, O’Connor GT, Rapoport DM, Robbins JA. Hormone replacement therapy and sleep-disordered breathing. American Journal of Respiratory and Critical Care Medicine. 2003;167:1186–1192. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- Soliz J, Joseph V. Perinatal steroid exposure and respiratory control during early postnatal life. Respiratory Physiology & Neurobiology. 2005;149:111–122. doi: 10.1016/j.resp.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Sparrenberger F, Cichelero FT, Ascoli AM, Fonseca FP, Weiss G, Berwanger O, Fuchs SC, Moreira LB, Fuchs FD. Does psychosocial stress cause hypertension[quest]. A systematic review of observational studies. Journal of Human Hypertension. 2008;23:12–19. doi: 10.1038/jhh.2008.74. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annual Review of Neuroscience. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tan W, Sherman D, Turesson J, Shao XM, Janczewski WA, Feldman JL. Reelin demarcates a subset of pre-Botzinger complex neurons in adult rat. Journal of Comparative Neurology. 2012;520:606–619. doi: 10.1002/cne.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker JG, Di S, Malcher-Lopes R. Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology. 2006;147:5549–5556. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth ZE, Gallatz K, Fodor M, Palkovits M. Decussations of the descending paraventricular pathways to the brainstem and spinal cord autonomic centers. The Journal of Comparative Neurology. 1999;414:255–266. [PubMed] [Google Scholar]
- Trotter A, Pohlandt F. The replacement of oestradiol and progesterone in very premature infants. Annals of Medicine. 2000;32:608–614. doi: 10.3109/07853890009002031. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennemann MM, Bajanowski T, Brinkmann B, Jorch G, Yucesan K, Sauerland C, Mitchell EA. Does breastfeeding reduce the risk of sudden infant death syndrome? Pediatrics. 2009;123:e406–e410. doi: 10.1542/peds.2008-2145. [DOI] [PubMed] [Google Scholar]
- White DP, Schneider BK, Santen RJ, McDermott M, Pickett CK, Zwillich CW, Weil JV. Influence of testosterone on ventilation and chemosensitivity in male subjects. Journal of Applied Physiology. 1985;59:1452–1457. doi: 10.1152/jappl.1985.59.5.1452. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiology and Behavior. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Haji A, Ohi Y, Takeda R. Effects of progesterone on apneic events during behaviorally defined sleep in male rats. Life Sciences. 2005;78:383–388. doi: 10.1016/j.lfs.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. American Journal of Respiratory and Critical Care Medicine. 2003;167:1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]