Abstract
Background
Previous economic analyses evaluating treatment of methicillin-resistant Staphylococcus aureus (MRSA) complicated skin and soft-tissue infections (cSSTI) failed to include all direct treatment costs such as outpatient parenteral antibiotic therapy (OPAT). Our objective was to develop an economic model from a US payer perspective that includes all direct inpatient and outpatient costs incurred by patients with MRSA cSSTI receiving linezolid, vancomycin, or daptomycin.
Methods
A 4-week decision model was developed for this economic analysis. Published literature and database analyses with validation by experts provided clinical, resource use, and cost inputs on data such as efficacy rate, length of stay, adverse events, and OPAT services. Base-case analysis assumed equal efficacy and equal length of stay for treatments. We conducted several sensitivity analyses where assumptions on resource use or efficacy were varied. Costs were reported in year-end 2011 US dollars.
Results
Total treatment costs in the base-case were lower for linezolid ($10,571) than vancomycin ($11,096), and daptomycin ($13,612). Inpatient treatment costs were $740 more, but outpatient costs, $1,266 less with linezolid than vancomycin therapy due to a switch to oral linezolid when the patient was discharged. Compared with daptomycin, both inpatient and outpatient treatment costs were lower with linezolid by $87 and $2,954 respectively. In sensitivity analyses, linezolid had lower costs compared with vancomycin and daptomycin when using differential length of stay data from a clinical trial, and using success rates from a meta-analysis. In a scenario without peripherally inserted central catheter line costs, linezolid became slightly more expensive than vancomycin (by $285), but remained less costly than daptomycin (by $2,316).
Conclusion
Outpatient costs of managing MRSA cSSTI may be reduced by 30%–50% with oral linezolid compared with vancomycin or daptomycin. Results from this analysis support potential economic benefit and cost savings of using linezolid versus traditional OPAT when total inpatient and outpatient medical costs are evaluated.
Keywords: economic model, OPAT, cost
Introduction
Treatment guidelines recently released by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus (MRSA) skin and soft-tissue infections (SSTI) list several options for treating hospitalized patients with complicated MRSA SSTI.1 Treatment recommendations based on evidence from one or more randomized, controlled studies include intravenous and oral linezolid and intravenous vancomycin, daptomycin, or telavancin for 7–14 days.
Linezolid, in comparison with these other recommended treatments, is 100% bioavailable when administered orally, meaning the same linezolid dosing regimen is used for intravenous and oral administration.2 The benefits of oral compared with intravenous administration are numerous and include decreased intravenous administration costs, intravenous line infections, length of hospital stay, and increased patient convenience.3,4
Results from clinical studies and meta-analyses show that linezolid is at least as effective as vancomycin or daptomycin in treating patients with MRSA SSTI.5–10 Patients prescribed linezolid compared with vancomycin for documented or presumed MRSA SSTI receive fewer days of intravenous therapy7,11–13 and have shorter hospital stays.7,9,11–15
In the year 2007, an estimated 570,000–600,000 patients in the US were hospitalized and discharged with a principal diagnosis of an SSTI;16 the inpatient length of stay was approximately 4.5 days.16 Results from data submitted to the SENTRY Antimicrobial Surveillance Program for the year 2004 show that S. aureus (51.6%) is the most common cause of SSTI in North America; oxacillin resistance is reported in 47.4% of S aureus isolates.17 Based on these data, approximately 135,500–146,750 patients are hospitalized and receive a primary diagnosis of MRSA SSTI in the United States annually.
Although the societal and economic costs of treating MRSA SSTI are currently unknown, the current health care climate demands utilization of the most cost-effective therapies in both the inpatient and outpatient settings. Costs that should be included in an overall evaluation of treatment strategy include not only drug acquisition costs, but also costs incurred for hospital room and board, drug administration and preparation, diagnostic and laboratory testing, and drug-related adverse events or allergic reactions.18 Study results show that the total cost of vancomycin therapy excluding room and board is three to four times higher than vancomycin acquisition cost.19
Several studies evaluated the economic effect of linezolid compared with vancomycin on the treatment of patients with MRSA complicated SSTI (cSSTI) from various perspectives.9,12,13,20–23 Linezolid was more cost-effective than vancomycin therapy for the treatment of patients with MRSA cSSTI in the majority of studies.9,12,13,21–23
Daptomycin is an intravenous therapy recommended1 and frequently used for MRSA cSSTI. Very few studies have been conducted on its economic comparison with vancomycin and/or linezolid.24,25 In studies that were reviewed, daptomycin was found cost-effective compared with vancomycin, but not versus linezolid.42
None of these studies, however, included all costs incurred by patients with MRSA cSSTI initially treated as a hospital inpatient and eventually treated as an outpatient for the remaining duration of therapy. Specifically, in many studies, the additional medical costs related to outpatient administration of parenteral vancomycin or daptomycin therapy were not included. Therefore, we conducted a pharmacoeconomic analysis to assess and compare the value of linezolid, vancomycin, and daptomycin in the treatment of MRSA cSSTI from a US third-party payer’s perspective using a decision analytic model. Our model uniquely includes the cost components of outpatient antibiotic treatment incurred by patients with MRSA cSSTI treated with either intravenous followed by oral linezolid, intravenous vancomycin or intravenous daptomycin.
Methods
Model overview
A decision tree was constructed based on information from clinical trials and other published literature such as retrospective database analyses. When gaps existed in the literature or when the literature information was contradictory or uncertain, it was complemented by assumptions from a panel of infectious disease physicians and a homecare intravenous therapy nurse. The model was developed using Microsoft Excel and Visual Basic.
The perspective of this analysis is that of US third-party payers. Our model includes direct medical costs only. A distinction was made between inpatient and outpatient costs. Inpatient costs include general ward, specialist, adverse event, and drug costs; outpatient costs include outpatient parenteral antibiotic therapy (OPAT), physician office visit, and peripherally inserted central catheter (PICC) placement and complication costs.
Model population
Our model population was equivalent to the population included in a linezolid Phase 4 study,7 and consisted of patients with various MRSA cSSTI such as surgical wound infections, traumatic wound infections, abscesses, other acutely infected ulcers, infected burn wounds (<20% body surface area), infected diabetic ulcers, and other cSSTIs requiring systemic antimicrobial therapy.
Model structure
Patients enter the model following diagnosis of a suspected or proven Gram-positive cSSTI (Figure 1). The model timeframe was 4 weeks to encompass the time needed for inpatient, outpatient, and second-line treatment of a Gram-positive cSSTI. The patient was initially treated for 2 days empirically (an average time per expert opinion that may be required to have culture and sensitivity results available from the laboratory) as a hospital inpatient with either vancomycin or cefazolin until confirmatory laboratory results were obtained. Hence, during this empiric treatment phase, 2 days of hospitalization costs and empiric drug treatment costs were generated. Following laboratory confirmation of MRSA cSSTI, patients received linezolid, vancomycin, or daptomycin for an additional 12 days.1,26 The total length of empiric and definitive antibiotic treatment was 14 days.1,10
Figure 1.
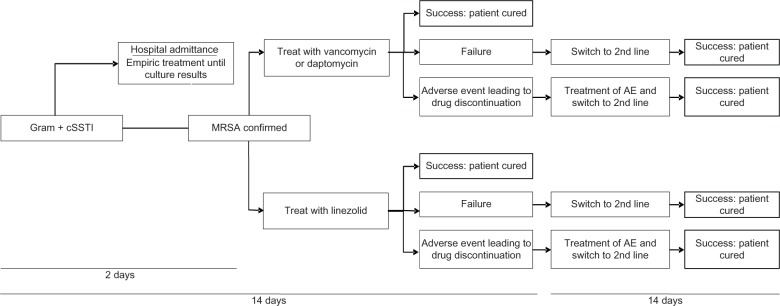
Model structure.
Abbreviations: cSSTI, complicated skin and soft tissue infection; AE, adverse events; MRSA, methicillin-resistant Staphylococcus aureus.
Subsequently, treatment can succeed, fail, or cause an adverse event leading to drug discontinuation. If the treatment succeeded, the patient received that one 14-day course of antibiotic treatment. If the treatment failed or was discontinued due to an adverse event, linezolid and vancomycin patients were switched to second-line daptomycin, and first-line daptomycin patients to second-line linezolid therapy based on expert opinion that if patients were failing initial therapy they would not be switched to vancomycin, but rather a newer agent with better tissue penetration. Economic consequences for patients failing first-line therapy or discontinuing first-line therapy due to an adverse event were 5 additional days of hospital stay27 and second-line drug therapy for 14 days.1
Model inputs
The model’s clinical inputs and resource use components are outlined in Table 1.
Table 1.
Model input data
| Linezolid | Vancomycin | Daptomycin | Data source | |
|---|---|---|---|---|
| Clinical inputs | ||||
| First-line treatment | ||||
| Success, % | 89.5 | 89.5 | 89.5a | Itani et al7,28 |
| Failure or discontinuation, %b | 10.5 | 10.5 | 10.5 | (Calculated from above) |
| Second-line treatment | ||||
| Success, % | 100 | 100 | 100 | Assumption |
| Resource inputs | ||||
| Hospital stay, days | 4.5 | 4.5 | 4.5 | Barrett et al16 |
| Hospital stay due to failure or discontinuation, additional daysb | 5 | 5 | 5 | Edelsberg et al27 |
| Empiric antibiotic therapy, days | 2 | 2 | 2 | De Cock et al,21 Schurmann et al23 |
| Definitive antibiotic therapy, days | 12 | 12 | 12 | Weigelt et al,10 Liu et al1 |
| IV therapy, days | 2.5 | 12 | 12 | |
| Oral therapy, days | 9.5 | 0 | 0 | |
| Intravenous doses per day | 2 | 2 | 1 | Product label,2 Tice46 |
| Oral doses per day | 2 | 0 | 0 | Product label2 |
| ID physician inpatient visits | 1 | 1 | 1 | Tice et al,43 assumption |
| ID physician office visits | 1 | 1 | 1 | Tice et al,43 assumption |
| Outpatient laboratory work | 1 | 1 | 1 | Tice46 |
| Outpatient parenteral antibiotic days | 0 | 9.5 | 9.5 | Calculated from above, also consistent with Itani et al7 |
| PICC placement | 0 | 1 | 1 | Tice46 |
| PICC complications | 0 | 1c | 1c | Tice et al,31 Moureau et al30 |
Notes:
Assumed to be same as linezolid/vancomycin for the base-case analysis; no direct trial comparison with linezolid and daptomycin;
discontinuation due to adverse event;
PICC complication costs will be added to all patients undergoing PICC placement. These costs are further detailed in Table 2.
Abbreviations: ID, infectious disease; IV, intravenous; PICC, peripherally inserted central catheter.
Clinical inputs
Drug efficacy was estimated from a randomized, open-label, controlled, multicenter, Phase 4 study (trial 1002) which compared linezolid with vancomycin therapy in patients with MRSA-confirmed cSSTI.7 The difference in clinical response at end of treatment between linezolid- and vancomycin-treated patients in this study was not statistically significant. Therefore, the linezolid and vancomycin results were pooled, and a weighted average efficacy rate of 89.5% was utilized.28 Noninferiority of daptomycin to comparator drugs (mainly vancomycin) has been reported in the daptomycin product label, thus for the base-case analysis, we assumed that daptomycin would have similar efficacy to vancomycin and linezolid. A meta-analysis for MRSA cSTTI was later used in sensitivity analyses of the model which provided differential efficacy rates.8
We assumed that the remaining 10.5% of patients were switched to daptomycin or linezolid second-line therapy1 due to treatment failure with first-line therapy or in the case of an adverse event requiring treatment discontinuation. All patients were assumed to successfully finish second-line therapy, an assumption also made by others.20 At the end of our 4-week model, all patients were assumed cured.
Resource inputs
In our model, we distinguished between resources used for empiric, first-line, and second-line treatments. Resources included in the model are the following:
Days of antibiotic treatment
Days of intravenous therapy
Hospital stay: general ward
Infectious disease specialist visits
Physician office visits
Outpatient laboratory tests
Days of OPAT homecare
Infusion center visits (for patients not receiving OPAT in the home)
PICC placement and complications.
All patients received antibiotic therapy for a minimum of 14 days, which included initial empiric treatment for 2 days (while waiting for culture results) followed by first-line treatment for 12 days. Empiric treatment assumed in the model was either intravenous vancomycin 1 g every 12 hours or cefazolin 1 g every 8 hours. First-line treatments in the model were either 2 days of inpatient and 10 days of outpatient intravenous vancomycin 1 g every 12 hours, or 2 days of inpatient intravenous linezolid 600 mg every 12 hours followed by 10 days of outpatient oral linezolid 600 mg every 12 hours, or 2 days of inpatient and 10 days outpatient intravenous daptomycin 4 mg/kg (300 mg for 75 kg patient) every 24 hours.
All patients were assumed treated as a hospital inpatient for 4.5 days based on the Healthcare Cost and Utilization Project data.16 This length of stay value was chosen over the global trial length of stay data (7.6 days linezolid versus 8.9 days vancomycin) because the trial data represented a mix of countries. In regional analyses of the trial data, the US-specific data also suggested a length of stay of ~5 days,29 thus confirming the nationally representative data. The hospital stay was assumed to be in a general ward. Patients receiving linezolid were switched from intravenous to oral therapy at hospital discharge. Patients receiving vancomycin or daptomycin were discharged home through an OPAT program.
Resource utilization was also attached to treatment failures and adverse events that resulted in drug discontinuation. Results from clinical studies suggested that drug failures and discontinuations due to adverse events would not differ substantially between the treatment arms.5,7,9,10 Additional resources for treatment of drug failures and adverse events resulting in drug discontinuation were deemed equal to the cost of five additional hospital days28 plus the cost of 14 days of second-line therapy. We assumed that each patient would require one infectious disease specialist consult during their inpatient stay. Vancomycin arm adverse events were assigned in the empiric phase of treatment and not again in the MRSA-confirmed phase of treatment. This was an assumption in favor of vancomycin, as patients assigned to the linezolid or daptomycin arm received vancomycin adverse event costs in the empiric phase, as well as linezolid/daptomycin adverse event costs in the definitive treatment phase.
Outpatient resource use was based on the published literature30–33 and supplemented with expert opinion when necessary for resource items such as frequency of outpatient visits (ie, all patients were expected to require one outpatient follow-up visit with a physician). Outpatient parenteral antibiotic therapy and a PICC line were required by patients receiving vancomycin or daptomycin, while linezolid patients received oral therapy as an outpatient.
Cost inputs
Wholesale acquisition price was utilized for drug costs (Table 2).34 Medical care resource unit costs were retrieved from the published literature30,32,33,35 and estimated based on current procedural terminology codes and corresponding fee schedules (Table 2).36 If needed, costs were adjusted to 2011 US dollars using the medical component of the Consumer Price Index.37
Table 2.
Cost inputs
| Cost inputs | Cost adjusted to 2011 (US$)a | Data source |
|---|---|---|
| Drug costs (wholesale acquisition cost per dose) | ||
| Linezolid IV 600 mg | $107.10 | First DataBank, Inc34 |
| Linezolid oral 600 mg | $89.39 | First DataBank, Inc34 |
| Vancomycin IV 1 g | $5.79 | First DataBank, Inc34 |
| Cefazolin IV 1 g | $2.19 | First DataBank, Inc34 |
| Daptomycin IV 300 mg (500 mg vial used) | $256.29 | First DataBank, Inc34 |
| Injection costs per dose | $7.21 | Tice et al33 |
| Medical costs | ||
| General ward, per day | $1,348.50 | Candrilli and Mauskopf35 |
| ID specialist, inpatient visit | $231.95 | CPT 99253, inpatient consult, 50th percentile, Ingenix36 |
| Physician outpatient visit | $222.40 | CPT 99215, office visit, 50th percentile, Ingenix36 |
| Laboratory tests, vancomycin | $101.06 | CPT 80069 renal function $64.92 + CPT 80202 vancomycin level $36.14, Ingenix36 |
| Laboratory tests, linezolid | $34.34 | CPT 85025 – complete blood count, Ingenix36 |
| OPAT daily cost | $193.67 | Tice et al32 (fee-for-service with medication related costs removed) |
| PICC placement | $656.80 | CPT 36569 for placement $561.58 + CPT 71010 for X-ray $95.22, 50th percentile, Ingenix36 |
| PICC complication cost per patient | $153.81 | |
| • Physician visit | • 17% (Tice et al31) @ CPT 99215, $222.40 (Ingenix36) | |
| • Declotting procedure with CathFlo | • 7% (Tice et al31) @ CPT 36593 $199.66 (Ingenix36) and drug cost $106.33 (First DataBank34) | |
| • Emergency room visit | • 2% (Moureau et al30) @ CPT 99284 $380.40 (Ingenix36) + facility fees of $222.58 | |
| • Rehospitalization | • 1% (Moureau et al30) @ DRG 920 $5,114.60 (2008 Median cost from HCUP adjusted to US$ 2011) | |
| • PICC replacement including chest X-ray | • 5% (Moureau et al30) @ CPT 36584 $532.42 + CPT 71010 $95.22 (Ingenix36) | |
Notes:
Drug prices reported as wholesale acquisition costs 2011, all other costs adjusted to 2011 US$.
Abbreviations: IV, intravenous; ID, infectious disease; OPAT, outpatient therapy; PICC, peripherally inserted central catheter; CPT, current procedural terminology; DRG, diagnosis-related group; HCUP, Healthcare Cost and Utilization Project.
Model assumptions
As information from the literature was lacking in several areas, the following assumptions were made that were subsequently verified by interviews with an infectious disease physician specializing in OPAT and a homecare intravenous therapy nurse familiar with OPAT. In some cases, the assumptions were also utilized in other pharmacoeconomic analyses:
Empiric treatment was with either vancomycin (50% of cases) or cefazolin (50% of cases) while culture results were pending.
Culture results confirming MRSA were obtained after 2 days of empiric treatment,23 at which point definitive treatment could be established with either linezolid vancomycin, or daptomycin.
Second-line treatment was administered for 14 days and was assumed to be effective.20
Resource use associated with drug failure or adverse events resulting in drug discontinuation was similar between all treatment arms.
Every patient survives during the period of antibiotic treatment.20 Furthermore, the Itani trial7 mortality rates were <1% and did not differ between treatments, thus mortality was not included in the analysis.
Base-case analysis
We conducted a cost-minimization analysis using our base-case data because no difference in efficacy was assumed. Total direct medical costs across the 4-week period were calculated for each drug. Additionally, we disaggregated costs by care setting (inpatient or outpatient), cost type (drug-related or medical), and resource component (eg, hospital stay, physician visits, PICC placement).
Sensitivity analyses
We conducted several scenario-based sensitivity analyses in which the assumptions for resource use or efficacy were varied. Scenarios were identified related to the variables found to have greatest influence on overall results.
Scenario 1: trial-based length of stay
In the first scenario, we altered length of stay by incorporating data from a Phase 4 study comparing linezolid with vancomycin therapy in patients with MRSA cSSTI.7 This scenario utilized a differential length of stay of 7.6 days for linezolid-treated patients and an 8.9-day length of stay for vancomycin and daptomycin (assumed to be similar to vancomycin) treated patients. In the base-case analysis, length of stay was 4.5 days for patients on all treatments.
Scenario 2: PICC costs excluded
In the second scenario, we excluded PICC placement costs and their associated complications. In an effort to capture the full cost of outpatient vancomycin or daptomycin therapy, we included PICC costs in our base-case model; to our knowledge, this is the only published economic study where such treatment comparisons for MRSA cSSTI include PICC cost data, therefore this sensitivity analysis evaluated the impact of use of PICC lines on economic outcomes related to OPAT
Scenario 3: efficacy rates from meta-analysis
In the final scenario we incorporated efficacy rates from a Bayesian meta-analysis8 into our model. Results from this meta-analysis suggested different efficacy rates for patients receiving linezolid (84.4%) compared with vancomycin (74.7%) or daptomycin (78.1%) for treatment of MRSA cSSTI. Thus, for this scenario we performed a cost-effectiveness analysis which incorporated quality-of-life assumptions. Differences in costs and quality-adjusted life years (QALYs) were determined. Utility values were not identified in the published literature for cSSTI. Therefore, we used proxies from diabetic foot infection, dermatologic disease, and other infections to estimate utility values.38–40 Utilities included in the current model were MRSA infection (0.44),40 treatment success (1, assumption), treatment failure (0.93),39 and drug discontinuation due to an adverse event (0.93).38 If a patient suffered from multiple health events at the same time, the corresponding utility values were calculated using the multiplicative method. For example, if a patient with an MRSA infection also experienced an adverse event resulting in drug discontinuation, the utility value was 0.44 × 0.93 = 0.41.
Results
Base-case analysis
The total cost per patient was US$10,571 for linezolid, US$11,096 for vancomycin, and US$13,612 for daptomycin treatment in the base-case analysis (Table 3). Treatment costs were lower by US$526 and US$3,042 per patient over the 4-week model timeframe for patients who received linezolid compared with vancomycin and daptomycin respectively.
Table 3.
Base-case analysis results
| Costs per patient (US$) | Linezolid (US$) | Vancomycin (US$) | Daptomycin (US$) |
|---|---|---|---|
| Total inpatient costs | $7,835 | $7,095 | $7,922 |
| Inpatient drug costs | $626 | $119 | $713 |
| Drug treatment | $554 | $47 | $659 |
| Drug administration | $72 | $72 | $54 |
| Inpatient medical costs | $7,209 | $6,976 | $7,209 |
| Hospital days | $6,068 | $6,068 | $6,068 |
| ID specialist | $232 | $232 | $232 |
| Adverse event costsa | $435 | $201 | $435 |
| Failure costs | $474 | $474 | $474 |
| Total outpatient costs | $2,736 | $4,001 | $5,690 |
| Outpatient drug costs | $2,086 | $634 | $2,766 |
| Drug treatment | $2,075 | $487 | $2,698 |
| Drug administration | $11 | $148 | $68 |
| Outpatient medical costs | $650 | $3,367 | $2,924 |
| Physician visit | $246 | $246 | $246 |
| Laboratory work | $34 | $101 | $28 |
| OPAT costb | $285 | $2,125 | $1,840 |
| PICC line and complicationsb | $85 | $896 | $811 |
| Total cost of treatment | $10,571 | $11,096 | $13,612 |
| Total drug costs | $2,712 | $754 | $3,479 |
| Total medical costs | $7,859 | $10,343 | $10,133 |
Notes: Costs may not tally due to rounding.
Adverse event costs are higher for linezolid and daptomycin arm because they are assigned the vancomycin adverse event costs from empiric treatment plus any linezolid/daptomycin adverse event costs. Vancomycin is only assigned adverse event costs once;
OPAT and PICC line costs are incurred in the linezolid arm through treatment failures and switching to other IV antibiotics.
Abbreviations: ID, infectious disease; OPAT, outpatient parenteral antibiotic therapy; PICC, peripherally inserted central catheter; IV, intravenous.
Analysis by site of care showed higher inpatient treatment costs with daptomycin and linezolid (US$7,922 and US$7,835 respectively) compared with vancomycin therapy (US$7,095). The higher inpatient linezolid and daptomycin treatment costs resulted from increased drug acquisition costs. In contrast, outpatient treatment costs were lower with linezolid (US$2,736) compared with vancomycin (US$4,001) and daptomycin (US$5,690) therapy, resulting from lower outpatient medical costs, specifically OPAT, PICC line, and laboratory costs.
Similarly, analysis by cost type found that total drug costs were US$1,958 higher but medical costs were US$2,484 lower with linezolid compared with vancomycin therapy. For daptomycin, both drug and medical costs were higher than linezolid and vancomycin. Once again, this decrease in medical costs for linezolid was derived from lower OPAT, PICC, and laboratory costs. Overall, outpatient medical costs were fivefold lower with linezolid than with vancomycin or daptomycin therapy.
Sensitivity analyses
Scenario 1: trial-based length of stay
In this scenario, the total cost per patient for linezolid treatment was US$14,906 compared with US$16,178 for vancomycin and US$18,694 for daptomycin (Table 4). Linezolid compared with vancomycin and daptomycin treatment saved US$1,272 and US$3,788, respectively, per patient over the 4-week model timeframe. The cost savings realized with linezolid therapy in this scenario were greater than in the base-case analysis. This additional cost savings resulted from a decrease in inpatient medical costs primarily due to a decrease in length of stay for linezolid compared with vancomycin- or daptomycin-treated patients.
Table 4.
Scenario-based sensitivity analyses
| Scenarios | Linezolid total cost (US$) | Vancomycin total cost (US$) | Daptomycin total cost (US$) |
|---|---|---|---|
| Scenario 1, trial-based length of stay7 | $14,906 | $16,178 | $18,694 |
| Linezolid, 7.6 days | |||
| Vancomycin and daptomycin, 8.9 days | |||
| Scenario 2, no PICC line costs | $10,486 | $10,201 | $12,802 |
| Scenario 3, meta analysis efficacy rates8 | $11,458 | $13,030 | $14,856 |
| Linezolid, 84.4% (credible interval: 76.6%–90.6%) | QALY = 0.063 | QALY = 0.060 | QALY = 0.061 |
| Vancomycin, 74.7% (credible interval: 64.1%–83.5%) | ICER = dominated by Linezolid | ICER = dominated by Linezolid | |
| Daptomycin, 78.1% (credible interval: 54.6%–93.2%) |
Abbreviations: ICER, incremental cost effectiveness ratio; PICC, peripherally inserted central catheter; QALY, quality-adjusted life years.
Scenario 2: PICC costs excluded
In this scenario, the total cost per patient for linezolid treatment was US$10,486, just slightly higher than the cost for vancomycin (US$10,201), but still substantially lower than daptomycin therapy (US$12,802) (Table 4). Thus, in some cases where a patient did not require a PICC line for vancomycin administration, linezolid therapy costs were similar to those of vancomycin: approximately US$285 more per patient over the 4-week model timeframe.
Scenario 3: efficacy rates from meta-analysis
In this scenario linezolid treatment was dominant, having more QALYs gained and a lower treatment cost compared with vancomycin and daptomycin treatment (Table 4). Gains in QALYs were very small due to the short model timeframe, and were higher for linezolid-treated patients by 0.002 QALYs and 0.001 QALYs compared with vancomycin and daptomycin respectively. Similarly, treatment costs were lower for linezolid compared with vancomycin and daptomycin (Table 4).
An assessment of the detailed cost breakdown indicated that the most influential model variables were success rate, followed by length of hospital stay, OPAT costs, and PICC line utilization and complications. These were the scenarios presented in Table 4. Drug prices and OPAT costs would be sensitive by definition as well; however, these would be fixed for a given health care system.
Discussion
The aim of our economic model was to provide a flexible framework capable of assessing and comparing the value of intravenous/oral linezolid, intravenous vancomycin, and intravenous daptomycin in the treatment of MRSA cSSTI.
In our conservative base-case analysis of equal efficacy, equal length of stay, and resource use, linezolid had lower total medical costs compared with vancomycin and daptomycin therapy. Linezolid’s higher drug price was offset by lower outpatient medical costs, specifically the cost of OPAT, PICC utilization and complications, and laboratory monitoring for vancomycin. On the contrary, vancomycin had significantly lower drug prices, but they were offset by higher outpatient medical costs. Daptomycin was found to have higher drug prices, and being only intravenous, had greater outpatient medical costs due to OPAT. As a result, its aggregated costs made it more expensive compared with linezolid and vancomycin.
Results were similar when efficacy rates and length of stay data from clinical studies were incorporated into the model. Linezolid was cost-saving compared with the other two therapies when length of stay was adjusted to 7.6 days for linezolid and 8.9 days for vancomycin and daptomycin based on previously published study results.7 Likewise, linezolid had lower total costs compared with vancomycin and daptomycin therapy when efficacy rates were adjusted based on results obtained from a Bayesian meta-analysis.8 In this scenario, linezolid had marginally higher QALY gains and when combined with lower costs, made it a in dominant strategy.
Our outcomes were similar to results from several studies reported in the published medical literature, where linezolid had overall lower costs compared with vancomycin, mainly due to fewer inpatient days and ability to switch to an oral formulation.9,12,13,21–24,41,43,44
In contrast, one published cost-effectiveness analysis in 2009 by Bounthavong and coworkers reported higher costs with linezolid than vancomycin in the treatment of MRSA cSSTI.20 The total direct costs for treatment with inpatient followed by outpatient intravenous vancomycin was US$9,750, inpatient intravenous vancomycin followed by outpatient oral linezolid was US$10,018, inpatient intravenous vancomycin for the treatment duration was US$16,686, and intravenous inpatient linezolid followed by oral inpatient and then outpatient linezolid was US$10,975.
There were several differences between our study and the 2009 study conducted by Bounthavong and coworkers.20 First, although Bounthavong and coworkers mentioned nursing labor costs for OPAT, these costs were not included in their list of base-case costs. In comparison, our model included the cost of vancomycin OPAT either administered at home or at an infusion center. Second, Bounthavong and coworkers did not incorporate the cost of a PICC line for outpatient administration of vancomycin into their model. Evidence suggested that vancomycin should be administered through a central catheter to decrease the risk of phlebitis.45 Last, hospital length of stay varied between our model and that by Bounthavong. In our base-case analysis, we used a 4.5-day length of stay for all patients. This timeframe was chosen based on the average length of stay for patients with SSTI.16 In our second scenario, we used a 1-day shorter length of stay for linezolid-compared with vancomycin-treated patients based on data from a published clinical study that evaluated linezolid and vancomycin treatment in patients with MRSA cSSTI.7 In contrast, Bounthavong used an approximately 2-day shorter inpatient stay for patients who received vancomycin rather than linezolid, a fact not supported by data in the published literature. Data from published studies suggested a shorter hospital stay for patients received linezolid than vancomycin for complicated MRSA SSTI.7,9,11–15 A shorter length of hospital stay with linezolid compared with vancomycin therapy was evident even when there were no barriers to a vancomycin OPAT program.7 From data published by Bounthavong, a 2-day increase in hospital length of stay for linezolid-treated patients increased costs by approximately US$2,500 (unexplained in the article), much more than the cost difference between intravenous inpatient followed by outpatient vancomycin (US$9,750) and inpatient intravenous than oral linezolid followed by outpatient oral linezolid (US$10,975).
Recently, these authors published another cost-effectiveness analysis using Bayesian methods and added daptomycin as a treatment comparator. They reported that linezolid was dominant (better outcomes, lower costs) compared with vancomycin and daptomycin when delivered in the inpatient setting.24 Our current analysis, which includes both inpatient and outpatient management of cSSTI, further added to the literature regarding potential cost aspects of vancomycin and daptomycin OPAT delivery.
As mentioned earlier, there were very few studies that have assessed the economic impact of daptomycin for the treatment of MRSA cSSTI. In addition to the study described above, a study conducted by Davis and colleagues in 2007 compared daptomycin with vancomycin and reported the former to be more cost-effective.25 However, this study had a nonrandomized prospective data analytical design (and not cost-effectiveness), did not correct success rates for the imbalance in MRSA cases (daptomycin 42% MRSA versus 75% MRSA in the vancomycin group, P < 0.001), and focused only on inpatient costs.
What are the implications of our analysis? First, this analysis highlights the value of including an appropriate analysis timeframe to include the full treatment period. In our analysis, we included all relevant inpatient and outpatient costs for the treatment period. Second, this analysis shows the continuing shift of costs from the inpatient to outpatient setting where some 20%–35% of costs were incurred, depending on drug used. Lastly, this analysis, similar to previously reported analyses,9,12,13,21–24 demonstrates that despite its higher acquisition cost, linezolid compared with vancomycin and daptomycin for the treatment of MRSA cSSTI has a more favorable economic profile from a US, third-party payer perspective.
Several limitations exist with this model, but are not uncommon in economic modeling studies. Due to lack of available evidence, this model is partly based on expert opinion. Clearly, expert opinion is not the highest level of evidence. However, combining expert opinion with trial data and managed care resource utilization data allows estimation of the cost savings and cost-effectiveness of the considered antibiotics.
The impact of adverse events on both clinical and economic outcomes is hard to assess. In our model, adverse events were assigned to the vancomycin arm in the empiric but not definitive phase of treatment. Whereas patients receiving empiric vancomycin followed by definitive linezolid had adverse event costs added in both the empiric and definitive treatment phases, those patients receiving empiric vancomycin followed by definitive vancomycin only had adverse event costs added during the empiric phase. This provided a slight cost advantage for vancomycin compared with linezolid therapy.
The assumption that second-line treatment is 100% effective is clearly a simplification of reality. However, this assumption was discussed and considered reasonable by clinical experts because there are no published efficacy data for second-line treatments in patients with MRSA cSSTI. This assumption was also used in a previously published model20 and required to avoid extrapolation of the time horizon beyond 4 weeks. Nevertheless, we believe the base-case analysis remains conservative.
The assumption that no patients died during the 4-week model timeframe is also a simplification of reality. However, evidence suggests that mortality rates are similar between patients with MRSA cSSTI treated with linezolid or vancomycin.5 Therefore, costs due to mortality would be similar for both treatment arms and were not included, a decision not unique to our study.20
Lastly, the true cost of OPAT is not known. Outpatient infusion of intravenous antibiotics is a complex process. In a study designed to map out an OPAT program, six processes, 67 sub-processes, and 217 possible failures were identified.46 In the US, several different models for OPAT are utilized. Patients can travel to an infusion center for drug administration, or the patient, a caregiver, or a visiting nurse or other health care professional can administer the drug at the patient’s home.47 Obviously, the costs associated with these models will differ. In one study, reimbursement for vancomycin OPAT primarily administered by the patient in their home ranged from US$103 to US$268 per day.29 Regardless of OPAT model utilized, all are more complex, costly, and time intensive than oral antibiotic administration.
Outpatient parenteral antibiotic therapy also requires patients to have intravenous access. There are numerous options for intravenous access, each associated with different costs and complications. We opted to include the cost of a PICC line into our model. In one study, more than 50% of patients receiving OPAT with vancomycin had a PICC line.32 Recommendations are for drugs with moderate phlebitis risk, such as vancomycin, to be administered through a central line.45 Regardless of intravenous catheter utilized, costs and complications associated with that catheter would be higher than those for patients not needing intravenous access.
Conclusion
This economic analysis is an update to a previously presented analysis, and it provides an important perspective on the inpatient and outpatient cost breakdown for the treatment of patients with cSSTI due to MRSA.48 Total treatment costs in the base-case analysis for patients with MRSA cSSTI were lower for linezolid than vancomycin and daptomycin therapy even though total drug acquisition costs were higher for linezolid versus vancomycin. When looking specifically at the total outpatient costs in our analysis, a linezolid oral strategy was 30%–50% less costly than OPAT with vancomycin or daptomycin. Additional benefits could be realized if indirect costs were evaluated. Recent medical literature suggests that patients would prefer oral compared with intravenous administration when outcomes are similar in order to maintain work productivity and freedom of activities once they leave the hospital.49
OPAT is a reasonable option for patients well enough to leave the hospital but still needing intravenous antibiotic therapy. However, OPAT treatment guidelines state that this method of drug administration be reserved for cases where other routes of drug administration are not an option.42 With linezolid approved as an effective oral option for treating patients with MRSA, the economic value should be reconsidered by managed care plans evaluating outpatient treatment strategies for completing antibiotic therapies. Our results, like those from most previously published studies,9,12,13,21–24 show that the budget impact of linezolid compared with vancomycin and daptomycin for the treatment of MRSA cSSTI is similar or cost saving when the total costs of inpatient and outpatient therapy are included.
Acknowledgments
The authors would like to thank Beth Lesher and Michelle Li from Pharmerit North America for assistance with literature review and synthesis and editorial assistance for the manuscript for submission.
Author contributions
JMS, XG, BGV, SH, and AS contributed to model conceptualization, design, and assumptions. JMS, XG, BGV, and DAP participated in development of the model inputs, programming, and analyses. DAP and JMS revised and updated the model for publication. All authors contributed to the review and interpretation of results and the development of the manuscript.
Disclosure
Pharmerit North America LLC received research funding from Pfizer Inc for development of the model. No funding was received for the development of the manuscript. JMS, XG, BGV, and DAP are employees of Pharmerit. SH and AS are employees of Pfizer Inc and hold employee stock options.
References
- 1.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 2.Zyvox® [package insert] New York, NY: Pharmacia and Upjohn Company; 2010. [Google Scholar]
- 3.Bartlett J. Impact of new oral antibiotics on the treatment of infectious diseases. Infect Dis Clin Pract. 1993;2:405–413. [Google Scholar]
- 4.Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006;90:1197–1222. doi: 10.1016/j.mcna.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Bounthavong M, Hsu DI. Efficacy and safety of linezolid in methicillin-resistant Staphylococcus aureus (MRSA) complicated skin and soft tissue infection (cSSTI): a meta-analysis. Curr Med Res Opin. 2010;26:407–421. doi: 10.1185/03007990903454912. [DOI] [PubMed] [Google Scholar]
- 6.Dodds TJ, Hawke CI. Linezolid versus vancomycin for MRSA skin and soft tissue infections (systematic review and meta-analysis) ANZ J Surg. 2009;79:629–635. doi: 10.1111/j.1445-2197.2009.05018.x. [DOI] [PubMed] [Google Scholar]
- 7.Itani KM, Dryden MS, Bhattacharyya H, Kunkel MJ, Baruch AM, Weigelt JA. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be caused by methicillin-resistant Staphylococcus aureus. Am J Surg. 2010;199:804–816. doi: 10.1016/j.amjsurg.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 8.Logman JF, Stephens J, Heeg B, et al. Comparative effectiveness of antibiotics for the treatment of MRSA complicated skin and soft tissue infections. Curr Med Res Opin. 2010;26:1565–1578. doi: 10.1185/03007995.2010.481251. [DOI] [PubMed] [Google Scholar]
- 9.Sharpe JN, Shively EH, Polk HC., Jr Clinical and economic outcomes of oral linezolid versus intravenous vancomycin in the treatment of MRSA-complicated, lower-extremity skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. Am J Surg. 2005;189:425–428. doi: 10.1016/j.amjsurg.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Weigelt J, Itani K, Stevens D, Lau W, Dryden M, Knirsch C. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother. 2005;49:2260–2266. doi: 10.1128/AAC.49.6.2260-2266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itani KM, Weigelt J, Li JZ, Duttagupta S. Linezolid reduces length of stay and duration of intravenous treatment compared with vancomycin for complicated skin and soft tissue infections due to suspected or proven methicillin-resistant Staphylococcus aureus (MRSA) Int J Antimicrob Agents. 2005;26:442–448. doi: 10.1016/j.ijantimicag.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 12.McCollum M, Sorensen SV, Liu LZ. A comparison of costs and hospital length of stay associated with intravenous/oral linezolid or intravenous vancomycin treatment of complicated skin and soft-tissue infections caused by suspected or confirmed methicillin-resistant Staphylococcus aureus in elderly US patients. Clin Ther. 2007;29:469–477. doi: 10.1016/s0149-2918(07)80085-3. [DOI] [PubMed] [Google Scholar]
- 13.McKinnon PS, Sorensen SV, Liu LZ, Itani KM. Impact of linezolid on economic outcomes and determinants of cost in a clinical trial evaluating patients with MRSA complicated skin and soft-tissue infections. Ann Pharmacother. 2006;40:1017–1023. doi: 10.1345/aph.1G728. [DOI] [PubMed] [Google Scholar]
- 14.Caffrey AR, Quilliam BJ, LaPlante KL. Comparative effectiveness of linezolid and vancomycin among a national cohort of patients infected with methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:4394–4400. doi: 10.1128/AAC.00200-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li JZ, Willke RJ, Rittenhouse BE, Rybak MJ. Effect of linezolid versus vancomycin on length of hospital stay in patients with complicated skin and soft tissue infections caused by known or suspected methicillin-resistant Staphylococci: results from a randomized clinical trial. Surg Infect (Larchmt) 2003;4:57–70. doi: 10.1089/109629603764655290. [DOI] [PubMed] [Google Scholar]
- 16.Barrett M, Wilson E, Whalen D. HCUP Methods Series report # 2010–2003. US Agency for Healthcare Research and Quality; 2010. 2007 HCUP Nationwide Inpatient Sample (NIS) comparison report. [Google Scholar]
- 17.Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004) Diagn Microbiol Infect Dis. 2007;57:7–13. doi: 10.1016/j.diagmicrobio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Nicolau DP. Containing costs and containing bugs: are they mutually exclusive? J Manag Care Pharm. 2009;15:S12–S17. doi: 10.18553/jmcp.2009.15.s2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah NP, Reddy P, Paladino JA, McKinnon PS, Klepser ME, Pashos CL. Direct medical costs associated with using vancomycin in methicillin-resistant Staphylococcus aureus infections: an economic model. Curr Med Res Opin. 2004;20:779–790. doi: 10.1185/030079904125003638. [DOI] [PubMed] [Google Scholar]
- 20.Bounthavong M, Hsu DI, Okamoto MP. Cost-effectiveness analysis of linezolid vs vancomycin in treating methicillin-resistant Staphylococcus aureus complicated skin and soft tissue infections using a decision analytic model. Int J Clin Pract. 2009;63:376–386. doi: 10.1111/j.1742-1241.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- 21.De Cock E, Sorensen S, Levrat F, et al. Cost-effectiveness of linezolid versus vancomycin for hospitalized patients with complicated skin and soft-tissue infections in France. Med Mal Infect. 2009;39:330–340. doi: 10.1016/j.medmal.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Patanwala AE, Erstad BL, Nix DE. Cost-effectiveness of linezolid and vancomycin in the treatment of surgical site infections. Curr Med Res Opin. 2007;23:185–193. doi: 10.1185/030079906X162700. [DOI] [PubMed] [Google Scholar]
- 23.Schurmann D, Sorensen SV, De Cock E, Duttagupta S, Resch A. Cost-effectiveness of linezolid versus vancomycin for hospitalised patients with complicated skin and soft-tissue infections in Germany. Eur J Health Econ. 2009;10:65–79. doi: 10.1007/s10198-008-0104-7. [DOI] [PubMed] [Google Scholar]
- 24.Bounthavong M, Zargarzadeh A, Hsu DI, Vanness DJ. Cost-effectiveness analysis of linezolid, daptomycin, and vancomycin in methicillin-resistant Staphylococcus aureus: complicated skin and skin structure infection using Bayesian methods for evidence synthesis. Value Health. 2011;14:631–639. doi: 10.1016/j.jval.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611–1618. doi: 10.1592/phco.27.12.1611. [DOI] [PubMed] [Google Scholar]
- 26.Weigelt J, Kaafarani HM, Itani KM, Swanson RN. Linezolid eradicates MRSA better than vancomycin from surgical-site infections. Am J Surg. 2004;188:760–766. doi: 10.1016/j.amjsurg.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 27.Edelsberg J, Berger A, Weber DJ, Mallick R, Kuznik A, Oster G. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29:160–169. doi: 10.1086/526444. [DOI] [PubMed] [Google Scholar]
- 28.Itani KMF, Weigelt J, Stevens MS, et al. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be due to methicillin-resistant Staphylococcus aureus (MRSA); Presentation at the 18th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); Reinach, Switzerland. April 2008. [Google Scholar]
- 29.Itani K, Sorensen S, Stokes M, Shelbaya A, McKinnon PS. A regional comparison of resource utilization in patients with methicillin-resistant Staphylococcus aureus (MRSA) complicated skin and soft tissue infections (cSSTI) treated with linezolid vs vancomycin; 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 12–15, 2009; San Francisco, CA. [Google Scholar]
- 30.Moureau N, Poole S, Murdock MA, Gray SM, Semba CP. Central venous catheters in home infusion care: outcomes analysis in 50,470 patients. J Vasc Interv Radiol. 2002;13:1009–1016. doi: 10.1016/s1051-0443(07)61865-x. [DOI] [PubMed] [Google Scholar]
- 31.Tice A, Bonstell R, Marsh PK, Craven PC, McEniry DW, Harding S. Peripherally inserted central venous catheters for outpatient intravenous antibiotic therapy. Infect Dis Clin Pract. 1993;2:186–190. [Google Scholar]
- 32.Tice AD, Hoaglund PA, Nolet B, McKinnon PS, Mozaffari E. Cost perspectives for outpatient intravenous antimicrobial therapy. Pharmacotherapy. 2002;22:63S–70S. doi: 10.1592/phco.22.4.63s.33653. [DOI] [PubMed] [Google Scholar]
- 33.Tice AD, Turpin RS, Hoey CT, Lipsky BA, Wu J, Abramson MA. Comparative costs of ertapenem and piperacillin-tazobactam in the treatment of diabetic foot infections. Am J Health Syst Pharm. 2007;64:1080–1086. doi: 10.1093/ajhp/64.10.1080. [DOI] [PubMed] [Google Scholar]
- 34.First Data Bank, Inc . Analy$ource Online. First DataBank, Inc; South San Francisco, CA: Apr 9, 2011. [Google Scholar]
- 35.Candrilli S, Mauskopf J. Value Health. 2006. How much does a hospital day cost? p. A56. [Google Scholar]
- 36.Ingenix . National Fee Analyzer. Eden Prairie, MN: Ingenix; 2011. [Google Scholar]
- 37.Consumer Price Index Medical Component [webpage on the Internet] Washington, DC: US Bureau of Labor Statistics; 2011Available from: http://www.bls.gov/CPIAccessed April 2011 [Google Scholar]
- 38.Chen SC, Bayoumi AM, Soon SL, et al. A catalog of dermatology utilities: a measure of the burden of skin diseases. J Investig Dermatol Symp Proc. 2004;9:160–168. doi: 10.1046/j.1087-0024.2003.09112.x. [DOI] [PubMed] [Google Scholar]
- 39.Ernst EJ, Ernst ME, Hoehns JD, Bergus GR. Women’s quality of life is decreased by acute cystitis and antibiotic adverse effects associated with treatment. Health Qual Life Outcomes. 2005;3:45. doi: 10.1186/1477-7525-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ragnarson Tennvall G, Apelqvist J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J Diabetes Complications. 2000;14:235–241. doi: 10.1016/s1056-8727(00)00133-1. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Medicare and Medicaid Services National Health Expenditure Web Tables Available from: http://www.cms.gov/NationalHealthExpendData/downloads/tables.pdfAccessed March 29, 2011
- 42.Menzin J, Marton JP, Meyers JL, Carson RT, Rothermel CD, Friedman M. Inpatient treatment patterns, outcomes, and costs of skin and skin structure infections because of Staphylococcus aureus. Am J Infect Control. 2010;38:44–49. doi: 10.1016/j.ajic.2009.04.287. [DOI] [PubMed] [Google Scholar]
- 43.Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651–1672. doi: 10.1086/420939. [DOI] [PubMed] [Google Scholar]
- 44.Marton JP, Jackel JL, Carson RT, Rothermel CD, Friedman M, Menzin J. Costs of skin and skin structure infections due to Staphylococcus aureus: an analysis of managed-care claims. Curr Med Res Opin. 2008;24:2821–2828. doi: 10.1185/03007990802365169. [DOI] [PubMed] [Google Scholar]
- 45.McKinnon PS, Carter CT, Girase PG, Liu LZ, Carmeli Y. The economic effect of oral linezolid versus intravenous vancomycin in the outpatient setting: the payer perspective. Manag Care Interface. 2007;20:23–34. [PubMed] [Google Scholar]
- 46.Tice A. Handbook of Outpatient Parenteral Antimicrobial Therapy. Tarrytown, NY: CRG Publishing; 2006. [Google Scholar]
- 47.Gilchrist M, Franklin BD, Patel JP. An outpatient parenteral antibiotic therapy (OPAT) map to identify risks associated with an OPAT service. J Antimicrob Chemother. 2008;62:177–183. doi: 10.1093/jac/dkn152. [DOI] [PubMed] [Google Scholar]
- 48.Stephens J, Gao X, Verheggen B, Shelbaya A, Haider S. Modeling the inpatient and outpatient costs of methicillin-resistant Staphyloccocus aureus (MRSA) complicated skin and soft tissue infections (cSSTI): a comparison of linezolid, vancomycin, daptomycin, and tigecycline; Poster presentation at the 14th Annual International Society for Pharmacoeconomics and Outcomes Research International Meeting; May 18–20, 2009; Orlando, FL, USA. [Google Scholar]
- 49.Paladino JA, Poretz D. Outpatient parenteral antimicrobial therapy today. Clin Infect Dis. 2010;51(Suppl 2):S198–S208. doi: 10.1086/653520. [DOI] [PubMed] [Google Scholar]


