Abstract
Planar and apical-basal cellular polarization of epithelia and endothelia are crucial during morphogenesis. The establishment of these distinct polarity states and their transitions are regulated by signaling networks that include polarity complexes, Rho GTPases, and phosphoinositides. The spatiotemporal coordination of signaling by these molecules modulates cytoskeletal remodeling and vesicle trafficking to specify membrane domains, a prerequisite for the organization of tissues and organs. Here we present an overview of how activation of the WASp/Arp2/3 pathway of actin remodeling by Nck coordinates directional cell migration and speculate on its role as a signaling integrator in the coordination of cellular processes involved in endothelial cell polarity and vascular lumen formation.
Keywords: Nck, actin polymerization, cell migration, cell polarity, adhesion dynamics, WASp, morphogenesis, endothelial cell
Introduction
Tyrosine phosphorylation is an essential posttranslational modification enabling signaling cascades driving development and disease.1 The status of tyrosine phosphorylation in metazoan cells is regulated by the interplay between protein tyrosine kinases and protein tyrosine phosphatases. A third, critical component of this signaling system consists of proteins containing the Src Homology 2 (SH2) domain.2 This modular domain, consisting of about ~100 residues, binds the phosphorylated state of tyrosine and achieves selectivity for phosphopeptides through the specific recognition of the three to five residues C-terminal to the phosphotyrosine.3 A subset of SH2 domain-containing proteins function exclusively as scaffolds that organize signaling networks by tethering relevant components or altering their subcellular distribution in such a way that facilitates their interaction.4 A group of adaptor proteins contains, in addition to an SH2 domain, one or multiple copies of SH3 domains but no intrinsic catalytic activity. The SH3 domain is another important protein interaction module, about 60 residues long, that specifically recognizes proline-rich ligands and regulates intramolecular interactions, the local concentration/subcellular distribution of binding partners, and the assembly of multiprotein complexes.5 The SH2/SH3 domain-containing adaptors, represented by Grb2, and the Crk and Nck (non-catalytic region of tyrosine kinase) families,6 play critical roles in developmental programs and disease. Whereas all of these adaptors are involved in signaling to the cytoskeleton, here we focus on modulation of actin dynamics by Nck and briefly review recently discovered and emerging roles of this family in cellular migration and membrane trafficking.
Nck Linking Tyrosine Phosphorylation with Localized Actin Polymerization
The Nck family of SH2/SH3 domain-containing adaptors, consisting of Nck1/α and Nck2/β (herein referred to as Nck),7,8 is required during development9 and involved in cytoskeletal remodeling underlying pathogen-host cell interactions,10-13 T-cell receptor activation,14 invadopodia formation,15,16 cell adhesion and motility,17-22 and intercellular junction organization in kidney podocytes.23,24 The two protein isoforms share an overall ~68% amino acid identity25 and their SH2 domains engage a common set of phosphopeptides with equivalent binding affinity.26 Although Nck1 and Nck2 are believed to have mostly overlapping functions,9 non-compensating roles depending on specific cellular and signaling contexts have been suggested.20,27,28
In addition to determining the architectural organization and morphology of cells, actin filaments (F-actin) provide support and protrusive force to the various structures involved in locomotion.29,30 Recognized among the critical players in F-actin assembly, the Arp2/3 complex30,31 binds preexisting filaments and directs nucleation of a branched actin network.32-36 The activity of the Arp2/3 complex is intrinsically weak, and therefore, its full activation is dependent on the presence of nucleation promoting factors.37,38 Type I nucleation promoting factors, typically represented by the WASp and WAVE protein families,39 contain a conserved VCA (verprolin-homology, cofilin-homology, and acidic domain) region that engages both monomeric actin and the Arp2/3 complex.38,40 Evidence from early studies suggested a model whereby Nck would promote Arp2/3-dependent actin polymerization through dissociation of the WAVE complex.41 This model has been revised and it is now appreciated that full activation of WAVE does not involve complex disruption but requires the presence of coincident signals.42 Notably, a pathway involving Nck-dependent recruitment/activation of the WAVE complex is involved in localized actin polymerization during pathogen-induced phagocytosis.43 In addition, Nck is recruited to phagocytic cups and cooperates with Cdc42 in N-WASp activation downstream of Fcγ receptor clustering.44
WASp proteins act as integrators and coincidence detectors of signals from Rho GTPases, SH2/SH3 domain-containing adaptors, and phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2).37,45 Members of the WASp family are activated through relief of autoinhibition by a mechanism that involves allosteric regulation and oligomerization.39 Pioneer studies showed that pathogens, including vaccinia virus10,46 and enteropathogenic E. coli,13 recruit Nck to promote localized, N-WASp/Arp2/3-stimulated actin polymerization. Clustering of Nck by a phosphopeptide from Tir, an Enteropathogenic E. coli effector protein, triggers actin tail formation in Xenopus egg extracts.12 In living cells, an increased local concentration of membrane-targeted Nck SH3 domains leads to the formation of actin comets.47 We showed that N-WASP recruitment/activation at the plasma membrane is elicited by a reciprocal interdependence between Nck and PtdIns(4,5)P2.48 Importantly, experimental evidence from our laboratory suggests that Nck provides a functional link between tyrosine phosphorylation and phosphoinositides in the activation of N-WASp.48
Recent studies also showed that Nck binding to WASp interacting protein (WIP) is essential for the recruitment of the WIP:N-WASp complex and full activation of localized actin polymerization.49,50 Notably, using a combination of quantitative experimentation and computational simulations Mayer at al.49 showed that the presence of WIP and the density of Nck molecules are crucial in activation of localized actin polymerization. Furthermore, their findings provide strong support for a model in which the Nck/N-WASP/Arp2–3 stoichiometry is 4:2:1.49 Although research over the last decade has established Nck as a central player in the regulation of localized actin polymerization by the Arp2/3 complex, important questions remain unanswered: What is the identity and regulation of the protein complex recruited by Nck that bridges phosphotyrosine and phosphoinositides signaling? How is the WIP:N-WASp complex activated upon recruitment by Nck? What critical cellular processes are regulated and integrated by this important cytoskeletal pathway? We anticipate that future research combining proteomics, superresolution microscopy, and computational biology will bring about exciting new discoveries on Nck-dependent regulation of cytoskeletal remodeling. A representation of structural organization and major interactions of Nck and WASp/WAVE proteins are shown in Figure 1.
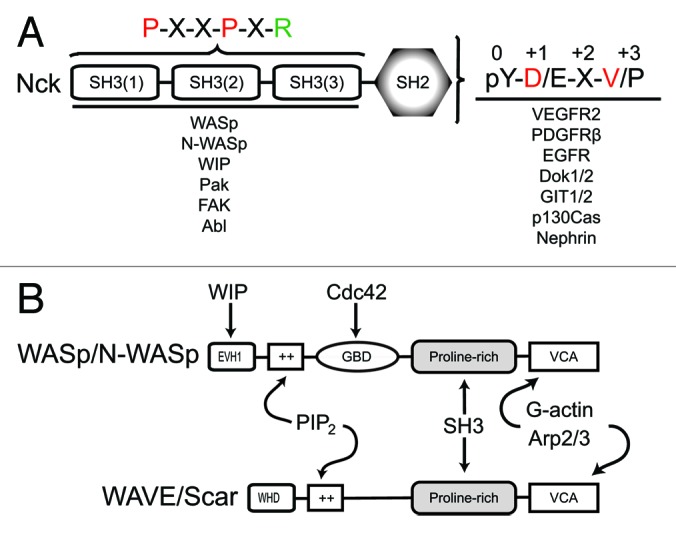
Figure 1. Representation of the structural domains of Nck, WASp/WAVE proteins and their critical interactions. (A) Nck consists of three N-terminal SH3 domains and a C-terminal SH2 domain. The consensus sequence of peptides found in proteins that bind Nck via SH2- or SH3-mediated interactions and well characterized Nck binding partners are shown. (B) Members of the WASP/WAVE families of nucleation promoting factors consist of a conserved C-terminus that enables interactions with G-actin and the Arp2/3 complex (VCA) and SH3 domains (proline-rich). Members from both families possess a polybasic motif (++) that mediates interaction/regulation by phosphoinositides. In cells, WASp proteins form a complex with WASp-interacting protein (WIP) whereas WAVE/Scar proteins are found as a complex that also includes Pir 121, Nap 1, Abi-1, and HSP300 (not shown).
Spatiotemporal Coordination of Signaling during Directional Cell Migration by Nck
Directional cell migration involves the establishment of a front-rear axis of polarity, successive cycles of membrane protrusion, adhesion to the substratum, forward propulsion of the cell body, and disengagement of the trailing edge.51 Morphological changes and asymmetric distribution of organelles, signaling and structural molecules underlie directional migration. For example, formation of new protrusions occurs at the cell front or lamellipodial edge whereas extension of lateral protrusion is limited during directional migration.51 Similarly, signaling molecules including Rho GTPases, phosphoinositides, and polarity complexes are more abundantly localized and activated at the leading edge of crawling cells.52 Endothelial cells exhibit planar polarity during migration and, therefore, the transition from apical-basal to planar polarity52 is one of the initial, important processes in sprouting angiogenesis.53
As in the case of apical-basal polarity, regulation of front-rear polarity is dependent on the modulation of cytoskeletal remodeling and vesicle trafficking by proteins of the polarity complexes, including Crumbs, Par, and Scribble,52,54 which are known downstream effectors of Rho GTPases. Using a combination of molecular genetics and quantitative live cell microscopy we showed that Nck is essential in the establishment of front-back polarity and directional migration of endothelial cells.55 Our study uncovered new mechanistic insights whereby Nck integrates signaling by tyrosine phosphorylation with precise spatiotemporal activation of the Rho GTPases in the coordination of cytoskeletal dynamics. The loss of planar cell polarity caused by silencing of Nck was accompanied by the formation of simultaneous, multidirectional protrusions linked to mislocalized activation of Cdc42 and Rac. In addition, the activity of RhoA and myosin II phosphorylation were reduced in Nck-depleted endothelial cells. These exciting findings stimulate new hypotheses and will prompt further research to address outstanding questions: How does Nck contribute to regulation of the spatiotemporal activation of Rho GTPases? Does Nck modulate the distribution/activation of guanine nucleotide-exchange factors (GEF) or GTPase activating proteins (GAP)? Is the subcellular distribution and activity of the polarity complexes dysregulated by abrogation of Nck signaling? Below, we speculate on signaling mechanisms potentially modulated by Nck. A cartoon highlighting known functions and hypothetical roles of Nck in the regulation of cell polarity and directional migration are shown in Figure 2.
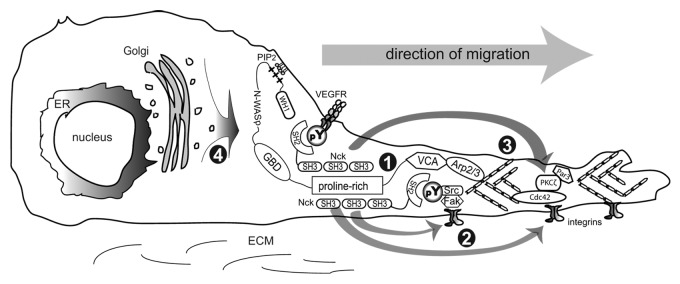
Figure 2. Cartoon highlighting known functions and hypothetical roles of Nck in the regulation of planar cell polarity and directional migration. (1) Nck stimulates Arp2/3-dependent polymerization of branched actin networks through activation of WASp/WAVE proteins, particularly at the leading edge/lamellipodium. (2) Nck links signaling by tyrosine phosphorylation induced by ligand-activated growth factor receptors and integrins with the cytoskeleton. Nck contributes to directional migration through the spatiotemporal regulation of Rho GTPases (only Cdc42 is represented) and stabilization of newly formed protrusions at the leading edge by strengthening cell-matrix adhesions. (3) Nck may play an indirect role in the differential distribution/activation of polarity complexes (only Par3/PKCζ is represented) at the leading edge through modulation of Rho GTPases. (4) Nck may also contribute to cell polarity through modulation of vesicular/membrane trafficking. The involvement of Nck in processes highlighted in 1 and 2 is supported by experimental data. The role of Nck in processes depicted in 3 and 4 remains speculative.
Modulation of the Subcellular Distribution/Activation of Rho GTPases and Polarity Complexes
Accumulating evidence suggests that myosin II contractility mediates the establishment of polarity in migrating cells by a mechanism that limits lateral protrusiveness through local depletion of β-Pix, a Cdc42/Rac GEF, and decreased Rac activation.56,57 Inhibition/depletion of p21 GTPase-activated kinase (Pak), an important cytoskeletal effector downstream of the small GTPases Rac and Cdc42,58 dislodges myosin IIA from the cell’s edge and decreases adhesion maturation.59 Nck directs the recruitment of Pak to the plasma membrane60,61 and modulates VEGF-stimulated endothelial cell migration through a cellular mechanism that involves regulation of adhesion assembly.62 Notably, the polarity protein Scribble interacts with integrin α5 and is required for endothelial polarization and directional migration.63 Because Scribble forms a complex with β-Pix and its binding partner GIT64 we speculate that the complex Nck-Pak-β-Pix-GIT,65-67 known to be recruited to focal adhesions, contributes to the localization of Scribble to newly formed adhesions at the cell front. Conceivably, alternative mechanisms may involve the selective recruitment of GTPase activating proteins that spatially restrict the activation of Rac/Cdc42, as recently shown for SH3BP1, an exocysts interaction partner that facilitates the cycling of Rac between inactive/active states to enable the formation/stabilization of protrusion at the leading edge of migrating cells.68
Seminal studies by Hall et al.69 showed that the wounding of a confluent monolayer of astrocytes induces polarized organelle distribution and protrusive activity of cells at the edge of the wound. These changes in morphology and architectural organization of cells are orchestrated by the polarized activation of Cdc42 and its downstream effector the polarity complex Par6/PKCζ.69 During angiogenic sprouting elicited by angiopoitin-1, which induces collective and directional migration of endothelial cells, a signaling complex that includes β-catenin and Par3/Par6/PKCζ is recruited to the cell front to coordinate localized activation of Rac by Tiam1, a Rac-specific GEF.70 Interestingly, this complex is also functional in adherens junctions where it might promote stabilization of cell-cell contacts.70 Consistent with this notion, a recent study demonstrated full activation of the VEGFR at the sprout tip but not in the stalk cells in which inactivation the VEGFR is mediated by Tie2-dependent targeting of vascular endothelia phosphotyrosine phosphatase (VE-PTP) to cell-cell junctions.71 Using a Cdc42 intramolecular Förster resonance energy transfer biosensor72 we determined polarized activation of Cdc42 in control endothelial cells undergoing directional migration but not in unpolarized, Nck-depleted cells.55 It has been reported that Nck binds directly to phosphorylated Tyr1214 within the activated VEGFR73 and phosphorylated Tyr351 within Dok-R/Dok2,74-76 an adaptor protein recruited to the activated Tek/Tie2 receptor. Furthermore, the Dok-R/Nck complex recruits and activates Pak at the cell membrane to drive angiopoietin-1 directed cell migration.75 Collectively, these findings suggest that further research is needed to address important questions: Does Nck play a differential role in migration of sprout tip vs. stalk endothelial cells through the engagement of VEGFR vis-à-vis Tek/Tie2? Is the activity of the Par3/Par6/ PKCζ complex modulated by Nck? Research aimed at understanding the role of Nck in regulation of the subcellular localization and activity of polarity complexes is ongoing in our laboratory.
Powering Directional Migration through Ca2+ flickers
An emerging concept in the field is that directionality of cell migration is also modulated by localized Ca2+ transients.77 Persistent migration of endothelial cells depends on the spatiotemporal coordination of the activation of polarity protein complexes downstream of receptor tyrosine kinases70,78 and integrins.63 Elegant studies by Cheng et al.79 showed that Ca2+ flickers occur more frequently at the leading edge of migrating cells and redirect cell polarization in response to a chemoattractant. Recent studies suggest that localized Ca2+ transients regulate critical processes during cell migration, including membrane protrusion, adhesion strength,80 and focal adhesion dynamics.81-85 What are the molecular mechanisms underlying the crosstalk between tyrosine phosphorylation and Ca2+ signaling during directional migration? Potential mechanisms of signaling integration are outlined below.
It is know that activation of tyrosine phosphorylation through integrins86 and VEGFR87 elicits Ca2+ transients in endothelial cells. Store-operated Ca2+ entry (SOCE) is a major Ca2+ regulatory pathway in non-excitable cells88-90 and, importantly, its molecular components are involved in regulation of Rho-dependent cytoskeletal tension.91,92 Ligand-activated VEGFR recruits phospholipase Cγ (PLCγ),93 an enzyme that hydrolyzes PtdIns(4,5)P2 to generate diacylglycerol and inositol-1,4,5-triphosphate (Ins(1,4,5)P3). Increased cytosolic levels of Ins(1,4,5)P3 stimulate endoplasmic reticulum Ins(1,4,5)P3 receptors and SOCE.88 Notably, Src-dependent phosphorylation of PLCγ plays a key role in stimulation of Ca2+ mobilization.94 Silencing of the endoplasmic reticulum stromal interaction molecule 1 (STIM1), a critical component of SOCE,90 abrogates VEGF-stimulated Ca2+ mobilization and cell migration.87 Unpublished data from our laboratory show that silencing of Nck leads to a significant decrease in VEGF-induced Ca2+ transients in endothelial cells. Consistent with these findings, Nck depletion was shown to decrease Ca2+ mobilization in T-cells.95 Importantly, the Nck binding partner GIT1 forms a complex with PLCγ and is required for Src-dependent activation of PLCγ, Ins(1,4,5)P3 production and Ca2+ mobilization downstream of receptor tyrosine kinase and G protein-coupled receptor activation.94 It is tempting to speculate that Nck regulates directional migration of endothelial cells during sprouting angiogenesis through modulation of SOCE and the generation of polarized Ca2+ flickers.
Vesicle Trafficking, Cell Polarity and Beyond: Is Nck Involved in Vascular Lumen Formation?
Three basic mechanisms have been proposed for lumen formation in tubular organs and networks, namely cavitation, hollowing, and focalized contact/membrane repulsion.54,96 The establishment of apical-basal polarity is an absolute requirement for normal vascular lumen organization.97 Although evidence for vascular lumen formation through cavitation is lacking, there is substantial support for lumenization of endothelial cords through hollowing98 or focalized contact/membrane repulsion,99 processes that may not be mutually exclusive. Regardless, the differentiation of apical vs. basolateral membrane domains entails coordination between the cytoskeleton and the membrane trafficking machinery.
Basic cellular mechanisms involving the establishment of cell-matrix contacts and cell-cell recognition are essential for symmetry breaking. These interactions provide essential spatial cues for the differentiation of membrane domains. Thus, the apical surface forms through the selective trafficking and delivery/fusion of vesicles containing apical proteins and lipids, particularly polarity complexes and phosphoinositides.96 Vascular endothelial cadherin (VE-cadherin)-mediated cell-cell contacts are involved in apical-basal polarization of endothelial cells and vascular lumen formation through the recruitment of Par3/PKCζ to adherens junctions.100 In humans, the stroke-predisposing disease cerebral cavernous malformation is characterized by vascular malformation and fragility caused by mutations in genes encoding the CCM protein family. CCM interacts with VE-cadherin and facilitates the assembly of cell-cell junctions and the recruitment of the polarity complex Par3/PKCζ.100 Abnormalities in the vasculature in this disease arise from disorganization of adherens junctions, loss of cell polarity and altered lumenization.100 Lumen formation also involves VE-cadherin-dependent vesicular trafficking of the sialomucin podocalyxin (PODXL), and anti-adhesive transmembrane protein, to areas of contact between adjacent endothelial cells.99 Unpublished data from our laboratory show that control and Nck-rescued, but not Nck-depleted endothelial cells, developed a robust, well interconnected network of cords on Matrigel and tubes in collagen three-dimensional matrices. In addition, our findings suggest that Nck silencing reduces apical surface localization of PODXL which appears to remain trapped in cytoplasmic vesicles. These findings point to abnormal trafficking of PODXL to the apical surface and incomplete polarization as the underpinnings of impaired tubulogenesis observed in Nck-depleted cells.
Lumen formation in endothelial cells cultured in three-dimensional environments depends on the polarity complex Par and involves reciprocity between Cdc42 signaling and MT1-MMP proteolysis.101 Notably, Nck silencing in a variety of tumor cells has been associated with decreased invasiveness and matrix degradation in vitro.15,16,102,103 Nck interacts with phosphorylated cortactin,15,104 an F-actin binding protein involved in regulation of tumor cell invasion and proteolysis of extracellular matrix components.103,105-108 Our recently published studies show that Nck modulates the polarized activation of Cdc42 in migrating cells.55 In addition, faciogenital dysplasia protein 1 (FGD1), a cortactin binding partner109 and Cdc42 GEF involved in post-Golgi cargo trafficking,110 has been implicated in invadopodia biogenesis and regulation of extracellular matrix remodeling.111,112 Whether the cortactin/Nck signaling axis modulates the Cdc42/MT1-MMP reciprocity during vascular morphogenesis remains an unanswered question.
Concluding Remarks
We have highlighted the functional versatility of Nck adaptors in cellular processes requiring localized actin polymerization. Over the last decade we have gained a functional understanding of how Nck modulates localized actin polymerization through activation of the WAVE/WASp/Arp2/3 complex. Essential aspects including molecular composition, stoichiometry, and critical interactions among complex components have been elucidated. In addition, novel functions of this major pathway of actin remodeling in various cellular processes are becoming apparent. Recent findings underscore an emerging role for Nck in cellular adhesion and polarization, processes intimately involved in morphogenesis. Combination of advanced imaging, proteomics, and computational modeling/simulation will enable the testing of new hypotheses to uncover a cohesive picture of signaling integration by Nck in the coordination of cytoskeletal remodeling during development and disease.
Acknowledgments
This work was supported by American Heart Association (AHA) grants (SDG0735282N and 12BGIA9030042), and Startup Funds from Texas A&M University to G.M.R.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/25744
References
- 1.Hunter T. Tyrosine phosphorylation: thirty years and counting. Curr Opin Cell Biol. 2009;21:140–6. doi: 10.1016/j.ceb.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim WA, Pawson T. Phosphotyrosine signaling: evolving a new cellular communication system. Cell. 2010;142:661–7. doi: 10.1016/j.cell.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machida K, Mayer BJ. The SH2 domain: versatile signaling module and pharmaceutical target. Biochim Biophys Acta. 2005;1747:1–25. doi: 10.1016/j.bbapap.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332:680–6. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer BJ. SH3 domains: complexity in moderation. J Cell Sci. 2001;114:1253–63. doi: 10.1242/jcs.114.7.1253. [DOI] [PubMed] [Google Scholar]
- 6.Birge RB, Knudsen BS, Besser D, Hanafusa H. SH2 and SH3-containing adaptor proteins: redundant or independent mediators of intracellular signal transduction. Genes Cells. 1996;1:595–613. doi: 10.1046/j.1365-2443.1996.00258.x. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Fan J, Woodley DT. Nck/Dock: an adapter between cell surface receptors and the actin cytoskeleton. Oncogene. 2001;20:6403–17. doi: 10.1038/sj.onc.1204782. [DOI] [PubMed] [Google Scholar]
- 8.Buday L, Wunderlich L, Tamás P. The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal. 2002;14:723–31. doi: 10.1016/S0898-6568(02)00027-X. [DOI] [PubMed] [Google Scholar]
- 9.Bladt F, Aippersbach E, Gelkop S, Strasser GA, Nash P, Tafuri A, et al. The murine Nck SH2/SH3 adaptors are important for the development of mesoderm-derived embryonic structures and for regulating the cellular actin network. Mol Cell Biol. 2003;23:4586–97. doi: 10.1128/MCB.23.13.4586-4597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreau V, Frischknecht F, Reckmann I, Vincentelli R, Rabut G, Stewart D, et al. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nat Cell Biol. 2000;2:441–8. doi: 10.1038/35017080. [DOI] [PubMed] [Google Scholar]
- 11.Scaplehorn N, Holmström A, Moreau V, Frischknecht F, Reckmann I, Way M. Grb2 and Nck act cooperatively to promote actin-based motility of vaccinia virus. Curr Biol. 2002;12:740–5. doi: 10.1016/S0960-9822(02)00812-6. [DOI] [PubMed] [Google Scholar]
- 12.Campellone KG, Rankin S, Pawson T, Kirschner MW, Tipper DJ, Leong JM. Clustering of Nck by a 12-residue Tir phosphopeptide is sufficient to trigger localized actin assembly. J Cell Biol. 2004;164:407–16. doi: 10.1083/jcb.200306032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruenheid S, DeVinney R, Bladt F, Goosney D, Gelkop S, Gish GD, et al. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat Cell Biol. 2001;3:856–9. doi: 10.1038/ncb0901-856. [DOI] [PubMed] [Google Scholar]
- 14.Lettau M, Pieper J, Janssen O. Nck adapter proteins: functional versatility in T cells. Cell Commun Signal. 2009;7:1. doi: 10.1186/1478-811X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oser M, Mader CC, Gil-Henn H, Magalhaes M, Bravo-Cordero JJ, Koleske AJ, et al. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J Cell Sci. 2010;123:3662–73. doi: 10.1242/jcs.068163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stylli SS, Stacey TT, Verhagen AM, Xu SS, Pass I, Courtneidge SA, et al. Nck adaptor proteins link Tks5 to invadopodia actin regulation and ECM degradation. J Cell Sci. 2009;122:2727–40. doi: 10.1242/jcs.046680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lapetina S, Mader CC, Machida K, Mayer BJ, Koleske AJ. Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. J Cell Biol. 2009;185:503–19. doi: 10.1083/jcb.200809085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antoku S, Saksela K, Rivera GM, Mayer BJ. A crucial role in cell spreading for the interaction of Abl PxxP motifs with Crk and Nck adaptors. J Cell Sci. 2008;121:3071–82. doi: 10.1242/jcs.031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivera GM, Antoku S, Gelkop S, Shin NY, Hanks SK, Pawson T, et al. Requirement of Nck adaptors for actin dynamics and cell migration stimulated by platelet-derived growth factor B. Proc Natl Acad Sci U S A. 2006;103:9536–41. doi: 10.1073/pnas.0603786103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan S, Fan J, Han A, Chen M, Woodley DT, Li W. Non-compensating roles between Nckalpha and Nckbeta in PDGF-BB signaling to promote human dermal fibroblast migration. J Invest Dermatol. 2009;129:1909–20. doi: 10.1038/jid.2008.457. [DOI] [PubMed] [Google Scholar]
- 21.Ruusala A, Pawson T, Heldin CH, Aspenström P. Nck adapters are involved in the formation of dorsal ruffles, cell migration, and Rho signaling downstream of the platelet-derived growth factor beta receptor. J Biol Chem. 2008;283:30034–44. doi: 10.1074/jbc.M800913200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abella JV, Vaillancourt R, Frigault MM, Ponzo MG, Zuo D, Sangwan V, et al. The Gab1 scaffold regulates RTK-dependent dorsal ruffle formation through the adaptor Nck. J Cell Sci. 2010;123:1306–19. doi: 10.1242/jcs.062570. [DOI] [PubMed] [Google Scholar]
- 23.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440:818–23. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 24.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116:1346–59. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, She H, Davis EM, Spicer CM, Kim L, Ren R, et al. Identification of Nck family genes, chromosomal localization, expression, and signaling specificity. J Biol Chem. 1998;273:25171–8. doi: 10.1074/jbc.273.39.25171. [DOI] [PubMed] [Google Scholar]
- 26.Frese S, Schubert WD, Findeis AC, Marquardt T, Roske YS, Stradal TE, et al. The phosphotyrosine peptide binding specificity of Nck1 and Nck2 Src homology 2 domains. J Biol Chem. 2006;281:18236–45. doi: 10.1074/jbc.M512917200. [DOI] [PubMed] [Google Scholar]
- 27.Guan S, Chen M, Woodley D, Li W. Nckbeta adapter controls neuritogenesis by maintaining the cellular paxillin level. Mol Cell Biol. 2007;27:6001–11. doi: 10.1128/MCB.01807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu T, Shi G, Larose L, Rivera GM, Mayer BJ, Zhou R. Regulation of process retraction and cell migration by EphA3 is mediated by the adaptor protein Nck1. Biochemistry. 2009;48:6369–78. doi: 10.1021/bi900831k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 30.Small JV, Stradal T, Vignal E, Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112–20. doi: 10.1016/S0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 31.Svitkina TM, Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol. 1999;145:1009–26. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machesky LM, Gould KL. The Arp2/3 complex: a multifunctional actin organizer. Curr Opin Cell Biol. 1999;11:117–21. doi: 10.1016/S0955-0674(99)80014-3. [DOI] [PubMed] [Google Scholar]
- 33.Mullins RD, Pollard TD. Structure and function of the Arp2/3 complex. Curr Opin Struct Biol. 1999;9:244–9. doi: 10.1016/S0959-440X(99)80034-7. [DOI] [PubMed] [Google Scholar]
- 34.Cooper JA, Wear MA, Weaver AM. Arp2/3 complex: advances on the inner workings of a molecular machine. Cell. 2001;107:703–5. doi: 10.1016/S0092-8674(01)00605-5. [DOI] [PubMed] [Google Scholar]
- 35.Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–26. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 36.Rotty JD, Wu C, Bear JE. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat Rev Mol Cell Biol. 2013;14:7–12. doi: 10.1038/nrm3492. [DOI] [PubMed] [Google Scholar]
- 37.Higgs HN, Pollard TD. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu Rev Biochem. 2001;70:649–76. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- 38.Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–51. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padrick SB, Rosen MK. Physical mechanisms of signal integration by WASP family proteins. Annu Rev Biochem. 2010;79:707–35. doi: 10.1146/annurev.biochem.77.060407.135452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 41.Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–3. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- 42.Lebensohn AM, Kirschner MW. Activation of the WAVE complex by coincident signals controls actin assembly. Mol Cell. 2009;36:512–24. doi: 10.1016/j.molcel.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pils S, Kopp K, Peterson L, Delgado Tascón J, Nyffenegger-Jann NJ, Hauck CR. The adaptor molecule Nck localizes the WAVE complex to promote actin polymerization during CEACAM3-mediated phagocytosis of bacteria. PLoS One. 2012;7:e32808. doi: 10.1371/journal.pone.0032808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dart AE, Donnelly SK, Holden DW, Way M, Caron E. Nck and Cdc42 co-operate to recruit N-WASP to promote FcγR-mediated phagocytosis. J Cell Sci. 2012;125:2825–30. doi: 10.1242/jcs.106583. [DOI] [PubMed] [Google Scholar]
- 45.Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci. 2001;114:1801–9. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- 46.Frischknecht F, Moreau V, Röttger S, Gonfloni S, Reckmann I, Superti-Furga G, et al. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999;401:926–9. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- 47.Rivera GM, Briceño CA, Takeshima F, Snapper SB, Mayer BJ. Inducible clustering of membrane-targeted SH3 domains of the adaptor protein Nck triggers localized actin polymerization. Curr Biol. 2004;14:11–22. doi: 10.1016/j.cub.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 48.Rivera GM, Vasilescu D, Papayannopoulos V, Lim WA, Mayer BJ. A reciprocal interdependence between Nck and PI(4,5)P(2) promotes localized N-WASp-mediated actin polymerization in living cells. Mol Cell. 2009;36:525–35. doi: 10.1016/j.molcel.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ditlev JA, Michalski PJ, Huber G, Rivera GM, Mohler WA, Loew LM, et al. Stoichiometry of Nck-dependent actin polymerization in living cells. J Cell Biol. 2012;197:643–58. doi: 10.1083/jcb.201111113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donnelly SK, Weisswange I, Zettl M, Way M. WIP Provides an Essential Link between Nck and N-WASP during Arp2/3-Dependent Actin Polymerization. Curr Biol. 2013;23:999–1006. doi: 10.1016/j.cub.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–49. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson WJ. Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb Perspect Biol. 2009;1:a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–78. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 54.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaki SP, Barhoumi R, Berginski ME, Sreenivasappa H, Trache A, Gomez SM, et al. Nck enables directional cell migration through the coordination of polarized membrane protrusion with adhesion dynamics. J Cell Sci. 2013;126:1637–49. doi: 10.1242/jcs.119610. [DOI] [PubMed] [Google Scholar]
- 56.Vicente-Manzanares M, Newell-Litwa K, Bachir AI, Whitmore LA, Horwitz AR. Myosin IIA/IIB restrict adhesive and protrusive signaling to generate front-back polarity in migrating cells. J Cell Biol. 2011;193:381–96. doi: 10.1083/jcb.201012159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13:383–93. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–81. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 59.Delorme-Walker VD, Peterson JR, Chernoff J, Waterman CM, Danuser G, DerMardirossian C, et al. Pak1 regulates focal adhesion strength, myosin IIA distribution, and actin dynamics to optimize cell migration. J Cell Biol. 2011;193:1289–303. doi: 10.1083/jcb.201010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galisteo ML, Chernoff J, Su YC, Skolnik EY, Schlessinger J. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996;271:20997–1000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- 61.Lu W, Katz S, Gupta R, Mayer BJ. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr Biol. 1997;7:85–94. doi: 10.1016/S0960-9822(06)00052-2. [DOI] [PubMed] [Google Scholar]
- 62.Stoletov KV, Ratcliffe KE, Spring SC, Terman BI. NCK and PAK participate in the signaling pathway by which vascular endothelial growth factor stimulates the assembly of focal adhesions. J Biol Chem. 2001;276:22748–55. doi: 10.1074/jbc.M009720200. [DOI] [PubMed] [Google Scholar]
- 63.Michaelis UR, Chavakis E, Kruse C, Jungblut B, Kaluza D, Wandzioch K, et al. The polarity protein Scrib is essential for directed endothelial cell migration. Circ Res. 2013;112:924–34. doi: 10.1161/CIRCRESAHA.112.300592. [DOI] [PubMed] [Google Scholar]
- 64.Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lécine P, Bellaiche Y, et al. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol. 2004;14:987–95. doi: 10.1016/j.cub.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 65.Manabe R, Kovalenko M, Webb DJ, Horwitz AR. GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J Cell Sci. 2002;115:1497–510. doi: 10.1242/jcs.115.7.1497. [DOI] [PubMed] [Google Scholar]
- 66.Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, et al. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J Cell Biol. 1999;145:851–63. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol Biol Cell. 2005;16:4316–28. doi: 10.1091/mbc.E05-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parrini MC, Sadou-Dubourgnoux A, Aoki K, Kunida K, Biondini M, Hatzoglou A, et al. SH3BP1, an exocyst-associated RhoGAP, inactivates Rac1 at the front to drive cell motility. Mol Cell. 2011;42:650–61. doi: 10.1016/j.molcel.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–98. doi: 10.1016/S0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 70.Oubaha M, Lin MI, Margaron Y, Filion D, Price EN, Zon LI, et al. Formation of a PKCζ/β-catenin complex in endothelial cells promotes angiopoietin-1-induced collective directional migration and angiogenic sprouting. Blood. 2012;120:3371–81. doi: 10.1182/blood-2012-03-419721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayashi M, Majumdar A, Li X, Adler J, Sun Z, Vertuani S, et al. VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation. Nat Commun. 2013;4:1672. doi: 10.1038/ncomms2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aoki K, Matsuda M. Visualization of small GTPase activity with fluorescence resonance energy transfer-based biosensors. Nat Protoc. 2009;4:1623–31. doi: 10.1038/nprot.2009.175. [DOI] [PubMed] [Google Scholar]
- 73.Lamalice L, Houle F, Huot J. Phosphorylation of Tyr1214 within VEGFR-2 triggers the recruitment of Nck and activation of Fyn leading to SAPK2/p38 activation and endothelial cell migration in response to VEGF. J Biol Chem. 2006;281:34009–20. doi: 10.1074/jbc.M603928200. [DOI] [PubMed] [Google Scholar]
- 74.Jones N, Chen SH, Sturk C, Master Z, Tran J, Kerbel RS, et al. A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function. Mol Cell Biol. 2003;23:2658–68. doi: 10.1128/MCB.23.8.2658-2668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Master Z, Jones N, Tran J, Jones J, Kerbel RS, Dumont DJ. Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak. EMBO J. 2001;20:5919–28. doi: 10.1093/emboj/20.21.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones N, Dumont DJ. The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R. Oncogene. 1998;17:1097–108. doi: 10.1038/sj.onc.1202115. [DOI] [PubMed] [Google Scholar]
- 77.Wei C, Wang X, Zheng M, Cheng H. Calcium gradients underlying cell migration. Curr Opin Cell Biol. 2012;24:254–61. doi: 10.1016/j.ceb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 78.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–94. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 79.Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–5. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsai FC, Meyer T. Ca2+ pulses control local cycles of lamellipodia retraction and adhesion along the front of migrating cells. Curr Biol. 2012;22:837–42. doi: 10.1016/j.cub.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giannone G, Rondé P, Gaire M, Beaudouin J, Haiech J, Ellenberg J, et al. Calcium rises locally trigger focal adhesion disassembly and enhance residency of focal adhesion kinase at focal adhesions. J Biol Chem. 2004;279:28715–23. doi: 10.1074/jbc.M404054200. [DOI] [PubMed] [Google Scholar]
- 82.Easley CA, 4th, Brown CM, Horwitz AF, Tombes RM. CaMK-II promotes focal adhesion turnover and cell motility by inducing tyrosine dephosphorylation of FAK and paxillin. Cell Motil Cytoskeleton. 2008;65:662–74. doi: 10.1002/cm.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kiyoshima D, Kawakami K, Hayakawa K, Tatsumi H, Sokabe M. Force- and Ca²⁺-dependent internalization of integrins in cultured endothelial cells. J Cell Sci. 2011;124:3859–70. doi: 10.1242/jcs.088559. [DOI] [PubMed] [Google Scholar]
- 84.Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, Habas R, et al. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem. 2006;281:11260–70. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–83. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 86.Schwartz MA. Spreading of human endothelial cells on fibronectin or vitronectin triggers elevation of intracellular free calcium. J Cell Biol. 1993;120:1003–10. doi: 10.1083/jcb.120.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li J, Cubbon RM, Wilson LA, Amer MS, McKeown L, Hou B, et al. Orai1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Circ Res. 2011;108:1190–8. doi: 10.1161/CIRCRESAHA.111.243352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis RS. Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harb Perspect Biol. 2011;3:3. doi: 10.1101/cshperspect.a003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pani B, Bollimuntha S, Singh BB. The TR (i)P to Ca²⁺ signaling just got STIMy: an update on STIM1 activated TRPC channels. Front Biosci. 2012;17:805–23. doi: 10.2741/3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carrasco S, Meyer T. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu Rev Biochem. 2011;80:973–1000. doi: 10.1146/annurev-biochem-061609-165311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen YT, Chen YF, Chiu WT, Wang YK, Chang HC, Shen MR. The ER Ca²⁺ sensor STIM1 regulates actomyosin contractility of migratory cells. J Cell Sci. 2013;126:1260–7. doi: 10.1242/jcs.121129. [DOI] [PubMed] [Google Scholar]
- 92.Gudermann T, Steinritz D. STIMulating stress fibers in endothelial cells. Sci Signal. 2013;6:pe8. doi: 10.1126/scisignal.2004051. [DOI] [PubMed] [Google Scholar]
- 93.Napione L, Pavan S, Veglio A, Picco A, Boffetta G, Celani A, et al. Unraveling the influence of endothelial cell density on VEGF-A signaling. Blood. 2012;119:5599–607. doi: 10.1182/blood-2011-11-390666. [DOI] [PubMed] [Google Scholar]
- 94.Haendeler J, Yin G, Hojo Y, Saito Y, Melaragno M, Yan C, et al. GIT1 mediates Src-dependent activation of phospholipase Cgamma by angiotensin II and epidermal growth factor. J Biol Chem. 2003;278:49936–44. doi: 10.1074/jbc.M307317200. [DOI] [PubMed] [Google Scholar]
- 95.Roy E, Togbe D, Holdorf AD, Trubetskoy D, Nabti S, Küblbeck G, et al. Nck adaptors are positive regulators of the size and sensitivity of the T-cell repertoire. Proc Natl Acad Sci U S A. 2010;107:15529–34. doi: 10.1073/pnas.1009743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Datta A, Bryant DM, Mostov KE. Molecular regulation of lumen morphogenesis. Curr Biol. 2011;21:R126–36. doi: 10.1016/j.cub.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abraham S, Yeo M, Montero-Balaguer M, Paterson H, Dejana E, Marshall CJ, et al. VE-Cadherin-mediated cell-cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol. 2009;19:668–74. doi: 10.1016/j.cub.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 98.Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16:222–31. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lammert E, Axnick J. Vascular lumen formation. Cold Spring Harb Perspect Med. 2012;2:a006619. doi: 10.1101/cshperspect.a006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, et al. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J Cell Sci. 2010;123:1073–80. doi: 10.1242/jcs.059329. [DOI] [PubMed] [Google Scholar]
- 101.Sacharidou A, Koh W, Stratman AN, Mayo AM, Fisher KE, Davis GE. Endothelial lumen signaling complexes control 3D matrix-specific tubulogenesis through interdependent Cdc42- and MT1-MMP-mediated events. Blood. 2010;115:5259–69. doi: 10.1182/blood-2009-11-252692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–52. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, et al. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–87. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc Natl Acad Sci U S A. 2007;104:11933–8. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–35. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 106.Clark ES, Weaver AM. A new role for cortactin in invadopodia: regulation of protease secretion. Eur J Cell Biol. 2008;87:581–90. doi: 10.1016/j.ejcb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tetè S, Luini A, et al. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci. 2008;121:369–78. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- 108.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–43. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 109.Hou P, Estrada L, Kinley AW, Parsons JT, Vojtek AB, Gorski JL. Fgd1, the Cdc42 GEF responsible for Faciogenital Dysplasia, directly interacts with cortactin and mAbp1 to modulate cell shape. Hum Mol Genet. 2003;12:1981–93. doi: 10.1093/hmg/ddg209. [DOI] [PubMed] [Google Scholar]
- 110.Egorov MV, Capestrano M, Vorontsova OA, Di Pentima A, Egorova AV, Mariggiò S, et al. Faciogenital dysplasia protein (FGD1) regulates export of cargo proteins from the golgi complex via Cdc42 activation. Mol Biol Cell. 2009;20:2413–27. doi: 10.1091/mbc.E08-11-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Genot E, Daubon T, Sorrentino V, Buccione R. FGD1 as a central regulator of extracellular matrix remodelling--lessons from faciogenital dysplasia. J Cell Sci. 2012;125:3265–70. doi: 10.1242/jcs.093419. [DOI] [PubMed] [Google Scholar]
- 112.Ayala I, Giacchetti G, Caldieri G, Attanasio F, Mariggiò S, Tetè S, et al. Faciogenital dysplasia protein Fgd1 regulates invadopodia biogenesis and extracellular matrix degradation and is up-regulated in prostate and breast cancer. Cancer Res. 2009;69:747–52. doi: 10.1158/0008-5472.CAN-08-1980. [DOI] [PubMed] [Google Scholar]


