Abstract
Background: Although in vitro and in vivo experiments have suggested that mesenchymal stromal cells (MSC) may have important immunomodulatory functions in allogeneic hematopoietic cell transplantation (HCT), results from clinical studies have been inconsistent. In the current study we investigate the safety of dog leukocyte antigen (DLA) identical or third party unrelated MSC in DLA-identical HCT.
Results: There were no differences between treatment groups in depth of granulocyte or platelet nadirs, time to granulocyte or platelet engraftment, rate of acute GVHD or rejection. All dogs tolerated the MSC infusion well, although 2 dogs treated with unrelated MSC were euthanized on day 9 due to complications unrelated to the MSC infusion. While no formation of ectopic tissue was observed, GFP positive signals in bone marrow, spleen or liver were detected at time of necropsy in 75% and 50% of dogs treated with DLA-identical or unrelated MSC, respectively.
Discussion: Treatment with DLA-identical or unrelated MSC in high dose DLA-identical HCT is safe, and provides a large animal HCT model in which to investigate immunological mechanisms and optimal treatment strategies for future human trials.
Methods: Fourteen dogs were treated with 920 cGy total body irradiation (TBI) followed by transplantation of marrow from DLA-identical littermates and immunosuppression with cyclosporine. Prior to infusion of marrow, dogs received infusions of DLA-identical MSC from the marrow donor (n = 4), unrelated MSC (n = 4), or culture medium (n = 6), within 1 h of TBI. MSC obtained from relevant donors were ex-vivo expanded and transduced with GFP-retrovirus before infusion.
Keywords: allogeneic hematopoietic cell transplantation, canine transplantation model, mesenchymal stem cells, third party, green fluorescent protein transduced
Introduction
In recent years the use of mesenchymal stromal cells (MSC) as adjuvant therapy has been the focus of novel strategies to enhance engraftment and ameliorate graft vs. host disease (GVHD) after allogeneic hematopoietic cell transplantation (HCT). MSC are bone-marrow derived non-hematopoietic cells that apart from their ability to differentiate into cell lineages of mesenchymal origin,1 also are powerful modulators of mammalian immune responses. In human, canine and murine in-vitro studies, MSC have been shown to inhibit T-cell proliferation in a dose-dependent, MHC-unrestricted, contact and antigen independent fashion.2-8 Based on these properties, the use of MSC has been explored in human patients. Initial reports from small case series and a phase II study were encouraging, showing complete resolution of steroid refractory acute GVHD without side effects.9-12 However in a double blind, placebo controlled randomized study of third party unrelated MSC for patients with steroid refractory acute GVHD, post hoc subgroup analysis suggested a possible therapeutic effect of MSC on gut and liver acute GVHD, with no difference between MSC treatment and placebo with respect to the primary endpoint of durable complete response ≥ 28 d.13 Attempts to use MSC prophylactically to prevent graft failure and GVHD have mainly been unsuccessful with GVHD and engraftment rates no different from historical non-MSC treated patients.14-17 In view of the inconsistent results observed in humans there is an urgent need for a reliable animal model where the molecular mechanisms governing the immunosuppressive effects of MSC can be investigated and basic questions such as optimal MSC donor source, cell dose and timing of treatment can be answered. The random-bred model of canine HCT is well established and has been the basis for the direct translation of pre-clinical studies into human patients.18 We have previously shown that canine MSC localize to the bone marrow and have similar in vitro properties as human and murine MSC.7,8,19 In the current study we demonstrate the safety of treatment with DLA identical or unrelated third party MSC in dog leukocyte antigen (DLA) identical HCT, and propose this model as a platform for further experiments.
Results
MSC product characteristics
Two different MSC preparation protocols were used. A long one, where the MSC were transduced in first passage and harvested in second (E645 and E647) and a short one were MSC were transduced in passage 0 and harvested in passage 1. Overall there was no difference observed in number of infused MSC between the two preparation schemes, as the 2 dogs treated with MSC harvested in second passage received 7.4 and 7.5 × 106 cells/kg, while the 6 dogs treated with MSC harvested in passage 1 received a median of 10 × 106 (range 4.8–10) cells/kg (Table 1). Transduction efficiency was only assayed in dogs treated with DLA-identical MSC, and showed an approximately twice as high efficiency in MSC transduced in passage 0 as compared with passage 1 (Table 1). The viability of the expanded MSC culture was similar after both protocols. However, the pre-infusion viability of unrelated donor derived MSC (median 81.75%; range 77.4–87.6%) was significantly lower (Mann-Whitney U-test, p = 0.03) than the viability of MSC from DLA-identical donors (95%, range 92.1–96.2%). Tests for bacterial or yeast contamination were negative in all MSC products, except one given to E645, which showed growth of a gram negative rod identified as Pasteurella or Actinobacillus on day 6 post infusion. Upon discovery of growth, an 8 d course of treatment with Ceftazidime and Gentamicin was initiated, although no clinical signs of infection or bacterial growth in blood cultures were observed at any time point during the study.
Table 1. Transplant and MSC product characteristics and tissue GFP positivity in dogs conditioned with 920 cGy prior DLA-identical marrow transplantation with or without MSC infusion.
| |
|
|
MSC treatment |
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recipient ID (sex) | Donor ID | Marrow cells dose (TNC × 108/kg) |
MSC source | Infused (cells x 106) |
Viability (%) |
Viable MC dose (×106 cells/kg) |
Transduction efficiency (%) |
Transduced dose (106 cells/kg) |
GFP (+/−) |
Survival (days) |
|
| E645(F) |
E647 |
4.3 |
DLA identical (E647) |
67.7 |
96.2 |
7.4 |
18.0 |
1.35 |
+ |
43 |
|
| E647(F) |
E645 |
3.5 |
DLA identical (E645) |
68.9 |
93.8 |
7.5 |
21.4 |
1.57 |
- |
43 |
|
| E612(M) |
E613 |
3.1 |
DLA identical (E613) |
110 |
97.6 |
10 |
50.4 |
5.04 |
+ |
100 |
|
| E613(M) |
E612 |
2.0 |
DLA identical (E612) |
129 |
92.1 |
10 |
51.8 |
5.18 |
+ |
100 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
| E705(F) |
E708 |
3.1 |
Unrelated (E450) |
116 |
87.6 |
10 |
|
|
+ |
9* |
|
| E708(M) |
E705 |
3.0 |
Unrelated (E450) |
102 |
86.1 |
10 |
|
|
- |
9* |
|
| E669(F) |
E672 |
4.0 |
Unrelated (E618) |
52.6 |
76.0 |
4.8 |
|
|
- |
43 |
|
| E672(M) |
E669 |
3.1 |
Unrelated (E618) |
82.8 |
77.4 |
5.1 |
|
|
+ |
43 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
| E626(F) |
E628 |
5.3 |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
104 |
|
| E628(M) |
E626 |
4.2 |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
104 |
|
| E683(F) |
E684 |
3.0 |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
98 |
|
| E684(M) |
E683 |
1.8 |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
98 |
|
| E709(M) |
E706 |
2.0 |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
102 |
|
| E706(F) | E709 | 2.7 | NA | NA | NA | NA | NA | NA | NA | 102 | |
Dogs were conditioned with 920 cGy total body irradiation followed by bone marrow transplantation and immunosuppression with cyclosporine (20 mg/kg twice daily from day 0 through 5 and 10 mg/kg from day 6 through the end of study). Mesenchymal stromal cells (MSC) were infused within 1 h of conditioning. *Euthanized on day 9 due to pulmonary edema (E705) and intussusception (E708). Tissue was collected at time of euthanasia and presence of green fluorescent protein (GFP) was detected by GFP specific PCR in bone marrow, spleen and liver (+, if GFP signal was positive in any of the three tested tissues; -, if GFP signal negative in all tissues tested). DLA, dog leukocyte antigen; TNC, total nucleated cells; NA, not applicable.
Transplantation outcome
In the current study, a total of 14 dogs were included. Four animals received MSC prepared from DLA-identical littermates, 4 from unrelated donors and 6 were designated as controls only receiving a sham treatment with infusion medium. All dogs tolerated the infusion of MSC, sham treatment and bone marrow well, with no signs of hypersensitivity or other immediate side effects. However, two dogs from the unrelated MSC group (E708 and E705) were euthanized, prematurely, on day 9, and thus not evaluable for engraftment and GVHD. E708 developed loose bloody stools on day 6, which, despite adequate treatment, developed into a clinical picture consistent with intussusception by day 9. E705 developed respiratory distress on day 5, which progressed despite treatment with antibiotics and supportive care to terminal pulmonary edema. Blood cultures from the day of euthanasia showed growth of gram-negative rods resembling Pseudomonas.
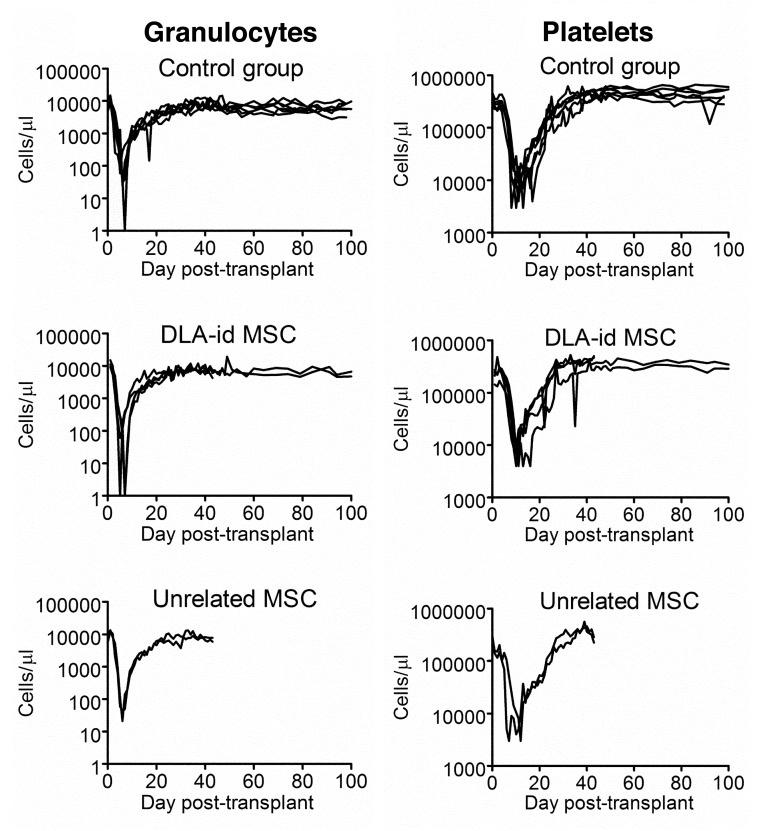
After transplantation the median time to granulocyte nadir was 6 d in all three groups with a narrow range of 5 to 8 d, while median granulocyte nadirs were 0 (range 0–60), 15 (range 0–43) and 12.5 (range 0–90) cells/μl in the groups treated with DLA-identical MSC, unrelated MSC and control, respectively (Fig. 1). Platelet nadirs were reached at a median of 10.5 (range10–11), 9 (range 7–12) and 10.5 (range 8–12) days with median nadirs of 4000 (range, 4000–8000), 3500 (range, 2000–5000) and 6000 (range, 3000–10,000) platelets/μl, in the groups treated with DLA-identical MSC, unrelated MSC and the controls, respectively (Fig. 1). Engraftment was observed in all 12 evaluable dogs. The median time to granulocyte recovery was 9 d in all 3 groups ranging between 8 to 10 d in dogs treated with DLA-identical MSC and 8 to 11 d in control dogs. The two evaluable dogs treated with unrelated MSC both recovered their granulocytes on day 9. Platelet recovery was observed after a median of 14.5 (range 13–18) days in the group treated with DLA-identical MSC and after a median of 16 (range 14–21) days in the control group. Both evaluable dogs treated with unrelated MSC recovered their platelet counts at day 15. No statistically significant differences between MSC treated groups and controls in time to engraftment of granulocytes or platelets were observed.
Figure 1. Granulocyte and platelet kinetics of individual dogs post-transplant. Infusion of mesenchymal stromal cells (MSC) or medium was given within 1 h of conditioning with 920 cGy total body irradiation. The control groups consisted of 6 dogs which received sham treatment with infusion medium only. Four dogs were treated with dog leukocyte antigen identical MSC. Of the 4 dogs treated with unrelated MSC, only two were evaluable for engraftment (E669 and E672).
Apart from E708 and E705, all dogs had clinically uneventful post-transplant recoveries without clinical evidence of GVHD. Serum chemistries revealed normal renal function in all animals as assessed by creatinine and blood urea nitrogen. There were abnormalities in liver function tests in animals in all groups. These consisted primarily of transient elevations of transaminases ranging from 1.5 to 5-fold the upper normal limit and alkaline phosphatase where all elevations were within 1.5 fold of the upper normal limit. Transient elevations of total bilirubin were observed without clinically evident jaundice.
Upon histopathological evaluation of 44 tissues obtained at necropsy from all MSC treated dogs, no ectopic tissue formation was identified. Pancreatic fibrosis, which is a recognized side effect of treatment with 920 cGy TBI20 was seen in MSC treated and control dogs. Pathological review of the remaining organs was unremarkable.
Detection of MSC in tissues
The presence of MSC in bone marrow, spleen and liver (only bone marrow and liver in E669) was investigated by GFP specific PCR on DNA extracted from paraffin-embedded tissue (Fig. 2). Apart from being tested for the presence of GFP, the integrity of the extracted DNA was also assessed by amplification of a small internal genomic control region (cMyc). As results were inconsistent with positive GFP signals appearing without amplification of cMyc, all samples were run twice for both GFP and cMyc. The stochastic amplification observed for cMyc suggested that integrity and presence of usable DNA template was extremely low. To avoid bias by low level contamination giving rise to false-positive results, results were interpreted cautiously with samples only considered positive for GFP when positive signals were observed for both first and second PCR. However, based on the complete lack of signal in the negative controls, it could be argued that tissues that are positive even only once for GFP were truly positive.
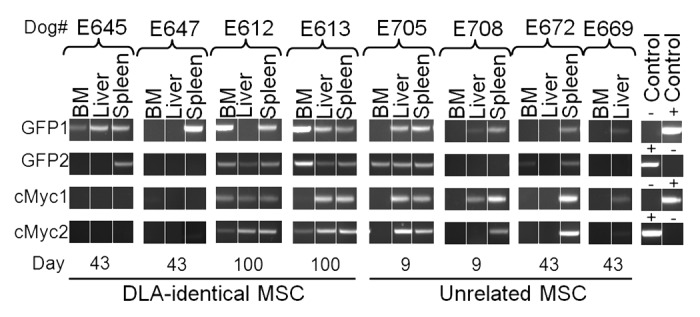
Figure 2. Detection of GFP positive MSC in bone marrow, liver and spleen from dogs treated with autologous or DLA-identical MSC. Autologous (n = 4) or dog leukocyte antigen (DLA)-identical (n = 4) mesenchymal stromal cells (MSC) were transfected with green fluorescent protein (GFP)-retrovirus, pOT-24, and infused into dogs treated with 920 cGy total body irradiation and DLA-identical bone marrow, within 1 h of conditioning. Tissue was collected at time of euthanasia (denoted by day in the figure) and presence of GFP and canine cMyc (internal control of DNA integrity) was detected by GFP and cMyc specific PCR, respectively. PCR was performed twice on each sample for both GFP (GFP1 and GFP2) and cMyc (cMyc1 and cMyc2). DNA from a canine testis fibroblast culture, transduced with a GFP-expressing lenti-virus was used as a positive control for GFP and cMyc. Water was used as a negative control. BM, bone marrow.
Using the cautious approach, 3 of 4 dogs treated with DLA-identical MSC were positive for GFP. The 2 with the longest follow-up (100 d) were positive in 3 of 3 (E613) and 2 of 3 (E612) tissues, while one with 43 d of follow-up was positive in the spleen, only. Of the dogs treated with unrelated MSC 2 of 4 were positive for GFP. E705 which was euthanized prematurely on day 9 was positive in the liver and spleen, while E672 which had 43 d of follow-up was positive in the spleen only.
Discussion
Encouraged by the immunosuppressive properties of MSC in-vitro, their effect on engraftment and efficacy in treatment and prevention of GVHD were explored in various settings of human allogeneic HCT. While the use of MSC was found to be safe with no increase in infection or relapse rates, the results for either therapy or prophylaxis of GVHD have been inconsistent with reports of both positive and neutral effects.10-14,16,17,21-24
Currently no consensus on the optimal MSC treatment regimen exists, and the ability to draw consistent conclusion from the human trials is limited by their small size and heterogeneity in MSC source, cell dose, patient cohort composition and transplantation regimen. The apparent lack of an ideal treatment paradigm can, in part, probably be attributed to the fast translation of in vitro MSC studies into clinical trials before optimal MSC treatment regimens could be investigated in relevant animal HCT models.5,25 Because the canine HCT model has been instrumental in the development and translation of HCT regimens into human trials, we chose this model to evaluate the safety and efficacy of ex-vivo expanded MSC. In the canine model we have previously shown that canine and human MSC share several characteristics, including expression of the most common MSC surface markers CD13, CD29, CD44, CD73, CD90, CD106 and CD166.8,26-28 Consistent with findings in humans and mice, canine MSC inhibit lymphocyte proliferation in a DLA-unrestricted dose-dependent fashion in mixed lymphocyte cultures.7,8,29 Experience with canine MSC in the HCT model have yielded results similar to human trials. In an acute GVHD model where dogs were transplanted with bone marrow from DLA-haploidentical littermates after 920 cGy TBI without post-transplant immunosuppression, concomitant infusion of immortalized clonally expanded canine MSC cell lines did not facilitate engraftment or prevent acute GVHD.7 In a transplant model with sub optimal conditioning with 100 cGy TBI, concomitant infusion of MSC from the DLA-identical marrow donor at the time of transplantation and after cessation of immunosuppression at day 35 post-transplant, did not prevent rejection.8
In the current study we demonstrate that concomitant transplantation of DLA-identical bone marrow and ex-vivo expanded DLA-identical or unrelated MSC is safe in a canine model of HCT. The two deaths that were observed were due to well recognized risks associated with HCT, and therefore probably not specifically linked to the MSC infusion. The MSC doses that were used were in the same range as both previous canine experiments and human trials.8,14-17 There was no ectopic tissue formation, side effects or adverse impact on rate of acute GVHD or engraftment related to treatment with MSC.
Based on the assumption that tissue GFP positivity was associated with the presence of MSC, we observed MSC in 3 of 4 dogs treated with DLA-identical MSC and in 2 of 4 treated with unrelated MSC. In line with previous biodistribution experiments, presence of MSC was observed in bone marrow, spleen or liver, with detectable GFP signal for at least 100 d in the group treated with DLA identical MSC.7,30,31 Although the presence of GFP-PCR positive tissues does imply the presence of MSC, the method does not convey information about cell frequency or whether the PCR product originates from viable cells or just DNA fragments. However, evidence exists that viable MSC are present at a low level weeks and months after treatment,7 and in a previous canine study with autologous GFP-transfected ex-vivo expanded MSC concomitantly with G-CSF mobilized autologous PBSC after 920 cGy TBI, MSC specific signals derived from both transgene DNA and RNA were observed in bone marrow at 6 mo after treatment, suggesting active transcription and thus the presence of viable cells.19
In conclusion, this is the first study that shows that co-transplantation of DLA-identical or unrelated in-vitro expanded MSC with DLA-identical bone marrow is safe and feasible. Although, the safe infusion of unrelated MSC is not a novel finding, our results establish the canine HCT model as a possible pre-clinical platform where both the immunosuppressive mechanisms of allogeneic MSC and treatment strategies can be investigated. Experiences in the randombred model of canine HCT have been directly translated into human trials since the 1960s, and with the inconsistent results that have been observed in clinical trials there is an urgent need to define the optimal paradigm for in-vivo treatment with MSC.
Material and Methods
Dogs
Fourteen dogs from litters of random-bred beagles and mini-mongrel cross-breeds, either raised at the Fred Hutchinson Cancer Research Center or purchased from commercial class A vendors licensed by the US Department of Agriculture, were enrolled in the study. The median weight and age were 11.1 (range 9.2–16.2) kg and 8 (range 7–10) months (Table 1). All dogs were enrolled in a routine preventive medicine program that included antihelminthic agents and standard immunization against Bordetella, canine parvo virus, canine papilloma virus, distemper, adeno virus type 2, leptospirosis, parainfluenza and rabies. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center. The study was performed in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources). The kennels were certified by the American Association for Accreditation of Laboratory Animal Care, International. DLA-identical littermates and random-bred unrelated MC donors were selected on the basis of identity for highly polymorphic major histocompatibility complex class I and class II microsatellite markers and identity for DLA-DRB1 alleles as determined by direct sequencing.32,33
Transplantation regimen
On day 0 dogs were conditioned with a single 920 cGy dose of total body irradiation (TBI) delivered at 7 cGy/min from 2 opposing cobalt-60 sources,34 with subsequent intravenous infusion of control media (50 ml DMEM-LG plus 30% autologous serum) (designated as control group, n = 6), MSC from DLA-identical (from the marrow donor; n = 4) or unrelated donors (n = 4). Within 1 h of MSC or control media infusion the dogs were given marrow grafts intravenously [median, 3.1 (range, 1.8–5.3) × 108 total nucleated cells/kg] from DLA-identical littermates (Table 1). Pharmacological immunosuppression consisted of cyclosporine 20 mg/kg twice daily from days 0 through 5 and 10 mg/kg twice daily from day 6 to the end of study. Supportive care after transplantation was given as previously described.35 Engraftment was assessed by the recovery of peripheral blood granulocyte and platelet counts following the irradiation nadirs and marrow cellularity at autopsy. Neutrophil engraftment was defined as the first day of 3 consecutive days with absolute neutrophil counts ≥ 500/μl and platelet engraftment was defined as the first day of 7 consecutive days with platelet counts ≥ 20,000/μl without transfusion. Dogs were planned to be euthanized at post-transplant days 43 (E647, E645, E669, E672), 100 (E613, E612, E708, E705) and 98–104 for the control group. Blood cell recovery and serum chemistries were assessed in blood samples obtained before TBI and at scheduled timepoints throughout the entire study period. Upon end of study, autopsies including histologic examinations of 44 tissues from each dog were performed to assess bone marrow cellularity, GVHD, potential toxicities and ectopic tissue formation. Samples obtained during necropsy were submitted to the Osiris Therapeutics, Inc. Histology Department, and Pathology Associates International and the Pathology Department at FHCRC where they were evaluated by a Faculty clinical pathologist. Histologic examinations were performed by light microscopy of paraffin embedded, sectioned and stained with Hematoxylin/Eosin or Periodic Acid Schiff’s stain (bone marrow).
Preparation and infusion of bone marrow derived MSC
Bone marrow for the preparation of DLA-identical MSC was obtained from appropriate donors, while bone marrows for unrelated MSC for E705 and E708 were obtained from unrelated dog E450 and for E669 and E672 from unrelated dog E618. The MSC preparation procedure has previously been published by Mosca et al.19 Briefly, 8 8 ml bone marrow aspirates were obtained from the donor’s humeri at least four weeks prior to transplantation and shipped to Osiris Therapeutics, Inc. (Baltimore, MD). Bone marrows diluted to a final concentration of 20 x 106 cells/ml in Dulbecco’s Phosphate Buffered Saline (DPBS) were subjected to density sedimentation through Percoll solution (sp Gr. 1.073 g/ml, Pharmacia Biotech, Piscataway, NJ). Cells collected at the interphase were cultured with 10–12 × 106 cells in 30 ml complete MSC canine culture medium (Dulbecco’s Modified Eagle Media-low glucose supplemented with 10% fetal bovine serum (Biocell Laboratories), 1% penicillin-streptomycin antibiotic solution (Life Technologies) and Glutamax-2 supplement (Life Technologies) in T-185 sized flasks. Cultures were incubated at 37°C at 5% CO2 with medium changes every 3 to 4 d and passage by trypsinization with subsequent replating in T80 flasks at a density of 0.4 × 108 cells in 18 ml complete medium on day 12 for E645 and E647, and on day 9 for the remaining dogs. Cultures from E645 and E647 were passaged again on day 18 and harvested on day 22, while cultures from the remaining dogs were passaged only once on day 9 and harvested on day 13.
Cultures were harvested by trypsinization and after washing with Hank’s Buffered Saline Solution (HBSS) resuspended at 107 cells/ml in cryoprotectant solution (85% Plasma-Lyte A (Baxter IV Therapy), 10% dimethyl sulfoxide and 5% autologous canine serum) and placed in a -70°C freezer or kept in the vapor phase of nitrogen until infusion. Two hours before infusion the vials with MSC where thawed in a 37°C water bath and resuspended in 50 ml Dulbecco’s moderate media-low glucose (DMEM-LG, Gibco) plus 30% autologous serum. The viability of the MSC product was determined by exclusion of trypan blue to determine the actual viable dose. MSC suspension or vehicle (control dogs) was infused over a 15–20 min period. An aliquot of each MSC product was submitted for yeast isolate, aerobic and non-aerobic growth.
The methods used to construct the GFP-retrovirus, pOT-24, and for centrifugal transduction of MSC have previously been published.19 Transduction efficiency was assessed upon cryopreservation in aliquots of 107 cells using anti-EGFP DNA PCR ELISA.19 Transductions were performed on days 15 and 17 on passage 2 cultures from E645 and E647 and on days 6 and 8 on passage 1 cultures from the remaining dogs.
DNA extraction from tissue and GFP PCR
The tissue used for DNA extraction was fixed in 10% neutral buffered formalin for at least 3 d and then paraffin processed. Bone samples were decalcified using Formical 4 (Fisher Scientific). The deparaffinization procedure was adapted from Shi et al.36 In short 100 mg of paraffin-embedded tissue was sequentially washed with 1 ml Xylene, 1ml 100% EtOH and 1ml 70% EtOH at 55°C for 20 min. for 2 changes and dried for 10 min at 55°C. DNA was extracted on silica-coated magnetic beads using a BioSprint 96 DNA Blood Kit (Qiagen, Valencia, CA, USA). Deparaffinized tissue was digested in 200 µl of ATL buffer and 20 µl of protease K (Qiagen kit) for 4 h at 55. After adding additional 200 µl of ATL buffer the samples were incubated at incubated at 100°C for 20 min, and DNA was purified according to the manufacturer’s instructions. Each DNA extract was used as template for PCR specific amplification of GFP (AAACGG CCACAAGTTCAGCGTGTC and CCTTGAAGAAGATGGTGCGCTCC) and canine cMyc (TGTCATTCTCCTCCGTGTCCGAAG and CTTAAGAGATGCCATGTGTCCACC) using Advantage II Polymerase Mix (Clontech Laboratories) under the following cycling conditions: 3 min hold at 94°C; 40 cycles of 94°C for 20 sec and 68°C for 2 min; 4 min hold at 68°C. As the aldehyde fixation of tissues used in the current study damages and only yields fragmented DNA, PCR only performs optimally with short amplicons. In the current study amplicons of 200 base pairs or less were used. DNA from a canine testis fibroblast culture, transduced with a GFP-expressing lenti-virus was used as a positive control for GFP and cMyc. About 70% of the cells were GFP-positive by fluorescence microscopy. Water was used as a negative control.
Statistical considerations
Time to engraftment was compared using the log-rank test and quantitative variables were compared using the Mann-Whitney U-test.
Disclosure of Potential Conflicts of Interest
All authors declare no existing conflicts of interest.
Acknowledgments
The authors are indebted to Osiris Therapeutics, Inc. (Baltimore, MD) for processing the MSC and providing research funding and to Michele Spector, D.V.M., the investigators who participated in the weekend treatments, and the technicians in the canine facilities. We also would like to thank Mike Harkey and Debe Higginbotham for DNA extraction/GFP PCR, Stacy Zellmer for DLA typing and Barry E. Storer for statistical help. We are grateful for the assistance of Helen Crawford, Bonnie Larson, and Sue Carbonneau for manuscript preparation.
Research funding was provided by Osiris Therapeutics, Inc. and the National Institutes of Health, Bethesda, MD grants P01HL036444, CA78902 and CA15704. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers. BK was supported by a fellowship from the Danish Cancer Society (DP08135), Anders Hasselbalchs fond til Leukæmiens Bekæmpelse and Frøken Amalie Jørgensens Mindelegat.
Authorship
BK drafted and revised the manuscript, analyzed and interpreted data. WML performed statistical calculations. EBS designed and conducted the study, drafted and revised manuscript, analyzed and interpreted data. RS designed study, revised manuscript. BMS conceived, designed, conducted and supervised the study, analyzed and interpreted data, drafted and revised manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/chimerism/article/25110
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 3.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 4.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 5.Sudres M, Norol F, Trenado A, Grégoire S, Charlotte F, Levacher B, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761–7. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 6.Sun S, Guo Z, Xiao X, Liu B, Liu X, Tang PH, et al. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells. 2003;21:527–35. doi: 10.1634/stemcells.21-5-527. [DOI] [PubMed] [Google Scholar]
- 7.Mielcarek M, Storb R, Georges GE, Golubev L, Nikitine A, Hwang B, et al. Mesenchymal stromal cells fail to prevent acute graft-versus-host disease and graft rejection after dog leukocyte antigen-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2011;17:214–25. doi: 10.1016/j.bbmt.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WS, Suzuki Y, Graves SS, Iwata M, Venkataraman GM, Mielcarek M, et al. Canine bone marrow-derived mesenchymal stromal cells suppress alloreactive lymphocyte proliferation in vitro but fail to enhance engraftment in canine bone marrow transplantation. Biol Blood Marrow Transplant. 2011;17:465–75. doi: 10.1016/j.bbmt.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 10.Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–7. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 11.Prasad VK, Lucas KG, Kleiner GI, Talano J-AM, Jacobsohn D, Szabolcs P, et al. Use of mesenchymal stem cells to treat pediatric patients with severe (grade III-IV) acute graft versus host disease refractory to steroid and other agents on a compassionate use basis [abstract] Blood. 2007;118:872A–873A, #2971. [Google Scholar]
- 12.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Developmental Committee of the European Group for Blood and Marrow Transplantation Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 13.Martin PJ, Uberti JP, Soiffer RJ, Klingemann H, Waller EK, Daly AS, et al. Prochymal improves response rates in patioents with steroid-refractory acute graft versus host disease (SR-GVHD) involving the liver and gut: results of a randomized, placebo-controlled, multicenter phase III trial in GVHD [abstract] Biol Blood Marrow Transplant. 2010;16:41. doi: 10.1016/j.bbmt.2009.12.057. [DOI] [Google Scholar]
- 14.Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–98. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J, et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733–8. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- 16.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764–7. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 17.Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008;22:593–9. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]
- 18.Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem H-P, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–54. [PubMed] [Google Scholar]
- 19.Mosca JD, Hendricks JK, Buyaner D, Davis-Sproul J, Chuang L-C, Majumdar MK, et al. Mesenchymal stem cells as vehicles for gene delivery. Clin Orthop Relat Res. 2000;379S(Suppl):S71–90. doi: 10.1097/00003086-200010001-00011. [DOI] [PubMed] [Google Scholar]
- 20.Storb R, Rudolph RH, Kolb HJ, Graham TC, Mickelson E, Erickson V, et al. Marrow grafts between DL-A-matched canine littermates. Transplantation. 1973;15:92–100. doi: 10.1097/00007890-197301000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Müller I, Kordowich S, Holzwarth C, Isensee G, Lang P, Neunhoeffer F, et al. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis. 2008;40:25–32. doi: 10.1016/j.bcmd.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Zhou H, Guo M, Bian C, Sun Z, Yang Z, Zeng Y, et al. Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol Blood Marrow Transplant. 2010;16:403–12. doi: 10.1016/j.bbmt.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Li JY, Cao K, Lu H, Hong M, Qian S, et al. Cotransplantation of HLA-identical mesenchymal stem cells and hematopoietic stem cells in Chinese patients with hematologic diseases. Int J Lab Hematol. 2010;32:256–64. doi: 10.1111/j.1751-553X.2009.01181.x. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalo-Daganzo R, Regidor C, Martín-Donaire T, Rico MA, Bautista G, Krsnik I, et al. Results of a pilot study on the use of third-party donor mesenchymal stromal cells in cord blood transplantation in adults. Cytotherapy. 2009;11:278–88. doi: 10.1080/14653240902807018. [DOI] [PubMed] [Google Scholar]
- 25.Tisato V, Naresh K, Girdlestone J, Navarrete C, Dazzi F. Mesenchymal stem cells of cord blood origin are effective at preventing but not treating graft-versus-host disease. Leukemia. 2007;21:1992–9. doi: 10.1038/sj.leu.2404847. [DOI] [PubMed] [Google Scholar]
- 26.Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M, et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006;97:744–54. doi: 10.1002/jcb.20681. [DOI] [PubMed] [Google Scholar]
- 27.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [Review] [DOI] [PubMed] [Google Scholar]
- 28.Bühring HJ, Treml S, Cerabona F, de Zwart P, Kanz L, Sobiesiak M. Phenotypic characterization of distinct human bone marrow-derived MSC subsets. Ann N Y Acad Sci. 2009;1176:124–34. doi: 10.1111/j.1749-6632.2009.04564.x. [DOI] [PubMed] [Google Scholar]
- 29.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 30.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 31.Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–7. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 33.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing. Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 34.Thomas ED, LeBlond R, Graham T, Storb R. Marrow infusions in dogs given midlethal or lethal irradiation. Radiat Res. 1970;41:113–24. doi: 10.2307/3572900. [DOI] [PubMed] [Google Scholar]
- 35.Ladiges WC, Storb R, Thomas ED. Canine models of bone marrow transplantation. Lab Anim Sci. 1990;40:11–5. [PubMed] [Google Scholar]
- 36.Shi SR, Cote RJ, Wu L, Liu C, Datar R, Shi Y, et al. DNA extraction from archival formalin-fixed, paraffin-embedded tissue sections based on the antigen retrieval principle: heating under the influence of pH. J Histochem Cytochem. 2002;50:1005–11. doi: 10.1177/002215540205000802. [DOI] [PubMed] [Google Scholar]