Abstract
The notochord is an evolutionarily conserved structure that has long been known to play an important role in patterning during embryogenesis. Structurally, the notochord is composed of two cell layers: an outer epithelial-like sheath, and an inner core of cells that contain large fluid-filled vacuoles. We have recently shown these notochord vacuoles are lysosome-related organelles that form through Rab32a and vacuolar-type proton-ATPase-dependent acidification. Disruption of notochord vacuoles results in a shortened embryo along the anterior-posterior axis. Interestingly, we discovered that notochord vacuoles are also essential for proper spine morphogenesis and that vacuole defects lead to scoliosis of the spine. Here we discuss the cellular organization of the notochord and how key features of its architecture allow the notochord to function in embryonic axis elongation and spine formation.
Keywords: zebrafish, notochord vacuole, lysosome-related organelle, axis elongation, spine formation, scoliosis
Introduction
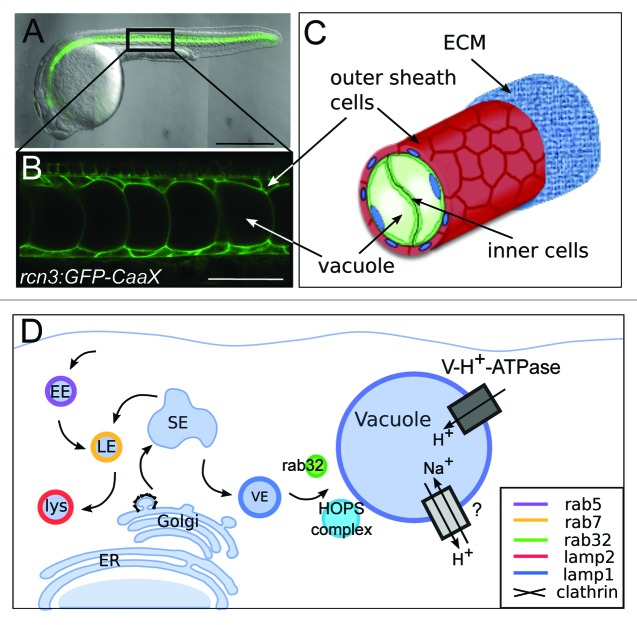
The notochord is a defining feature of all chordates, including our subphylum vertebrata. This evolutionarily conserved structure provided a scaffold to early chordate embryos that enabled locomotion and allowed for our ancestors to evade predation. In zebrafish the notochord begins as a rod of stacked cells that differentiates into two cell layers, an outer epithelial-like layer and an inner core of vacuolated cells. During development, notch signaling is important for specification of the outer sheath cell fate.1 Upon specification, the sheath cells leave the notochord midline and move to enclose the inner core of cells. Simultaneously, the inner cells begin to inflate their vacuoles and expand to occupy the space left behind by the movement of the sheath cells. Each inner cell contains a single fluid-filled vacuole that is up to 40 µm in diameter and occupies the majority of the cell volume. The entire notochord is encased in a fibrous extracellular matrix (ECM) that is secreted from the outer cell layer (Fig. 1). The sheath and vacuoles have been described in all species in which the notochord has been examined histologically,2,3 suggesting that both of these components are essential for the notochord to perform its roles during development.
Figure 1. Notochord vacuoles form through biosynthesis via rab32a and the HOPS complex. (A) 24 h post fertilization (hpf) zebrafish embryo expressing membrane GFP in the notochord. Anterior is left, posterior is right, dorsal is top, and ventral is down. (B) Higher magnification of membrane GFP in the notochord. (C) Cartoon depicting notochord structure of inner vacuolated cells surrounded by an epithelial-like sheath of cells. (D) Cartoon depicting trafficking requirements of vacuole inflation. EE = early endosome, LE = late endosome, lys = lysosome, SE = sorting endosome, VE = vacuolar endosome. Presumptive channels are found on the vacuole membrane. Scale bars: (A) 500 µm. (B) 50 µm. All panels adapted from Ellis et al. JCB 2013.
Modeling of growing notochords suggests that the notochord growth can act as a morphogenetic driving force mediating the elongation of the anterior-posterior embryonic axis. These modeling studies concluded that the elongating force provided by the notochord arises from the pressure of the inflating vacuoles being constricted by the surrounding ECM sheath.4,5 The ECM forms a rigid container surrounding the notochord, as this structure does not deform as the vacuoles expand, and it retains its overall shape in the absence of vacuoles.6 Thus, as the vacuoles inflate and expand within the cell, the notochord sheath ultimately restricts their radial expansion while permitting expansion along the anterior-posterior axis of the notochord. Therefore, in normal conditions vacuole expansion is distributed along the long anterior-posterior axis of the notochord resulting in the elongation of the embryonic axis.
In our recent work6 we investigated the cellular and molecular mechanisms controlling notochord vacuole biogenesis in zebrafish. These studies also provided new insights into the role the notochord vacuoles play during embryonic development. We started by defining the origin of the membranes contributing to the expanding vacuole. Using a combination of mutants, chemical inhibitors and dominant negative constructs we concluded that biosynthetic trafficking but not endocytosis is required for vacuole formation. The requirement for clathrin indicated that post-Golgi trafficking is involved in vacuole biogenesis and led us to investigate the role of the late endosomal machinery. Here we benefited from previous work that identified several mutants in genes encoding members of the HOPS (homotypic fusion and vacuole protein sorting) complex and V-H+-ATPase complexes.7-9 We found that both the HOPS complex genes vps11 and vps18, and V-H+-ATPase-dependent acidification are necessary for vacuole biogenesis and maintenance. However, we also determined that the classical regulator of late endosomes, Rab7, is not necessary for vacuole formation. Therefore, we next investigated other rab proteins that function at the late endosomal, lysosome, or lysosome-related organelle (LRO) stages of vesicular transport such as Rab32 and Rab38, which have been shown to be involved in post-Golgi traffic to melanosomes, a well-characterized LRO.10 Interestingly, expression of dominant negative (DN) Rab32a caused vacuole fragmentation. The involvement of an LRO rab led us to investigate whether lysosomal proteins localize to the vacuole. We found that lysosomal membrane protein Lamp2 labeled lysosomes, whereas Lamp1 co-localized with Rab32a on the vacuole membrane. Taken together, these data showed that the notochord vacuole is a unique fluid-filled LRO (Fig. 1).
We next sought to determine what mechanical functions the notochord vacuoles serve during embryonic development. Disrupting inner vacuolated cell differentiation through overexpression of DN Rab5c or the Notch intracellular domain (NICD) early during development results in notochords without vacuoles.6 Larvae whose notochords have no vacuoles or fragmented vacuoles within the inner cells are dramatically shorter along the anterior-posterior axis at 5 d post fertilization (dpf). When these short larvae were raised to juvenile stages we discovered that defects in notochord vacuoles leads to scoliosis of the spine later during development.
Altogether, our findings demonstrated that in vivo notochord vacuole expansion provides the driving force for embryonic axis elongation. Surprisingly, we also discovered that the notochord has an important structural role later in development during spine formation. This commentary will further examine the architecture of the notochord and discuss what makes this cellular arrangement most effective for elongating the embryonic axis. We will also discuss potential mechanical constraints determining the generation of a single vacuole per inner cell. Finally, we will examine the architecture at a tissue level and propose how two rows of vacuolated cells along the length of the notochord are generated.
Cellular Architecture: What Makes a Single Vacuole More Efficient than Multiple Vacuoles?
When vacuole formation or maintenance is disrupted, there are many small fragmented vacuoles in a given inner cell and these cells occupy less volume than neighboring cells with single fully inflated vacuoles. Moreover, multiple small vacuoles also allow for more irregular cell shapes. Thus, the smaller cellular volume and higher deformability allows cells to pack more tightly into the column of the notochord sheath. In notochords where the inner cells are packed more densely and the overall inner cell number stays the same, the resulting embryo is shorter in the anterior-posterior axis. This can been seen in vps18 mutants who have fragmented vacuoles in many of the inner cells at 5 dpf. In cross section more inner vacuolated cells are seen in a given plane and the vacuoles are smaller overall. The cells are packed more densely in the notochords of these mutants and the larvae are shorter than wild-type siblings at 5 dpf (unpublished data).
Our work has also shown that fragmentation of vacuoles results in a kinking of the spine as it develops.6 Because the fluid within the vacuoles, water, is incompressible, the fully inflated vacuoles in the notochord provide a stable structure that is able to resist the compressive forces of the vertebrae as they grow concentrically around the notochord during spine formation. This concept can be illustrated by thinking of a bag filled with sand compared with a bag filled with large pebbles. The bag of sand can more readily be deformed because the sand grains are tiny and can move past one another within the bag. The bag full of large pebbles, however, cannot be deformed as much in response to a given load because it is more difficult for the large pebbles to rearrange. When multiple small vacuoles are present, compression from growing vertebrae results in a deformation of the overall notochord shape. This occurs because multiple small vacuoles can be displaced and are able to slide past one another within the inner cells of the notochord.
Our findings have raised additional questions about vacuole inflation. One area of interest is to determine the types of channels and transporters found on the vacuole membrane that allow for its rapid inflation. We found that notochord vacuoles are very sensitive to perturbations in the V-H+-ATPase; therefore, it is highly likely that this pump is located on the vacuole membrane and transports protons into the lumen. However, we know that the lumen of the vacuole is not acidic.6 Thus, there must be a system that removes protons from the lumen of the vacuole at the same rate as the V-H+-ATPase pumps them inside. A possible candidate for this proton exchanger could be a Na+/H+ exchanger that neutralizes the luminal pH. The positively charged sodium ions could then function as an osmolyte to draw water into the vacuole during inflation. Alternatively, other positively charged small molecules might serve a similar role. Future work will be aimed at identifying such transporters.
Another area of interest is investigating how vacuole expansion and final size are determined. The rigid ECM encasing the notochord ultimately restricts the expansion of the single vacuole within inner cells. This raises the interesting question of whether the vacuole is at an equilibrium where the final volume is obtained when the channels are all at a steady-state or if there are mechanisms that downregulate the activity of transporters and channels involved in vacuole inflation to limit its expansion. A candidate mechanosensory element limiting vacuole growth is a member of the transient receptor potential (TRP) family of ion channels, TRPV4. TRPV4 is an osmotically activated calcium ion channel that has been shown to be important for osmoregulation in mice11 and cell volume regulation in vitro.12 Importantly, TRPV4 is expressed in the notochord of zebrafish at the time of vacuole inflation.13 Future work should investigate the role of this channel in regulating notochord vacuole volume.
Tissue Architecture: What Determines the Organization of Two Rows of Vacuolated Cells?
Along the length of the anterior-posterior axis of the notochord, inner vacuolated cells are organized in rows of two. When viewed dorsally, the two rows of inner vacuolated cells are aligned such that two vacuoles are not exactly side-by-side, but are slightly offset, with one row being more anterior than the other. The same structure viewed laterally is seen as a single layer of vacuolated cells. This arrangement seen in cross-section shows two vacuolated cells that occupy the entire ~80 µm diameter of the notochord (Fig. 2).
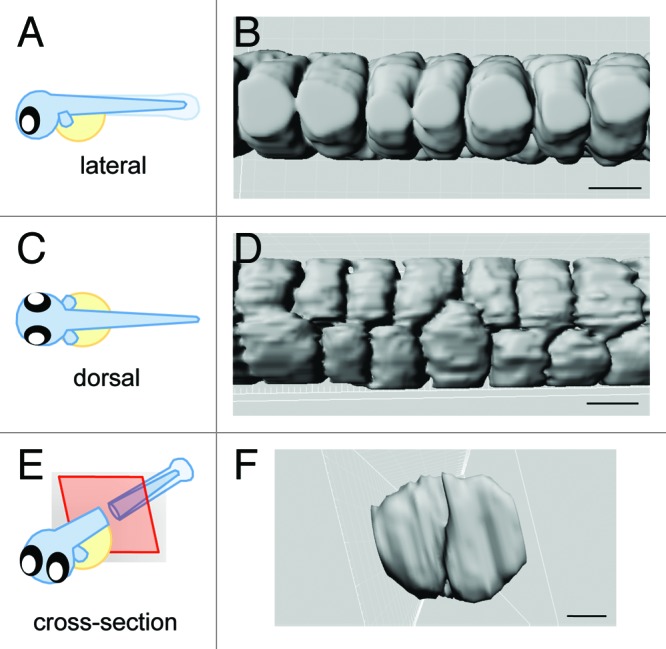
Figure 2. 3-Dimensional model of vacuolated cell arrangement. Imaris software was used to generate a 3-D model of vacuolated cells using a transgenic line that expresses GFP in the cytosol of the inner vacuolated cells, Gt(Gal4FF)nksagff214a; Tg(UAS:GFP).(A, C, E) Cartoons depicting orientation of the embryo for the following panels. (B, D, F) 3-D rendering of vacuolated cells. Scale bars = 30 µm.
Two rows of vacuolated cells filling up the notochord allows for the vacuoles to be a manageable size. If there were instead a single row of large vacuolated cells that occupied the width of the notochord, the vacuoles would be nearly 80 µm in diameter. This would require the vacuoles to be two times larger in diameter and 8 times larger in volume. With 40 µm in diameter at their widest point, notochord vacuoles are the largest known fluid-filled organelle. Perhaps this is nearing the physiological limit for how large a vacuole can be in terms of membrane stability and capacity to transport osmolytes and water within a short developmental window.
The notochord architecture with two rows of vacuolated cells can also be explained by considering the rate of vacuole inflation and the rate of cellular rearrangement required for notochord expansion. As described previously, the notochord begins as a rod of cells in a line like a stack of coins due to the intercalation of chordamesoderm cells that form the notochord during late gastrulation.14,15 The outer sheath cells then leave the midline of the notochord to fully enclose the vacuolated layer in an epithelial-like sheath while the inner cells rearrange and inflate their characteristic vacuoles. The notochord develops in an anterior to posterior gradient; therefore, inner vacuolated cell rearrangement and rate of vacuole inflation are coupled temporally. That is, the inner vacuolated cells are rearranging and inflating simultaneously in discrete regions of the notochord in a wave that starts anterior and moves posteriorly.
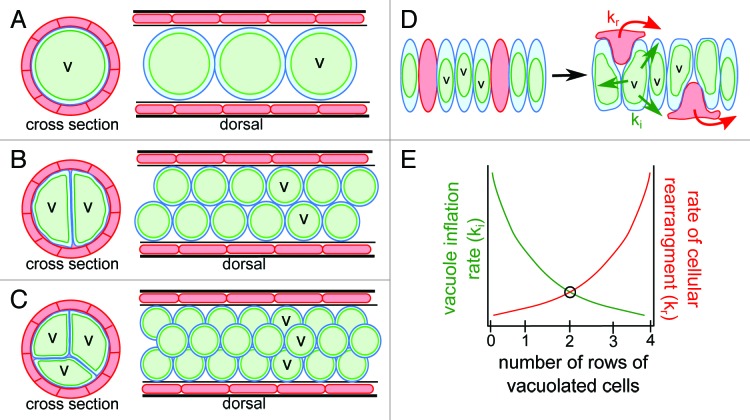
In Figure 3, we propose three hypothetical notochord arrangements: a hypothetical notochord with a single row of vacuolated cells, the actual zebrafish notochord with two rows of vacuolated cells, and a hypothetical notochord with three rows of vacuolated cells. In the first hypothetical notochord where there is only a single row of vacuolated cells, a low rate of cellular rearrangement is required during vacuole inflation because the inner cells are already present in a single file arrangement. In this notochord the vacuoles must also have a rapid inflation rate, otherwise there would be an opportunity for the vacuolated cells to settle and pack next to one another during inflation. In an actual larval zebrafish notochord there are two rows of vacuolated cells. This organization requires a higher rate of cellular rearrangement than the previous scenario so that two vacuolated cells can move to occupy the same plane in cross-section, but requires a slower inflation rate. Finally, in a hypothetical notochord with three rows of vacuolated cells there must be an even higher rate of cellular rearrangement of inner cells during vacuole inflation to allow for three cells to migrate and occupy the same plane in cross-section. In this type of notochord the vacuoles must also inflate much more slowly in order to allow for the packing of three vacuoles per plane to take place. Therefore, given our three scenarios of notochord structure, the ultimate organization that balances vacuole inflation rate with cellular rearrangement is favored (Fig. 3). Future work can test this hypothesis by adjusting the activity of channels on the vacuole membrane and examining the resulting inner vacuolated cell arrangement.
Figure 3. Proposed model of relationship between rate of vacuole inflation and rate of cellular rearrangement. (A) Cartoons of cross section and dorsal views of a hypothetical notochord consisting of a single row of vacuolated inner cells. (B) Cartoons of cross section and dorsal views of a zebrafish notochord consisting of two rows of inner vacuolated cells. (C) Cartoons of cross section and dorsal views of a hypothetical notochord consisting of three rows of vacuolated inner cells. (D) Cartoon depicting the organization of the notochord before vacuole inflation as a single stack of intercalated sheath and vacuolated cells. During development the sheath cells leave the midline to surround the vacuolated cells at a rate of kr while the vacuoles inflate simultaneously at a rate of ki. (E) Graph of the relationship between the rate of cell rearrangement (kr) and rate of vacuole inflation (ki). We hypothesize a balance between the two rates is seen with an organization of two rows of vacuolated cells.
The notochord has long been studied for its signaling roles early in development. Its ability to perform mechanical roles during axis elongation and withstand compressive forces during spine formation brings a new dimension to this organ. Notochord vacuoles represent a unique example of an intracellular structure that has a profound effect on an organ and is critical for shaping the entire embryo.
Acknowledgments
This work was funded by an NIH innovator grant (1DP2OD006486) to M.B. and the Basil O’Connor Starter Scholar from the March of Dimes to B.D.H..
Glossary
Abbreviations:
- ECM
extracellular matrix
- LRO
lysosome-related orgnalle
- DN
dominant negative
- WT
wild type
- dpf
days post fertilization
- hpf
hours post fertilization
- HOPS
homotypic vacuole protein sorting
- V-H+-ATPase
vacuolar-type proton ATPase
- NICD
notch intracellular domain
- TRP
transient receptor potential
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/25503
References
- 1.Yamamoto M, Morita R, Mizoguchi T, Matsuo H, Isoda M, Ishitani T, et al. Mib-Jag1-Notch signalling regulates patterning and structural roles of the notochord by controlling cell-fate decisions. Development. 2010;137:2527–37. doi: 10.1242/dev.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bancroft M, Bellairs R. The development of the notochord in the chick embryo, studied by scanning and transmission electron microscopy. J Embryol Exp Morphol. 1976;35:383–401. [PubMed] [Google Scholar]
- 3.Leeson TS, Leeson CR. Observations on the histochemistry and fine structure of the notochord in rabbit embryos. J Anat. 1958;92:278–85. [PMC free article] [PubMed] [Google Scholar]
- 4.Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–30. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- 5.Koehl MA, Quillin KJ, Pell CA. Mechanical design of fiber-wound hydraulic skeletons: the stiffening and straightening of embryonic notochords. Am Zool. 2000;40:28–41. doi: 10.1668/0003-1569(2000)040[0028:MDOFWH]2.0.CO;2. [DOI] [Google Scholar]
- 6.Ellis K, Bagwell J, Bagnat M. Notochord vacuoles are lysosome-related organelles that function in axis and spine morphogenesis. J Cell Biol. 2013;200:667–79. doi: 10.1083/jcb.201212095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas JL, Vihtelic TS, denDekker AD, Willer G, Luo X, Murphy TR, et al. The loss of vacuolar protein sorting 11 (vps11) causes retinal pathogenesis in a vertebrate model of syndromic albinism. Invest Ophthalmol Vis Sci. 2011;52:3119–28. doi: 10.1167/iovs.10-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadler KC, Amsterdam A, Soroka C, Boyer J, Hopkins N. A genetic screen in zebrafish identifies the mutants vps18, nf2 and foie gras as models of liver disease. Development. 2005;132:3561–72. doi: 10.1242/dev.01918. [DOI] [PubMed] [Google Scholar]
- 9.Nuckels RJ, Ng A, Darland T, Gross JM. The vacuolar-ATPase complex regulates retinoblast proliferation and survival, photoreceptor morphogenesis, and pigmentation in the zebrafish eye. Invest Ophthalmol Vis Sci. 2009;50:893–905. doi: 10.1167/iovs.08-2743. [DOI] [PubMed] [Google Scholar]
- 10.Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J Cell Biol. 2006;175:271–81. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci U S A. 2003;100:13698–703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker D, Blase C, Bereiter-Hahn J, Jendrach M. TRPV4 exhibits a functional role in cell-volume regulation. J Cell Sci. 2005;118:2435–40. doi: 10.1242/jcs.02372. [DOI] [PubMed] [Google Scholar]
- 13.Mangos S, Liu Y, Drummond IA. Dynamic expression of the osmosensory channel trpv4 in multiple developing organs in zebrafish. Gene Expr Patterns. 2007;7:480–4. doi: 10.1016/j.modgep.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glickman NS, Kimmel CB, Jones MA, Adams RJ. Shaping the zebrafish notochord. Development. 2003;130:873–87. doi: 10.1242/dev.00314. [DOI] [PubMed] [Google Scholar]
- 15.Sepich DS, Calmelet C, Kiskowski M, Solnica-Krezel L. Initiation of convergence and extension movements of lateral mesoderm during zebrafish gastrulation. Dev Dyn. 2005;234:279–92. doi: 10.1002/dvdy.20507. [DOI] [PubMed] [Google Scholar]