Abstract
The mTOR inhibitor everolimus is currently approved for the treatment of renal cell carcinoma (RCC) and several Toll-like receptor 9 (TLR9) agonists, including immunomodulatory oligonucleotides (IMOs), have been tested for their therapeutic potential against advanced RCC. However, no clinical trials investigating the combination of mTOR inhibitors with TLR9 agonists in RCC patients have been performed to date. Our results may pave the way to translate this combinatorial approach to the clinical setting.
Keywords: mTOR, TLR9, everolimus, renal cell carcinoma, microenvironment
Several antiangiogenic agents have been approved in the last few years for the treatment of renal cell carcinoma (RCC), including the mammalian target of rapamycin (mTOR) inhibitors everolimus and temsirolimus. mTOR is a serine-threonine kinase that regulates cell growth, metabolism, proliferation, and motility by integrating a variety of signals that reflect mitogenic stimuli, nutrient availability, and energy status. The PI3K/AKT1/mTOR signal transduction pathway plays an important role in the response of cells to hypoxia and energy depletion, and these functions are highly relevant for the growth of RCC, which is often characterized by alterations of the von Hippel-Lindau (VHL) gene.1 Recent preclinical and clinical studies have demonstrated the antineoplastic activity of so-called “immunomodulatory oligonucleotides” (IMOs), second-generation Toll-like receptor 9 (TLR9) agonists.2 Beside exerting immunomodulatory functions, these agents mediate direct antineoplastic and antiangiogenic effects and appear to cooperate with both epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) inhibitors.3,4
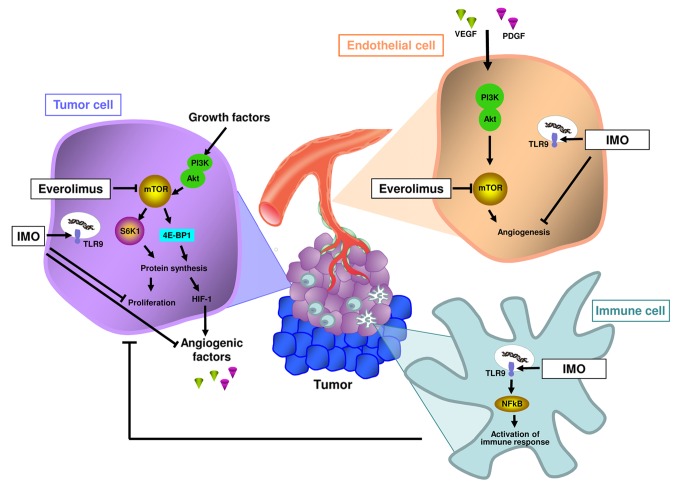
We have recently tested the combination of IMOs and everolimus in models of human RCC differing from each other with the respect to the VHL gene status, in vitro and in vivo.5 At least 2 pieces of evidence support the clinical use of such a combinatorial approach for the therapy of RCC. First, RCCs frequently express TLR9, which is associated with improved disease outcomes. A TLR9-stimulated immune response directed against RCC cells may explain the favorable influence of TLR9 on the course of RCC.6 Second, everolimus as well as IMOs exert antineoplastic effects not only as they directly target malignant cells, but also as they interfere with the functions of different cell populations of the tumor microenvironment, including immune, stromal and endothelial cells (Fig. 1).4,7 Of note, the contribution of the tumor microenvironment to the growth of RCCs may be particularly relevant in lesions that bear VHL mutations, resulting in the hyperactivation of hypoxia-inducible factor 1 (HIF-1) and hence a consistent production of pro-angiogenic factors.
Figure 1. Effects of immunomodulatory oligonucleotides and everolimus on neoplastic lesions. Mechanisms of action of immunomodulatory oligonucleotides (IMOs) and the mTOR inhibitor everolimus on different cell populations of the tumor microenvironment, including malignant, endothelial as well as immune cells.
Our findings indicate that both IMOs and everolimus inhibit the growth and survival of RCC cells as standalone therapeutic interventions, while their combinatorial administration generates a synergistic effect. Consistently with the notion that IMOs interfere with EGFR signaling3 and that mTOR is a key signal transducer downstream of PI3K/AKT1 pathway,1 the combination of IMOs and everolimus efficiently interfered with the EGFR pathway. Moreover, while everolimus induced the activation of AKT1 and mitogen-activated protein kinases (MAPKs) in some RCC cell lines, mostly due to loss of mTOR-S6K-dependent negative feedback loops,1 the concomitant administration of IMO robustly counteracted this process. As hypothesized on the basis of the well-known antiangiogenic effects of IMOs and everolimus, the combined administration of these agents efficiently inhibited the secretion of VEGF from all RCC cell lines tested. Moreover, IMO plus everolimus promoted long-lasting cooperative antitumor effects against RCC xenografts, irrespective of their VHL gene status, featuring a robust inhibition in tumorigenic signal transduction pathways, potent inhibition of tumor growth and significant increases in the survival of RCC-bearing mice. The antitumor activity of IMOs was particularly evident in the VHL mutant 786-O model, probably reflecting the effects of IMOs on the tumor microenvironment rather than on malignant cells. Functional studies on human umbilical vein endothelial cells (HUVECs), investigating their adhesion to basal membranes, migratory activity, and ability to form capillaries, clarified that the antiangiogenic effects observed upon the administration of everolimus and IMOs to tumor-bearing mice could be related not only to the reduction of VEGF secretion by cancer cells but also to inhibitory effects on endothelial cells.
Our study demonstrates that the combination of everolimus and IMOs is effective against several models of RCC, irrespective of their VHL gene status, as it interferes with tumor growth and angiogenesis, hence representing a promising therapeutic approach. In the last few years, the availability of new therapeutic agents caused a significant prolongation in the survival of RCC patients. Indeed, many RCC patients, after obtaining a clinical benefit from first-line chemotherapy (generally based on the multi-kinase inhibitor sunitinib), can initiate a second-line treatment and, upon further progression, a considerable fraction of them maintains a performance status good enough to receive a third-line therapy. Our findings suggest that novel, rational combinatorial approaches such as the co-administration of everolimus and IMOs may further ameliorate the course of RCC.5 Everolimus is currently approved for the treatment of RCC,8 and TLR9 agonists including IMOs have been tested in multicenter phase I/II studies for their therapeutic activity in advanced RCC.9,10 To date, however, no clinical trials investigating the combination of mTOR inhibitors with TLR9 agonists have been launched. Our results may pave the way to translate this combinatorial approach to clinical settings, perhaps even to situations in which patients are resistant to everolimus employed as standalone therapeutic interventions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25123
References
- 1.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal S, Kandimalla ER. Synthetic agonists of Toll-like receptors 7, 8 and 9. Biochem Soc Trans. 2007;35:1461–7. doi: 10.1042/BST0351461. [DOI] [PubMed] [Google Scholar]
- 3.Damiano V, Caputo R, Bianco R, D’Armiento FP, Leonardi A, De Placido S, et al. Novel toll-like receptor 9 agonist induces epidermal growth factor receptor (EGFR) inhibition and synergistic antitumor activity with EGFR inhibitors. Clin Cancer Res. 2006;12:577–83. doi: 10.1158/1078-0432.CCR-05-1943. [DOI] [PubMed] [Google Scholar]
- 4.Damiano V, Caputo R, Garofalo S, Bianco R, Rosa R, Merola G, et al. TLR9 agonist acts by different mechanisms synergizing with bevacizumab in sensitive and cetuximab-resistant colon cancer xenografts. Proc Natl Acad Sci U S A. 2007;104:12468–73. doi: 10.1073/pnas.0705226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damiano V, Rosa R, Formisano L, Nappi L, Gelardi T, Marciano R, et al. Toll-like receptor 9 agonist IMO cooperates with everolimus in renal cell carcinoma by interfering with tumour growth and angiogenesis. Br J Cancer. 2013;108:1616–23. doi: 10.1038/bjc.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronkainen H, Hirvikoski P, Kauppila S, Vuopala KS, Paavonen TK, Selander KS, et al. Absent Toll-like receptor-9 expression predicts poor prognosis in renal cell carcinoma. J Exp Clin Cancer Res. 2011;30:84. doi: 10.1186/1756-9966-30-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan F, Schimming A, Jaeger D, Podar K. Targeting the tumor microenvironment: focus on angiogenesis. J Oncol. 2012;2012:281261. doi: 10.1155/2012/281261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. RECORD-1 Study Group Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 9.Kuzel T, Dutcher J, Ebbinghaus S, Gordon M, Grubbs S, Khan K, et al. A phase 2 multicenter, randomized, open-label study of two dose levels of IMO-2055 in patients with metastatic or recurrent renal cell carcinoma. Proc 8th Intern Kidney Cancer Symposium 2009, Sep 25–26 2009, Chicago, IL, USA [Google Scholar]
- 10.Thompson JA, Kuzel T, Drucker BJ, Urba WJ, Bukowski RM. Safety and efficacy of PF-3512676 for the treatment of stage IV renal cell carcinoma: an open-label, multicenter phase I/II study. Clin Genitourin Cancer. 2009;7:E58–65. doi: 10.3816/CGC.2009.n.025. [DOI] [PubMed] [Google Scholar]