Abstract
Thousands of women with organ transplantation have undergone successful pregnancies, however little is known about how the profound immunologic changes associated with pregnancy might influence tolerance or rejection of the allograft. Pregnant women with a solid organ transplant are complex chimeras with multiple foreign cell populations from the donor organ, fetus, and mother of the pregnant woman. We consider the impact of complex chimerism and pregnancy-associated immunologic changes on tolerance of the allograft both during pregnancy and the postpartum period. Mechanisms of allograft tolerance are likely dynamic during pregnancy and affected by the influx of fetal microchimeric cells, HLA relationships (between the fetus, pregnant woman and/or donor), peripheral T cell tolerance to fetal cells, and fetal minor histocompatibility antigens. Further research is necessary to understand the complex immunology during pregnancy and the postpartum period of women with a solid organ transplant.
Keywords: pregnancy, transplantation, transplant, tolerance, graft rejection, microchimerism, chimerism, liver, kidney
Introduction
The first solid organ transplantation occurred over 60 years ago, and only a few years later the first pregnancy occurred in an organ transplant recipient.1,2 Since then, thousands of women with organ transplantation have undergone successful pregnancies with live birth rates of approximately 70%. Pregnancy is a unique immunologic phenomenon and although several mechanisms suppress maternal immunity to the semi-allogeneic fetus, there is evidence that maternal T cells actively respond to fetal antigens and can recognize and kill fetal cells in maternal blood.3-5
Maintaining tolerance in a pregnant woman becomes even more complex if she has previously had a solid organ transplant, because multiple allogeneic or semi-allogeneic cell populations are likely to come into contact with one another during the pregnancy. These small foreign cell populations are called microchimerism (Mc) and make a pregnant woman with a solid organ transplant a complex chimera. The primary sources of Mc in this case are from the donor organ and the fetus. During pregnancy, fetal cells routinely enter the maternal circulation and may persist long-term in multiple immune cell subsets similar to cells from the donor organ allograft.6,7 Fetal microchimerism (fetal Mc; FMc) refers to this small foreign cell population that may persist for decades, and has been implicated in autoimmune disease and immune surveillance.8,9 A third source of Mc is from maternal cells that entered the pregnant woman when she herself was a fetus (maternal Mc; MMc) and have persisted for decades.10 Additional Mc cell populations may be present in women with a solid organ transplant as a result of a transfusion, a prior pregnancy or from cell transfer between twins in utero.11-14 Male cells have also been identified in the apheresis products for hematopoietic stem cell transplants from female donors, suggesting that male Mc (presumably FMc) is also transferred.15 Therefore, there exist at least three and possibly more sources of Mc in the pregnant woman, which include FMc, MMc and from the transplant itself. If we consider that antigen-presenting cells (APC) from the pregnant woman or donor organ can present antigens from each other or Mc cell populations, there are at least 16 (24) combinations of Mc, donor, or recipient antigen presentation by either the donor, recipient Mc.
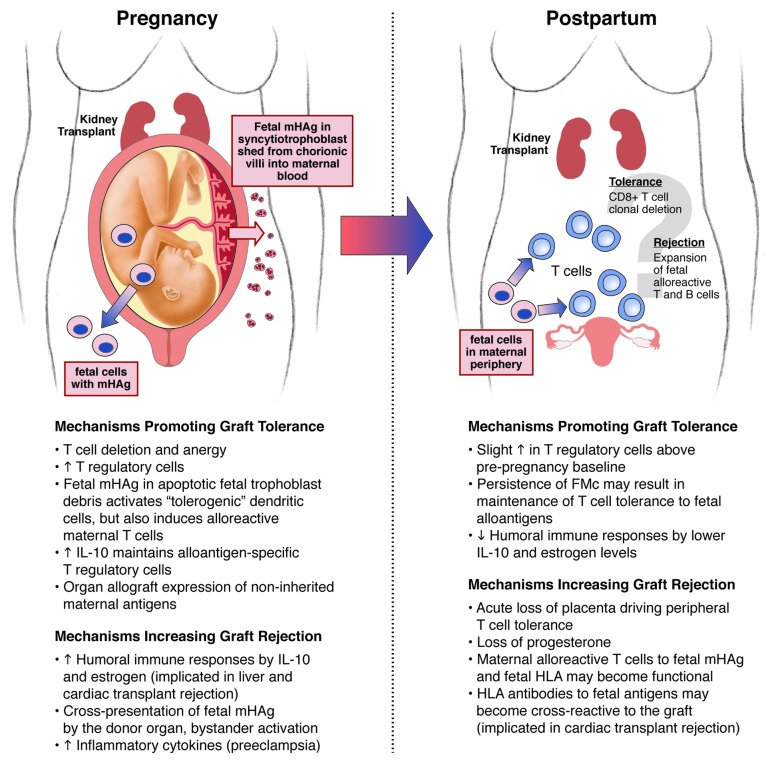
The immunologic complexity presented by this unique situation presents several questions worthy of consideration and further investigation. What are the mechanisms responsible for organ allograft rejection during pregnancy, and could Mc play a role? There is also evidence that the postpartum period is immunologically unique with greater T cell autoreactivity than during pregnancy. The allograft may be at increased risk for rejection postpartum if there is greater T cell allo- and auto-reactivity. The characteristic postpartum flare of certain autoimmune diseases and frequent development of thyroiditis suggests that an immunologic shift from a pregnant to a non-pregnant state is not always smooth.16-18 Here, we explore potential mechanisms of immune tolerance and rejection within a pregnant woman and postpartum of a solid organ transplant and implications of this complex chimerism (Fig. 1).
Figure 1. Possible mechanisms for solid organ transplant rejection or tolerance during pregnancy and postpartum.
Solid Organ Transplant Rejection during Pregnancy
Many mechanisms of transplant rejection may act over time to damage the graft and include hyperacute, acute and chronic rejection that involve both B and T cell-mediated responses.19 Most pregnant women with an organ transplant are more than one year out from their transplant and are at greatest risk of chronic allograft rejection, but late cases of antibody-mediated acute rejection can also occur. Chronic rejection refers to the long-term loss of organ function from the inability to suppress antibodies or T cells alloreactive for the graft. When kidney rejection is antibody-mediated, donor-specific antibodies bind to and injure the capillary endothelium leading to cellular hypertrophy, fibrin deposition, and characteristic changes in the glomerular basement membrane. T cells alloreactive for the graft invade the renal parenchyma and interstitium, causing widespread damage.
The cumulative data from the National Transplantation Pregnancy Registry (NTPR) in 2011 for rejection and graft loss are shown in Table 1.20 The NTPR is a voluntary registry and does not capture all US pregnancies in women with a prior organ transplant, but is currently the best source of data in this population. Data for transplant rejection and graft loss is greatest for women with a prior kidney or liver transplant with less data on outcomes for pancreas-kidney, cardiac and lung transplants. In Table 1, graft rejection for the kidney represents acute, biopsy-proven rejection and for other organs reflect a mix of acute and chronic rejection that was not always confirmed by biopsy. Not all cases of kidney rejection were captured if the physicians treated for suspected rejection without performing a biopsy. Some cardiac rejections reflected histologic evidence of rejection found on a protocol-driven biopsy that was not treated.
Table 1. Rejection and graft loss in pregnant and non-pregnant women with an organ transplant.
| Organ Transplant Subgroup | Pregnant women with an organ transplant | Non-pregnant women with organ transplant | ||||
|---|---|---|---|---|---|---|
| |
N |
Rejection during pregnancy |
Graft loss within 2 y of delivery |
N |
Rejection within 1 y of transplant |
Graft loss within 2 y of transplant |
|
Kidney* |
|
|
|
|
|
|
| CsA |
517 |
1% |
9% |
17,379 |
11% |
11% |
| CsA mod |
241 |
2% |
6% |
|||
| Tacrolimus |
278 |
2% |
6% |
|||
|
Liver |
|
|
|
|
|
|
| CsA |
100 |
11% |
8% |
2,891 |
19% |
23% |
| CsA modified |
64 |
2% |
3% |
|||
| Tacrolimus |
140 |
5% |
5% |
|||
|
Pancreas-Kidney |
|
|
|
|
|
|
| CsA |
23 |
14% |
13% |
1,855 |
10% |
12% |
| CsA modified |
23 |
0% |
17% |
|||
| Tacrolimus |
42 |
5% |
10% |
|||
|
Heart |
|
|
|
|
|
|
| CsA |
43 |
21% |
0% |
1,259 |
31% |
21% |
| CsA modified |
25 |
4% |
4% |
|||
| Tacrolimus |
35 |
3% |
3% |
|||
| Lung | 31 | 16% | 14% | 1,257 | 32% | 28% |
n = number of pregnancies or number of non-pregnant women. Data was obtained from the NTPR (2011 Annual Report)18 and the Organ Procurement and Transplantation Network on May 24, 2013. The non-pregnant women were 18–39 y old and received their transplant between 2000–2010. CsA: Sandimmune® brand cyclosporine A; CsA modified: newer formulation of CsA became available in 1994 with improved absorption over CsA. *The NTPR data on rejection in kidney transplants during pregnancy is acute, biopsy-proven rejection. Rejection for other organ transplants represents both acute and chronic rejection and is not always biopsy-proven.
A direct comparison of rejection rates in pregnancy across organ transplants is difficult due to the many confounding variables associated with the diagnosis of graft rejection during pregnancy. The first factor is that physicians have a lower threshold to biopsy a heart or liver transplant to diagnose rejection in pregnant women, because these organs are truly “life-saving” and dialysis is not an option. Renal biopsies during pregnancy may be delayed until postpartum and if rejection is suspected, treatment may be initiated without biopsy. This would bias toward a relatively higher rate of rejection in cardiac and liver transplants during pregnancy and a higher rejection of kidney transplants postpartum. Comparison of these rates to non-pregnant women in Table 1 is challenging for slightly different reasons. For pregnant women, rejection and graft loss are reported during the pregnancy, which may be several years after the initial transplantation. In non-pregnant women, rejection and graft loss are reported with respect to time of transplantation, which captures a high-risk time early after transplantation of acute rejection and graft loss. Many other factors not categorized here also influence rates of graft loss including age, immunosuppressant regimen, degree of HLA matching (kidney), and primary disease leading to organ transplant (liver).
An interesting question is whether the mechanisms of allograft rejection during pregnancy are somewhat different than in non-pregnant women with greater tolerance imparted to organ transplants requiring T cell tolerance due to more robust HLA expression (e.g., kidney). For kidney transplants, HLA class I and II antigens are expressed and greater HLA matching is associated with superior outcomes.21,22 Alloreactive T cells for the donor organ may be downregulated during pregnancy, thus decreasing the risk of chronic rejection in kidney transplants. In contrast, liver and cardiac transplants require no HLA matching and normal hepatocytes and cardiac myocytes either do not or weakly express HLA class I and class II; rejection in these organs is often acute and B-cell mediated.23-26 We hypothesize that a possible increase in cardiac and liver transplant rejection during pregnancy could be primarily antibody-mediated as a result of placental IL-10 secretion, which activates B cells (see “Other mechanisms” section); higher IL-10 is known to be associated with cardiac transplant rejection in non-pregnant patients.27,28 This hypothesis has not been previously tested, but could be investigated using immunohistochemistry in a retrospective study using tissue biopsies from pregnant and non-pregnant women with organ rejection.
During Pregnancy: Possible Mechanisms of Organ Transplant Tolerance or Rejection
Peripheral T cell tolerance established during pregnancy results in T cell anergy, deletion and induction of Treg cells, which we have previously hypothesized to be a result of the interaction between fetal antigens or cells and the maternal immune system.29,30 One possible mechanism for graft rejection or tolerance during pregnancy would involve cross-presentation of fetal antigens by donor (graft) antigen-presenting cells that would lead to production of maternal alloreactive CD8+ T cells (cross-priming).31 Large quantities of fetal antigens become systemic due to the daily shedding of apoptotic syncytiotrophoblast debris from the placenta. Antigens shed likely include minor histocompatibility antigens, since several minor antigens that are found in the syncytiotrophoblast correspond to identified populations of expanded maternal T cells specific for fetal minor antigens.32-34 During normal pregnancy, the uptake of these antigens by immature dendritic cells in the context of apoptotic debris would bias toward a tolerogenic phenotype and induction of T cell tolerance.29,35 However, in the setting of heightened systemic inflammation this process may be more likely to incite an alloreactive response; inflammation is critical for implantation, but also occurs with preterm birth and preeclampsia.36-38 Alloreactivity to fetal antigens presented by donor APCs in the graft might then lead to inflammation with bystander damage, widespread organ injury and rejection. This mechanism is consistent with murine data suggesting that the maternal immune system becomes “aware” of a fetal minor antigen through indirect presentation by maternal antigen presenting cells.39
The organ transplant may benefit from the presence of microchimeric cells within the allograft during pregnancy, which may have tolerogenic effects. Starzl first proposed that after liver transplantation, the detection of Mc donor cells in the recipient was beneficial and correlated with long-term tolerance of the organ allograft.40 In the setting of multiple sources of Mc, the role of donor Mc from the organ allograft is unknown, but is postulated to have a beneficial effect on tolerance if present. We hypothesize that both FMc and MMc also interact with the donor organ and may become incorporated in the tissue. Studies have shown that FMc and MMc are found in many internal organs and even express tissue-specific markers (e.g., sarcomeric α-actin in cardiac myocytes, insulin staining in pancreatic islet β cells).41-46 Higher levels of MMc in the blood, heart and spleen have recently been correlated with cardiac transplant survival in mice.47 The interaction of multiple sources of Mc in this setting may be analogous to studies of Mc in parous women or after cord blood transplantation (two donors), where Mc populations may compete resulting in predominance of Mc from one source.48,49 If the dominant Mc population expresses an HLA shared by the transplanted organ, this may be a factor increasing long-term tolerance of the organ allograft.
Consideration of HLA relationships between the recipient, her Mc cells, and the transplanted organ are likely a major factor in determining the balance between tolerance and rejection of a solid organ transplant during pregnancy. A significant difference in 10-y survival of a kidney transplant from a sibling was found if the organ expressed non-inherited maternal antigens (NIMA) vs. the non-inherited paternal antigen (NIPA; 77% vs 49%).50 The transplant recipients were thought to become tolerant to NIMA through exposure to maternal antigens or MMc, which begins in fetal life. Tolerance associated with NIMA exposure has now been shown to correlate with NIMA-specific regulatory T cells (Tregs) within the allograft of a murine model.51 We also believe that HLA disparity between the pregnant woman and her fetus is another important factor in achieving “tolerogenic” effects for the allograft associated with a more robust peripheral T cell tolerance. Amelioration or remission of both rheumatoid arthritis and inflammatory bowel disease (T cell mediated) is greatest with maternal-fetal HLA-disparity and increasing disparity at multiple HLA loci.52,53
Other Mechanisms of Organ Transplant Tolerance or Rejection during Pregnancy
Some mechanisms that promote maternal-fetal tolerance during pregnancy may paradoxically increase allograft rejection. The placenta secretes interleukin-10 (IL-10) and estrogen, which enhances B cell survival and antibody production.54,55 Higher levels of IL-10 are also associated with liver and cardiac transplant rejection.56-58 Pregnancy has been implicated in two case reports as a factor for acute antibody-mediated rejection; in both cases there were new antibodies to fetal inherited paternal antigens (IPA) and the allograft detected following pregnancy.59,60 Parous women are also known to frequently (~30%) have antibodies against fetal HLA molecules (paternally inherited), minor histocompatibility antigens (mHA), and ABO blood-group antigens, which may cross-react with the allograft.61
Preeclampsia, a pregnancy complication associated with production of inflammatory cytokines, has been thought to increase the risk of rejection and occurs frequently in pregnant women with kidney transplants (20–27%).62-66 However, preeclampsia was not correlated with graft rejection either during pregnancy, 3 months postpartum or within 2 years of pregnancy among pregnant women with a kidney transplant on tacrolimus (n = 54 with vs. n = 94 without preeclampsia).67 Another explanation for higher rates of rejection during pregnancy could be hemodynamic stress on the graft associated with a 30–50% increase in cardiac output during pregnancy and peripartum fluid shifts. Both of these factors may contribute to endothelial dysfunction or oxidative stress within the graft.68-70
Postpartum: Possible Mechanisms of Organ Transplant Tolerance or Rejection
The lack of research on immunologic shifts and allo- or autoreactivity in the postpartum period represents a scientific “black box.” There is very little data on graft rejection in the first 3 months postpartum with the exception of recent data presented for women with a kidney transplant on tacrolimus-based immunosuppression. In this analysis, women were compared who had their first post-transplant pregnancy after a first transplant (T1, n = 129) or a retransplantation (T2; n = 26).67 Kidney graft rejection was more common in the first 3 months postpartum (T1: 6%, T2: 8%) than during pregnancy (T1: 2%, T2: 4%). We recognize that renal biopsies are sometimes delayed until after delivery, which would bias toward a higher rejection rate postpartum. However, the idea that the maternal immune system is more allo- or autoreactive postpartum is consistent with the classic flares of autoimmune disease typical for women with rheumatoid arthritis,18 Graves disease,71 and multiple sclerosis72 in the first few months after delivery. We hypothesize that the delicate immunologic balance required for solid organ allograft tolerance is perturbed in the first few months postpartum due mainly to the acute loss of the placenta driving multiple mechanisms of peripheral T cell tolerance.29,30 The rapid decline in progesterone is another potential factor in graft rejection postpartum; progesterone is known to have immunomodulatory effects on several immune cell populations (B cells, macrophages, NK cells, production of Th2 cytokines).73 Estrogen withdrawal may also be a factor favoring graft rejection in breastfeeding women, because estrogen expands Treg populations.74 In preliminary data, no adverse neonatal outcomes (n = 98 infants) were found by the NTPR in breastfeeding women on a variety of immunosuppressants, but postpartum graft rejection in this population has not been fully evaluated.75
HLA sensitization can occur following (or during) pregnancy and has been described in two cardiac transplant recipients who developed new cross-reactive HLA class II paternal and donor-specific antibodies. In one case, the antibodies developed following a normal pregnancy and stable immunosuppressant regimen 17 years after transplantation.59 At three months postpartum, she presented with allograft dysfunction and was noted to have developed new HLA donor-specific antibodies. HLA typing performed on her baby and the baby’s father showed paternal antibodies presented by the fetus likely led to cross-reactivity to donor allograft antibodies leading to a cytotoxic response. She had clinical improvement with intravenous immunoglobulin, but ultimately required retransplantation at five months postpartum. Her failed allograft showed severe cardiac vasculopathy with mild acute cellular rejection. A second case of antibody-mediated rejection of a cardiac transplant was reported following an 8-week miscarriage 6 years after transplantation.60 Incidence of HLA sensitization in pregnant women with organ transplants is unknown, but may be exacerbated by elevated IL-10 and estrogen. With more than 100 pregnancy outcomes in cardiac transplant recipients in the NTPR database, it would be of further interest to assay for HLA antibodies specific for the donor and fetal IPA in cardiac transplant and other solid organ transplant recipients as a mechanism for postpartum allograft rejection or graft loss within the first few years after delivery.67
In addition to de novo generation of maternal anti-paternal HLA antibodies during pregnancy, maternal T lymphocytes specific for fetal minor histocompatibility antigens expand.4,32,33,76 Although these cells can be shown to possess effector cytokine and cytotoxic activity once stimulated in vitro,4,33,77 it is highly likely that in vivo expansion is blunted, they are hyporesponsive to antigen, and they upregulate inhibitory receptors.3,39,78,79 Interestingly, the long-term persistence of maternal T cells specific for fetal minor antigens, including HA-1, HA-2 and HY, suggests that these cells could interact with the same alloantigens in a different context, such as transplantation, during or following pregnancy. Whether these T cells might become alloreactive to or protective for the organ transplant during pregnancy or postpartum is uncertain, but in mouse studies it is observed that T cell tolerance during pregnancy to male skin grafts is induced as a result of multiparity.76,80,81 However, in another study using tumor cells expressing paternal Major Histocompatibility Complex (MHC) genes, the cells were tolerated during pregnancy but rejected immediately following pregnancy.3 This difference in alloreactivity may depend upon whether the microchimeric cells engraft into the spleen or bone marrow; one study described lifelong maintenance of Mc after engraftment of splenocytes with a single foreign MHC class I–restricted epitope that correlated with CD8+ T cell unresponsiveness, likely from clonal deletion.82 Further mechanistic studies on the short- and long-term impact of the pregnancy-associated maternal immune response to major and minor histocompatibility antigens shared by the fetoplacental unit and grafted organs will be informative of the sequelae of events associated with transplantation outcome following pregnancy.
Summary
A pregnant woman with an organ transplant is a complex chimera with cell populations deriving from the donor organ, fetus, her own mother and potentially other sources (i.e., blood transfusion or a prior pregnancy). By the third trimester, the maternal immune system is exposed daily to gram-quantities of apoptotic syncytiotrophoblast material containing fetal antigens, which is thought to promote peripheral T cell tolerance through uptake by immature dendritic cells. Whether fetal Mc persisting postpartum in a T- or B-cell lineage might change functionally after the delivery to then cross-react with the organ transplant may be a function of exposure to inflammation (e.g., preeclampsia) and HLA relationships between the mother, fetus and donor. Exposure to NIMA, either in fetal life or through MMc, is known to be associated with superior outcomes in kidney transplants that also express NIMA. Factors other than Mc are likely very important for driving acute antibody-mediated rejection during pregnancy for cardiac and liver transplants; placental secretion of IL-10 and estrogen promotes humoral immune responses and may exacerbate antibody responses to the graft during pregnancy.
The postpartum period is immunologically unique with relatively little evidence to determine if this period is characterized by greater graft rejection and relatively more alloreactive than at other times in a woman’s life. A major factor that could influence graft rejection is the acute loss of the placenta, which was a primary driver of peripheral T cell tolerance through shedding of fetal antigens in apoptotic syncytiotrophoblast. Interestingly, a few T cell mediated autoimmune diseases that may ameliorate or remit during pregnancy have a characteristic flare postpartum suggesting that this period may be characterized by greater autoreactivity. At the same time, IL-10 and estrogen levels also decrease, which may decrease the humoral immune responses that play a role in antibody-mediated rejection. Although there are limited data to confirm or refute hypotheses regarding immunology of the postpartum period, consideration of interactions between these pathways has highlighted areas for future investigation that have relevance for Mc, transplantation, and women’s health. Encouraging pregnant women with a solid organ transplant to register with the NTPR will allow further study of these questions.
Financial Disclosures
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Allergy and Infectious Diseases, and National Center for Research Resources of the National Institutes of Health under award numbers [R01AI100989, K08AI067910, K12HD001264, P30HD002274, UL1RR025014]. The authors are also grateful for support past and present from the March of Dimes (21-FY06-77, 21-FY08-562) University of Washington Institute for Translational Health Research, and the Washington State Obstetrical Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders. The NTPR is supported by grants from Novartis Pharmaceuticals Corp., Astellas Pharma, US Inc., Pfizer Inc. and Bristol-Myers Squibb, Co.
Acknowledgments
I am grateful to Dr Lee Nelson for the many thought-provoking discussions about microchimerism, transplantation and the postpartum “black box.” We gratefully acknowledge Jan Hamanishi for her help in preparing Figure 1. We also thank the Organ Procurement and Transplantation Network, who provided us with customized data on non-pregnant women shown in Table 1.
Submitted
04/16/13
Revised
06/13/13
Accepted
06/13/13
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/chimerism/article/25401
References
- 1.Murray JE, Merrill JP, Dammin GJ, Dealy JB, Jr., Walter CW, Brooke MS, et al. Study on transplantation immunity after total body irradiation: clinical and experimental investigation. Surgery. 1960;48:272–84. [PubMed] [Google Scholar]
- 2.Murray JE, Reid DE, Harrison JH, Merrill JP. Successful pregnancies after human renal transplantation. N Engl J Med. 1963;269:341–3. doi: 10.1056/NEJM196308152690704. [DOI] [PubMed] [Google Scholar]
- 3.Tafuri A, Alferink J, Möller P, Hämmerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–3. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 4.Lissauer D, Piper K, Goodyear O, Kilby MD, Moss PA. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J Immunol. 2012;189:1072–80. doi: 10.4049/jimmunol.1200544. [DOI] [PubMed] [Google Scholar]
- 5.Bonney EA, Matzinger P. The maternal immune system’s interaction with circulating fetal cells. J Immunol. 1997;158:40–7. [PubMed] [Google Scholar]
- 6.Lambert NC, Evans PC, Hashizumi TL, Maloney S, Gooley T, Furst DE, et al. Cutting edge: persistent fetal microchimerism in T lymphocytes is associated with HLA-DQA1*0501: implications in autoimmunity. J Immunol. 2000;164:5545–8. doi: 10.4049/jimmunol.164.11.5545. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA.Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum Proc Natl Acad Sci U S A 199693705–8.PMID:8570620 10.1016/j.it.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson JL.The otherness of self: microchimerism in health and disease Trends Immunol 201233421–7.PMID:22609148 10.1073/pnas.93.2.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams KM, Nelson JL. Microchimerism: an investigative frontier in autoimmunity and transplantation. JAMA. 2004;291:1127–31. doi: 10.1001/jama.291.9.1127. [DOI] [PubMed] [Google Scholar]
- 10.Maloney S, Smith A, Furst DE, Myerson D, Rupert K, Evans PC, et al. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999;104:41–7. doi: 10.1172/JCI6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Moor G, De Bock G, Noens L, De Bie S. A new case of human chimerism detected after pregnancy: 46,XY karyotype in the lymphocytes of a woman. Acta Clin Belg. 1988;43:231–5. doi: 10.1080/17843286.1988.11717936. [DOI] [PubMed] [Google Scholar]
- 12.Lee TH, Paglieroni T, Ohto H, Holland PV, Busch MP. Survival of donor leukocyte subpopulations in immunocompetent transfusion recipients: frequent long-term microchimerism in severe trauma patients. Blood. 1999;93:3127–39. doi: 10.1172/JCI6611. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi DW, Farina A, Weber W, Delli-Bovi LC, Deriso M, Williams JM, et al. Significant fetal-maternal hemorrhage after termination of pregnancy: implications for development of fetal cell microchimerism. Am J Obstet Gynecol. 2001;184:703–6. doi: 10.1067/mob.2001.111072. [DOI] [PubMed] [Google Scholar]
- 14.Peterson SE, Nelson JL, Guthrie KA, Gadi VK, Aydelotte TM, Oyer DJ, et al. Prospective assessment of fetal-maternal cell transfer in miscarriage and pregnancy termination. Hum Reprod. 2012;27:2607–12. doi: 10.1093/humrep/des244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams KM, Lambert NC, Heimfeld S, Tylee TS, Pang JM, Erickson TD, et al. Male DNA in female donor apheresis and CD34-enriched products. Blood. 2003;102:3845–7. doi: 10.1182/blood-2003-05-1570. [DOI] [PubMed] [Google Scholar]
- 16.Davies TF. The thyroid immunology of the postpartum period. Thyroid. 1999;9:675–84. doi: 10.1089/thy.1999.9.675. [DOI] [PubMed] [Google Scholar]
- 17.Chan GW, Mandel SJ. Therapy insight: management of Graves’ disease during pregnancy. Nat Clin Pract Endocrinol Metab. 2007;3:470–8. doi: 10.1038/ncpendmet0508. [DOI] [PubMed] [Google Scholar]
- 18.Hench PS. The ameliorating effect of pregnancy on chronic atrophic (infectious rheumatoid) arthritis, fibrositis, and intermittent hydrarthrosis. Mayo Clin Proc. 1938;13:161–7. [Google Scholar]
- 19.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363:1451–62. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 20.National Transplantation Pregnancy Registry (NTPR)2011Annual Report. Philadelphia, PA: Thomas Jefferson University;2011.
- 21.Müller CA, Markovic-Lipkovski J, Risler T, Bohle A, Müller GA. Expression of HLA-DQ, -DR, and -DP antigens in normal kidney and glomerulonephritis. Kidney Int. 1989;35:116–24. doi: 10.1038/ki.1989.16. [DOI] [PubMed] [Google Scholar]
- 22.Daar AS, Fuggle SV, Fabre JW, Ting A, Morris PJ. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984;38:287–92. doi: 10.1097/00007890-198409000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Navarro V, Herrine S, Katopes C, Colombe B, Spain CV. The effect of HLA class I (A and B) and class II (DR) compatibility on liver transplantation outcomes: an analysis of the OPTN database. Liver Transpl. 2006;12:652–8. doi: 10.1002/lt.20680. [DOI] [PubMed] [Google Scholar]
- 24.Fleming KA, McMichael A, Morton JA, Woods J, McGee JO. Distribution of HLA class 1 antigens in normal human tissue and in mammary cancer. J Clin Pathol. 1981;34:779–84. doi: 10.1136/jcp.34.7.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukusato T, Gerber MA, Thung SN, Ferrone S, Schaffner F. Expression of HLA class I antigens on hepatocytes in liver disease. Am J Pathol. 1986;123:264–70. [PMC free article] [PubMed] [Google Scholar]
- 26.Chih S, Chruscinski A, Ross HJ, Tinckam K, Butany J, Rao V. Antibody-mediated rejection: an evolving entity in heart transplantation. J Transplant. 2012;2012:210210. doi: 10.1155/2012/210210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Neil DA, Hübscher SG. Current views on rejection pathology in liver transplantation. Transpl Int. 2010;23:971–83. doi: 10.1111/j.1432-2277.2010.01143.x. [DOI] [PubMed] [Google Scholar]
- 29.Adams KM, Yan Z, Stevens AM, Nelson JL. The changing maternal “self” hypothesis: a mechanism for maternal tolerance of the fetus. Placenta. 2007;28:378–82. doi: 10.1016/j.placenta.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Taglauer ES, Adams Waldorf KM, Petroff MG. The hidden maternal-fetal interface: events involving the lymphoid organs in maternal-fetal tolerance. Int J Dev Biol. 2009:15. doi: 10.1387/ijdb.082800et. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bevan MJ. Cross-priming. Nat Immunol. 2006;7:363–5. doi: 10.1038/ni0406-363. [DOI] [PubMed] [Google Scholar]
- 32.van Halteren AG, Jankowska-Gan E, Joosten A, Blokland E, Pool J, Brand A, et al. Naturally acquired tolerance and sensitization to minor histocompatibility antigens in healthy family members. Blood. 2009;114:2263–72. doi: 10.1182/blood-2009-01-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verdijk RM, Kloosterman A, Pool J, van de Keur M, Naipal AM, van Halteren AG, et al. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103:1961–4. doi: 10.1182/blood-2003-05-1625. [DOI] [PubMed] [Google Scholar]
- 34.Holland OJ, Linscheid C, Hodes HC, Nauser TL, Gilliam M, Stone P, et al. Minor histocompatibility antigens are expressed in syncytiotrophoblast and trophoblast debris: implications for maternal alloreactivity to the fetus. Am J Pathol. 2012;180:256–66. doi: 10.1016/j.ajpath.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taglauer ES, Adams Waldorf KM, Petroff MG. The hidden maternal-fetal interface: events involving the lymphoid organs in maternal-fetal tolerance. Int J Dev Biol. 2010;54:421–30. doi: 10.1387/ijdb.082800et. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–7. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–93. doi: 10.1016/S0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 38.Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol. 2012;95:1–14. doi: 10.1016/j.jri.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117:1399–411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–82. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan WF, Gurnot C, Montine TJ, Sonnen JA, Guthrie KA, Nelson JL. Male microchimerism in the human female brain. PLoS One. 2012;7:e45592. doi: 10.1371/journal.pone.0045592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevens AM, Hermes HM, Rutledge JC, Buyon JP, Nelson JL. Myocardial-tissue-specific phenotype of maternal microchimerism in neonatal lupus congenital heart block. Lancet. 2003;362:1617–23. doi: 10.1016/S0140-6736(03)14795-2. [DOI] [PubMed] [Google Scholar]
- 43.Stevens AM, McDonnell WM, Mullarkey ME, Pang JM, Leisenring W, Nelson JL. Liver biopsies from human females contain male hepatocytes in the absence of transplantation. Lab Invest. 2004;84:1603–9. doi: 10.1038/labinvest.3700193. [DOI] [PubMed] [Google Scholar]
- 44.Nelson JL, Gillespie KM, Lambert NC, Stevens AM, Loubiere LS, Rutledge JC, et al. Maternal microchimerism in peripheral blood in type 1 diabetes and pancreatic islet beta cell microchimerism. Proc Natl Acad Sci U S A. 2007;104:1637–42. doi: 10.1073/pnas.0606169104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gammill HS, Nelson JL. Naturally acquired microchimerism. Int J Dev Biol. 2010;54:531–43. doi: 10.1387/ijdb.082767hg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gadi VK. Fetal microchimerism in breast from women with and without breast cancer. Breast Cancer Res Treat. 2010;121:241–4. doi: 10.1007/s10549-009-0548-1. [DOI] [PubMed] [Google Scholar]
- 47.Dutta P, Burlingham WJ. Correlation between post transplant maternal microchimerism and tolerance across MHC barriers in mice. Chimerism. 2011;2:78–83. doi: 10.4161/chim.18083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutman JA, Turtle CJ, Manley TJ, Heimfeld S, Bernstein ID, Riddell SR, et al. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T-cell response against the nonengrafted unit. Blood. 2010;115:757–65. doi: 10.1182/blood-2009-07-228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gammill HS, Guthrie KA, Aydelotte TM, Adams Waldorf KM, Nelson JL. Effect of parity on fetal and maternal microchimerism: interaction of grafts within a host? Blood. 2010;116:2706–12. doi: 10.1182/blood-2010-02-270942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339:1657–64. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 51.Molitor-Dart ML, Andrassy J, Kwun J, Kayaoglu HA, Roenneburg DA, Haynes LD, et al. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol. 2007;179:6749–61. doi: 10.4049/jimmunol.179.10.6749. [DOI] [PubMed] [Google Scholar]
- 52.Nelson JL, Hughes KA, Smith AG, Nisperos BB, Branchaud AM, Hansen JA. Maternal-fetal disparity in HLA class II alloantigens and the pregnancy-induced amelioration of rheumatoid arthritis. N Engl J Med. 1993;329:466–71. doi: 10.1056/NEJM199308123290704. [DOI] [PubMed] [Google Scholar]
- 53.Kane S, Kisiel J, Shih L, Hanauer S. HLA disparity determines disease activity through pregnancy in women with inflammatory bowel disease. Am J Gastroenterol. 2004;99:1523–6. doi: 10.1111/j.1572-0241.2004.30472.x. [DOI] [PubMed] [Google Scholar]
- 54.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993;151:4562–73. [PubMed] [Google Scholar]
- 55.Lang TJ. Estrogen as an immunomodulator. Clin Immunol. 2004;113:224–30. doi: 10.1016/j.clim.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Segal BM, Glass DD, Shevach EM. Cutting Edge: IL-10-producing CD4+ T cells mediate tumor rejection. J Immunol. 2002;168:1–4. doi: 10.4049/jimmunol.168.1.1. [DOI] [PubMed] [Google Scholar]
- 57.Wan R, Tang L, Shan R, Zeng L, Chen H, Gao L. Humoral immunity-mediated chronic rejection in liver transplantation is associated with predominant IL-10 expression. Front Biosci (Elite Ed) 2012;4:2121–30. doi: 10.2741/529. [Elite Ed] [DOI] [PubMed] [Google Scholar]
- 58.Qian S, Li W, Li Y, Fu F, Lu L, Fung JJ, et al. Systemic administration of cellular interleukin-10 can exacerbate cardiac allograft rejection in mice. Transplantation. 1996;62:1709–14. doi: 10.1097/00007890-199612270-00002. [DOI] [PubMed] [Google Scholar]
- 59.Ginwalla M, Pando MJ, Khush KK. Pregnancy-related human leukocyte antigen sensitization leading to cardiac allograft vasculopathy and graft failure in a heart transplant recipient: a case report. Transplant Proc. 2013;45:800–2. doi: 10.1016/j.transproceed.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Boyle PJ, Smith JD, Danskine AJ, Lyster HS, Burke MM, Banner NR. De novo HLA sensitization and antibody mediated rejection following pregnancy in a heart transplant recipient. Am J Transplant. 2010;10:180–3. doi: 10.1111/j.1600-6143.2009.02875.x. [DOI] [PubMed] [Google Scholar]
- 61.Van Rood JJ, Eernisse JG, Van Leeuwen A. Leucocyte antibodies in sera from pregnant women. Nature. 1958;181:1735–6. doi: 10.1038/1811735a0. [DOI] [PubMed] [Google Scholar]
- 62.Deshpande NA, James NT, Kucirka LM, Boyarsky BJ, Garonzik-Wang JM, Cameron AM, et al. Pregnancy outcomes of liver transplant recipients: a systematic review and meta-analysis. Liver Transpl. 2012;18:621–9. doi: 10.1002/lt.23416. [DOI] [PubMed] [Google Scholar]
- 63.Deshpande NA, James NT, Kucirka LM, Boyarsky BJ, Garonzik-Wang JM, Montgomery RA, et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant. 2011;11:2388–404. doi: 10.1111/j.1600-6143.2011.03656.x. [DOI] [PubMed] [Google Scholar]
- 64.Madazli R, Aydin S, Uludag S, Vildan O, Tolun N. Maternal plasma levels of cytokines in normal and preeclamptic pregnancies and their relationship with diastolic blood pressure and fibronectin levels. Acta Obstet Gynecol Scand. 2003;82:797–802. doi: 10.1034/j.1600-0412.2003.00206.x. [DOI] [PubMed] [Google Scholar]
- 65.Takacs P, Green KL, Nikaeo A, Kauma SW. Increased vascular endothelial cell production of interleukin-6 in severe preeclampsia. Am J Obstet Gynecol. 2003;188:740–4. doi: 10.1067/mob.2003.134. [DOI] [PubMed] [Google Scholar]
- 66.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102:20–5. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 67.2011 Annual Report: National Transplantation Pregnancy Registry. Philadelphia, PA: Thomas Jefferson University;2011. [Google Scholar]
- 68.Gabbe SG, Niebyl JR, Simpson JL, et al. Obstetrics: Normal and Problem Pregnancies Philadelphia, PA: Elsevier, Inc.; 2012. [Google Scholar]
- 69.Davies PF, Dewey CF, Jr., Bussolari SR, Gordon EJ, Gimbrone MA., Jr. Influence of hemodynamic forces on vascular endothelial function. In vitro studies of shear stress and pinocytosis in bovine aortic cells. J Clin Invest. 1984;73:1121–9. doi: 10.1172/JCI111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li R, Jen N, Yu F, Hsiai TK. Assessing mitochondrial redox status by flow cytometric methods: vascular response to fluid shear stress. Curr Protoc Cytom. 2011;Chapter 9:37. doi: 10.1002/0471142956.cy0937s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nicholson WK, Robinson KA, Smallridge RC, Ladenson PW, Powe NR. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;16:573–82. doi: 10.1089/thy.2006.16.573. [DOI] [PubMed] [Google Scholar]
- 72.Finkelsztejn A, Brooks JB, Paschoal FM, Jr., Fragoso YD. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta-analysis of the literature. BJOG. 2011;118:790–7. doi: 10.1111/j.1471-0528.2011.02931.x. [DOI] [PubMed] [Google Scholar]
- 73.Miller L, Alley EW, Murphy WJ, Russell SW, Hunt JS. Progesterone inhibits inducible nitric oxide synthase gene expression and nitric oxide production in murine macrophages. J Leukoc Biol. 1996;59:442–50. doi: 10.1002/jlb.59.3.442. [DOI] [PubMed] [Google Scholar]
- 74.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173:2227–30. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 75.Thiagarajan KM, Arakali SR, Mealey KJ, Cardonick EH, Gaughan WJ, Davison JM, et al. Safety considerations: breastfeeding after transplant. Prog Transplant. 2013;23:137–46. doi: 10.7182/pit2013803. [DOI] [PubMed] [Google Scholar]
- 76.James E, Chai JG, Dewchand H, Macchiarulo E, Dazzi F, Simpson E. Multiparity induces priming to male-specific minor histocompatibility antigen, HY, in mice and humans. Blood. 2003;102:388–93. doi: 10.1182/blood-2002-10-3170. [DOI] [PubMed] [Google Scholar]
- 77.Piper KP, McLarnon A, Arrazi J, Horlock C, Ainsworth J, Kilby MD, et al. Functional HY-specific CD8+ T cells are found in a high proportion of women following pregnancy with a male fetus. Biol Reprod. 2007;76:96–101. doi: 10.1095/biolreprod.106.055426. [DOI] [PubMed] [Google Scholar]
- 78.Jiang SP, Vacchio MS. Multiple mechanisms of peripheral T cell tolerance to the fetal “allograft”. J Immunol. 1998;160:3086–90. [PubMed] [Google Scholar]
- 79.Taglauer ES, Yankee TM, Petroff MG. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J Reprod Immunol. 2009;80:12–21. doi: 10.1016/j.jri.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Billingham RE, Silvers WK, Wilson DB. A Second Study on the H-Y Transplantation Antigen in Mice. Proc R Soc Lond B Biol Sci. 1965;163:61–89. doi: 10.1098/rspb.1965.0060. [DOI] [PubMed] [Google Scholar]
- 81.Smith RN, Powell AE. The adoptive transfer of pregnancy-induced unresponsiveness to male skin grafts with thymus-dependent cells. J Exp Med. 1977;146:899–904. doi: 10.1084/jem.146.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonilla WV, Geuking MB, Aichele P, Ludewig B, Hengartner H, Zinkernagel RM. Microchimerism maintains deletion of the donor cell-specific CD8+ T cell repertoire. J Clin Invest. 2006;116:156–62. doi: 10.1172/JCI26565. [DOI] [PMC free article] [PubMed] [Google Scholar]